SUMMARY
Neural stem cells in the subventricular zone (SVZ) of the adult mammalian forebrain are a potential source of neurons for neural tissue repair after brain insults such as ischemic stroke and traumatic brain injury (TBI). Recent studies show that neurogenesis in the ventricular zone (VZ) of the adult zebrafish telencephalon has features in common with neurogenesis in the adult mammalian SVZ. Here, we established a zebrafish model to study injury-induced neurogenesis in the adult brain. We show that the adult zebrafish brain possesses a remarkable capacity for neuronal regeneration. Telencephalon injury prompted the proliferation of neuronal precursor cells (NPCs) in the VZ of the injured hemisphere, compared with in the contralateral hemisphere. The distribution of NPCs, viewed by BrdU labeling and ngn1-promoter-driven GFP, suggested that they migrated laterally and reached the injury site via the subpallium and pallium. The number of NPCs reaching the injury site significantly decreased when the fish were treated with an inhibitor of γ-secretase, a component of the Notch signaling pathway, suggesting that injury-induced neurogenesis mechanisms are at least partly conserved between fish and mammals. The injury-induced NPCs differentiated into mature neurons in the regions surrounding the injury site within a week after the injury. Most of these cells expressed T-box brain protein (Tbr1), suggesting they had adopted the normal neuronal fate in this region. These results suggest that the telencephalic VZ contributes to neural tissue recovery following telencephalic injury in the adult zebrafish, and that the adult zebrafish is a useful model for regenerative medicine.
INTRODUCTION
The adult mammalian brain retains two regions of constitutive neurogenesis: the subventricular zone (SVZ) of the lateral wall of the lateral ventricles, and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (Altman, 1969; Kaplan and Hinds, 1977; Doetsch and Alvarez-Buylla, 1996; Alvarez-Buylla and Garcia-Verdugo, 2002; Kempermann, 2002; Taupin and Gage, 2002; Garcia et al., 2004; Sun et al., 2005; Chojnacki et al., 2009; Kaneko and Sawamoto, 2009). Neural stem cells (NSCs) in the SVZ generate neural precursor cells (NPCs) that supply the olfactory bulb (OB). These SVZ-derived NPCs rapidly migrate through the rostral migratory stream (RMS) into the OB (Doetsch et al., 1999; Jankovski and Sotelo, 1996). After their long journey, most of the NPCs differentiate into interneurons in the OB. Therefore, these endogenous NSCs in the adult brain have been proposed as a potential source of neurons for neural tissue repair after various brain insults, such as ischemic stroke or traumatic brain injury (TBI) (Kernie and Parent, 2010). Previous studies have shown that the SVZ does indeed contribute to brain remodeling following these injuries (Arvidsson et al., 2002; Parent et al., 2002; Goings et al., 2004; Salman et al., 2004; Sundholm-Peters et al., 2005; Ohab et al., 2006; Yamashita et al., 2006; Thored et al., 2007; Kojima et al., 2010). The regenerative ability of the adult brain requires a series of coordinated cellular processes: NPC proliferation and migration to injury sites, neuronal differentiation, cell survival, and the integration of the new neurons into existing neural circuits. However, the regeneration efficiency of neurons in the injured mammalian brain is extremely low (Arvidsson et al., 2002).
By contrast, in non-mammalian animals, neurogenesis occurs in numerous regions of the adult brain (Chapouton et al., 2007; Kaslin et al., 2008). The telencephalic ventricular zone (VZ) is a well-defined neurogenic region in the adult brain of non-mammalian vertebrates, including reptiles, birds and fish (Alvarez-Buylla et al., 1998; Zupanc, 2001; Font et al., 2001; Doetsch and Scharff, 2001; Garcia-Verdugo et al., 2002). Recent studies show that the telencephalic VZ in the adult zebrafish brain generates NPCs that share characteristics with the NPCs in the mammalian SVZ: they migrate tangentially into the OB via an RMS-like route and differentiate into mature neurons (Grandel et al., 2006; Adolf et al., 2006; Lam et al., 2009; März et al., 2010; Ganz et al., 2010; Kishimoto et al., 2011). In contrast to mammals, the adult central nervous system (CNS) of teleost fish exhibits a high capacity for neuronal regeneration after injury (Zupanc, 2001). Thus, comparative studies in zebrafish and mammals should reveal both general and divergent properties of adult neurogenesis. The zebrafish has been successfully used for the in vivo genetic dissection of neural circuits (Asakawa et al., 2008) and for drug discovery (Taylor et al., 2010), and it provides a uniquely useful model for studying adult neurogenesis and neuronal regeneration.
Here, to investigate the cellular and molecular mechanisms underlying the strong ability of zebrafish to undergo neuronal regeneration, we developed a zebrafish model of adult telencephalic injury. Using this model, we revealed a series of regenerative processes in the injured telencephalon: the proliferation of endogenous NPCs in the telencephalic VZ, the lateral migration of NPCs from the telencephalic VZ towards the injury site, and neuronal differentiation at the injury site. Treatment of the zebrafish to inhibit Notch 1 signaling, which is required for injury-induced neurogenesis in mammals (Wang et al., 2009), impaired neurogenesis at the injury site. We also demonstrate that the adult zebrafish telencephalon can regenerate mature neurons that have a similar marker-expression profile to the preexisting neurons in the same region. This study provides a novel zebrafish disease model for studying neuronal regeneration.
RESULTS
Self repair of adult telencephalic tissue in a zebrafish model of brain injury
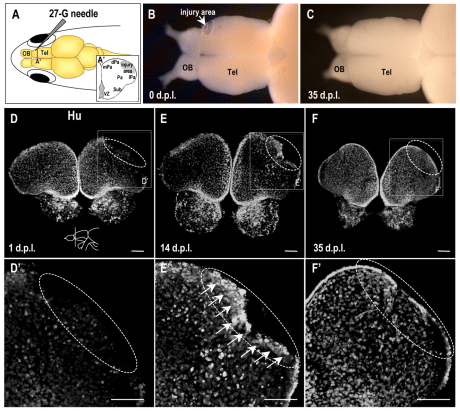
To induce TBI in the adult zebrafish, we inserted a 27-gauge needle from the side into the dorsolateral domain of one telencephalic hemisphere (Fig. 1A,A′). The size of the resultant lesion was consistent in brains dissected immediately after the surgery (n=35) (Fig. 1B). Interestingly, the injury was no longer clearly visible on the brain surface at 35 days post-lesion (dpl) in ∼80% of the animals studied (n=20, P<0.05; Fig. 1C). To examine whether neurons in the adult zebrafish brain could be replaced, we followed the expression of Hu protein, which is a specific marker for post-mitotic neurons (Mueller and Wullimann, 2002), from 1 to 35 dpl (n=5; Fig. 1D–F). At 1 dpl, neuron loss was observed at the injury site (n=5; Fig. 1D,D′). At 14 dpl, Hu-positive cells were accumulated in the region adjacent to the injury site (n=5; Fig. 1E,E′), followed by a gradual reduction in lesion size (Fig. 1D–F). The telencephalic injury showed considerable recovery at 35 dpl (n=5; Fig. 1F,F′). These results indicate that the adult zebrafish telencephalon can repair itself.
Fig. 1.
The healing process in the adult zebrafish telencephalon. (A) A zebrafish model of adult telencephalon lesions. A lesion was formed by inserting a needle into the dorsolateral domain of one hemisphere of the telencephalon. (A′) The inset is an illustration of the coronal hemisphere section obtained by a cut at the red line. Tel, telencephalon; mPa, medial pallium; dPa, dorsal pallium; Pa, pallium; lPa, lateral pallium; Sub, subpallium. (B,C) Dorsal views of an injured adult zebrafish brain at 0 days post-lesion (dpl) (B) and 35 dpl (C). Note the reduction of the lesion at 35 dpl. (D–F) Hu protein detected in coronal brain sections at the level indicated in the illustration in panel D (dorsal up) of zebrafish at 1 (D), 14 (E) and 35 (F) dpl. Higher magnifications of the boxed regions in panels D–F are shown in D′–F′, respectively. Arrows indicate accumulated Hu-positive cells at the injury site, which is circled in white. Scale bars: 100 μm.
Injury transiently stimulates cell proliferation in the telencephalic VZ and in regions adjacent to the injury site
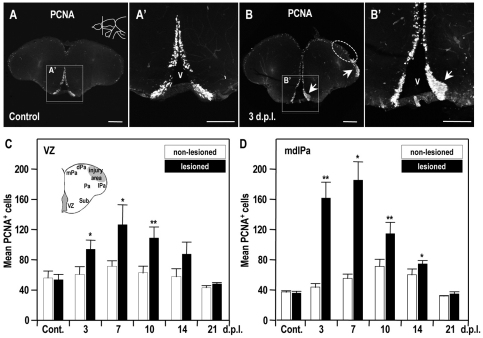
To examine the cell proliferation induced by the telencephalon injury, we observed dividing cells between 3 and 21 dpl. Dividing cells were identified by their expression of proliferating cell nuclear antigen (PCNA) (Fig. 2). In the normal adult zebrafish telencephalon, proliferating cells are located in the VZ and the medial-dorsal-lateral pallium (mdlPa) (Zupanc et al., 2005; Grandel et al., 2006; Adolf et al., 2006; Kishimoto et al., 2011) (n=5; Fig. 2A,A′). Compared with the contralateral hemisphere, the number of PCNA-positive cells in the injured hemisphere increased in both the telencephalic VZ and the regions adjacent to the injury site (mdlPa) (n=5; Fig. 2B,B′) until 7 dpl (n=5; Fig. 2C,D). From 7 dpl onwards, the number of PCNA-positive cells in these regions gradually decreased, reaching normal levels at 21 dpl (Fig. 2C,D). These data indicate that telencephalon injury in adult zebrafish transiently stimulates cell proliferation, both in the telencephalic VZ and in the regions adjacent to the injury site.
Fig. 2.
Cell proliferation induced by telencephalon injury. (A,B) Immunodetection of PCNA in coronal brain sections at the level indicated in the illustration in panel A (dorsal up): (A) control; (B) 3 dpl. Higher magnifications of panels A and B are shown in A’ and B’, respectively. Notice the injury-induced cell proliferation in the vicinity of the injury site (B) and in the telencephalic ventricular zone (B′). Arrows show the zones where cell proliferation was induced by injury. White dotted circles indicate the injury site. V, telencephalic ventricle. Scale bars: 100 μm. (C,D) Histograms showing the PCNA-positive cell counts in the telencephalic ventricular zone (C), and in the medial-dorsal-lateral domain of the telencephalic pallium (mdlPa) (D) over time. Student’s t-test was used to determine significant differences in expression. Error bars represent s.e.m. *P<0.05, **P<0.01. VZ, ventricular zone; Sub, subpallium; Pa, pallium; mPa, medial pallium; dPa, dorsal pallium; lPa, lateral pallium.
Injury activates Notch signaling in VZ cells to produce migratory NPCs
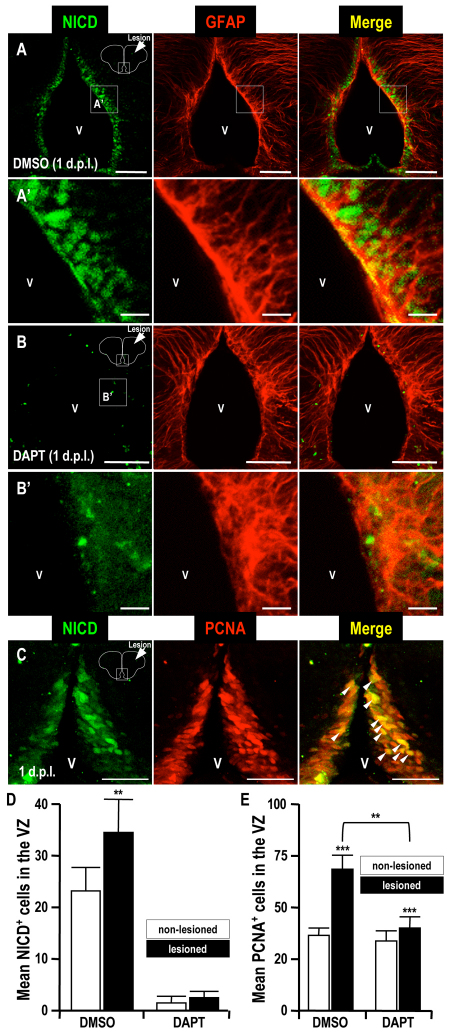
Notch 1 signaling is known to play a key role in mammalian injury-induced proliferation and neurogenesis (Givogri et al., 2006; Wang et al., 2009; Tatsumi et al., 2010). To identify brain cells in which the Notch signal was activated, we stained brain sections for the Notch intracellular domain (NICD) using an antibody against the active form of Notch (Phiel et al., 2003; Ishikura et al., 2005; Palomero et al., 2006; Yang et al., 2009). Interestingly, the number of NICD-positive cells among the PCNA-positive proliferating cells increased in the VZ of the injured hemisphere (n=5; Fig. 3A,A′,C,D). To study the role of Notch 1 signaling in the injury-induced responses in our zebrafish model, we blocked Notch 1 signaling in vivo by adding DAPT, a γ-secretase inhibitor that impairs the generation of NICD, to the aquarium water (Geling et al., 2002). The numbers of NICD- or PCNA-positive cells in the VZ were significantly decreased by the DAPT treatment, compared with the control DMSO-treated group (n=5; Fig. 3B,B′,D,E), indicating that the injury-induced Notch 1 activation in the VZ was efficiently blocked.
Fig. 3.
Notch intracellular domain immunoreactivity after telencephalic injury. (A,B) Immunodetection of the Notch intracellular domain (NICD) in coronal brain sections (dorsal up) at 1 dpl. Higher magnifications of panels A and B are shown in panels A’ and B’, respectively. (C) Immunodetection of a proliferation marker (PCNA) and the NICD in a coronal brain section (dorsal up) at 1 dpl. Arrowheads indicate NICD and PCNA double-positive cells in the telencephalic VZ. V, telencephalic ventricle. (D,E) Histograms showing the counts of NICD-positive cells (D) and PCNA-positive cells (E) in the telencephalic VZ. Notice the accumulation of NICD in proliferating cells in the telencephalic VZ induced in the injured hemisphere, compared with the uninjured one (A,C,D). This accumulation was prevented by treatment with the Notch inhibitor DAPT (B,D,E). Student’s t-test was used to determine significant differences in expression. Error bars represent s.e.m. **P<0.01, ***P<0.001. Scale bars: 50 μm (A,B,C); 10 μm (A′,B′).
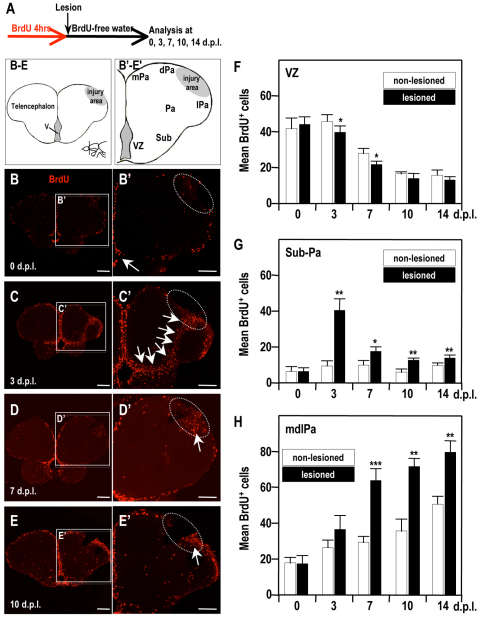
Under normal conditions, the adult zebrafish telencephalic VZ continuously supplies new neurons to the OB (Byrd and Brunjes, 2001; Grandel et al., 2006; Adolf et al., 2006; Kishimoto et al., 2011). We next performed a BrdU pulse-chase experiment to trace the migrating newly born progeny of ventricular proliferative cells from the telencephalic VZ to the injury site (Zupanc et al., 2005; Grandel et al., 2006; Adolf et al., 2006; Kishimoto et al., 2011). We followed the BrdU-labeled cells in three telencephalic regions – the VZ, the subpallium and the mdlPa (the region surrounding the injury site) – on 0, 3, 7, 10 and 14 dpl (Fig. 4A). At 0 dpl, a large number of BrdU-labeled cells was detected in the telencephalic VZ (n=5; Fig. 4B,B′,F). At 3 dpl, BrdU-labeled cells appeared in the subpallium and pallium along the pathway connecting the telencephalic VZ and the injury site in the injured hemisphere (n=5; Fig. 4C,C′,G). From 3 dpl onwards, the number of BrdU-labeled cells decreased in the telencephalic VZ, pallium and subpallium (n=5; Fig. 4D,D′,G), whereas labeled cells gradually accumulated in the region adjacent to the injury site (n=5; Fig. 4D–E′,H). Interestingly, these BrdU-labeled cells expressed PSA-NCAM, a marker for migrating young neurons, in the VZ and in the vicinity of the VZ, but not in the region adjacent to the injury site (supplementary material Fig. S1). The number of BrdU-labeled cells in the uninjured side of the VZ decreased over time, because of their relocation to the OB (Adolf et al., 2006). The number of BrdU-labeled cells in the mdlPa showed a slight increase over time, because these cells divide slowly, even in uninjured brain (Adolf et al., 2006) (data not shown). Our results suggest that progenitors generated in the telencephalic VZ relocate to the injury site through a pathway that includes the subpallium and pallium.
Fig. 4.
Distribution of BrdU-labeled cells in the injured adult zebrafish telencephalon. (A) Adult zebrafish were placed in water containing 10 mM BrdU for 4 hours, and then in BrdU-free water. After creating the lesion, they were then sacrificed at the indicated time points. (B–E′) Immunodetection of BrdU in the coronal brain sections (dorsal up) at 0 (B,B′), 3 (C,C′), 7 (D,D′) and 10 (E,E′) dpl. Higher magnifications of the boxed areas in panels BE are shown in B′–E′, respectively. Arrows show injury-induced accumulations of BrdU-positive cells. White dotted circles indicate the injury site. VZ, ventricular zone; Sub, subpallium; Pa, pallium; mPa, medial pallium; dPa, dorsal pallium; lPa, lateral pallium. Scale bars: 100 μm. (F–H) Histograms showing the counts of BrdU-positive cells in the telencephalic VZ (F), in the subpallium (Sub) and pallium (Pa) (G), and in the medial-dorsal-lateral domain of the telencephalic pallium (mdlPa) (H). Student’s t-test was used to determine significant differences in expression. Error bars represent s.e.m. *P<0.05, **P<0.01, ***P<0.001.
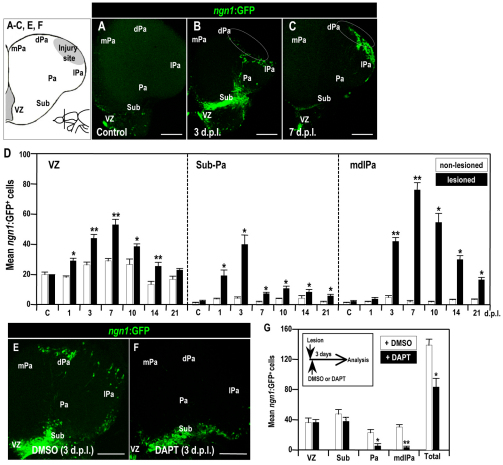
To further confirm the contribution of telencephalic NPCs to neural tissue repair in the injured hemisphere, we used a transgenic zebrafish expressing ngn1:gfp, a marker for young migrating NPCs, and monitored the number of GFP-containing cells in the telencephalic VZ (Blader et al., 2003; Adolf et al., 2006; Kishimoto et al., 2011) (Fig. 5A). The number of ngn1:gfp-expressing cells in the telencephalic VZ progressively increased until 7 dpl (n=5; Fig. 5D). Interestingly, up until 3 dpl, these ngn1:gfp-expressing cells were present in the pathway leading from the telencephalic VZ through the subpallium to the regions adjacent to the injury site (Fig. 5B,D), the same pathway that was identified in our BrdU pulse-chase experiment (Fig. 4C,C′). From 3 dpl onwards, however, these cells diminished in the pathway and appeared in the region adjacent to the injury site (Fig. 5C,D). Notably, ngn1:gfp expression was reduced in the region surrounding the lesion from 21 dpl onwards (Fig. 5D). The number of ngn1:gfp-expressing cells in the region surrounding the injury site (mdlPa) was significantly decreased by DAPT treatment, compared with the control-treated group (n=5; Fig. 5E–G). These results suggest that Notch 1 signaling in the VZ is involved in the production of NPCs that migrate to the injured site.
Fig. 5.
Distribution of neuronal precursor cells in the injured adult zebrafish telencephalon. (A–C,E,F) Distribution of GFP-positive cells in the lesioned brain of adult Tg(ngn1:gfp) fish (coronal views, dorsal up). (A) Control; (B) 3 dpl; (C) 7 dpl; (E) 3 dpl treated with DMSO; (F) 3 dpl treated with DAPT. White dotted circles indicate the injury site. VZ, ventricular zone; Sub, subpallium; Pa, pallium; mPa, medial pallium; dPa, dorsal pallium; lPa, lateral pallium. Scale bars: 100 μm. (D,G) Histograms showing the GFP-positive cell counts in the VZ, in the subpallium (Sub) and pallium (Pa), and in the medial-dorsal-lateral domain of the telencephalic pallium (mdlPa) in the injured brains of adult Tg(ngn1:gfp) fish, with (D) no treatment, and (G) DMSO or DAPT treatment. Student’s t-test was used to determine significant differences in expression. Error bars represent s.e.m. *P<0.05, **P<0.01.
Taken together, these results suggest that the telencephalic injury activates Notch signaling in proliferating cells in the VZ, and the resulting NPCs can migrate through the pallial tissues, towards the region adjacent to the injury site.
Neuronal regeneration at the injury site
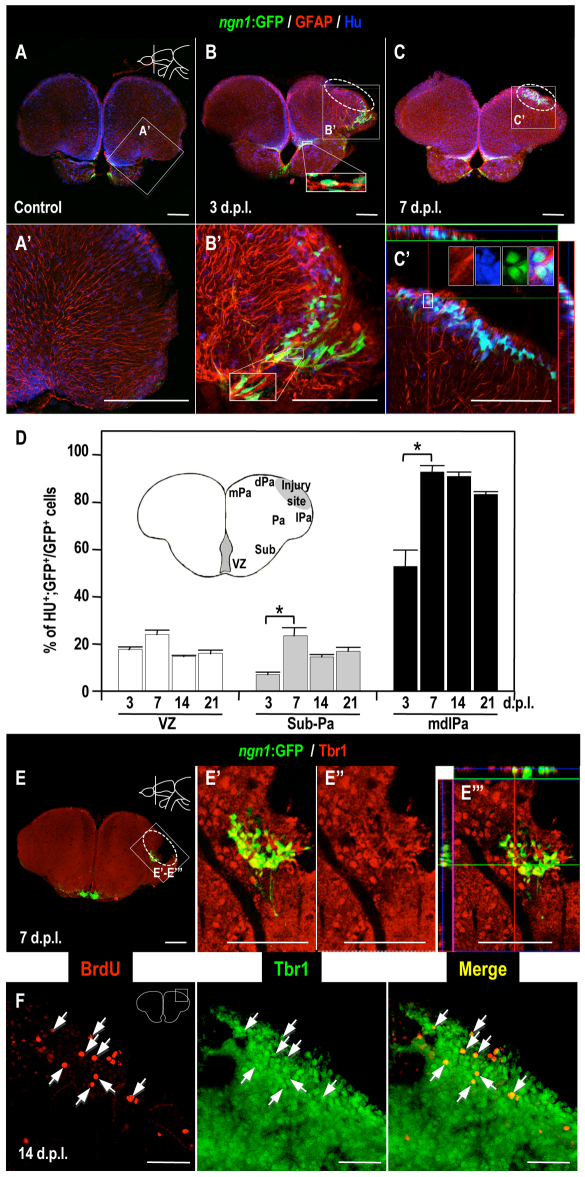
Finally, we studied the neuronal differentiation of NPCs in the region surrounding the lesion. We compared the morphology of ngn1:gfp-expressing cells in the migratory pathway with those in the region surrounding the injury site (Fig. 6). The cells that were closely apposed to radial glial fibers in the migratory pathway had an elongated shape (Fig. 6B,B′). By contrast, the cells in the injured region were round (Fig. 6C,C′), suggesting that they had stopped migrating to differentiate. To monitor the neuronal differentiation of these NPCs, we analyzed the expression of the neuronal marker Hu in the ngn1:gfp-expressing cells. The majority of ngn1:gfp-expressing cells in the telencephalic VZ and in the migratory pathway were negative for Hu (n=5; Fig. 6B,B′, D). By contrast, most ngn1:gfp-expressing cells at the injury site prominently expressed Hu protein (Fig. 6C–D).
Fig. 6.
Neuronal precursor cells differentiate into neurons adjacent to the injury site. (A–C) Immunodetection of Hu (blue), GFAP (red) and GFP (green) in coronal brain sections of the lesioned adult Tg(ngn1:gfp) fish brain (dorsal up): (A) control; (B) 3 dpl; (C) 7 dpl. Higher magnifications of the boxed areas in panels A–C are shown in A′–C′, respectively. Insets in B, B′ and C′ show high-magnification views of the boxed areas in B, B′, and C′, respectively. (D) The total count of GFP-positive cells, and the percentage that was also positive for Hu, in the telencephalic VZ, subpallium (Sub) and pallium (Pa), and the medial-dorsal-lateral domain of the telencephalic pallium (mdlPa). Student’s t-test was used to determine significant differences in expression. Error bars represent s.e.m. *P<0.05. (E) Immunodetection of Tbr1 (red) and GFP (green) in coronal brain sections (dorsal up) of injured adult Tg(ngn1:gfp) zebrafish at 7 dpl. Higher magnifications of the boxed areas in panel E are shown in panels E′–E″′. The ngn1:gfp-positive cells expressed Tbr1 protein. (F) Immunodetection of BrdU (red) and Tbr1 (green) in coronal brain sections (dorsal up) of the injured adult wild-type fish at 14 dpl. White arrows indicate BrdU and Tbr1 double-positive cells. BrdU-labeling protocol: see Fig. 4A. White dotted circles indicate the injury site. Scale bars: 100 μm (A–C,E); 50 μm (F).
We also examined ngn1:gfp-expressing cells in the region surrounding the injury site for the expression of Tbr1 protein (Fig. 6E–E″′) and tyrosine hydroxylase (TH). Tbr1 is a transcription factor that is expressed at high levels in postmitotic glutamatergic cortical neurons (Englund et al., 2005), and was abundant in the pallium and mdlPa (supplementary material Fig. S2). TH is a marker for dopaminergic neurons, including a subset of OB periglomerular neurons (Kosaka et al., 1995). We observed that the telencephalic injury stimulated apoptosis in the region adjacent to the injury site (data not shown). Tbr1-positive cells were destroyed by the telencephalic injury, but their numbers recovered within 1 month (supplementary material Fig. S2). We also observed BrdU+Tbr1+ and BrdU+Hu+ cells at the injury site, suggesting that newly born cells had differentiated into mature neurons (Fig. 6F; supplementary material Fig. S3). The ngn1:gfp-expressing cells in the region surrounding the injury site were positive for Tbr1 (Fig. 6E–E″′) and negative for TH (data not shown), suggesting that they differentiated into the neuronal subtype lost by the lesion. Taken together, these data indicate that the migrating NPCs are immature, and that they differentiate into mature neurons after reaching the region adjacent to the injury site.
DISCUSSION
The adult mammalian brain harbors NSCs, which are a potential source of neurons for repairing injured brain tissue. These endogenous NSCs are located in the SVZ and the SGZ, and are stimulated by various insults such as ischemic stroke and TBI (Kernie and Parent, 2010) to contribute to neuronal repair. However, the adult mammalian brain has a low ability to regenerate, making it difficult to fully recover the lost neurons and their functions. The cellular and molecular mechanisms of neuronal regeneration in the injured brain remain unclear. Here, we show the cellular processes of neuronal regeneration in a novel zebrafish model of adult brain injury.
Teleost fish have an enormous potential for neurogenesis and neuronal regeneration after brain injury (Kirsche, 1965; Zupanc and Zupanc, 2006; Ito et al., 2010), including injuries to the cerebellum (Zupanc et al., 1998; Zupanc and Ott, 1999; Zupanc and Zupanc, 2006; Zupanc, 2001; Zupanc, 2006; Zupanc et al., 2006; Zupanc, 2008) and to the telencephalon striatum (Ayari et al., 2010). For the past 20 years, the zebrafish, which is a teleost fish, has served as an excellent model for examining molecular and cellular mechanisms of the CNS. Recent studies have revealed some conserved features between neurogenesis in the adult zebrafish telencephalic VZ and mammalian adult neurogenesis, for which the zebrafish has become a new model (Grandel et al., 2006; Adolf et al., 2006; März et al., 2010; Ganz et al., 2010; Kishimoto et al., 2011). A recent study also suggests that zebrafish can regenerate neurons after telencephalic striatum injuries (Ayari et al., 2010). However, the potential of zebrafish VZ NPCs to regenerate neurons after adult telencephalon cortical injury has not been demonstrated. In this study, we used a stab wound to mimic the cellular phenomena of adult TBI, which is usually caused by an impact to the head that results in a mechanical insult to the brain. Unlike models for neuron-specific degenerative diseases, the stab wound affects not only mature neurons, but also on other surrounding structures, such as the meninges, roof plate, blood vessels, and radial glial cells in the dorsolateral domain of the adult zebrafish telencephalon. Using this model of adult brain injury, we showed that telencephalic injury induces coordinated cellular processes that underlie neuronal regeneration: the upregulated proliferation of NPCs in the telencephalic VZ and the differentiation of NPCs into mature neurons at the injury site. In contrast to the limited regenerative responses in mammalian brains, the adult zebrafish brain appeared fully repaired within a month after the lesion, and its regenerative processes were even able to recover lost Tbr1-positive neurons.
Previous studies have shown that brain injuries, such as experimental stroke or TBI, stimulate cell proliferation and neurogenesis in the adult rodent SVZ (Arvidsson et al., 2002; Jin et al., 2001; Parent et al., 2002; Zhang et al., 2001; Itoh et al., 2010). Similarly, we observed injury-induced cell proliferation and neurogenesis in the telencephalic VZ and the regions surrounding the injury site of the adult zebrafish brain. Our results suggest that injury-induced NPCs in the telencephalic VZ migrate to the injury site, and then express neuronal markers. In addition, we found that PSA-NCAM, a marker for migrating young neurons, is expressed by newborn cells in the VZ and in the vicinity of the VZ, but not by those in the regions adjacent to the injury site (supplementary material Fig. S1), supporting our idea that the telencephalic VZ cells are a potential source of neurons for repair after brain injury. We also observed that BrdU-positive cells expressed Hu protein in the injury site at 21 dpl (supplementary material Fig. S3), indicating that the proliferating neuronal NPCs could differentiate into mature neurons. However, it is unlikely that all of the newborn cells that reach the injury site differentiate into neurons. Indeed, a subpopulation of BrdU-positive cells at the injury site were negative for ngn1:GFP (data not shown), suggesting that they represent non-neuronal cell lineages, including blood cells. Furthermore, we have not ruled out the possibility that cells born near the injury site contribute to the replacement of neurons lost in the injury. It is also possible that simple tissue rearrangement and inflammation at the injury site play roles in the healing process. In addition, we cannot rule out the possibility that the distribution of ngn1:GFP-positive cells from the VZ to the injury site reflects the timing of ngn1 expression.
Previous studies have demonstrated that ischemia-induced cell proliferation in the mammalian SVZ is blocked by inhibiting the Notch signaling pathway, and that the level of NICD increases in the SVZ after this type of injury (Givogri et al., 2005; Wang et al., 2009). We observed similar characteristics in our zebrafish model of adult brain injury. These findings indicate that Notch 1 signaling might play a crucial role in injury-induced neurogenesis. In adult mice, Notch 1 is expressed in migratory NPCs within the SVZ and the RMS, as well as in SVZ astrocytes (Givogri et al., 2006; Wang et al., 2009). We demonstrated that inhibiting the Notch 1 signaling pathway with DAPT prevented the injury-induced proliferation of NPCs. By contrast, two recent studies have shown Notch to have the opposite effect on the mdlPa cells in the adult zebrafish telencephalon (Chapouton et al., 2010; Rothenaigner et al., 2011). These distinct outcomes of Notch signaling might be owing to differential Notch functioning between the normal and injured brain and/or between the VZ and the mdlPa. In addition, our results cannot rule out the possibility that Notch signaling plays crucial roles in later cellular events, such as the neuronal migration and differentiation. Interestingly, NPCs were observed in the VZ and the subpallium of DAPT-treated fish, but not in the regions surrounding the injury site. It is possible that the migrating NPCs could not maintain their undifferentiated state in the DAPT-treated fish brains because Notch 1 signaling plays a role in keeping NPCs undifferentiated during their exit from the mammalian SVZ (Givogri et al., 2006).
Previous studies have shown that radial glial fibers play an important role in guiding migration, both in the migration of young cells from the proliferation zones to injury sites in another cerebellum lesion teleost model (Clint and Zupanc, 2001), and in various neuronal migration processes occurring during neural development and regeneration in mammals. In our model of adult telencephalic injury, we determined the migratory pathway followed by telencephalic VZ-derived progeny migrating to the injury site via the subpallium and pallium (Fig. 4C, Fig. 5B). Radial glial fibers extend from the telencephalic VZ to the cortical regions (i.e. the medial, dorsal and lateral pallium) (März et al., 2010; Ganz et al., 2010), and this orientation parallels the route of the migrating NPCs (Fig. 4C, Fig. 5B). Indeed, NPCs were closely apposed to radial glial fibers in the injured brain (Fig. 6B,B′), suggesting that the NPCs use radial glial fibers as a scaffold for their radial migration towards the injury site. In addition, radial glial cells can divide to produce neurons and, upon injury in adult teleost fish, they increase their generation of young neurons (Zupanc and Ott, 1999; Zupanc and Zupanc, 2006). By producing new neurons and guiding them to the lesion site, radial glial cells might be responsible for the relatively strong ability of the adult fish brain to regenerate neural tissues. By contrast, in mammals, radial glial cells transform into multipolar astrocytes in the adult brain, and no longer exist as radially oriented cells (Voigt, 1989), consistent with the idea that neuronal regeneration depends on the maintenance of radial glial cells.
What cues guide the directional migration of NPCs towards the injury site? Angiopoietin 1 (Ang1) and stromal-derived factor 1 (Sdf1) regulate the migration of SVZ NPCs in mice after stroke (Ohab et al., 2006), although the expression of orthologs of these factors in the adult zebrafish brain has not been reported. Prokineticin 2 (PROK2) is a chemoattractant that guides the migration of SVZ-derived NPCs towards the OB in mammals (Ng et al., 2005; Prosser et al., 2007), and it has neurotrophic activity (Melchiorri et al., 2001). Telencephalon injury induces the ectopic expression of PROK2 from the subpallium to the injury site from 3 to 7 dpl (Ayari et al., 2010). This is quite similar to the distribution pattern of both the BrdU-labeled (Fig. 4C) and the ngn1:gfp-expressing (Fig. 5B) cells, and to the timing of their appearance after telencephalic injury, suggesting that PROK2 is involved in NPC migration towards the injury site.
In conclusion, we have developed a novel zebrafish model of adult brain injury. The sophisticated live-imaging and genetic techniques available in this model animal should help make it a powerful tool for studying the cellular and genetic basis of injury-induced adult neurogenesis. In addition, our pharmacological studies suggest that this model is applicable to in vivo screening for drugs that promote injury-induced adult neurogenesis, which might be used to treat TBI and other brain lesions.
METHODS
Fish strains
All experiments were performed on adult (5- to 10-month-old) zebrafish (Danio rerio). Zebrafish were maintained by standard procedures (Westerfield, 2000). Wild-type AB zebrafish were obtained from the Zebrafish International Resource Center (ZIRC). The Tg(−8.4ngn1:gfp) (Blader et al., 2003) strain was provided by Uwe Strähle.
Telencephalon injury
Adult zebrafish were anesthetized in Tricaine. A sterile 27-gauge needle was inserted into the dorsolateral domain of the telencephalic hemisphere to create a less than 0.1-mm-deep stab wound in the telencephalon (Fig. 1A). Immediately after creating the lesion, the animals were put into fresh water to recover.
BrdU labeling
To label newborn cells in the adult zebrafish brain, adult animals were placed in water containing 10 mM 5-bromo-2′-deoxyuridine (BrdU) for 4 hours, and then were released into fresh water. The fish were sacrificed at various time points after treatment, as indicated in the figures.
TRANSLATIONAL IMPACT.
Clinical issue
Traumatic brain injury (TBI) is the most common type of brain injury in humans. Owing to the temporary or permanent disruption of normal brain functions, individuals affected by TBI frequently suffer from neurological symptoms such as limb paralysis; loss of vision, hearing and long-term memory; seizures; and nausea. Although rehabilitation therapy is considered important for TBI, it is anticipated that regenerative therapy will soon be available as a promising treatment for these patients. It has been shown in animal models that endogenous neural stem cells that persist in the subventricular zone are stimulated by TBI in adults, suggesting that these adult stem cells might have therapeutic potential. However, the cellular and molecular mechanisms that could be exploited therapeutically to recover neural tissue function following TBI remain to be elucidated.
Results
In this study, the authors establish a zebrafish model for an adult telencephalon injury that is similar to TBI in humans. The authors use a transgenic zebrafish line that enables visualization of neuronal precursor cells (NPCs) to show that lesions in the adult zebrafish telencephalon stimulate the proliferation of NPCs in the telencephalic ventricular zone (VZ). These telencephalic NPCs seem to migrate laterally from the VZ towards the injury site, where they differentiate into mature neurons. The pharmacological inhibition of Notch 1 signaling prevents injury-induced neurogenesis, suggesting that Notch 1 signaling is required for neuronal repair. These results demonstrate that the telencephalic VZ contributes to neural tissue recovery.
Implications and future directions
These data show that the adult zebrafish telencephalon has a remarkable ability to regenerate neurons. This zebrafish model for adult TBI can be used to further elucidate the cellular and molecular mechanisms underlying the injury-induced proliferation of NPCs, their migration from the VZ to the injury site, their differentiation into neurons and their integration into existing neural circuits following TBI. Adult zebrafish with fluorescently labeled NPCs will also be useful for screening new drugs and small molecules to treat TBI, including those that stimulate neuronal regeneration.
DAPT treatment
To block Notch signaling, the animals were placed in water containing DAPT {N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester} (Nacalai Tesque) (Geling et al., 2002) at a final concentration of 10 μM. Control animals were treated with DMSO.
Immunohistochemistry and microscopy
The fish were anesthetized with Tricaine. The brains were dissected and fixed in 4% paraformaldehyde at 4°C overnight. Immunostaining was performed on 50-μm vibratome sections. To prepare the sections, whole brains were embedded in 3% agarose in PBS and cut serially using a vibrating microtome (Leica VT-1200S). The sections were blocked for 1 hour at room temperature with 0.3% Triton X-100 and 10% normal donkey serum in PBS; they were then incubated overnight at 4°C with primary antibodies diluted in the blocking buffer. For BrdU immunodetection, the sections were pre-treated with 2 M HCl at 37°C for 30 minutes prior to incubating with primary antibodies. The primary antibodies were rabbit anti-GFAP (1:500; Dako), mouse anti-GFAP (zrf1, 1:200; ZIRC), mouse anti-PCNA (PC-10, 1:500; Dako), rat anti-BrdU (1:300; Abcam), rabbit anti-GFP (1:500; MBL), mouse anti-HuC/D (1:400; Invitrogen), rabbit anti-Tbr1 (1:300; Abcam), mouse anti-TH (LNC1, 1:500; Millipore), rabbit anti-cleaved Notch 1 (1:50; Cell Signaling), mouse anti-PSA-NCAM (1:1000; a kind gift from Tatsunori Seki, Tokyo Medical University, Japan) and rabbit anti-ssDNA (1:200; IBL). Secondary antibodies were from the Alexa Fluor series (Alexa Fluor 488, 568, 633; 1:500; Invitrogen). Sections were mounted on glass slides in PermaFluor (Thermo Scientific).
For laser-scanning confocal microscopy, we used an LSM5 PASCAL microscope (Zeiss). Three vibratome sections per animal were photographed as stacks of ten optical sections using the Zeiss LSM Image Examiner software (n=5 animals). The cells in each optical section were counted manually, using images generated by the same software. Images of the whole zebrafish brain were captured using a Leica microscope (MZ 16FA) with a connected camera (DFC300FX; Leica Microsystems) and associated computer software (Leica Application Suite V3). Pictures were acquired in TIFF format with analysis software, and were processed with Adobe Photoshop.
Supplementary Material
Acknowledgments
We are grateful to Uwe Strähle for providing the Tg(ngn1:gfp) fish, Tatsunori Seki for providing the anti-PSA-NCAM antibody, and to members of the Sawamoto laboratory for useful discussions.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
N.K. and K. Sawamoto designed the experiments and wrote the paper. N.K. and K. Shimizu performed the experiments and analyzed the data.
FUNDING
This work was supported by the Funding Program for Next Generation World-Leading Researchers [LS104] (to K. Sawamoto); a Grant-in-Aid for Young Scientists (S) [21670003] (to K. Sawamoto); a Grant-in-Aid for Scientific Research on Priority Areas-Molecular Brain Science-from the Ministry of Education, Culture, Sports, Science and Technology of Japan [20022035] (to K. Sawamoto); by the Uehara Memorial Foundation (to K. Sawamoto); by The International Human Frontier Science Program Organization (to K. Sawamoto); by Global COE Programs at Keio University (to K. Sawamoto, N.K.); and by a Grant-in-Aid for Scientific Research (C) [20509005] (to N.K.).
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.007336/-/DC1
REFERENCES
- Adolf B., Chapouton P., Lam C. S., Topp S., Tannhauser B., Strahle U., Gotz M., Bally-Cuif L. (2006). Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–293 [DOI] [PubMed] [Google Scholar]
- Altman J. (1969). Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 137, 433–457 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Garcia-Verdugo J. M. (2002). Neurogenesis in adult subventricular zone. J. Neurosci. 22, 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Garcia-Verdugo J. M., Tramontin A. D. (1998). Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J. Neurosci. 18, 1020–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970 [DOI] [PubMed] [Google Scholar]
- Asakawa K., Suster M. L., Mizusawa K., Nagayoshi S., Kotani T., Urasaki A., Kishimoto Y., Hibi M., Kawakami K. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 105, 1255–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayari B., El Hachimi K. H., Yanicostas C., Landoulsi A., Soussi-Yanicostas N. (2010). Prokineticin 2 expression is associated with neural repair of injured adult zebrafish telencephalon. J. Neurotrauma 27, 959–972 [DOI] [PubMed] [Google Scholar]
- Blader P., Plessy C., Strahle U. (2003). Multiple regulatory elements with spatially and temporally distinct activities control neurogenin1 expression in primary neurons of the zebrafish embryo. Mech. Dev. 120, 211–218 [DOI] [PubMed] [Google Scholar]
- Byrd C. A., Brunjes P. C. (2001). Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience 105, 793–801 [DOI] [PubMed] [Google Scholar]
- Chapouton P., Jagasia R., Bally-Cuif L. (2007). Adult neurogenesis in non-mammalian vertebrates. BioEssays 29, 745–757 [DOI] [PubMed] [Google Scholar]
- Chapouton P., Skupien P., Hesl B., Coolen M., Moore J. C., Madelaine R., Kremmer E., Faus-Kessler T., Blader P., Lawson N. D., et al. (2010). Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J. Neurosci. 30, 7961–7974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki A. K., Mak G. K., Weiss S. (2009). Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat. Rev. Neurosci. 10, 153–163 [DOI] [PubMed] [Google Scholar]
- Clint S. C., Zupanc G. K. (2001). Neuronal regeneration in the cerebellum of adult teleost fish, Apteronotus leptorhynchus: guidance of migrating young cells by radial glia. Brain Res. Dev. Brain Res. 130, 15–23 [DOI] [PubMed] [Google Scholar]
- Doetsch F., Alvarez-Buylla A. (1996). Network of tangential pathways for neuronal migration in adult mammalian brain. Proc. Natl. Acad. Sci. USA 93, 14895–14900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Scharff C. (2001). Challenges for brain repair: Insight from adult neurogenesis in birds and mammals. Brain Behav. Evol. 58, 306–322 [DOI] [PubMed] [Google Scholar]
- Doetsch F., Caille I., Lim D. A., Garcia-Verdugo J. M., Alvarez-Buylla A. (1999). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 [DOI] [PubMed] [Google Scholar]
- Englund C., Fink A., Lau C., Pham D., Daza R. A., Bulfone A., Kowalczyk T., Hevner R. F. (2005). Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 25, 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font E., Desfilis E., Perez-Canellas M. M., Garcia-Verdugo J. M. (2001). Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav. Evol. 58, 276–295 [DOI] [PubMed] [Google Scholar]
- Ganz J., Kaslin J., Hochmann S., Freudenreich D., Brand M. (2010). Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia 58, 1345–1363 [DOI] [PubMed] [Google Scholar]
- Garcia A. D., Doan N. B., Imura T., Bush T. G., Sofroniew M. V. (2004). GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 7, 1233–1241 [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo J. M., Ferron S., Flames N., Collado L., Desfilis E., Font E. (2002). The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res. Bull. 57, 765–775 [DOI] [PubMed] [Google Scholar]
- Geling A., Steiner H., Willem M., Bally-Cuif L., Haass C. (2002). A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 3, 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givogri M. I., de Planell M., Galbiati F., Superchi D., Gritti A., Vescovi A., de Vellis J., Bongarzone E. R. (2006). Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev. Neurosci. 28, 81–91 [DOI] [PubMed] [Google Scholar]
- Goings G. E., Sahni V., Szele F. G. (2004). Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 996, 213–226 [DOI] [PubMed] [Google Scholar]
- Grandel H., Kaslin J., Ganz J., Wenzel I., Brand M. (2006). Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev. Biol. 295, 263–277 [DOI] [PubMed] [Google Scholar]
- Ishikura N., Clever J. L., Bouzamondo-Bernstein E., Samayoa E., Prusiner S. B., Huang E. J., DeArmond S. J. (2005). Notch-1 activation and dendritic atrophy in prion disease. Proc. Natl. Acad. Sci. USA 102, 886–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tanaka H., Okamoto H., Ohshima T. (2010). Characterization of neural stem cells and their progeny in the adult zebrafish optic tectum. Dev. Biol. 342, 26–38 [DOI] [PubMed] [Google Scholar]
- Itoh T., Imano M., Nishida S., Tsubaki M., Hashimoto S., Ito A., Satou T. (2010). Exercise increases neural stem cell proliferation surrounding the area of damage following rat traumatic brain injury. J. Neural. Transm. 118, 193–202 [DOI] [PubMed] [Google Scholar]
- Jankovski A., Sotelo C. (1996). Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J. Comp. Neurol. 371, 376–396 [DOI] [PubMed] [Google Scholar]
- Jin K., Minami M., Lan J. Q., Mao X. O., Batteur S., Simon R. P., Greenberg D. A. (2001). Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 98, 4710–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N., Sawamoto K. (2009). Adult neurogenesis and its alteration under pathological conditions. Neurosci. Res. 63, 155–164 [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Hinds J. W. (1977). Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 197, 1092–1094 [DOI] [PubMed] [Google Scholar]
- Kaslin J., Ganz J., Brand M. (2008). Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 101–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. (2002). Neuronal stem cells and adult neurogenesis. Ernst Schering Res. Found. Workshop 35, 17–28 [DOI] [PubMed] [Google Scholar]
- Kernie S. G., Parent J. M. (2010). Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol. Dis. 37, 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsche W. (1965). Regenerative processes in the brain and spinal cord. Ergeb. Anat. Entwicklungsgesch. 38,143–194 [PubMed] [Google Scholar]
- Kishimoto N., Alfaro-Cervelloc C., Shimizu K., Asakawa K., Urasaki A., Nonaka S., Kawakami K., Garcia-Verdugo J. M., Sawamoto K. (2011). Migration of neuronal precursors from the telencephalic ventricular zone into the olfactory bulb in adult zebrafish. J. Comp. Neurol. 519, 3549–3565 [DOI] [PubMed] [Google Scholar]
- Kojima T., Hirota Y., Ema M., Takahashi S., Miyoshi I., Okano H., Sawamoto K. (2010). Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 28, 545–554 [DOI] [PubMed] [Google Scholar]
- Kosaka K., Aika Y., Toida K., Heizmann C. W., Hunziker W., Jacobowitz D. M., Nagatsu I., Streit P., Visser T. J., Kosaka T. (1995). Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb. Neurosci. Res. 23, 73–88 [PubMed] [Google Scholar]
- Lam C. S., Marz M., Strahle U. (2009). gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain. Dev. Dyn. 238, 475–486 [DOI] [PubMed] [Google Scholar]
- März M., Chapouton P., Diotel N., Vaillant C., Hesl B., Takamiya M., Lam C. S., Kah O., Bally-Cuif L., Strahle U. (2010). Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 58, 870–888 [DOI] [PubMed] [Google Scholar]
- Melchiorri D., Bruno V., Besong G., Ngomba R. T., Cuomo L., De Blasi A., Copani A., Moschella C., Storto M., Nicoletti F., et al. (2001). The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways. Eur. J. Neurosci. 13, 1694–1702 [DOI] [PubMed] [Google Scholar]
- Mueller T., Wullimann M. F. (2002). BrdU-, neuroD (nrd)- and Hu-studies reveal unusual non-ventricular neurogenesis in the postembryonic zebrafish forebrain. Mech. Dev. 117, 123–135 [DOI] [PubMed] [Google Scholar]
- Ng K. L., Li J. D., Cheng M. Y., Leslie F. M., Lee A. G., Zhou Q. Y. (2005). Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science 308, 1923–1927 [DOI] [PubMed] [Google Scholar]
- Ohab J. J., Fleming S., Blesch A., Carmichael S. T. (2006). A neurovascular niche for neurogenesis after stroke. J. Neurosci. 26, 13007–13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero T., Barnes K. C., Real P. J., Glade Bender J. L., Sulis M. L., Murty V. V., Colovai A. I., Balbin M., Ferrando A. A. (2006). CUTLL1, a novel human T-cell lymphoma cell line with t(7;9) rearrangement, aberrant NOTCH1 activation and high sensitivity to gamma-secretase inhibitors. Leukemia 20, 1279–1287 [DOI] [PubMed] [Google Scholar]
- Parent J. M., Vexler Z. S., Gong C., Derugin N., Ferriero D. M. (2002). Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 52, 802–813 [DOI] [PubMed] [Google Scholar]
- Phiel C. J., Wilson C. A., Lee V. M., Klein P. S. (2003). GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 423, 435–439 [DOI] [PubMed] [Google Scholar]
- Prosser H. M., Bradley A., Chesham J. E., Ebling F. J., Hastings M. H., Maywood E. S. (2007). Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc. Natl. Acad. Sci. USA 104, 648–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenaigner I., Krecsmarik M., Hayes J. A., Bahn B., Lepier A., Fortin G., Gotz M., Jagasia R., Bally-Cuif L. (2011). Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development 138, 1459–1469 [DOI] [PubMed] [Google Scholar]
- Salman H., Ghosh P., Kernie S. G. (2004). Subventricular zone neural stem cells remodel the brain following traumatic injury in adult mice. J. Neurotrauma 21, 283–292 [DOI] [PubMed] [Google Scholar]
- Sun D., Colello R. J., Daugherty W. P., Kwon T. H., McGinn M. J., Harvey H. B., Bullock M. R. (2005). Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J. Neurotrauma 22, 95–105 [DOI] [PubMed] [Google Scholar]
- Sundholm-Peters N. L., Yang H. K., Goings G. E., Walker A. S., Szele F. G. (2005). Subventricular zone neuroblasts emigrate toward cortical lesions. J. Neuropathol. Exp. Neurol. 64, 1089–1100 [DOI] [PubMed] [Google Scholar]
- Tatsumi K., Okuda H., Makinodan M., Yamauchi T., Makinodan E., Matsuyoshi H., Manabe T., Wanaka A. (2010). Transient activation of Notch signaling in the injured adult brain. J. Chem. Neuroanat. 39, 15–19 [DOI] [PubMed] [Google Scholar]
- Taupin P., Gage F. H. (2002). Adult neurogenesis and neural stem cells of the central nervous system in mammals. J. Neurosci. Res. 69, 745–749 [DOI] [PubMed] [Google Scholar]
- Taylor K. L., Grant N. J., Temperley N. D., Patton E. E. (2010). Small molecule screening in zebrafish: an in vivo approach to identifying new chemical tools and drug leads. Cell Commun. Signal. 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P., Wood J., Arvidsson A., Cammenga J., Kokaia Z., Lindvall O. (2007). Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38, 3032–3039 [DOI] [PubMed] [Google Scholar]
- Voigt T. (1989). Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes, J. Comp. Neurol. 289, 74–88 [DOI] [PubMed] [Google Scholar]
- Wang X., Mao X., Xie L., Greenberg D. A., Jin K. (2009). Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J. Cereb. Blood Flow Metab. 29, 1644–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. Eugene: University of Oregon Press [Google Scholar]
- Yamashita T., Ninomiya M., Hernandez Acosta P., Garcia-Verdugo J. M., Sunabori T., Sakaguchi M., Adachi K., Kojima T., Hirota Y., Kawase T., et al. (2006). Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci. 26, 6627–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Chan C. Y., Jiang B., Yu X., Zhu G. Z., Chen Y., Barnard J., Mei W. (2009). hnRNP I inhibits Notch signaling and regulates intestinal epithelial homeostasis in the zebrafish. PLoS Genet. 5, e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. L., Zhang Z. G., Zhang L., Chopp M. (2001). Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 105, 33–41 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K. (2001). Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Brain Behav. Evol. 58, 250–275 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K. (2006). Neurogenesis and neuronal regeneration in the adult fish brain. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 192, 649–670 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K. (2008). Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J. Physiol. Paris 102, 357–373 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K., Ott R. (1999). Cell proliferation after lesions in the cerebellum of adult teleost fish: time course, origin, and type of new cells produced. Exp. Neurol. 160, 78–87 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K., Zupanc M. M. (2006). New neurons for the injured brain: mechanisms of neuronal regeneration in adult teleost fish. Regen. Med. 1, 207–216 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K., Kompass K. S., Horschke I., Ott R., Schwarz H. (1998). Apoptosis after injuries in the cerebellum of adult teleost fish. Exp. Neurol. 152, 221–230 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K., Hinsch K., Gage F. H. (2005). Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 488, 290–319 [DOI] [PubMed] [Google Scholar]
- Zupanc M. M., Wellbrock U. M., Zupanc G. K. (2006). Proteome analysis identifies novel protein candidates involved in regeneration of the cerebellum of teleost fish. Proteomics 6, 677–696 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.