SUMMARY
PTEN is an essential tumor suppressor that antagonizes Akt/PKB signaling. The zebrafish genome encodes two Pten genes, ptena and ptenb. Here, we report that zebrafish mutants that retain a single wild-type copy of ptena or ptenb (ptena+/−ptenb−/− or ptena−/−ptenb+/−) are viable and fertile. ptena+/−ptenb−/− fish develop tumors at a relatively high incidence (10.2%) and most tumors developed close to the eye (26/30). Histopathologically, the tumor masses were associated with the retrobulbar vascular network and diagnosed as hemangiosarcomas. A single tumor was identified in 42 ptena−/−ptenb+/− fish and was also diagnosed as hemangiosarcoma. Immunohistochemistry indicated that the tumor cells in ptena+/−ptenb−/− and ptena−/−ptenb+/− fish proliferated rapidly and were of endothelial origin. Akt/PKB signaling was activated in the tumors, whereas Ptena was still detected in tumor tissue from ptena+/−ptenb−/− zebrafish. We conclude that haploinsufficiency of the genes encoding Pten predisposes to hemangiosarcoma in zebrafish.
INTRODUCTION
Over the last decade, zebrafish has emerged as a powerful model organism to study tumor and cancer progression. The experimental accessibility, the broad range of zebrafish mutants and the highly conserved genetic and biochemical pathways between zebrafish and mammals lifted zebrafish to become one of the most attractive vertebrate models to study gene function and to model human diseases. Several tumor and cancer models were generated, by expression of an oncogene driven by a tissue-specific or ubiquitously expressed promoter (for a review, see Mione and Trede, 2010). In addition, two mutant models have been established with inactivated tumor suppressor genes. Inactivation of p53 results in development of malignant peripheral nerve sheath tumors (Berghmans et al., 2005) and we have previously reported ocular tumors in ptenb−/−mutants (Faucherre et al., 2008).
PTEN (phosphatase and tensin homolog from chromosome 10) is one of the most frequently mutated tumor suppressor genes in the progression of cancer and was discovered by several independent groups in the late nineties (Li et al., 1997; Podsypanina et al., 1999; Steck et al., 1997; Suzuki et al., 1998). Fundamental research during the last decade revealed the imperative cellular role of PTEN. Germline mutation of PTEN is associated with rare autosomal dominant cancer syndromes such as Cowden disease and Lhermitte-Duclos disease, and leads to an increased susceptibility to cancer (Eng, 2003; Liaw et al., 1997; Marsh et al., 1998; Marsh et al., 1997; Nelen et al., 1997).
PTEN belongs to the superfamily of protein-tyrosine phosphatases. Although it exhibits some protein phosphatase activity, PTEN functions predominantly as a lipid phosphatase (Mounir et al., 2009; Myers et al., 1998; Raftopoulou et al., 2004; Tamura et al., 1998). PTEN removes a phosphate specifically from the D3 position of the second messenger, PIP3 [phosphatidylinositol (3,4,5)-triphosphate; also known as PtdIns(3,4,5)P3], which is formed by PI3-kinase (PI3K) (Maehama and Dixon, 1998; Myers et al., 1998). Thus, PTEN is one of the few known lipid phosphatases counteracting the PI3K pathway. An important downstream target of PI3K signaling is Akt, also known as PKB. Akt/PKB signaling modulates a variety of cellular functions, including inhibition of GSK-3 by phosphorylation (Cross et al., 1995; Vivanco and Sawyers, 2002) and activation of cell proliferation by inactivation of p27 (Fujita et al., 2002), exerting an anti-apoptotic effect (Biggs et al., 1999; Brunet et al., 1999; del Peso et al., 1997; Guo et al., 1999; Kops et al., 1999; Nakae et al., 1999; Rena et al., 1999; Takaishi et al., 1999; Tang et al., 1999). Akt/PKB signaling also regulates a subset of proteins involved in growth, metabolism and angiogenesis (Maehama and Dixon, 1998; Myers et al., 1998; Salmena et al., 2008).
Functional analysis in mouse, Drosophila and Caenorhabditis elegans revealed the significance of PTEN, because loss of function leads to embryonic lethality (Di Cristofano et al., 1998; Gao et al., 2000; Goberdhan et al., 1999; Suzuki et al., 1998). Somatic deletion of PTEN function in various tissues leads to tumor formation and cancer (Ali et al., 1999; Freeman et al., 2006; Li and Sun, 1997; Li et al., 1997). Heterozygous Pten+/− mice display various hyperplasias and even Pten hypomorphs with a 20% reduction in PTEN protein expression display enhanced tumor incidence, indicating that subtle reductions in the dose of PTEN predispose to tumorigenesis (Alimonti et al., 2010).
Zebrafish possess two Pten-encoding genes, referred to as ptena and ptenb, which are both functional and encode proteins that exhibit lipid phosphatase activity (Croushore et al., 2005; Faucherre et al., 2008). Single mutants (ptena−/− or ptenb−/−) are viable and fertile and lack visible morphological defects during embryonic development. However, embryos deficient for Ptena and Ptenb die at 5–6 days post-fertilization (dpf) and display major hyperplastic-dysplastic changes in several organs. These observations indicate that Ptena and Ptenb are necessary, but have redundant functions, during embryonic development (Faucherre et al., 2008).
Here, we report that adult fish lacking three Pten alleles, ptena+/−ptenb−/− or ptena−/−ptenb+/−, are viable and fertile. These fish spontaneously develop tumors, frequently with retrobulbar localization. Histopathological analysis revealed that these tumors are hemangiosarcomas that consist of blood-filled spaces lined by plump, neoplastic invading cells. Immunohistochemistry confirmed the presence of CD31-positive endothelial cells within the tumor blood vessels in ptena+/−ptenb−/− as well as ptena−/−ptenb+/− fish. Moreover, these cells were strongly positive for proliferating cell nuclear antigen (PCNA), an S-phase marker, indicating that the majority of tumor cells were cycling. Finally, we demonstrate that Akt/PKB signaling was activated in tumors from ptena+/−ptenb−/−fish. We conclude that haploinsufficiency of the genes encoding Pten predisposes to hemangiosarcoma in zebrafish.
RESULTS
Tumor development in ptena+/−ptenb−/− and ptena−/−ptenb+/−zebrafish
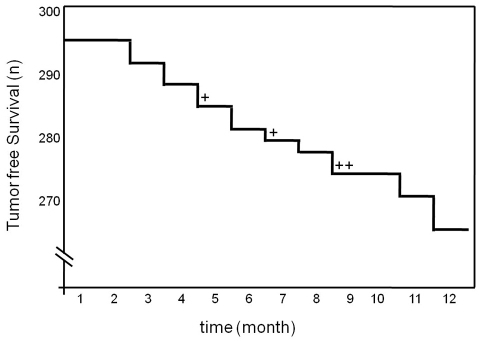
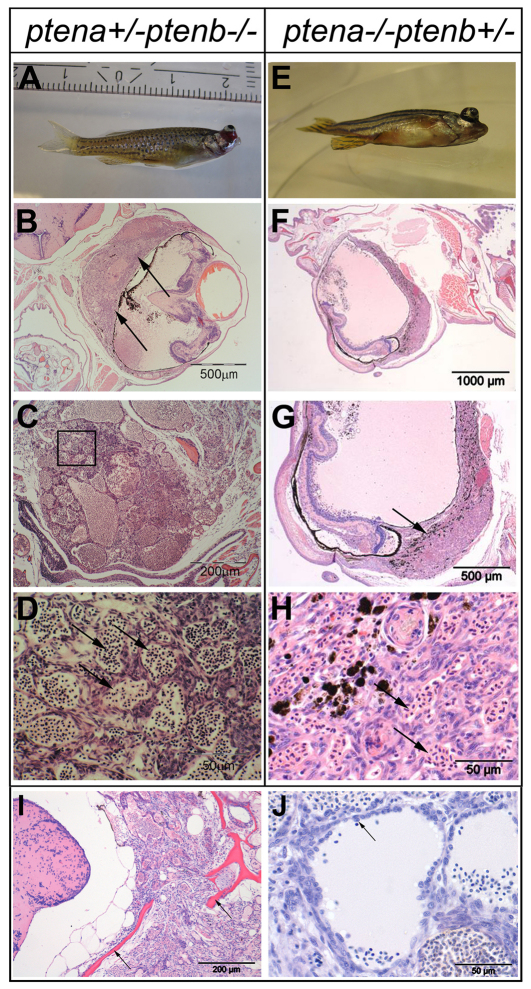
To investigate whether zebrafish mutants with one wild-type ptena allele (ptena+/−ptenb−/−) develop tumors during their life span, we monitored around 300 ptena+/−ptenb−/− mutant fish. In a time frame of 12 months, we observed tumor formation in 30 out of 294 individuals (10.2%). Tumor development was not strictly correlated to age, in that tumors occurred randomly from 3 months onwards (Fig. 1). It is noteworthy that tumor formation is extremely rare in wild-type fish, with an incidence of <0.01% in 12-month-old fish. Furthermore, we have monitored 495 ptena+/−ptenb+/− fish for more than 12 months and never observed a tumor in any of these fish, indicating that tumor formation is specific for fish harboring a single wild-type Pten allele. In 26 of the 30 ptena+/−ptenb−/− fish with tumors, the pathological mass was localized close to the eye (Fig. 2A). The other four tumors were localized in various tissues, mostly adipose connective tissue (data not shown). For histopathological diagnosis, we fixed the entire fish, prepared consecutive sections including the tumor mass and performed hematoxylin and eosin (H&E) staining, which clearly showed the formation of an ocularly located mass (Fig. 2B,C). In all 26 cases, the tumor was located in, or replaced, the retrobulbar vascular network and frequently expanded into the eye; the growth pattern was remarkably reproducible. The neoplasias consisted of variably sized blood-filled spaces, lined by plump to spindle-shaped cells indicative of angiogenic origin (Fig. 2D). Plump cell morphology, protrusion into the neoplastic vascular lumen and local tissue invasion (Fig. 2I,J) favored a diagnosis of hemangiosarcoma. Next to the ptena+/−ptenb−/− fish, we also monitored a family of ptena−/−ptenb+/− fish, consisting of 42 individual fish. We observed an ocular tumor in a 9-month-old fish (Fig. 2E), which we histologically also classified as hemangiosarcoma (Fig. 2F–H). Taken together, these results suggest that loss of three of the four Pten alleles predisposes to hemangiosarcoma.
Fig. 1.
Ocular tumor incidence in ptena+/−ptenb−/− zebrafish over time. Kaplan-Meier plot of tumor incidence. Ptena+/−ptenb−/− zebrafish (n=294) were monitored over time. Number of tumor-free fish are plotted on the y-axis (n). Most tumors (26/30) developed close to the eye. The tumors in other locations (4/30) are indicated as +.
Fig. 2.
Ocular tumor of ptena+/−ptenb−/− and ptena−/−ptenb+/− mutants, diagnosed as hemangiosarcoma. (A–D) 3-month-old ptena+/−ptenb−/− and (E-H) 9-month-old ptena−/−ptenb+/− mutant with ocular tumor. The entire intact fish was fixed and embedded in paraffin. (B–D) Transversal sections and (F–H) sagittal sections were stained with H&E. Arrows indicate tumor mass, which is associated with the eye bulbs. (B,G) Higher-power magnifications of the tumor mass; (D) magnification of the boxed area in C. The tumor consists of cells that form different sizes of blood-filled spaces (arrows in D,H).(I,J) H&E staining of sections from two individuals revealed hemangiosarcoma formation. (I) The tumor was invasive and penetrated into the brain region with enclosing scull elements (arrows). (J) Cells with plump morphology (arrow) are detaching from surrounding tissue and protrude into the vessel lumen. Sections of representative tumors are depicted here.
High cell proliferation and endothelial cell fate in hemangiosarcomas from Pten mutants
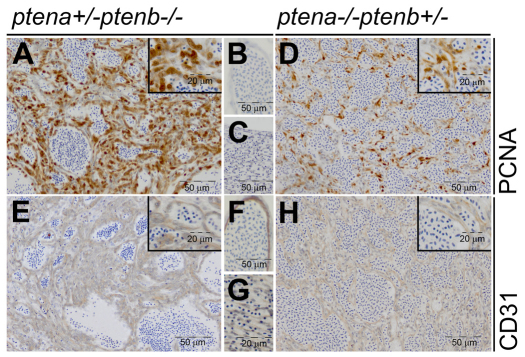
To examine cell proliferation in the tumors, immunohistochemistry was performed. Tumor samples from ptena+/−ptenb−/− and ptena−/−ptenb+/− mutants exhibited elevated nuclear PCNA staining (Fig. 3A,D). As a control, individual blood vessels and vessels in the rete mirabile, a collection of arteries and veins lying very close to each other (Barnett, 1951; Wittenberg and Wittenberg, 1974), located contralaterally in the same section outside the tumor mass were examined. PCNA staining was clearly less prominent in the vessels outside the tumor mass (Fig. 3B,C). Histological analysis of H&E-stained slides suggested that the tumor consisted of blood-filled vessel-like structures that were presumably formed by neoplastic endothelial cells. To confirm the endothelial origin of these cells, we used a panel of antibodies that are routinely used for human endothelial cells, including anti-CD31 (PECAM-1), anti-CD133 and anti-Factor VIII-related antigen. Anti-CD31 detected zebrafish endothelial cells reliably, whereas antibodies against CD133 and Factor VIII-related antigen did not stain endothelial cells of blood vessels or tumor cells. All three antibodies stained mouse endothelial cells in sections of adult mouse gut, liver and pancreas that were processed in parallel (data not shown). Comparable to human hemangioma (Boscolo and Bischoff, 2009), we detected CD31 in the cells of the tumor masses from ptena+/−ptenb−/− and ptena−/−ptenb+/− fish (Fig. 3E,H). Blood vessels and rete mirabile at the contralateral side in the same sections outside the tumor masses stained positive for CD31 in a similar manner (Fig. 3F,G). Cells from non-endothelial origin, such as muscle and ovaries, did not stain positive for CD31 (supplementary material Fig. S1), demonstrating specificity of the antibody. Taken together, our results demonstrate that the cells in the tumors are proliferating (PCNA positive) and of endothelial origin (CD31 positive).
Fig. 3.
Low-dose Pten tumors display elevated cell proliferation and have endothelial cell origin. ptena+/−ptenb−/− (n=6) (A–C,E–G) and ptena−/−ptenb+/− (n=1) (D,H) mutants with tumors were fixed, paraffin embedded and sectioned transversally or sagitally. Immunohistochemistry using PCNA, a cell proliferation marker, showed clearly enhanced nuclear PCNA staining in tumor cells (A,D) compared with control tissue in the same sections (B,C). CD31, an endothelial cell marker, was expressed in the tumor tissue (E,H) in a similar manner as in control blood vessels in the same sections (F,G). Representative sections are depicted here.
Tumor progression in Pten mutants is associated with activated Akt/PKB signaling
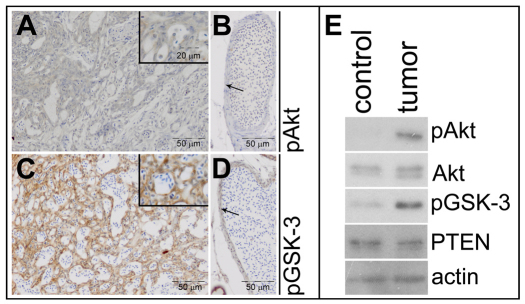
Loss of Pten results in activation of the Akt/PKB signaling pathway. The first step in this pathway is activation of Akt/PKB by phosphorylation. To investigate whether Akt/PKB signaling is activated in the tumors, we stained sections of ptena+/−ptenb−/−fish with antibodies specific for phospho-Akt (pAkt; pThr308). We observed a weak signal for pAkt in most of the endothelial tumor cells (Fig. 4A). Endothelial cells of a blood vessel outside the tumor in the same section were negative for pAkt (Fig. 4B). GSK-3 is a direct target of Akt signaling and we examined GSK-3β phosphorylation on Ser9 using a phospho-GSK-3β (pGSK-3)-specific antibody. All endothelial cells in the tumor mass of ptena+/−ptenb−/− fish stained positive for pGSK-3 (Fig. 4C), whereas endothelial cells in a control vessel in the same section only stained weakly positive for pGSK-3 (Fig. 4D). To confirm these immunohistochemistry results, we analyzed Akt/PKB signaling by immunoblotting. To this end, part of the head of a tumor-bearing ptena+/−ptenb−/− fish, including the tumor, was dissected and tissue from the contralateral side was isolated as a control. The tissues were lysed and the proteins were separated on SDS-polyacrylamide gels, blotted and probed with antibodies that detect Akt/PKB signaling. Akt/PKB Ser473 phosphorylation was elevated in the tumor sample, compared with the contralateral control, whereas Akt protein levels were similar (Fig. 4E). GSK-3β Ser9 phosphorylation was also elevated in the tumor samples, confirming the immunohistochemistry data. Taken together, these results demonstrate that Akt/PKB signaling is activated in endothelial cells of hemangiosarcomas of Pten mutant fish.
Fig. 4.
Elevated Akt/PKB signaling in Pten mutant tumors. Immunohistochemistry on transversal sections from a ptena+/−ptenb−/− mutant fish, using pAkt- and pGSK-3β-specific antibodies. (A) Tumor area stains weakly positive for pAkt, whereas (B) control vessel (arrow) from the same section is not stained (n=10). (C) Tumor area is highly positive for pGSK-3β, whereas (D) cells from a control vessel (arrow) from the same section only stain mildly positive (n=6). (E) The cranial part of a ptena+/−ptenb−/− mutant that developed a tumor was dissected into two fragments, one harboring tumor tissue (tumor) and the other representing control tissue (control). The samples were lysed and the lysates were run on a denaturing SDS-polyacrylamide gel. The proteins were transferred to a PVDF membrane and after blocking the blot was probed with anti-pAkt antibody, stripped and sequentially probed with anti-Akt, anti-pGSK-3, anti-PTEN and, as a loading control, anti-actin.
To investigate the possibility that tumor development was due to loss of heterozygosity for ptena in ptena+/−ptenb−/− fish, we probed the blots with an antibody that was raised against human PTEN. This antibody specifically recognized zebrafish Ptena, but not Ptenb (supplementary material Fig. S2). Tumor material from ptena+/−ptenb−/− zebrafish stained positive for PTEN, as did control material (Fig. 4E), suggesting that tumor development in ptena+/−ptenb−/− zebrafish was not due to loss of heterozygosity. Unfortunately, immunohistochemistry with the Ptena-specific antibody resulted in non-specific background staining. Although we cannot exclude the possibility that the Ptena signal in the immunoblots (Fig. 4E) is due to non-tumor cells, the level of Ptena expression is very similar in the tumor and contralateral tissue, suggesting that Ptena expression is not lost in the tumor cells.
DISCUSSION
The zebrafish genome contains two Pten-encoding genes, referred to as ptena and ptenb, with redundant roles during development (Faucherre et al., 2008). Zebrafish mutants harboring one wild-type Pten allele are viable and fertile, yet they spontaneously develop tumors relatively frequently in a reproducible manner. Histological analysis of the tumors indicated that these were large masses consisting of blood-filled lumens that displayed local tissue invasion. The endothelial cell type was confirmed histochemically using an endothelial-cell-specific marker (CD31). The tumors displayed high levels of cell proliferation, visualized by PCNA-positive nuclei, reflecting uncontrolled cell growth. Taken together, these tumors bear all the hallmarks of hemangiosarcoma.
Previously, we reported that ptenb−/− zebrafish developed eye tumors around 7 months of age (Faucherre et al., 2008). These tumors were diagnosed as neuroepithelioma. The cells seemed to be of neurogenic origin and were organized in rosette-like structures. The tumors we observed in ptenb−/− fish are distinct from the hemangiosarcomas we observed in ptena+/−ptenb−/− and ptena−/−ptenb+/− fish. The incidence of tumors in ptenb−/− zebrafish has decreased dramatically over time. The ptenb−/− line has been outcrossed several times and the decrease in eye tumors in ptenb−/−zebrafish might be due to loss of enhancers of the ptenb−/− phenotype.
Using immunohistochemistry and immunoblotting, we observed elevated Akt/PKB signaling in the tumor mass compared with control tissue. Apparently, loss of three of the four Pten alleles is sufficient to raise Akt/PKB signaling to some extent. Whether human hemangiosarcomas display defects in Akt/PKB signaling remains to be determined. It is interesting to note that canine hemangiosarcomas displayed mildly enhanced pAkt levels (Chandler et al., 2009). In addition, reduced Pten expression was reported in 12 cases of canine hemangiosarcoma (Dickerson et al., 2005), which is consistent with a role for partial loss of Pten in hemangiosarcoma formation. On the basis of our results and on published reports in canine hemangiosarcomas, it will be interesting to investigate PTEN expression levels in human hemangiosarcoma. Tumor development was not fully penetrant in zebrafish. Therefore, it is likely that a second genetic lesion is required for tumor development in ptena+/−ptenb−/−and ptena−/−ptenb+/− fish. The identity of the second lesion remains to be determined.
Interestingly, Ptena expression was very similar in the tumor and contralateral control tissue, suggesting that the tumors in ptena+/−ptenb−/− fish were not caused by loss of heterozygosity. Immunohistochemical analysis of Pten in tumor sections, using several different PTEN-specific antibodies, unfortunately did not result in specific staining and we cannot rule out the possibility that the observed Ptena expression is due to non-tumor cells. However, we have derived an endothelial-like cell line from a tumor of a ptena+/−ptenb−/− fish, and genotyping of this cell line indicated that these cells have retained a wild-type ptena allele. Moreover, immunoblotting of whole-cell lysates of this cell line demonstrated that Ptena protein is expressed (data not shown), supporting the notion that the tumors in ptena+/−ptenb−/− fish are not caused by loss of heterozygosity.
In humans, germline mutations of PTEN are associated with Cowden disease, Bannayan-Zonana syndrome and Lhermitte-Duclos disease (Eng, 2003; Liaw et al., 1997; Marsh et al., 1998; Marsh et al., 1997; Nelen et al., 1997). Patients share pathological features, including development of hamartomas, benign hyperplasias or tumors of different tissue origin. Mice that are heterozygous for Pten genes develop various kinds of hyperplasias and tumors during their lifetime (Di Cristofano et al., 1998; Li et al., 1997; Podsypanina et al., 1999; Suzuki et al., 1998). Interestingly, Freeman et al. reported vascular abnormalities in PtenΔ5/+ mice that resembled angiomatosis or hemangiomas, and the onset and spectrum of tumor formation were dependent on the genetic background (Freeman et al., 2006). In addition, transgenic murine models with sustained Akt activation display vascular malformation. Expression of myr-Akt1 in endothelial cells results in abnormal blood vessel formation (Phung et al., 2006; Sun et al., 2005). It remains to be determined in the ptena+/−ptenb−/− and ptena−/−ptenb+/− fish why endothelial cells are particularly susceptible to tumor formation associated with loss of three of the four Pten alleles.
The tumors that we observed in zebrafish were predominantly (26/30) ocular hemangiosarcomas. Interestingly, ocular hemangioma and hemangiosarcoma has been documented as a common tumor in dogs (Chandler et al., 2009), suggesting that this location is prone to the formation of hemangiomas and hemangiosarcomas. We hypothesize that the location of tumor origin in zebrafish Pten mutants is the choroidal gland, an anatomical structure that is known as rete mirabile (Barnett, 1951; Wittenberg and Wittenberg, 1974). The function of the rete mirabile in fish is not yet known.
It is well established that PI3-K signaling has a prominent role in angiogenesis. Expression of an oncogenic form of PI3-K in chicks results in an increased number of blood vessels, sprouting of new vessels and enlargement of pre-existing vessels (Jiang et al., 1999). PI3-K signaling is activated in response to Angiopoietin-mediated activation of Tie2 (Jones et al., 1999), which in turn leads to activation of Akt/PKB signaling. Pten mutant fish are hypersensitive to Akt/PKB signaling, because they express reduced levels of Pten, the antagonist of Akt/PKB signaling. It is likely that angiogenic factors are abundant in the rete mirabile, close to the eye in zebrafish, and loss of three of the four Pten alleles in mutant fish might result in elevated Akt/PKB signaling particularly at, or close to, the rete mirabile, and hence induce hemangiosarcoma formation locally.
To our knowledge, this is the first report of genetic mutations that reproducibly result in development of hemangiosarcoma in zebrafish. The advantages of zebrafish as an experimental model system facilitate further elucidation of the molecular and cell biological origin of hemangiosarcoma. We conclude that haploinsufficiency of the genes encoding the tumor suppressor Pten predisposes to uncontrolled proliferation of endothelial cells, resulting in hemangiosarcoma in zebrafish, which is accompanied by moderate activation of Akt/PKB signaling.
METHODS
Zebrafish mutant lines and maintenance
Zebrafish Pten mutant lines were generated by target selected gene inactivation. For maintenance of zebrafish lines, ptena+/−ptenb−/−or ptena−/−ptenb+/− mutants were intercrossed and offspring genotyped as described by Faucherre et al. (Faucherre et al., 2008). All procedures involving experimental animals were approved by the local animal experiments committee and performed in compliance with local animal welfare laws, guidelines and policies, according to national and European law.
Immunohistochemistry
Euthanized fish were fixed with Bouin’s fixative solution overnight at room temperature and subsequently washed with 70% ethanol. Immunohistochemistry (IHC) was performed according to standard protocols. Briefly, fish were embedded in paraffin, sectioned (6 μm) transversally or sagittally, placed on charged slides and deparaffinized. As a control for efficacy of the antibodies, sections of adult mouse gut were used in parallel to the zebrafish tumor sections. Peroxidases were blocked by incubating the slides for 15 minutes at room temperature in 0.4 M citric acid, 1.2 M di-sodium-hydrogen-phosphate-2-hydrate, 0.02% (w/v) sodium azide and 1.5% peroxide. For antigen retrieval, the sections were boiled for 20 minutes in 0.01 M tri-sodium-citrate-2-hydrate, pH 6, and cooled down at room temperature for at least 1 hour. Sections were blocked for 20 minutes in 1% BSA/PBS (w/v) and incubated with primary antibody in blocking buffer at 4°C overnight. Details of antibodies used for IHC are listed in Table 1. Slides were washed three times in PBS (5 minutes each) and incubated for 1 hour with biotinylated secondary anti-rabbit or anti-mouse antibodies. Sections were rinsed four times in PBS and developed with 3.3′-diaminobenzidine tetrahydrochloride-H2O2 solution for 10 minutes. Slides were washed subsequently and counterstained with hematoxylin. Serial sections were used and one section from each tumor sample was stained with H&E. Pictures were taken using an Olympus SZX9 connected to a microscope with 4×, 10×, 20× and 40× objectives.
Table 1.
Primary antibodies used for immunohistochemistry
Immunoblotting
Tumor mass and control tissue from a ptena+/−ptenb−/− mutant were dissected after euthanizing fish and immediately lysed with cell lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 10% glycerol, 1% Triton X-100, 1% sodium orthovanadate and protease inhibitors, including 5 mM betaglycerophosphate, 1 μg/ml aprotinin, 5 mM NaF, 1 mM Na3VO4 and 1 μg/ml leupeptin). To validate specificity of the anti-PTEN antibody, 3-dpf-old ptena−/−and ptenb−/− (Faucherre et al., 2008) and control embryos were lysed in cell lysis buffer. Samples were sonicated, mixed with 2× Laemmli sample buffer and boiled for 5 minutes. Western blotting was performed according to standard procedure using antibodies listed in Table 2.
Table 2.
Primary antibodies used for immunoblotting
TRANSLATIONAL IMPACT.
Clinical issue
PTEN is one of the most important known tumor suppressor genes, and is frequently inactivated by mutation in human tumors of many different types. PTEN is a lipid phosphatase that functions as an antagonist of PI3-kinase, thereby inhibiting Akt/PKB signaling. Studies of model systems in which the homolog of PTEN is mutated or inactivated should clarify the role of PTEN mutations in human cancer. Complete inactivation of PTEN in model systems (including mice, flies, worms and fish) is incompatible with life, and mice that are heterozygous for Pten inactivation are tumor-prone. The zebrafish genome contains two Pten-encoding genes (ptena and ptenb); inactivation of both genes results in embryonic lethality.
Results
This paper reports that zebrafish with a single wild-type Pten allele develop tumors with high incidence. The authors monitor 294 ptena+/−ptenb−/− and 42 ptena−/−ptenb+/− fish over 12 months for development of tumors and find a relatively high tumor incidence (10.2% and 2.4%, respectively). Histopathological analysis reveals tumor masses that are associated with the retrobulbar vascular network, and are diagnosed as hemangiosarcomas composed of rapidly proliferating cells of endothelial origin. Interestingly, Akt/PKB signaling is activated in the tumors. Thus, altered gene dosage of the genes encoding Pten predisposes to hemangiosarcoma in zebrafish.
Implications and future directions
This is the first report of a genetic mutation that results in hemangiosarcoma in zebrafish. The observation that moderate Akt/PKB activation is a feature of hemangiosarcoma in this model indicates that it might be of interest to investigate the role of Akt/PKB signaling in human hemangiosarcoma. In addition, the finding that haploinsufficiency of the genes encoding Pten predisposes to uncontrolled proliferation of endothelial cells in particular suggests that further investigation of the role of PTEN in endothelial cells is warranted. The advantages of using zebrafish as an experimental model system should facilitate pursuit of this issue, as well as the further elucidation of the molecular and cellular origins of hemangiosarcoma.
Supplementary Material
Acknowledgments
The authors thank Jeroen Korving for excellent histotechnical support and Petra van Duijn for support in the maintenance of the Pten mutant lines.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
S.C. and J.d.H. conceived the experiments; S.C., R.V.K., A.d.B. and J.d.H. designed the experiments; S.C. and R.V.K. performed the experiments; S.C., R.V.K., A.d.B. and J.d.H. analyzed the data and wrote the manuscript.
FUNDING
This work was supported in part by an EU (FP7) grant [HEALTH-F2-2008-201439 (ZF-CANCER)].
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.008326/-/DC1
REFERENCES
- Ali I. U., Schriml L. M., Dean M. (1999). Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J. Natl. Cancer Inst. 91, 1922–1932 [DOI] [PubMed] [Google Scholar]
- Alimonti A., Carracedo A., Clohessy J. G., Trotman L. C., Nardella C., Egia A., Salmena L., Sampieri K., Haveman W. J., Brogi E., et al. (2010). Subtle variations in Pten dose determine cancer susceptibility. Nat. Genet. 42, 454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett C. H. (1951). The structure and function of the choroidal gland of teleostean fish. J. Anat. 85, 113–119 [PMC free article] [PubMed] [Google Scholar]
- Berghmans S., Murphey R. D., Wienholds E., Neuberg D., Kutok J. L., Fletcher C. D., Morris J. P., Liu T. X., Schulte-Merker S., Kanki J. P., et al. (2005). tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA 102, 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs W. H., 3rd, Meisenhelder J., Hunter T., Cavenee W. K., Arden K. C. (1999). Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96, 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo E., Bischoff J. (2009). Vasculogenesis in infantile hemangioma. Angiogenesis 12, 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999). Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- Chandler H. L., Newkirk K. M., Kusewitt D. F., Dubielzig R. R., Colitz C. M. (2009). Immunohistochemical analysis of ocular hemangiomas and hemangiosarcomas in dogs. Vet. Ophthalmol. 12, 83–90 [DOI] [PubMed] [Google Scholar]
- Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- Croushore J. A., Blasiole B., Riddle R. C., Thisse C., Thisse B., Canfield V. A., Robertson G. P., Cheng K. C., Levenson R. (2005). Ptena and ptenb genes play distinct roles in zebrafish embryogenesis. Dev. Dyn. 234, 911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Peso L., Gonzalez-Garcia M., Page C., Herrera R., Nunez G. (1997). Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278, 687–689 [DOI] [PubMed] [Google Scholar]
- Di Cristofano A., Pesce B., Cordon-Cardo C., Pandolfi P. P. (1998). Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19, 348–355 [DOI] [PubMed] [Google Scholar]
- Dickerson E. B., Thomas R., Fosmire S. P., Lamerato-Kozicki A. R., Bianco S. R., Wojcieszyn J. W., Breen M., Helfand S. C., Modiano J. F. (2005). Mutations of phosphatase and tensin homolog deleted from chromosome 10 in canine hemangiosarcoma. Vet. Pathol. 42, 618–632 [DOI] [PubMed] [Google Scholar]
- Eng C. (2003). PTEN: one gene, many syndromes. Hum. Mutat. 22, 183–198 [DOI] [PubMed] [Google Scholar]
- Faucherre A., Taylor G. S., Overvoorde J., Dixon J. E., den Hertog J. (2008). Zebrafish pten genes have overlapping and non-redundant functions in tumorigenesis and embryonic development. Oncogene 27, 1079–1086 [DOI] [PubMed] [Google Scholar]
- Freeman D., Lesche R., Kertesz N., Wang S., Li G., Gao J., Groszer M., Martinez-Diaz H., Rozengurt N., Thomas G., et al. (2006). Genetic background controls tumor development in PTEN-deficient mice. Cancer Res. 66, 6492–6496 [DOI] [PubMed] [Google Scholar]
- Fujita N., Sato S., Katayama K., Tsuruo T. (2002). Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 277, 28706–28713 [DOI] [PubMed] [Google Scholar]
- Gao X., Neufeld T. P., Pan D. (2000). Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev. Biol. 221, 404–418 [DOI] [PubMed] [Google Scholar]
- Goberdhan D. C., Paricio N., Goodman E. C., Mlodzik M., Wilson C. (1999). Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 13, 3244–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Rena G., Cichy S., He X., Cohen P., Unterman T. (1999). Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J. Biol. Chem. 274, 17184–17192 [DOI] [PubMed] [Google Scholar]
- Jiang B. H., Aoki M., Zheng J. Z., Li J., Vogt P. K. (1999). Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc. Natl. Acad. Sci. USA 96, 2077–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Master Z., Jones J., Bouchard D., Gunji Y., Sasaki H., Daly R., Alitalo K., Dumont D. J. (1999). Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J. Biol. Chem. 274, 30896–30905 [DOI] [PubMed] [Google Scholar]
- Kops G. J., de Ruiter N. D., De Vries-Smits A. M., Powell D. R., Bos J. L., Burgering B. M. (1999). Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 [DOI] [PubMed] [Google Scholar]
- Li D. M., Sun H. (1997). TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 57, 2124–2129 [PubMed] [Google Scholar]
- Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R., et al. (1997). PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 [DOI] [PubMed] [Google Scholar]
- Liaw D., Marsh D. J., Li J., Dahia P. L., Wang S. I., Zheng Z., Bose S., Call K. M., Tsou H. C., Peacocke M., et al. (1997). Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 16, 64–67 [DOI] [PubMed] [Google Scholar]
- Maehama T., Dixon J. E. (1998). The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Dahia P. L., Zheng Z., Liaw D., Parsons R., Gorlin R. J., Eng C. (1997). Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat. Genet. 16, 333–334 [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Coulon V., Lunetta K. L., Rocca-Serra P., Dahia P. L., Zheng Z., Liaw D., Caron S., Duboue B., Lin A. Y., et al. (1998). Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum. Mol. Genet. 7, 507–515 [DOI] [PubMed] [Google Scholar]
- Mione M. C., Trede N. S. (2010). The zebrafish as a model for cancer. Dis. Model. Mech. 3, 517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounir Z., Krishnamoorthy J. L., Robertson G. P., Scheuner D., Kaufman R. J., Georgescu M. M., Koromilas A. E. (2009). Tumor suppression by PTEN requires the activation of the PKR-eIF2alpha phosphorylation pathway. Sci. Signal. 2, ra85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. P., Pass I., Batty I. H., Van der Kaay J., Stolarov J. P., Hemmings B. A., Wigler M. H., Downes C. P., Tonks N. K. (1998). The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc. Natl. Acad. Sci. USA 95, 13513–13518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J., Park B. C., Accili D. (1999). Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J. Biol. Chem. 274, 15982–15985 [DOI] [PubMed] [Google Scholar]
- Nelen M. R., van Staveren W. C., Peeters E. A., Hassel M. B., Gorlin R. J., Hamm H., Lindboe C. F., Fryns J. P., Sijmons R. H., Woods D. G., et al. (1997). Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum. Mol. Genet. 6, 1383–1387 [DOI] [PubMed] [Google Scholar]
- Phung T. L., Ziv K., Dabydeen D., Eyiah-Mensah G., Riveros M., Perruzzi C., Sun J., Monahan-Earley R. A., Shiojima I., Nagy J. A., et al. (2006). Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 10, 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K., Ellenson L. H., Nemes A., Gu J., Tamura M., Yamada K. M., Cordon-Cardo C., Catoretti G., Fisher P. E., Parsons R. (1999). Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. USA 96, 1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou M., Etienne-Manneville S., Self A., Nicholls S., Hall A. (2004). Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303, 1179–1181 [DOI] [PubMed] [Google Scholar]
- Rena G., Guo S., Cichy S. C., Unterman T. G., Cohen P. (1999). Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 274, 17179–17183 [DOI] [PubMed] [Google Scholar]
- Salmena L., Carracedo A., Pandolfi P. P. (2008). Tenets of PTEN tumor suppression. Cell 133, 403–414 [DOI] [PubMed] [Google Scholar]
- Steck P. A., Pershouse M. A., Jasser S. A., Yung W. K., Lin H., Ligon A. H., Langford L. A., Baumgard M. L., Hattier T., Davis T., et al. (1997). Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 [DOI] [PubMed] [Google Scholar]
- Sun J. F., Phung T., Shiojima I., Felske T., Upalakalin J. N., Feng D., Kornaga T., Dor T., Dvorak A. M., Walsh K., et al. (2005). Microvascular patterning is controlled by fine-tuning the Akt signal. Proc. Natl. Acad. Sci. USA 102, 128–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., de la Pompa J. L., Stambolic V., Elia A. J., Sasaki T., del Barco, Barrantes I., Ho A., Wakeham A., Itie A., Khoo W., et al. (1998). High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8, 1169–1178 [DOI] [PubMed] [Google Scholar]
- Takaishi H., Konishi H., Matsuzaki H., Ono Y., Shirai Y., Saito N., Kitamura T., Ogawa W., Kasuga M., Kikkawa U., et al. (1999). Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc. Natl. Acad. Sci. USA 96, 11836–11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Gu J., Matsumoto K., Aota S., Parsons R., Yamada K. M. (1998). Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617 [DOI] [PubMed] [Google Scholar]
- Tang E. D., Nunez G., Barr F. G., Guan K. L. (1999). Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274, 16741–16746 [DOI] [PubMed] [Google Scholar]
- Vivanco I., Sawyers C. L. (2002). The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B., Wittenberg B. A. (1974). The choroid rete mirabile of the fish eye. I. Oxygen secretion and structure: comparison with the swimbladder rete mirabile. Biol. Bull. 146, 116–136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.