Abstract
Objective
To investigate how birthplace influences the incidence of papillary thyroid cancer among Asian American women.
Methods
Birthplace- and ethnic-specific age-adjusted and age-specific incidence rates were calculated using data from the California Cancer Registry for the period 1988–2004. Birthplace was statistically imputed for 30% of cases using a validated imputation method based on age at Social Security number issuance. Population estimates were obtained from the US Census. Incidence rate ratios (IRR) and 95% confidence intervals (CI) were estimated for foreign-born vs. US-born women.
Results
Age-adjusted incidence rates of papillary thyroid cancer among Filipina (13.7 per 100,000) and Vietnamese (12.7) women were more than double those of Japanese women (6.2). US-born Chinese (IRR=0.48, 95% CI: 0.40–0.59) and Filipina women (IRR=0.74, 95% CI: 0.58–0.96) had significantly higher rates than those who were foreign-born; the opposite was observed for Japanese women (IRR=1.55, 95% CI: 1.17–2.08). The age-specific patterns among all foreign-born Asian women and US-born Japanese women showed a slow steady increase in incidence until age 70. However, among US-born Asian women (except Japanese), substantially elevated incidence rates during the reproductive and menopausal years were evident.
Conclusions
Ethnic- and birthplace-variation in papillary thyroid cancer incidence can provide insight into the etiology of this increasingly common and understudied cancer.
Keywords: papillary thyroid cancer, incidence rates, birthplace, Asian American women, cancer surveillance
INTRODUCTION
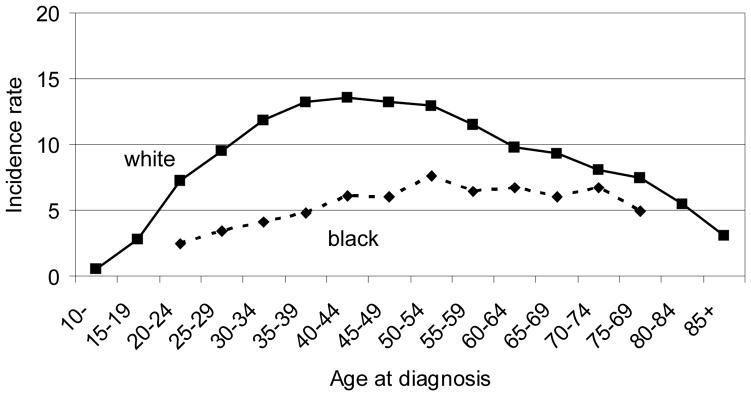
Despite its relative rarity among many population groups worldwide, thyroid cancer ranks among the five most commonly occurring cancers in some Asian female populations (1–3). Papillary carcinoma (including its variant, mixed papillary/follicular) is the most common type of thyroid cancer accounting for up to 85% of all thyroid cancers, particularly in areas of iodine sufficiency; follicular carcinoma is more common in iodine deficient areas where endemic goiter is common (4, 5). Papillary thyroid cancer is about three times more common in women than men with the earliest diagnoses occurring around the time of puberty (5). Two distinct patterns of age-specific papillary thyroid cancer incidence rates have been observed. In non-Hispanic (NH) white women, rates increase rapidly through the reproductive years, plateau through menopause and the 60s, and decline steadily thereafter (Figure 1) (5, 6). This pattern differs from that observed among men and NH black women, in whom, incidence rates are substantially lower and increase steadily with age, showing the same decline after age 70.
Figure 1.
Age-specific papillary thyroid cancer incidence rates (per 100,000) in white and black women, California 1988–2004.
Age-adjusted incidence rates of thyroid cancer in Asian American women vary by ethnicity, with the highest rates observed among those of Southeast Asian ancestry (including Vietnamese, Cambodian, Filipina, and other ancestries) (1, 7, 8). In the US, incidence rates are significantly higher in Filipina women (17.7 per 100,000, 95% CI: 16.4–19.2) than NH white women (11.8 per 100,000, 95% CI: 10.6–12.0) and elevated in US Vietnamese (13.3 per 100,000) and Kampuchean (Cambodian) women (13.4) (1). Rates among US Chinese (10.0 per 100,000), Korean (9.8), Asian Indian/Pakistani, (8.3) and Japanese (8.2) women are lower than among NH white women (1). Among Asian women internationally, there is a more than 20-fold variation in the incidence of thyroid cancer (3). In general, rates in Filipina and Chinese women residing in the US are higher than their counterparts in the Philippines and China (3, 9). The opposite is true for Korean women where rates in the US are generally lower than those in Korea (3).
Within an ethnic group, understanding how cancer rates vary with migration patterns can provide valuable insights into the etiology and prevention of that cancer. However, only a single paper has evaluated the incidence of thyroid cancer among US Asians by birthplace (10). Using data from the US Surveillance, Epidemiology, and End Results (SEER) program for the period 1973–86, those authors found that Chinese women born in the US had papillary thyroid cancer incidence rates that were 42% higher than rates among those who were born in China and immigrated to the US. Among Filipina and Japanese American women, the opposite pattern was observed; those born in the US experienced rates 69% and 62%, respectively, lower than those born in Asia (10). These estimates, however, may be somewhat biased since they rely on the random imputation of birthplace for between 15% and 27% of the ethnic-specific birthplace data. Because birthplace data are more likely to be missing for US-born persons than those who are foreign-born (11, 12), comparisons that ignore this information may introduce bias. To overcome this limitation, we augmented data from the California Cancer Registry through imputation of immigrant status based on patients’ Social Security numbers (SSN) for cancer cases with missing birthplace and used robust demographic methods to compute corresponding population estimates. Using these enhanced surveillance data, the present analysis examines the effects of birthplace on papillary thyroid cancer rates in five ethnic groups of Asian women residing in California during the period 1988–2004.
MATERIALS AND METHODS
Cancer incidence data for this analysis were obtained from the California Cancer Registry (CCR), which is part of the National Cancer Institute’s SEER Program and adheres to the highest quality standards. Reporting of cancer cases to the CCR is mandated by state law and data sharing agreements with 13 states yield an estimated completeness rate exceeding 98 percent. Women with thyroid cancer (International Classification of Diseases for Oncology version 3 (ICD-O-3) site code C73.9) were included in the present analysis if they were diagnosed between January 1, 1988 and December 31, 2004, were residents of California at the time of diagnosis, were diagnosed with an invasive tumor of papillary or mixed papillary-follicular histology (ICD-O-3 histology codes 8050, 8260, or 8340–8344), and were Japanese (n=219), Chinese (n=623), Korean (n=255), Vietnamese (n=370), or Filipina (n=1100). Also included for comparison are non-Hispanic white (n=12,045) and non-Hispanic black (n=659) women meeting these same criteria.
Information on birthplace is routinely collected by the CCR from hospital medical records and death certificates. Our prior research shows that these data, when available, are highly accurate at the level of US- and foreign-born (11, 13); however, the completeness of these data is less than optimal. Among Asian women in the present analysis, medical record information regarding a specific birthplace was available for 67% of women (ranging from 60% among Chinese to 78% among Koreans) with death certificate information on birthplace available for an additional 1%. For women whose birthplace was not available from these two sources, we used the first five digits of the SSN to impute birthplace; these first five digits correspond with the year of issuance. Women whose SSN was issued prior to age 25 were assumed to be US-born, while women who were age 25 or older at issuance were assumed to be born outside the US (14, 15). This age cut-point was determined based on a comparison with self-reported birthplace derived from a series of epidemiologic studies of cancer patients (11), and maximizes predictive value and minimizes misclassification as determined by examining receiver operating characteristic curves, which plots sensitivity by (1-specificity). Using this method, the age cut-point was the same across Asian ethnic subgroups. The single age cut-point was also confirmed by using a logistic regression model with age at SSN issue as a continuous predictor of foreign-born status. Thirty percent (ranging from 19% among Koreans to 37% among Chinese) of the birthplace data were determined by this method. For the remaining 2% of women for whom SSN information was not available, birthplace was randomly assigned based on ethnic- and birthplace-specific distributions of all cancer cases reported to the registry.
Population estimates by sex, ethnicity, and five-year age groups were obtained from the 1990 and 2000 Census Summary Files 3 (SF-3) for the state of California. Data from the 20% Integrated Public-Use Microdata Sample (IPUMS) of the censuses were used to estimate the percent of foreign-born persons, by age, for each sex and ethnic subgroup; estimates were smoothed using the smooth spline function in the R statistical software package (16–19). For intercensal years, the percent of foreign-born persons was estimated using linear interpolation and extrapolation methods. Estimates were adjusted to match total population estimates, by age and year, as provided by the California Department of Finance (for years 1988–1989) and the US Census (for years 1990–2004).
California data were uploaded into SEER*Stat software (20) which was used to compute age-adjusted and age-specific incidence rates per 100,000 women, standardized to the 2000 US standard million population, and 95% confidence intervals (CI). Incidence rate ratios (IRR) and corresponding CIs were calculated to compare incidence rates for foreign-born compared to US-born women.
RESULTS
For women diagnosed with thyroid cancer during the 1988–2004 study period, the proportions born in the US were: 29% for Chinese, 44% for Japanese, 13% for Filipina, 9% for Korean, and 16% for Vietnamese. By comparison, for the general female California population, the proportions were: 30% for Chinese, 60% for Japanese, 29% for Filipina, 21% for Korean, and 23% for Vietnamese. Among these five Asian ethnic groups, age-adjusted papillary thyroid cancer incidence rates varied 2.2-fold, from 6.18 per 100,000 (95% CI: 5.37–7.08) for Japanese women to 13.70 per 100,000 (95% CI: 12.89–14.54) for Filipina women (Table 1). Vietnamese and Filipina women experienced incidence rates that were significantly greater than women in the other three Asian ethnic groups and NH white women (8.01 per 100,000). Women in all Asian ethnic groups experienced rates that were substantially greater than those observed among NH black women (3.73 per 100,000).
Table 1.
Age-adjusteda papillary thyroid cancer incidence rates (per 100,000) in women, California 1988–2004.
| Ethnicity |
|
|||
|---|---|---|---|---|
| Casesb | Populationb | Rate | 95% CI | |
| White (non-Hispanic) | 12,045 | 142,863,244 | 8.01 | 7.86 – 8.15 |
| Black (non-Hispanic) | 659 | 19,418,799 | 3.73 | 3.44 – 4.03 |
| Asian | ||||
| Japanese | 219 | 3,066,109 | 6.18 | 5.37 – 7.08 |
| Chinese | 623 | 7,985,862 | 7.33 | 6.76 – 7.94 |
| Korean | 255 | 2,914,486 | 8.76 | 7.69 – 9.94 |
| Vietnamese | 370 | 3,264,276 | 12.66 | 11.32 – 14.12 |
| Filipina | 1,100 | 7,809,502 | 13.70 | 12.89 – 14.54 |
Standardized to the 2000 United States population.
17-year aggregate.
Japanese women born outside the US had a significantly higher rate of thyroid cancer than US-born Japanese women (IRR=1.55, 95% CI: 1.17–2.08), whereas the opposite pattern was observed among Chinese (IRR=0.48, 95% CI: 0.40–0.59) and Filipina women (IRR=0.74, 95% CI: 0.58–0.96), among whom US-born women had significantly higher thyroid cancer rates (Table 2). US-born Korean women also experienced a higher incidence of thyroid cancer than foreign-born women; however, this difference was not statistically significant. A similar pattern was observed for Vietnamese women but the rate among those born in the US was unstable due to the small number of cases and is not shown.
Table 2.
Age-adjusteda papillary thyroid cancer incidence rates (per 100,000) in Asian American women by birthplace, California 1988-2004.
| Ethnicity | Foreign-born | US-born | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Casesb | Populationb | Rate | 95% CI | Casesb | Populationb | Rate | 95% CI | Rate ratioc | 95% CI | p-value | |
| Japanese | 123 | 1,236,742 | 7.61 | 6.29 – 9.21 | 96 | 1,829,367 | 4.90 | 3.94 – 6.01 | 1.55 | 1.17 – 2.08 | 0.002 |
| Chinese | 440 | 5,626,259 | 6.22 | 5.64 – 6.89 | 183 | 2,359,603 | 12.90 | 10.97 – 15.06 | 0.48 | 0.40 – 0.59 | <0.001 |
| Korean | 231 | 2,306,719 | 8.48 | 7.39 – 9.77 | 24 | 607,767 | 14.08 | 7.49 – 23.77 | 0.60 | 0.35 – 1.16 | 0.13 |
| Vietnamese | 311 | 2,524,663 | 11.31 | 10.01 – 12.82 | * | * | |||||
| Filipina | 956 | 5,577,737 | 13.26 | 12.42 – 14.18 | 144 | 2,231,765 | 17.84 | 13.97 – 22.47 | 0.74 | 0.58 – 0.96 | 0.02 |
Standardized to the 2000 United States standard million population.
17 year aggregate.
Ratio of incidence rate for foreign-born to rate for US-born.
Rates are unstable due to the small number of cases.
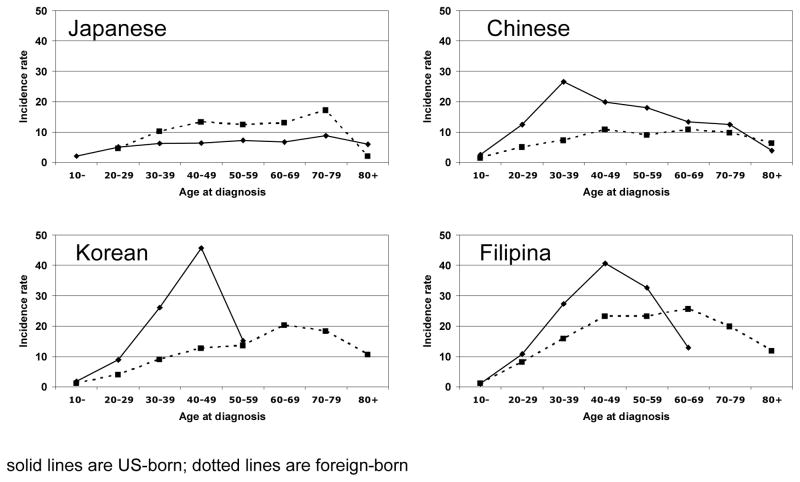
Figure 2 illustrates age-specific incidence rates for US- and foreign-born women by ethnic group. For Japanese women, the age-specific patterns among both US- and foreign-born were similar with a slow steady increase in rates until age 70, followed by a decline. For Chinese, Korean, Vietnamese (data not shown), and Filipina women born outside the US, we saw a similar pattern of increasing incidence until age 70, after which rates declined. However, among women from the same ethnic groups but born in the US, we observed a substantially elevated incidence during the reproductive and menopausal years. The age-specific incidence rates for US-born Chinese women age 20–59 are statistically significantly greater than those observed for foreign-born Chinese women. For Korean and Filipina women, incidence rates among US-born (compared to foreign-born) women were significantly higher for the 30–49 year old age groups.
Figure 2.
Age-specific papillary thyroid cancer incidence rates (per 100,000) in Asian American women by birthplace, California 1988–2004. solid lines are US-born; dotted lines are foreign-born
DISCUSSION
In the present study, we found marked differences in the age-specific incidence of papillary thyroid cancer according to birthplace among Chinese, Korean, Filipina, and Vietnamese women residing in the US. Among foreign-born women, rates increased until about age 70 and declined thereafter. In US-born women, incidence rates peaked during the reproductive years and, for Chinese women, plateaued during the menopausal period, mirroring the pattern seen in NH white women. The difference in age-specific patterns between immigrants and subsequent generations suggests that modifiable early and young-adult exposures, particularly those specific to immigration and acculturation of these Asian ethnic groups (e.g., goitrogenic exposures, diet, body size, and menstrual and reproductive events), may have a strong influence on the development of papillary thyroid cancer. In Japanese women, the incidence patterns were similar for US- and foreign-born women and followed the pattern observed for men and NH black women. Different incidence patterns between US-born Japanese and other US-born Asian women may reflect dissimilarities in migration patterns, acculturation, or culture-specific exposures and behaviors; however, we have no specific hypothesis at present as to specifically what these factors may be.
Differences in the prevalence of immigration- and acculturation-specific exposures and life events in young Asian American women and their correspondence with the observed incidence rates may provide clues as to the relative importance of various exposures in influencing disease risk. A case-control study of breast cancer among young (age<56) Chinese, Japanese, and Filipina women in California and Hawaii examined differences in risk factors among controls by birthplace and migration patterns. In that study, the prevalence of early (age ≤12) menarche was substantially higher in US-born Asian women (and young white women from a comparable study) than in foreign-born Asian American women (21). Weight and body mass index (BMI) were also found to be significantly higher in US-born than foreign-born Asian women (22). The relationship between age at menarche and papillary thyroid cancer risk has proven to be complex, with possible modification by race and age (6); however, it appears to be an important thyroid cancer risk factor (6, 23). Greater BMI has been fairly consistently associated with increased risk (24–28). Reproductive history has also been increasingly implicated in the etiology of thyroid cancer. Parous women are at greater risk of thyroid cancer than nulliparous women (6, 23, 29). Among the Asian American controls in the previous breast cancer study, US-born women and those immigrating to the US before age 36 were more likely to be parous than older immigrants (21). Age at first full-term pregnancy, has not been associated with thyroid cancer risk in most studies (23, 27, 30, 31), nor did it differ by nativity or age at immigration in the previous case-control study (21). Age at last full-term pregnancy and recency of the last pregnancy have both been associated with thyroid cancer risk (6, 27, 29); however, we have no data on how these factors differ by nativity and immigration status. Thus, it may be these later aspects of reproductive history that are influencing the birthplace-specific incidence patterns we observe.
Ethnic and nativity differences in incidence patterns may also be related to differences in sex steroid hormone levels. Populations which experience low thyroid cancer rates during early and mid-adulthood (i.e., men, black women, and foreign-born Asian women), tend to have higher androgen levels (overall, premenopausally, or during pregnancy) than white women and US-born Asian women (22, 32–34). In addition, estrogen levels have been observed to be 50% lower among the least westernized Asian women residing in the US compared to 3rd generation US-born Asian women (22). While a hypothesis of high estrogen levels being associated with the higher thyroid cancer rates among young women is not consistent with the higher total and free estradiol levels that have been observed among black compared to white women (33, 34), one study found no black-white difference in estrogen levels during pregnancy (32), perhaps suggesting that a relatively greater transient increase in estrogen levels during this critical period may somehow play a role in the increase incidence rates. However, while the availability of these limited observations may provide clues to the role of hormones and menstrual and reproductive history in thyroid cancer etiology, further research is clearly needed to understand the complex relationships between these exposures and the observed differences in ethnic- and birthplace-specific papillary thyroid cancer incidence rate patterns.
We also found significantly higher age-adjusted incidence rates among US-born Chinese and Filipina women compared to their foreign-born counterparts, whereas the converse was true among Japanese. The only previous study to examine these associations found a similar pattern among Japanese (i.e., higher rates in foreign-born) and Chinese women (i.e., higher rates in US-born; albeit a much less pronounced difference than we observed) but the opposite among Filipina women (i.e., higher rates among foreign-born) (10). This discrepancy may be explained by the different time periods and regional populations included in the studies. The present study covered a period, on average, 16 years later than the study by Rossing et al. (10), and included a substantially larger and perhaps more homogeneous population in terms of immigration, whereas the study by Rossing et al. (10) included populations from Hawaii, the San Francisco Bay Area, and western Washington. However, the discrepancies are also likely to result, at least in part, from methodologic differences. Rossing et al. (10) randomly imputed birthplace for the 19% of Asian cancer cases who had unknown birthplace (compared to 2% random imputation in the present study), thereby assuming the same birthplace distribution for women with missing data as for those with known data, taking into account age, race, and area of residence. However, our research has shown that cancer cases with missing birthplace data are more likely to be alive, of younger age, and US-born than those with known data, and thus, random imputation of birthplace will underestimate rates in US-born and overestimate them in foreign-born persons (11, 13, 35, 36). Indeed, our rates for US-born Chinese and Filipina women were substantially higher than those observed in the previous study and our rates for foreign-born Japanese and Filipina women were substantially lower.
The state of California has the largest and fastest-growing Asian population in the nation (37), as well as anywhere outside of Asia, making our enhanced population-based cancer surveillance data a powerful resource for examining patterns of cancer among racial/ethnic groups by immigration status. Understanding the factors influencing the differences in the ethnic- and birthplace-specific incidence patterns of thyroid and other cancers is important for cancer prevention efforts. The present study of these differences is based on a validated imputation method and uses a substantially larger population-based sample than was used in the previous report (10), resulting in more precise rate estimates. Nonetheless, data are still imputed for 30% of cancer cases and, while an improvement over prior imputation methods, our method, with sensitivity and specificity of 84% and 80%, respectively, still results in some misclassification. In addition, despite the large population, some rates could not be precisely estimated due to the small number of cases in some age-specific US-born ethnic groups. These age-specific patterns, however, have not been previously reported by birthplace and provide important insight into the incidence of papillary thyroid cancer.
In summary, we used enhanced surveillance data to examine the influence of birthplace on papillary thyroid cancer incidence rates in Asian women residing in California. We observed that the pattern of age-specific rates is strongly influenced both by ethnicity and birthplace, with most US-born Asian women having a substantially increased incidence of this cancer during early and mid adult life. Further research is needed to elucidate how immigration and acculturation experiences influence other life events and exposures which may account for these elevated rates as this will be critical for cancer prevention efforts.
Acknowledgments
The authors thank Dr. Tim Miller, Ms. Rita Leung, Ms. Sarah Shema, and Ms. Jane Pham for their help with data collection for this study.
This research was supported by the National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results (SEER) Program under contract N01-PC-35136 awarded to the Northern California Cancer Center (NCCC) and by a SEER Rapid Response Surveillance Study under contracts N01-PC-35136 and N01-PC-35139. The collection of cancer incidence data used in this study was supported by the California Department of Health Services (CDHS) as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the NCI’s SEER Program under contract N01-PC-35136 awarded to the NCCC, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute (PHI); and the Centers for Disease Control and Prevention’s (CDCP) National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to PHI. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, CDHS, NCI, and CDCP or their contractors and subcontractors is not intended nor should be inferred.
References
- 1.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19:227–56. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cresswell SL, Gomez SL, Clarke CA, Chang ET, Keegan THM, McClure LA, et al. Cancer Incidence and Mortality in the Greater Bay Area, 1988–2005. Fremont, CA: Northern California Cancer Center; 2008. Contract No.: Document Number. [Google Scholar]
- 3.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. IARC Scientific Publications No 160. IX. Lyon: IARC; 2007. Cancer Incidence in Five Continents. Contract No.: Document Number. [Google Scholar]
- 4.Ron E. Thyroid cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 2. New York: Oxford University Press; 1996. pp. 1000–21. [Google Scholar]
- 5.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER website. [updated 2010; cited]; Available from. [Google Scholar]
- 6.Sakoda LC, Horn-Ross PL. Reproductive and menstrual history and papillary thyroid cancer risk: the San Francisco Bay Area Thyroid Cancer Study. Cancer Epidemiol Biomarkers Prev. 2002;11(1):51–7. [PubMed] [Google Scholar]
- 7.Kem R, Chu KC. Cambodian cancer incidence rates in California and Washington, 1998–2002. Cancer. 2007;110:1370–5. doi: 10.1002/cncr.22914. [DOI] [PubMed] [Google Scholar]
- 8.Kwong SL, Chen MS, Snipes KP, Bal DG, Wright WE. Asian subgroups and cancer incidence and mortality rates in California. Cancer. 2005;104:2975–81. doi: 10.1002/cncr.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prehn A, Lin S, Clarke C, Packel L, Lum R, Lui S, et al. Cancer Incidence in Chinese, Japanese and Filipinos in teh US and Asia, 1988–1992. Union City, CA: Northern California Cancer Center; 1999. [Google Scholar]
- 10.Rossing MA, Schwartz SM, Weiss NS. Thyroid cancer incidence in Asian migrants to the United States and their descendents. Cancer Causes and Control. 1995;6(5):439–44. doi: 10.1007/BF00052184. [DOI] [PubMed] [Google Scholar]
- 11.Gomez SL, Glaser SL, Kelsey JL, Lee MM. Bias in completeness of birthplace data for Asian groups in a population-based cancer registry (United States) Cancer Causes Control. 2004;15:243–53. doi: 10.1023/B:CACO.0000024244.91775.64. [DOI] [PubMed] [Google Scholar]
- 12.Lin SS, Clarke CA, O'Malley CD, Le GM. Studying cancer incidence and outcomes in immigrants: methodologic concerns. Am J Public Hlth. 2002;92:1757–9. doi: 10.2105/ajph.92.11.1757-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez SL, Glaser SL. Quality of birthplace information obtained from death certificates for Hispanics, Asians, and Pacific Islanders. Ethn Dis. 2004;14:292–5. [PubMed] [Google Scholar]
- 14.Block G, Matanoski GM, Seltser RS. A method for estimating year of birth using social security number. Am J Epidemiol. 1983;118:377–95. doi: 10.1093/oxfordjournals.aje.a113645. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates D, Chambers J, Dalgaard P, et al. R Program [R] 2.8.0. The R Foundation for Statistical Computing; [Google Scholar]
- 17.Chang ET, Keegan TH, Gomez SL, Le GM, Clarke CA, So SK, et al. The burden of liver cancer in Asians and Pacific Islanders in the Greater San Francisco Bay Area, 1990 through 2004. Cancer. 2007;109:2100–8. doi: 10.1002/cncr.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez SL, Le GM, Miller T, et al. Cancer Incidence Among Asians in the Greater Bay Area, 1990–2002. Fremont, CA: Northern California Cancer Center; 2005. [Google Scholar]
- 19.Keegan TH, Gomez SL, Clarke CA, Chan JK, Glaser SL. Recent trends in breast cancer incidence among 6 Asian groups in the Greater Bay Area of Northern California. Int J Cancer. 2007;120:1324–9. doi: 10.1002/ijc.22432. [DOI] [PubMed] [Google Scholar]
- 20.Surveillance Research Program. SEER*Stat Software. 6.1.4. 2005. [Google Scholar]
- 21.Wu AH, Ziegler RG, Pike MC, Nomura AMY, West DW, Kolonel LN, et al. Menstrual and reproductive factors and risk of breast cancer in Asian-Americans. Br J Cancer. 1996;73(5):680–6. doi: 10.1038/bjc.1996.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk RT, Fears TR, Hoover RN, Pike MC, Wu AH, Nomura AMY, et al. Does place of birth influence endogenous hormone levels in Asian-American women? Br J Cancer. 2002;87:54–60. doi: 10.1038/sj.bjc.6600339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negri E, Dal Maso L, Ron E, La Vecchia C, Mark SD, Preston-Martin S, et al. A pooled analysis of case-control studies of thyroid cancer II: Menstrual and reproductive factors. Cancer Causes Control. 1999;10(2):143–55. doi: 10.1023/a:1008880429862. [DOI] [PubMed] [Google Scholar]
- 24.Dal Maso L, La Vecchia C, Franceschi S, Preston-Martin S, Ron E, Levi F, et al. A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control. 2000 Feb;11(2):137–44. doi: 10.1023/a:1008938520101. [DOI] [PubMed] [Google Scholar]
- 25.Engeland A, Tretli S, Akslen LA, Bjorge T. Body size and thyroid cancer in two million Norwegian men and women. British Journal of Cancer. 2006:1–5. doi: 10.1038/sj.bjc.6603249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guenel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. Am J Epidemiol. 2007 Nov 15;166(10):1140–9. doi: 10.1093/aje/kwm204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossing MA, Voigt LF, Wicklund KG, Daling JR. Reproductive factors and risk of papillary thyroid cancer in women. Am J Epidemiol. 2000;151(8):765–72. doi: 10.1093/oxfordjournals.aje.a010276. [DOI] [PubMed] [Google Scholar]
- 28.Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6:863–73. [PubMed] [Google Scholar]
- 29.Brindel P, Doyon F, Rachedi F, Boissin J-L, Sebbag J, Shan L, et al. Menstrual and reproductive factors in the risk of differentiated thyroid carcinoma in native women in French Polynesia: a population-based case-control study. Am J Epidemiol. 2008;15:219–29. doi: 10.1093/aje/kwm288. [DOI] [PubMed] [Google Scholar]
- 30.Memon A, Darif M, Al-Saleh K, Suresh A. Epidemiology of reproductive and hormonal factors in thyroid cancer: evidence from a case-control study in the Middle East. Int J Cancer. 2002;97:82–9. doi: 10.1002/ijc.1573. [DOI] [PubMed] [Google Scholar]
- 31.Truong T, Orsi L, Dubourdieu D, Rougier Y, Hemon D, Guenel P. Role of goiter and of menstrual and reproductive factors in thyroid cancer: a population-based case-control study in New Caledonia (South Pacific), a very high incidence area. Am J Epidemiol. 2005;161:1056–65. doi: 10.1093/aje/kwi136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potischman N, Troisi R, Thadhani R, Hoover RN, Dodd K, Davis WW, et al. Pregnancy hormone concentrations across ethnic groups: implications for later cancer risk. Cancer Epidem Biomarkers Prev. 2005;14:1514–20. doi: 10.1158/1055-9965.EPI-04-0869. [DOI] [PubMed] [Google Scholar]
- 33.Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE. Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomarkers Prev. 2005;14:2147–53. doi: 10.1158/1055-9965.EPI-04-0944. [DOI] [PubMed] [Google Scholar]
- 34.Ukkola O, Gagnon J, Rankinen T, Thompson PA, Hong Y, Leon AS, et al. Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol. 2001;145:1–9. doi: 10.1530/eje.0.1450001. [DOI] [PubMed] [Google Scholar]
- 35.Gomez SL, Kelsey JL, Glaser SL, Lee MM, Sidney S. Immigration and acculturation in relation to health and health-related risk factors among specific Asian subgroups in a health maintenance organization. Am J Public Hlth. 2004;94:1977–84. doi: 10.2105/ajph.94.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SS, O'Malley CD, Lui SW. Factors associated with missing birthplace information in a population-based cancer registry. Ethn Dis. 2001;11:598–605. [PubMed] [Google Scholar]
- 37.Barnes JS, Bennett CE. US Census Bureau. 2002. [Google Scholar]