Abstract
Background:
Migraine is a common, disabling headache disorder that leads to lost quality of life and productivity. We investigated whether a proactive approach to patients with migraine, including an educational intervention for general practitioners, led to a decrease in headache and associated costs.
Methods:
We conducted a pragmatic randomized controlled trial. Participants were randomized to one of two groups: practices receiving the intervention and control practices. Participants were prescribed two or more doses of triptan per month. General practitioners in the intervention group received training on treating migraine and invited participating patients for a consultation and evaluation of the therapy they were receiving. Physicians in the control group continued with usual care. Our primary outcome was patients’ scores on the Headache Impact Test (HIT-6) at six months. We considered a reduction in score of 2.3 points to be clinically relevant. We used the Kessler Psychological Distress Scale (K10) questionnaire to determine if such distress was a possible effect modifier. We also examined the interventions’ cost-effectiveness.
Results:
We enrolled 490 patients in the trial (233 to the intervention group and 257 to the control group). Of the 233 patients in the intervention group, 192 (82.4%) attended the consultation to evaluate the treatment of their migraines. Of these patients, 43 (22.3%) started prophylaxis. The difference in change in score on the HIT-6 between the intervention and control groups was 0.81 (p = 0.07, calculated from modelling using generalized estimating equations). For patients with low levels of psychological distress (baseline score on the K10 ≤ 20) this change was −1.51 (p = 0.008), compared with a change of 0.16 (p = 0.494) for patients with greater psychological distress. For patients who were not using prophylaxis at baseline and had two or more migraines per month, the mean HIT-6 score improved by 1.37 points compared with controls (p = 0.04). We did not find the intervention to be cost-effective.
Interpretation:
An educational intervention for general practitioners and a proactive approach to patients with migraine did not result in a clinically relevant improvement of symptoms. Psychological distress was an important confounder of success. (Current Controlled Trials registration no. ISRCTN72421511.)
Migraine is a common, disabling headache disorder that results in lost quality of life and productivity, both during and between attacks.1–8 Many patients with migraine suffer unnecessarily because they are not using their medications appropriately, or they are unaware of the possibility of prophylactic treatment. In the Netherlands, 3% of patients who take triptans consume 12 or more doses of the drug each month.9 These patients account for almost half of the costs associated with triptan use.10 In addition, although more than 25% of patients with migraine have two or more attacks each month, making them eligible for preventive treatment, only 8%–12% of patients use prophylaxis.2,3,11–13 More than half of the patients with migraine in Dutch primary care who have an indication for prophylaxis have not discussed that option with their general practitioner.13
We investigated whether a proactive approach to identifying patients with migraine who are receiving suboptimal treatment (i.e., inviting them to a consultation to evaluate their current treatment regimen and advising them about the options available for treating their migraine) could increase the use of preventive treatment and reduce the overuse of triptans, thereby reducing headache recurrence and associated costs. Our intervention involved educational sessions for general practitioners. Earlier studies aimed at reducing the overuse of other medications in primary care, such as benzodiazepines and acid-repressive drugs, showed that a proactive intervention led to a reduction in the use of medications.14,15
Because most patients with migraine in the Netherlands are treated by their general practitioner, we evaluated the costs and effects of a proactive approach to migraine in primary care. We included patients who had two or more attacks per month, because improvement could be reasonably expected in this group.
Methods
This study involved 64 general practices in a semiurban area in the Netherlands, between 2007 and 2009. We used a cluster randomized design with general practice as the unit of randomization to prevent contamination.16
The study was approved by the Ethical Committee of the Leiden University Medical Center.
Participants
General practitioners were asked to participate in a trial aimed at improving the treatment of migraine in primary care. We provided the physicians with as little information as possible about the intervention to avoid changes in the behaviour of participants in the control group.
Patients aged 18 years or older were selected from the electronic patient record by the researchers in consultation with the general practitioners. Patients were selected using their prescription data. Patients were eligible for the study if they had received prescriptions for 24 or more doses of triptan in the previous 12 months, or 12 or more doses in the previous 6 months. Exclusion criteria were cluster headaches, cognitive impairment, a severe psychiatric disorder, terminal illness or insufficient understanding of the Dutch language.
General practitioners sent eligible patients an invitation letter, together with an information booklet, an informed consent form and a baseline questionnaire. Patients were informed that the aim of the study was to improve the treatment of migraine in primary care and that they might be invited for an evaluation and consultation with their general practitioner. Participants returned the consent form and baseline questionnaire to the researchers.
Randomization
We randomized the practices using a computer-generated list after eligible patients had been selected. The randomization was done by a statistician who was unaware of the characteristics of the practices. We stratified practices based on the median percentage of the population of all practices who used two or more doses of triptans per month (i.e., practices where ≥ 5.9% of the population used ≥ 2 doses of triptan per month, and practices where < 5.9% of the population used ≥ 2 doses of triptan per month).
Intervention
Physicians in the intervention group received two training sessions, each three hours in length, from two general practitioners with experience in the management of migraine (Jeanet Blom and Frans Dekker). The protocol was based on the headache guideline of the Dutch College of General Practitioners.17 The training included diagnostic criteria for headache (migraine, tension headache, and medication-overuse headache), acute and prophylactic treatment, and treatment of medication-overuse headache (see Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110908/-/DC1).
General practitioners were asked to invite patients who had agreed to meet to an evaluation/consultation. During this meeting, the physicians followed a step-wise approach. First, they reconsidered the diagnosis of migraine. If the diagnosis was confirmed, they advised the patient on the appropriate use of medication. Patients who had two or more migraines per month were offered prophylactic treatment. On agreement between the patient and physician, a β-blocker was started at a low dose and gradually increased to the dose with the greatest effect and acceptable adverse effects. If this treatment was not effective, patients were prescribed sodium valproate.18,19 In cases of medication-overuse headache, patients were advised to stop all pain medication for three months, after which their headaches were re-evaluated.9 Notwithstanding these guidelines, physicians were free to conduct the intervention in their own way because of the pragmatic character of our trial.
Usual care
Physicians in the control group were asked to continue to provide their usual care.16 These physicians received no additional information on the diagnosis and treatment of headache, and they were not informed as to which patients had agreed to participate in the study. Control patients were not told to which group they had been allocated.
Outcome measures
The primary outcome for the effectiveness of the intervention was patients’ scores on the Dutch version of the Headache Impact Test (HIT-6), a six-item questionnaire measuring the severity and impact of headache on a patient’s life.20,21 The minimal clinically important difference on the HIT-6 is a reduction of 2.3 points on a scale ranging from 36 to 78, with a higher score representing more complaints of headache.22
We preplanned two subgroup analyses because we expected to see the largest effect among patients not using prophylactic medication at baseline, and among patients having two or more attacks each month.
One of our secondary outcomes was change in patients’ scores on the EuroQol questionnaire. This questionnaire measures quality of life in five domains (mobility, self-care, main activity, social relationships and pain), with the total score ranging from −0.33 (poor quality of life) to 1.0 (good quality of life).23,24
In addition to completing the questionnaire, patients were asked to keep a headache diary in which they recorded their medication use, the frequency, severity and duration of migraine attacks, and absences from work due to migraine during a four-week period.
We used the Kessler Psychological Distress Scale (the “K10” questionnaire) to determine whether such distress was a possible modifier of effect. Scores on this scale range from 10 (no distress) to 50 (severe distress).25
To assess study-induced changes in migraine treatment by the physicians in the control group, data from the electronic patient record on prescriptions and consultation parameters were compared with those from 12 months earlier (baseline).
Follow-up
Questionnaires were sent to all selected patients at baseline. Participants received follow-up questionnaires 3, 6 and 12 months after their evaluation/consultation. Questionnaires could be completed on paper or on a website. Nonrespondents received a postal reminder after five weeks and a telephone reminder after six weeks. At baseline and at 12 months, prescription data and data on consultation frequency were collected from the electronic patient record.
Sample size calculation
We assumed that 50% of the selected patients would participate and that loss to follow-up would be 10%.26 To detect a change in score of 2.3 points on the HIT-6 with 80% power at a 5% significance level and an intracluster coefficient of 0.01–0.02, we needed to recruit 60 primary care practices with 900 potential patients.27
Statistical analysis
Statistical analysis was done using the intention-to-treat principle, and results are reported at the level of the individual patient. We used generalized estimating equations, correcting for age, sex, baseline scores and clustering of patients per primary care practice.28 We used independent-sample t tests to analyze the nonparticipants (people who did not give informed consent) and nonresponders (people who did not complete the questionnaire after having given informed consent), and to compare attendees (patients who attended follow-up) and nonattendees (patients who did not attend follow-up). In the case of skewed data, we used the nonparametric independent samples test.
For missing data, we used multiple imputation by chained equations, with five iterations for the switching regression model.29 Results from the the imputed data were compared with analyses of the measured data for a sensitivity analysis exploring selective loss to follow-up.30
We analyzed the cost-effectiveness of the intervention from a societal perspective during the one-year follow-up (see Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110908/-/DC1).
Results
Participants
We approached 205 primary care practices, 64 of which (31.2%) agreed to participate. Concern about the workload involved was the main barrier to participation.
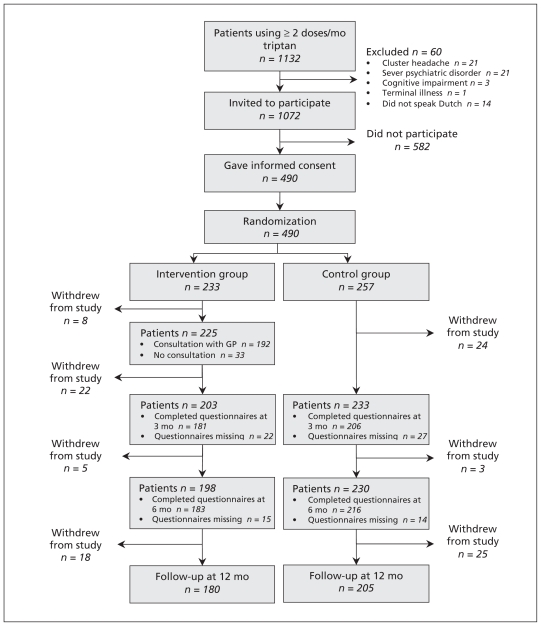
Of the 1072 patients eligible for our study, 490 (45.7%) participated. Figure 1 shows the flow of patients through the study. The baseline characteristics of the participants in the control and intervention groups were similar (Table 1).
Figure 1:
The flow of patients through the trial.
Table 1:
Baseline characteristics of the 490 patients who participated in the trial
| Characteristic | Mean (SD)* | |
|---|---|---|
| Intervention n = 233 |
Control n = 257 |
|
| Age, yr | 47.5 (10.7) | 47.0 (9.4) |
| Female, no. (%) | 195 (83.7) | 223 (86.8) |
| Triptans used per month during past year | 5.2 (4.3) | 5.1 (3.8) |
| Consultations for migraine in past year | 0.81 (1.2) | 0.80 (1.3) |
| Prophylaxis, no. (%)† | 76 (32.2) | 71 (27.6) |
| β-blocker | 54 (23.6) | 54 (20.6) |
| Sodium valproate | 8 (4.3) | 4 (1.9) |
| Other prophylaxis | 9 (5.2) | 11 (5.4) |
| Headache | n = 229 | n = 254 |
| HIT-6 score | 61.6 (5.5) | 61.9 (5.0) |
| Depressive/anxiety symptoms | n = 228 | n = 250 |
| K10 score | 19.6 (6.8) | 18.4 (5.7) |
| Quality of life | n = 230 | n = 254 |
| EurQol score | 0.80 (0.21) | 0.83 (0.18) |
| Migraine attacks during previous month | n = 219 | n = 235 |
| < 2 attacks/mo, % | 81 (34.8) | 89 (34.6) |
| ≥ 2 attacks/mo, % | 152 (65.2) | 168 (65.4) |
| Absences from work, d/mo | n = 205 | n = 224 |
| 0.47 (1.12) | 0.59 (1.28) | |
| Absences from work in previous 3 months | n = 220 | n = 251 |
| 1.29 (3.13) | 1.48 (2.60) | |
| Triptans used in previous month | n = 198 | n = 212 |
| 5.11 (4.37) | 4.88 (3.61) | |
Note: HIT-6 = Headache Impact Test, K10 = Kessler Psychological Distress Scale, SD = standard deviation.
Unless stated otherwise.
Data missing for five patients in the intervention group and four patients in the control group.
Nonparticipants and nonresponders
Participants did not differ from nonparticipants in terms of age, sex, triptan use at baseline, triptans prescribed during the study period, consultations for headache in the previous year, HIT-6 score, EuroQol score and use of prophylactic medication at baseline or at 12 months (data not shown).
Compared with responders, nonresponders were slightly younger (44.7 v. 47.8 yr at 6-months follow-up [p = 0.009], and 44.5 v. 47.9 yr at 12-months follow-up [p = 0.003]).
Nonresponders at the six-month follow-up had a slightly higher score on the baseline K10 questionnaire than responders (20.8 v. 18.6, p = 0.007), suggesting a higher level of psychological distress.
No differences were found between nonresponders and responders in terms of sex, number of triptans prescribed at baseline, HIT-6 or EuroQol scores at baseline, use of prophylactic medication at 12 months, and number of triptan doses prescribed during the study period (data not shown).
Nonresponders at six months in the intervention group had a slightly lower baseline EuroQol score than nonresponders in the control group (median score 0.78 v. 0.84, p = 0.015). No differences were found on any of the other parameters at 6 and 12 months (data not shown).
Outcomes
The evaluation/consultation was attended by 192 (82.4%) of the patients in the intervention group (Figure 1). There were no differences between the baseline characteristics of attendees and those of nonattendees (data not shown).
In the intervention group, 28.3% (43/152) of patients not using prophylaxis before the trial received a prescription for a prophylactic agent, compared with 14.2% (25/176) of patients not using prophylaxis in the control group (Table 2). However, the number of prescriptions for triptan was comparable between the two groups (Table 2). Medication-overuse headache was diagnosed in seven patients, each of whom was advised to stop all pain medications. Five of these patients lowered their use of pain medications, but none of the patients stopped using them completely (data not shown).
Table 2:
Medications used by participants during the trial
| Medication | Intervention, no. (%)* n = 226† |
Control, no. (%)* n = 247† |
p value |
|---|---|---|---|
| Prophylactic agent | |||
| Prophylaxis at baseline | 74 (32.7) | 71 (28.7) | |
| Stopped prophylaxis during trial | 18 (24.3) | 15 (21.1) | 0.40‡ |
| No prophylaxis at baseline | 152 (67.3) | 176 (71.3) | |
| Started prophylaxis during trial | 43 (28.3) | 25 (14.2)‡ | 0.001‡ |
| Triptans | |||
| Doses per month, mean (SE) | 4.80 (0.32) | 5.30 (0.29) | 0.72§ |
Note: SE = standard error.
Unless otherwise indicated.
Data not available for 7 patients in the intervention group and 10 patients in the control group.
χ2 test.
t test.
Intracluster coefficient
The intracluster coefficient of our study was −0.048, meaning that there was no clustering of headache complaints by general practice.9 Post hoc, we calculated a power of 87% for this study.
Primary outcome
At six months, the HIT-6 questionnaire was completed correctly by 80.2% (393/490) of the participants. The change in HIT-6 scores did not differ between the intervention and control groups (Table 3). Subgroup analyses showed some significant, but not clinically relevant, differences.22
Table 3:
Change in score on the headache inventory test at 3, 6 and 12 months for all participants and subgroups corrected for clustering, age, sex and score at baseline
| Subgroup/follow-up, mo | Intervention group | Control group | Adjusted between- group differences (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| No. of respondents | Mean change (SE) | No. of respondents | Mean change (SE) | |||
| Total group | ||||||
| 3 | 181 | −2.64 (0.44) | 205 | −2.11 (0.39) | −0.72 (−1.80 to 0.36) | 0.19 |
| 6 | 181 | −3.12 (0.40) | 212 | −2.55 (0.36) | −0.81 (−1.68 to 0.06) | 0.07 |
| 12 | 181 | −4.01 (0.47) | 202 | −2.97 (0.41) | −1.23 (−2.45 to −0.00) | 0.05 |
| Patients not using prophylaxis at baseline | ||||||
| 3 | 115 | −2.83 (0.55) | 135 | −2.24 (0.46) | −1.22 (−2.15 to −0.29) | 0.01 |
| 6 | 118 | −3.51 (0.48) | 142 | −2.69 (0.44) | −1.18 (−2.14 to −0.21) | 0.02 |
| 12 | 120 | −4.14 (0.54) | 137 | −2.81 (0.48) | −1.59 (−2.85 to −0.32) | 0.01 |
| Patients with > 2 attacks/mo at baseline | ||||||
| 3 | 130 | −3.14 (0.51) | 139 | −2.13 (0.46) | −1.01 (−2.40 to 0.39) | 0.16 |
| 6 | 128 | −3.48 (0.45) | 140 | −2.35 (0.44) | −1.26 (−2.28 to −0.24) | 0.02 |
| 12 | 124 | −4.33 (0.56) | 137 | −2.37 (0.48) | −2.02 (−3.35 to −0.70) | 0.003 |
| Patients with no prophylaxis who had ≥ 2 attacks/mo at baseline | ||||||
| 3 | 82 | −3.05 (0.66) | 88 | −1.92 (0.51) | −1.31 (−2.83 to 0.20) | 0.09 |
| 6 | 82 | −3.82 (0.52) | 90 | −2.61 (0.54) | −1.37 (−2.64 to −0.20) | 0.04 |
| 12 | 81 | −4.15 (0.61) | 91 | −2.15 (0.57) | −2.16 (−3.72 to −0.60) | 0.007 |
Note: CI = confidence interval, SE = standard error.
At 12 months, the HIT-6 questionnaire was completed by 78.2% (383/490) of participants. The intervention group reported a greater decrease in HIT-6 score than the control group (−4.01 v. −2.97). A similar difference was seen in all subgroups (Table 3).
For patients with low levels of psychological distress (baseline K10 score ≤ 20), the change in HIT-6 score at six months was −1.51 (p = 0.008), compared with 0.16 (p = 0.494) for patients with increased psychological distress,25 suggesting that psychological distress acted as an effect modifier.
No significant differences were found between the intervention and control groups in terms of the frequency, severity or duration of attacks, the number of days per month on which patients had headaches and absences from work (Table 4).
Table 4:
Adjusted difference in average change in score for secondary outcomes between the intervention and control groups at follow-up at 3, 6 and 12 months corrected for clustering, age, sex and baseline score
| Outcome at follow-up | Intervention group | Control group | Adjusted between- group differences (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| No. of respondents | Mean change (SE) | No. of respondents | Mean change (SE) | |||
| Migraine attacks, no./mo* | ||||||
| 3 | 173 | −0.43 (0.16) | 192 | −0.41 (0.13) | −0.02 (−0.41 to 0.38) | 0.94 |
| 6 | 171 | −0.37 (0.17) | 195 | −0.48 (0.13) | 0.07 (−0.33 to 0.46) | 0.75 |
| 12 | 172 | −0.83 (0.17) | 193 | −0.61 (0.15) | −0.29 (−0.63 to 0.04) | 0.08 |
| Headache, d/mo† | ||||||
| 3 | 170 | −0.44 (0.26) | 190 | −0.37 (0.25) | −0.06 (−0.67 to 0.54) | 0.84 |
| 6 | 168 | −0.51 (0.30) | 195 | −0.59 (0.20) | 0.20 (−0.37 to 0.77) | 0.49 |
| 12 | 168 | −1.08 (−0.26) | 191 | −0.32 (0.26) | −0.72 (−1.37 to −0.06) | 0.03 |
| Severity attacks, mean†‡ | ||||||
| 3 | 146 | −0.07 (0.05) | 164 | −0.08 (0.04) | −0.04 (−0.14 to 0.06) | 0.44 |
| 6 | 145 | −0.71 (0.05) | 163 | −0.11 (0.04) | 0.04 (−0.08 to 0.15) | 0.53 |
| 12 | 136 | −0.11 (0.05) | 166 | −0.03 (0.04) | −0.07 (−0.18 to 0.04) | 0.22 |
| Triptans used in previous month, no. | ||||||
| 3 | 122 | −0.44 (0.34) | 151 | −0.47 (0.30) | 0.10 (−0.70 to 0.90) | 0.81 |
| 6 | 117 | −0.81 (0.37) | 145 | −0.40 (0.35) | −0.14 (−0.72 to 0.44) | 0.64 |
| 12 | 116 | −1.72 (0.44) | 144 | −0.83 (0.32) | −0.79 (−1.68 to 0.104) | 0.08 |
| Absence from work, d/mo | ||||||
| 3 | 149 | −0.08 (0.11) | 165 | −0.11 (0.13) | −0.02 (−0.28 to 0.23) | 0.85 |
| 6 | 162 | −0.01 (0.17) | 185 | −0.24 (0.09) | 0.15 (−0.16 to 0.46) | 0.33 |
| 12 | 142 | 0.04 (0.12) | 166 | −0.06 (0.11) | 0.02 (−0.28 to 0.32) | 0.88 |
Note: CI = confidence interval, SE = standard error.
Attacks of maximum 72 h, attacks recurring within 24 h are considered to be a recurrence of the earlier attack.
Days with at least four hours of headache.
On a three-point scale (1 = mild, 2 = moderate, 3 = severe).
Sensitivity analyses after imputation of missing data did not substantially change the results (data not shown).
The intervention was not cost-effective (see Appendix 2).
Interpretation
We found no clinically relevant effect of a proactive approach to migraine in primary care for patients who were taking two or more doses of triptan per month. Among patients not using prophylactic medication at baseline who had two or more attacks per month, we saw a significant but not clinically relevant difference. Patients with a low level of psychological distress appeared to derive more benefit from the intervention, but the effect was not clinically relevant. The intervention was not cost-effective.
We saw no effect of the intervention on headache complaints at six months. The negative results could not be explained by selection bias, selective loss to follow-up of patients or behavioural changes in the physicians in the control group. However, there are some possible explanations for our results. First, the threshold of two or more prescribed doses of triptan per month proved too low when targeting patients who have two or more attacks of migraine each month. Second, many patients were already using prophylaxis, and room for improvement among these patients was less than expected. Third, the threshold of two or more attacks of migraine per month might have been considered too low by either the patient or the general practitioner, resulting in a decision not to start prophylaxis. Finally, the effectiveness of the currently available prophylactic agents has not been convincingly proven. It is estimated that fewer than 50% of patients respond well to these treatments.18,19
Other studies suggested that an educational program for general practitioners leads to better diagnostic accuracy and better treatment of migraine. However, these studies did not measure the effect in terms of patient outcomes,31 or they did not include a control group.32 A recent study evaluating an educational intervention for general practitioners with the aim of increasing the use of prophylactic medications led to 30% of the patients receiving at least one prescription for a prophylactic agent after six months.33 This result is similar to our findings.
Limitations
A possible limitation of our study is that the general practitioners and participants could not be blinded. Although physicians in the control group were not informed as to the content of the intervention, their participation in our study may have triggered them to update their knowledge on the treatment of migraine. In addition, patients may have been triggered to seek help for their condition. This attention bias may have led to an underestimation of the effect of the intervention.16
Another limitation is the pragmatic design of our study, which we chose to ensure external validity and to facilitate subsequent implementation. However, because we chose not to monitor the treatment given by the physicians, we were unable to influence the actual treatment that patients received. As such, participants may have received suboptimal treatment.
Conclusion
This intervention resulted in an increase in the prescribing of prophylactic treatments for migraine. However, we saw no clinically relevant beneficial effect of this approach on headache complaints at six months. It is possible that the intervention resulted in better treatment for patients not using prophylactic medication at baseline who had two or more attacks of migraine per month. Future interventions in primary care should target these patients. Also, it should be considered that an intervention might have less effect for patients with higher levels of psychological distress.
Supplementary Material
Acknowledgements
The authors thank all of the patients and general practitioners who contributed to this research, Professor R. Brand for performing the randomization procedure and Professor T. Stijnen for advice during the analyses. The study was funded by a grant from the Netherlands Organisation for Health Research and Development (grant no. 80-007022-98-07602) and a grant from NutsOhra health care grants (project no. SNO-T-0601-106). The project’s sponsors had no role in the study design nor in the collection, analysis, interpretation and reporting of the data.
See also research article by Li and colleagues at www.cmaj.ca/lookup/doi/10.1503/cmaj.110551 and commentary by Molsberger at www.cmaj.ca/lookup/doi/10.1503/cmaj.112032.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Jeanet Blom, Frans Dekker, Arie Knuistingh Neven, Frans Zitman, Michel Ferrari and Pim Assendelft conceived and designed the study. Antonia Smelt, Jeanet Blom and Pim Assendelft acquired the data. Antonia Smelt, Jeanet Blom, Frans Dekker, M. Elske van den Akker, Frans Zitman, Michel Ferrari and Pim Assendelft analyzed and interpreted the data. Antonia Smelt, Jeanet Blom, M. Elske van den Akker, Michel Ferrari and Pim Assendelft drafted the manuscript. All of the authors critically revised the manuscript for important intellectual content and approved the final version submitted for publication.
References
- 1.Brown JS, Neumann PJ, Papadopoulos G, et al. Migraine frequency and health utilities: findings from a multisite survey. Value Health 2008;11:315–21 [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME. The epidemiology of migraine. Am J Med 2005;118(Suppl 1):3S–10S [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–9 [DOI] [PubMed] [Google Scholar]
- 4.Terwindt GM, Ferrari MD, Tijhuis M, et al. The impact of migraine on quality of life in the general population: the GEM study. Neurology 2000;55:624–9 [DOI] [PubMed] [Google Scholar]
- 5.Stewart WF, Wood GC, Razzaghi H, et al. Work impact of migraine headaches. J Occup Environ Med 2008;50:736–45 [DOI] [PubMed] [Google Scholar]
- 6.Freitag FG. The cycle of migraine: patients’ quality of life during and between migraine attacks. Clin Ther 2007;29:939–49 [DOI] [PubMed] [Google Scholar]
- 7.Burton WN, Landy SH, Downs KE, et al. The impact of migraine and the effect of migraine treatment on workplace productivity in the United States and suggestions for future research. Mayo Clin Proc 2009;84:436–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandes JL. The migraine cycle: patient burden of migraine during and between migraine attacks. Headache 2008;48:430–41 [DOI] [PubMed] [Google Scholar]
- 9.Wiendels NJ, Knuistingh NA, Rosendaal FR, et al. Chronic frequent headache in the general population: prevalence and associated factors. Cephalalgia 2006;26:1434–42 [DOI] [PubMed] [Google Scholar]
- 10.Dekker F, Wiendels NJ, de Valk V, et al. Triptan overuse in the Dutch general population: a nationwide pharmacoepidemiology database analysis in 6.7 million people. Cephalalgia 2011;31: 943–52 [DOI] [PubMed] [Google Scholar]
- 11.Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47:355–63 [DOI] [PubMed] [Google Scholar]
- 12.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology 1999;53:537–42 [DOI] [PubMed] [Google Scholar]
- 13.Kol CM, Dekker F, Neven AK, et al. Acceptance or rejection of prophylactic medicine in patients with migraine: a cross-sectional study. Br J Gen Pract 2008;58:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorgels WJ, Oude Voshaar RC, Mol AJ, et al. Discontinuation of long-term benzodiazepine use by sending a letter to users in family practice: a prospective controlled intervention study. Drug Alcohol Depend 2005;78:49–56 [DOI] [PubMed] [Google Scholar]
- 15.Krol N, Wensing M, Haaijer-Ruskamp F, et al. Patient-directed strategy to reduce prescribing for patients with dyspepsia in general practice: a randomized trial. Aliment Pharmacol Ther 2004; 19:917–22 [DOI] [PubMed] [Google Scholar]
- 16.Smelt AF, van der Weele GM, Blom JW, et al. How usual is usual care in pragmatic intervention studies in primary care? An overview of recent trials. Br J Gen Pract 2010;60:e305–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knuisting Neven A, Bartelink MEL, De Jongh TOH, et al. NHG-standaard Hoofdpijn [article in Dutch]. Huisarts Wet 2004; 46:411–22 [Google Scholar]
- 18.Linde K, Rossnagel K. Propranolol for migraine prophylaxis. Cochrane Database Syst Rev 2004;(2):CD003225. [DOI] [PubMed] [Google Scholar]
- 19.Mulleners WM, Chronicle EP. Anticonvulsants in migraine prophylaxis: a Cochrane review. Cephalalgia 2008;28:585–97 [DOI] [PubMed] [Google Scholar]
- 20.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 2003;12:963–74 [DOI] [PubMed] [Google Scholar]
- 21.Gandek B, Alacoque J, Uzun V, et al. Translating the short-form Headache Impact Test (HIT-6) in 27 countries: methodological and conceptual issues. Qual Life Res 2003;12:975–9 [DOI] [PubMed] [Google Scholar]
- 22.Coeytaux RR, Kaufman JS, Chao R, et al. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol 2006;59:374–80 [DOI] [PubMed] [Google Scholar]
- 23.EuroQol — a new facility for the measurement of health-related quality of life. The EuroQol Group Health Policy 1990;16:199–208 [DOI] [PubMed] [Google Scholar]
- 24.Lamers LM, Stalmeir PFM, McDonnell J, et al. Kwaliteit van leven meten in economische evaluaties: het Nederlands EQ-5D-tarief [article in Dutch]. Ned Tijdschr Geneeskd 2005;149:1574–8 [PubMed] [Google Scholar]
- 25.Donker T, Comijs H, Cuijpers P, et al. The validity of the Dutch K10 and extended K10 screening scales for depressive and anxiety disorders. Psychiatry Res 2010;176:45–50 [DOI] [PubMed] [Google Scholar]
- 26.van der Wouden JC, Blankenstein AH, Huibers MJ, et al. Survey among 78 studies showed that Lasagna’s law holds in Dutch primary care research. J Clin Epidemiol 2007;60:819–24 [DOI] [PubMed] [Google Scholar]
- 27.Killip S, Mahfoud Z, Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. Ann Fam Med 2004;2:204–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubbard AE, Ahern J, Fleischer NL, et al. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010;21:467–74 [DOI] [PubMed] [Google Scholar]
- 29.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681–94 [DOI] [PubMed] [Google Scholar]
- 30.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karli N, Zarifoglu M, Erer S, et al. The impact of education on the diagnostic accuracy of tension-type headache and migraine: a prospective study. Cephalalgia 2007;27:41–5 [DOI] [PubMed] [Google Scholar]
- 32.Smith TR, Nicholson RA, Banks JW. Migraine education improves quality of life in a primary care setting. Headache 2010;50:600–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owens C, Pugmire B, Owens K. A migraine prophylaxis educational intervention in a Medicaid population. Headache 2008;48: 267–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.