Abstract
Cardiac optical mapping has proven to be a powerful technology for studying cardiovascular function and disease. The development and scientific impact of this methodology are well documented. Because of its relevance in cardiac research, this imaging technology advances at a rapid pace. Here we review technological and scientific developments during the past several years and look also towards the future.
First we explore key components of a modern optical mapping setup, focusing on 1) new camera technologies, 2) powerful light-emitting-diodes (from ultraviolet to red) for illumination, 3) improved optical filter technology, 4) new synthetic and optogenetic fluorescent probes, 5) optical mapping with motion and contraction, 6) new multi-parametric optical mapping techniques and 7) photon scattering effects in thick tissue preparations. We then look at recent optical mapping studies in single cells, cardiomyocyte monolayers, atria and whole hearts. Finally, we briefly look into the possible future roles of optical mapping in the development of regenerative cardiac research, cardiac cell therapies, and molecular genetic advances.
Keywords: Optical mapping, Optogenetics, Arrhythmia, Fluorescence, Multi-Parametric
Introduction
The study of the electrophysiological properties of the healthy and diseased heart, as well as the coupling between excitation and contraction in its component cells and tissues provides important mechanistic insights into how life-threatening cardiac arrhythmias can occur.1 This information may be used to aid in the generation of computer models to make predictions about arrhythmogenesis and ultimately for design of rational anti-arrhythmic therapies.2 Traditionally, surface electrodes have been, and continue to be employed to measure extracellular cardiac electrical potentials. However, this surface contact mapping suffers from low spatial resolution, low depth of field, far-field effects and interference from electrical stimulation electrodes. Optical mapping using fluorescent probes to look at physiological parameters offers a higher resolution and less invasive method to study the electrical activity of the heart and cardiac cells. In addition, fluorescent methods have revolutionized the analysis of the molecular dynamics underlying the coupling between cardiac excitation and contraction.
Fluorescence techniques are widely used in cardiac research. Immunocytochemistry using immunolabelled fluorescent probes provides subcellular molecular stucture data while parameter-sensitive probes (i.e. ion selective and/or voltage) loaded into live cells and tissue provide measurement of physiological function.3,4 Hence, the use of fluorescence has led to detailed mechanistic insight into the molecular and cellular processes that are vital to cardiac function. For example, the use of calcium sensitive fluorescent probes led to discovery of the precise cellular and molecular mechanisms of cardiomyocyte excitation-contraction coupling5 and how this vital coupling is altered in pathophysiological states such as heart failure.6,7 The heart works as a functional syncytium of many electrically and mechanically connected cardiomyocytes, so it is imperative to study physiology of the whole heart or multicellular tissue preparations to provide a conceptual framework for single cell data.
Fluorescence imaging using voltage sensitive dyes was first used by Cohen et al. in 1974 to record changes in axonal impulse propagation.8 Since then, fluorescent dyes have been used to study a wide array of electrically excitable organs and tissues. In cardiac tissues, simultaneous potentiometric dye fluorescence recordings from multiple sites have been made by arrays of photodiodes, photomultiplier tubes (PMTs), laser scanning systems, charge-coupled device (CCD) cameras and their more recent derivatives.9–11 Furthermore, whole heart optical mapping techniques have been developed and utilized to study electrophysiological mechanisms of cardiac arrhythmias.12,13 Since the early 1990s optical mapping with CCD cameras has demonstrated to be an extremely useful tool in the analysis of the complex patterns of electrical wave propagation in both atrial and ventricular arrhythmias in isolated animal hearts.14
Now, optical mapping makes it possible to measure action potential and calcium wave propagation at high spatiotemporal resolution.15 The complex dynamic coupling between these two electrophysiological parameters is critical for proper cardiac function, thus underscoring the vital need to monitor multiple parameters simultaneously. New techniques are being developed to extend multi-parametric imaging to include fluorescent monitoring of the metabolic state of the heart together with electrophysiological monitoring.16 Fluorescent reporters have been developed more recently for magnesium, sodium, potassium, pH, nitric oxide, redox state and oxygen content. Even subcellular organelle electrophysiology can now be probed optically.17,18 Simultaneous multi-parametric imaging of membrane voltage and pH, for example, will provide new insights into cellular and molecular mechanisms of arrhythmias in ischemia. Indeed, the general goal of cardiac optical mapping is to provide a better understanding of cardiac electrophysiological function during health and disease. Cardiac optical mapping techniques have been reviewed extensively.11,19 Here we discuss some of the new optically-based developments for cardiac mapping since that time and present emerging technologies that will prove pivotal in the advancement of the cardiac optical mapping field.
Optical Mapping Instrumentation & Fluorescent Probes
Photodetectors for Optical Mapping
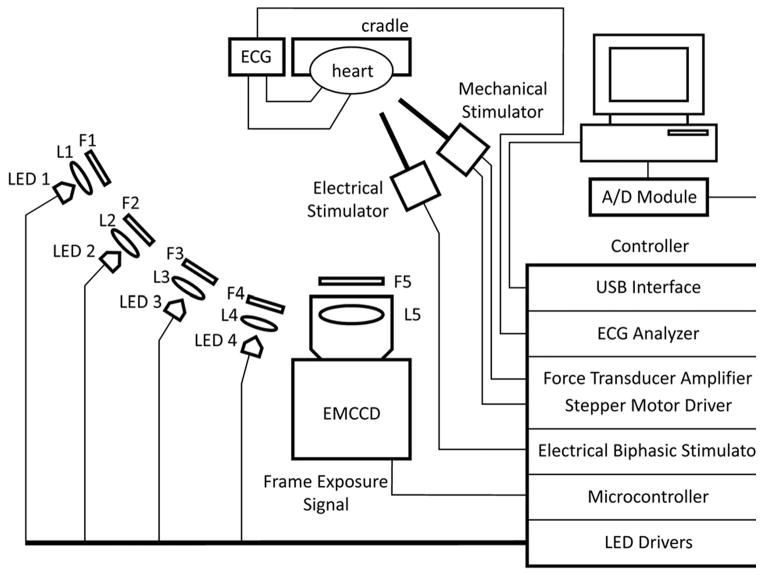
Optical mapping experiments require a light source to illuminate cardiac preparations treated with a fluorescent reporter and a detector to sense the changes in fluorescence that represent physiological changes in transmembrane potential (Vm) or intracellular ion concentrations (e.g. Ca2+). An optical mapping set up is displayed schematically in Figure 1. The optical detector is perhaps the most essential component of the setup.10 In this section we review the various options for high-resolution optical mapping detectors (cameras) and focus on technologies that have emerged and are being utilized.
Figure 1.
Components of a cardiac optical mapping set up. LED illumination is emerging as a leading technology for optical imaging applications. A high-speed microcontroller interface between LED excitation and camera acquisition allows precise timing for multi-parameter optical mapping. The microcontroller also enables precise timing of external electrical and mechanical stimulators relative to the ECG. Multi-band optical filter technology enables the use of a single camera. Adapted from Lee et al.28 by permission.
The propagating electrical impulse that triggers every heart beat is rapid and short lived. Due to the low signal strength (i.e. ΔF/F) of current voltage sensitive fluorescent dyes and the high-speed of electrical wave propagation, high-speed low-noise photodetectors are required. There are currently only a limited number of useful detectors that offer high spatiotemporal resolution for cardiac mapping. The available technologies include: photodiode arrays (PDAs), photomultiplier tubes (PMT), CCD cameras, and complementary metal-oxide semiconductor (CMOS) cameras. PMTs offer high temporal resolution acquisition of fluorescence signals, but little to no spatial information. PDA detectors and CCD cameras have predominantly been used for cardiac imaging applications due to their high spatial and temporal resolutions. 20–22
For optimal optical mapping of the heart and other cardiac preparations CCD/CMOS technologies are proving to be far superior to PDAs in terms of spatiotemporal resolution due to the massive number of pixels and fast data streaming rates to a computer. However, CCD sensors offer a slower full-frame-rate than PDAs (due to the much larger number of pixels) but this can be overcome by increased pixel binning. Pixel binning, however, decreases spatial resolution and thus it is imperative to utilize the optimal resolution relative to the preparation being used or the question being asked. For very low light level signals such as those from cell culture systems using monolayers or strands newer CCD technology has been developed that provides increased sensitivity with low noise. The two CCD derivatives are: 1) electron-multiplying CCDs (EMCCDs), and 2) intensified camera systems (I-CCD/CMOS).10
The most recently developed concept for increasing sensitivity of CCDs is on-chip electron multiplication, referred to as EMCCD technology.23 EMCCDs utilize impact ionization (an avalanche process) to produce secondary electrons prior to conversion to a voltage signal.24 An on-board fully chip-incorporated “gain register” provides electron multiplication in a serial process that involves the application of high electric fields. Major benefits of EMCCDs over older CCD technology include: 1) lower readout noise at higher acquisition rates, 2) lower multiplicative noise, and 3) improved quantum efficiency (QE) compared to intensifiers. New EMCCDs show promise as high-resolution cameras, especially for cell monolayers that require high sensitivity and temporal resolution,25 and have recently been suggested to provide the highest promise for fast imaging at very low light levels, as encountered in mapping of voltage and calcium waves in cultured cells.26 The latest thermoelectrically-cooled EMCCD cameras permit user-specified EM gain, with minimal decrease in the signal-to-noise ratio, for low light level applications. For the whole heart, which emits high fluorescence, large pixel well-depths and dynamic ranges are critical. Modern EMCCD cameras (ex. Evolve cameras from Photometrics and SciMOS cameras from Fairchild Imaging) with these features makes for a camera that is versatile enough to be used for either whole heart optical mapping where signal intensity is strong or cell culture systems where fluorescence signal intensity is weak and dynamic fluorescence is fast (i.e. action potential propagation). As photodetector and CCD camera technologies advance, so will the cardiac optical mapping field and our understanding and treatment of cardiac arrhythmias.
Illumination
Proper illumination is pivotal to successful optical mapping experiments. Whether the preparation is a Langendorff-perfused heart or a monolayer of cells, stable and even illumination during recording of fluorescence changes is key for high signal-to-noise recordings of physiological parameters. Multi-parameter cardiac imaging often relies on switching between distinct wavelengths of light, thus making the choice of illuminating sources essential for these technically challenging experiments.
Traditional lighting methods have relied on xenon, mercury or halogen lamps for illumination and excitation of fluorescent reporters.11 Illumination duration has been controlled by shuttering devices and moving parts which can be impractical when stable and fast multi-color imaging is required (i.e., multi-parametric imaging). Stable illumination requires a highly regulated voltage supply and feedback control system, and multi-color illumination requires filter wheels, galvonometers and shutters to switch between excitation wavelengths. Arc lamps are unstable due to plasma oscillations and thermal runaway.27 Newer illumination technology, such as light-emitting-diodes (LEDs) offer a more stable and easy-to-use alternative to traditional lighting sources for standard cardiac optical mapping.11,27,28
LED illumination is cost effective, energy efficient, portable and flexible.10 The main concern with LED use is adequate cooling and sufficient output power. New high-power LEDs from UV to red wavelengths are now available with sufficient power and stability for more light-demanding preparations like the whole-heart. Computer control of multiple LED lights allows for complex and rapid wavelength switching, thus enabling multi-parametric and ratiometric imaging for quantitative fluorescence measurements.28, 29 To date, the majority of optical mapping studies have utilized single illumination sources to study a single physiological parameter, typically using a potentiometric dye. LED illumination, which easily enables multi-parametric and ratiometric imaging due to the ability to rapidly change between excitation sources in real-time and during the experimental procedure, will increase the rate and breadth of knowledge acquisition in the cardiac optical mapping field.
Potentiometric dyes
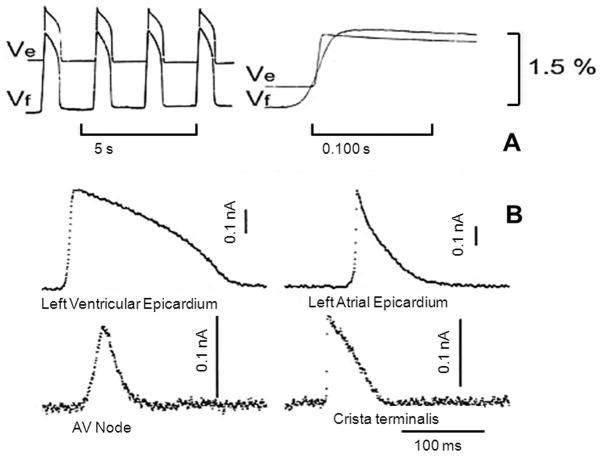
Several decades ago molecular probes were discovered that embedded into the plasma membrane of neurons and cardiac cells and exhibited interactions with the electric field. The resulting molecular rearrangement of the probe within the membrane led to a change in fluorescence or absorption that reported alterations of membrane potential.30,31 Potentiometric probes enable high spatial resolution measurements of membrane potential in subcellular compartments (e.g., mitochondria),32 single cells, monolayers of cardiac cells33,34 and in the whole heart.35 Fluorescence of these dye molecules changes linearly with membrane potentials in the physiological range36 and modern probes can yield over 10% fractional changes in fluorescence. Figure 2 illustrates the tight correlation between fluorescence changes induced by transmembrane changes in voltage and sharp microelectrode recordings in various anatomical regions of the heart.
Figure 2.
Optical recordings of cardiac action potentials (Vf) compared to simultaneous microelectrode recordings (Ve). Recordings demonstrate reproduction of transmembrane potential by fluorescence changes. From Cohen et al.37 by permission.
Potentiometric dyes are classified into fast-response and slow-response dyes based on their response times and mechanism of voltage sensitivity,37 where fast-response probes report voltage changes on the order of microseconds.38 The most typically used are the styryl dyes developed by Loew et al.39 of which the most commonly used are the ANEP (aminonaphthylethenylpyridinium) dyes, di-4-ANEPPS and di-8-ANEPPS. Both dyes yield a uniform 10% change in fluorescence per 100mV change in membrane potential but di-4-ANEPPS rapidly internalizes in cells, thus making it useful only for short-term experiments. di-8-ANEPPS is better retained in the outer leaflet (longer membrane anchor) of the plasma membrane, which also contributes to its reported lower phototoxicity (related to higher photostability) to cells. 40, 41 Although di-4-ANEPPS is more commonly used for whole heart optical mapping, di-8-ANEPPS is also used for that purpose in some laboratories.42 Both ANEP dyes respond to increases of membrane potential (hyperpolarization) with a decrease in fluorescence emission when excited at their optimal wavelengths, which depends on the synthetic/biological preparation where the dyes are loaded (i.e., the dyes’ properties are sensitive to the local environment). According to Loew et al., the change in fluorescence over the background fluorescence (ΔF/F) ranges between 7 and 10%, depending on the preparation.43 Ratiometric cardiac action potential recording using di-4-ANEPPS and pulsed LED excitation has recently been described.44 Styryl voltage-sensitive dyes have been widely and successfully used in cardiac cells and tissues. However, their utility has been somewhat limited because their excitation wavelengths are restricted to the short wavelength (blue to green) range. Longer excitation/emission wavelength probes (near infrared) can minimize interference from endogenous chromophores and improve recording from deeper tissue layers. Voltage dyes in multi-cellular preparations have been used extensively with much success because tissue is robust and provides fluorescence past the lens focus, providing a large ΔF/F. Single cell and monolayer imaging, however, suffers from dye phototoxicity and low ΔF/F. New voltage sensitive dyes that are less cytotoxic and provide larger ΔF/F are required to extend optical mapping to the single cell level.
New longer wavelength voltage-sensitive dyes are red-shifted, which can be advantageous for combining with other dyes (e.g., Ca2+), seeing through blood and having bigger ΔF/F signals. Recently generated long wavelength voltage-sensitive dyes are designed to record cardiac action potentials (APs) from deeper layers in the heart. To do this, the emission spectrum of styryl voltage-sensitive dyes was red-shifted by incorporating a thienyl group in the polymethine bridge to lengthen and retain the rigidity of the chromophore.45 Seven distinct dyes, called Pittsburgh I to IV and VI to VIII (PGH I–VIII) were synthesized in the Salama laboratory and characterized with respect to their spectral properties in organic solvents and heart muscles.45 PGH voltage sensitive dyes exhibited two absorption, two excitation and two voltage-sensitive emission peaks, each with very large Stokes shifts (> 100 nm). As reported, hearts (rabbit, guinea pig and Rana pipiens) were effectively stained by injecting a bolus (10–50 μL) of stock solution of voltage sensitive dye (2–5 mmol/L) dissolved in dimethylsulfoxide (DMSO) plus low molecular weight Pluronic (16% of L64). Other preparations were better stained with a bolus of voltage-sensitive dye (2–5 mM) Tyrode’s solution at pH 6.0. APs measured with a fast CCD camera showed that PGH I exhibited an increase in fractional fluorescence, ΔF/F=17.5%, per action potential at 720 nm (emission) with 550 nm excitation and ΔF/F=−6% per AP at 830 nm with 670 nm excitation. The long wavelengths, large Stokes shifts, high fluorescence change upon membrane depolarization and low baseline fluorescence make PGH dyes a valuable tool in optical mapping and for simultaneous mapping of APs and intracellular Ca2+. The spectra for potentiometric dyes have been tested almost exclusively in mammalian systems. As optical mapping techniques are extended to studies using genetically malleable Zebrafish and Drosophila hearts, it will be critical to determine the spectra of these dyes in non-mammalian systems.
The Loew laboratory recenty reported46 developing new potentiometric styryl dyes with red excitation wavelengths and near-infrared emission. Three dyes for cardiac optical mapping were investigated in depth from several hundred dyes containing 47 variants of the styryl chromophores. They recorded absorbance and emission spectra in ethanol and multilamellar vesicles, as well as voltage-dependent spectral changes in a model lipid bilayer. Optical action potentials were recorded in tissues of rats, guinea pigs and pigs and compared with those of di-4-ANEPPS. The voltage sensitivities of these new potentiometric indicators were shown to be as high as those of the widely used ANEP series of probes. In addition, because of molecular engineering of the chromophore, the new dyes provide a wide range of dye loading and washout time constants. These dyes will enable a series of new experiments requiring the optical probing of thick and/or blood-perfused cardiac tissues as well as deeper recording in the myocardial tissue.46
In 2007, the first characterization of two new infrared styryl dyes di-4-ANBDQPQ and di-4-ANBDQBS optimized for blood-perfused tissue and intramural optical mapping was reported.47 These dyes had excitation/emission wavelengths shifted >100nm toward the red. Voltage-dependent spectra were recorded in a model lipid bilayer and a comprehensive examination of the new dyes was conducted in multiple cardiac preparations, including Langendorff-perfused pig hearts using Tyrode’s solution and a blood-saline mix, and coronary-perfused pig right ventricular wall preparations. In cardiac tissues the optimal excitation (650nm) was >70nm beyond the absorption maximum of hemoglobin, pointing to the potential utility to optically map through blood. Signal decay half-life due to dye internalization was 80–210 minutes, which is 5–7 times slower than for di-4-ANEPPS. In transillumination mode, ΔF/F was as high as 20%. In blood-perfused tissues, ΔF/F reached 5.5% (1.8 times higher than for di-4-ANEPPS). In effect, these dyes provide both high voltage-sensitivity, and 5–7 times slower internalization rate compared to conventional dyes. They are optimized for deeper tissue probing and optical mapping of blood-perfused tissue, and can also be used for conventional applications. In the future, the continued development of new red-shifted voltage-sensitive dyes will pave the way for endoscopic mapping in-vivo using cardiac catheterization, which may one day become a useful clinical tool to optically map and precisely diagnose arrhythmias and perhaps guide cardiac ablation procedures.
Calcium sensitive dyes
Calcium cycling in cardiomyocytes is a vital component of cardiac excitation-contraction coupling.48,49 Cardiac excitation-contraction coupling is crucial for proper heart function and the ubiquitous second messenger, Ca2+, is central to this elegant coupling.50 The action potential causes Ca2+ influx through activation of L-type voltage gated Ca2+ channels. This Ca2+ triggers release of Ca2+ from intracellular stores of the sarcoplasmic reticulum (SR) that activates contraction. Ca2+ release from the SR is mediated by Ca2+ release channels (ryanodine receptors) that are activated by localized sub-sarcolemmal Ca2+ entry into the cell via L-type Ca2+ channels and this process is commonly referred to as Ca2+ induced Ca2+ release (CICR).5 In pathological conditions such as heart failure, dysregulation of cellular Ca2+ homeostasis may activate Ca2+ dependent currents that can influence action potential duration and trigger spontaneous membrane depolarizations.51,52 In fact, mishandling of intracellular Ca2+ in cardiomyocytes contributes to contractile dysfunction and arrhythmogenesis in failing hearts.53, 54 Therefore, simultaneous measurement of action potential and Ca2+ wave propagation are essential to provide mechanistic insight into acquired arrhythmias associated with heart failure and inherited Ca2+ mediated arrhythmias such as catecholaminergic polymorphic ventricular tachycardia (CPVT).55–57
To minimize perturbation of the [Ca2+]i dynamics in cardiac cells and tissue, the choice of Ca2+ dye is critical for acquiring accurate measurements of the amplitude and time course of [Ca2+]i transients. For cardiomyocytes and tissue, which show large and rapid changes in [Ca2+]i, a low-affinity and rapidly responding dye is necessary.58 Other widely-used Ca2+dyes, such as Fluo-4, Fluo-3 and Fura-2,59 have a relatively high affinity for Ca2+. This can artificially prolong the Ca2+ transient and confound interpretation (i.e., the dye acts as a chelator and clings on to Ca2+ ions for too long). Low-affinity calcium dyes provide more accurate measurement of calcium dynamics.60 The most ideal Ca2+ indicator molecule would combine the option of ratiometry for amplitude quantification with low Ca2+ affinity, such as the newly developed Fura-4F dye.61 Ratiometric optical mapping has been technically challenging using traditional light sources that require moving parts for filter switching between excitation lights. Recently, this technological challenge has been overcome by the use of electronically controlled LED illumination thus enabling quantitative assessment of calcium wave amplitudes and dynamics in whole hearts.28 Small molecule dyes are very useful due to their high signal-to-noise ratio; there is a wide range of indicators with various excitation/emission spectra and affinities for Ca2+. Any untoward effects of small-molecule calcium dyes are easily overcome because of the ability to control the concentration of dye that enters cardiac cells. Thus small molecule calcium dyes are most commonly used for optical mapping experiments and this will likely continue into the future.
Genetically encoded Ca2+ indicator proteins (GECIs) represent a new generation of calcium sensing molecules. GECIs offer nominal advantages over small molecule indicators such as Fura-2 and Fluo-4, which include cell specific calcium mapping and the possibility for chronic imaging over days and weeks.62 A well-known limitation of GECIs, however, is their slow response time because of the slow on and off kinetics of calcium binding. This attribute makes GECIs less suitable for cardiac optical mapping, but development of genetically encoded proteins with faster response times will provide powerful new tools for cell specific Ca2+ imaging within the whole heart.
Optical Mapping Limitations and Solutions
Motion artifacts
One limitation of optical mapping of the heart is imaging artifact caused by cardiac contraction. Motion artifacts obscure the optical recordings of voltage or calcium transients and thus prevent accurate measurement of parameter dynamics. Mechanical, pharmacological and imaging methods have been developed to overcome this limitation.19 Mechanical restriction of heart motion without physiological implication reportedly works for small rodent hearts.20 However, the heart is well known to be sensitive to mechanical distortion,63 thus limiting the utility of this approach. Pharmacological approaches have included the use of calcium channel blockers64 or myosin inhibitors such as 2,3-butanedione monoxime (BDM) and blebbistatin.65,66 BDM is non-specific; it also blocks function of membrane ion pumps including SERCA2, thus having significant effects on cardiac electrophysiology and limiting its usefulness. Blebbistatin is a specific myosin II inhibitor that reportedly prevents contraction but does not have any noticeable effects on cardiac electrophysiology,67 thus making it a more useful tool for cardiac optical mapping. However, it is important to consider that inhibition of the primary consumer of intracellular ATP (myosin) may confound experiments studying mechanisms of arrhythmias that can be influenced by the intracellular metabolic state and any mechano-electric-feedback is also eliminated that may play a critical role in arrhythmias.63 Additionally, the contribution of the myofilaments to cardiac arrhythmias is beginning to be realized and eliminating their function in optical mapping experiments may have profound effects on the study of arrhythmia mechanisms.68,69 Despite their inherent limitations, motion blockers/uncouplers have helped optical mapping studies tremendously. Use of ratiometric imaging techniques29 and post-acquisition motion tracking70 may permit the mapping of contracting tissue.
Photon Scattering Effects
The majority of optical mapping studies to date have performed epicardial recording of transmembrane voltage signals. However, because of the thickness of the ventricular wall and large depth-of-focus inherent in typical macroscopic optical mapping setups, fluorescence is collected not only at the surface, but also from deeper tissue (Figure 3).71,72 Activation wavefronts initiated below the epicardial surface can reach the surface at various angles. Thus, caution must be used when comparing epicardial predictions to measurements obtained using optical mapping techniques.73 The optical signal should not be compared to the transmembrane potential calculated at the surface of the tissue, but instead to the transmembrane potential averaged over depth. Indeed, results suggest that optical mapping underestimates the surface transmembrane potential during electrical stimulation.74 Depth and radius of regions interrogated by cardiac optical mapping with a laser beam depend on photon travel through tissue. It would be useful to limit the range of depth and radius interrogated. Ramshesh et al.75 simulated the effects of using a condensing lens to concentrate laser light at a specific depth within the heart, and near infrared excitation to increase penetration and produce two-photon absorption. The results indicated that mapping at depths up to 300 μm under the epicardial surface can provide significant improvement in localization over existing cardiac optical mapping.75 Mathematical modeling taking into account photon scattering effects has the potential to extrapolate useful 3-D information about intramural impulse propagation.76–78 Combined experimental/simulation studies that take photon scattering and optical signal distortion into account provides an interpretation of optical recordings during ventricular tachycardia and defibrillation.72 Laminar Optical Tomography (LOT) provides a view of the 3-D propagation of electrical waves in the heart by taking into account the effects of fluorescence depth of field and light scattering.79,80
Figure 3.
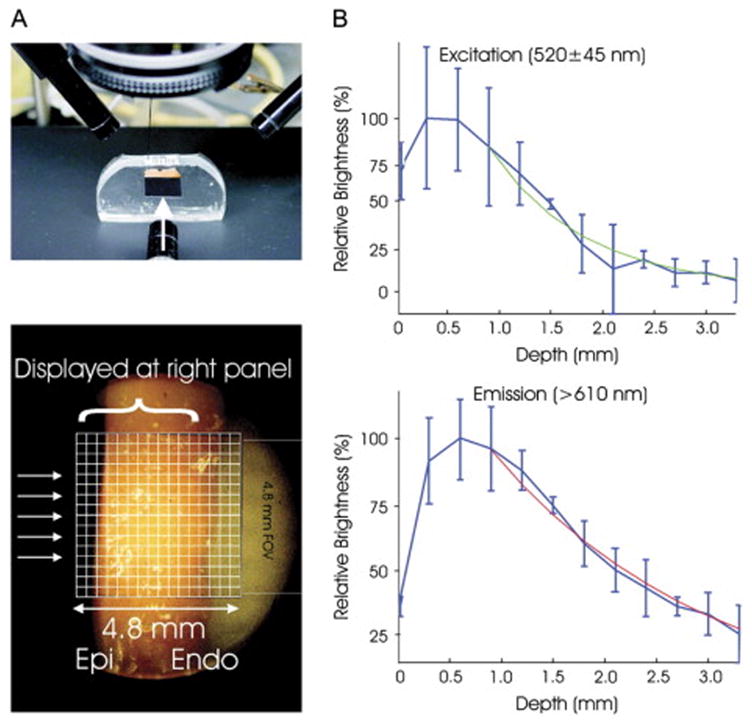
Illustration of photon scattering effects. In di-4-ANEPPS loaded rabbit hearts, the optimal fluorescence signal is 0.5–1mm deep below the epicardial surface. From Bishop et al.72 by permission.
Multi-parametric Optical Mapping
Simultaneous Voltage and Calcium Optical Mapping
In 2004 multi-parametric imaging was considered to be a field “…in its infancy”.19 Today this field has advanced extensively due to technological developments in LED illumination, optical filters and fluorescent dyes. Yet the bulk of cardiac optical mapping data to date has been generated by measuring only a single dye for a single parameter, typically monitoring either Vm or intracellular Ca2+. The complex interrelationship between these physiological parameters is critical for cardiac function and alterations of the normal dynamics of either can lead to cardiac arrhythmias.81,82 Membrane depolarization triggers intracellular Ca2+ transients and changes of intracellular Ca2+ can affect Vm by modulating the function of multiple ionic currents such as L-type Ca2+, chloride and sodium-calcium exchanger currents.83–85 Therefore, to gain a precise understanding of the complex nature of the electrical activity of every heart beat and to precisely identify mechanisms of arrhythmias it is ideal to view both parameters simultaneously.60,86
In 2000 Choi et al.87 developed and reported a method to simultaneously map Vm and Ca2+ on the epicardium of perfused hearts by staining cells with a voltage-sensitive dye (RH-237) and loading also the cytosol with a fluorescent Ca2+ indicator (rhod-2). The fluorescence characteristics of these two dyes are such that one illumination wavelength was used to excite each dye simultaneously, and through the use of dichroic mirrors, the unique emission wavelength of light was directed to one of two PDAs. In these experiments the image of the anterior region of the heart was focused on the array and each photodiode recorded activity from a 0.8mm×0.8mm area of epicardium, thus greatly limiting the area of view to a small portion of the heart. The use of multiple PDAs required a complicated 6 step procedure to precisely focus and align the arrays, thus making this technology cumbersome and limiting its applicability to only the very engineering savvy cardiac researcher. An important feature of this optical apparatus is its extremely fast kinetic resolution (4000 frames s−1; due to the small number of pixels) for each parameter to very accurately determine the temporal relationship between Vm and Ca2+. Though a tour-de-force at the time, this technology provided low spatial resolution (16×16 PDA), and was challenging to set up and align.
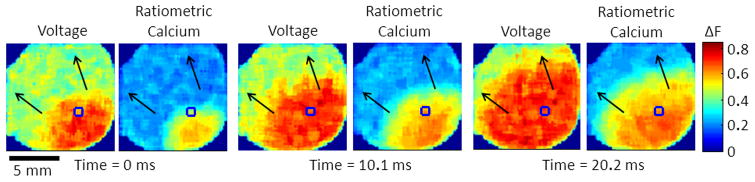
Since that time new detector configurations have been developed in an attempt to utilize higher resolution CCD cameras and to simplify detector alignment. In 2004, Omichi et al.15 employed a two CCD camera system to increase spatial resolution of dual Vm-Ca2+ mapping for the study of dynamics during ventricular fibrillation (VF). In this configuration the two cameras, with appropriate emission filters, were placed side-by-side and were manually aligned to view approximately the same area of the heart. Although the setup provided higher spatial resolution using CCD detectors, the manual alignment of the multiple sensors introduced inaccuracies to the measurements. In 2009 Holcomb et al.88 described a simplified method for detector alignment. The advantage of this system over others is in the software camera calibration routine that eliminates the need for precise camera alignment for acquisition and processing of data, as is the case with complicated PDA alignment. Figure 1 shows an optical mapping set up that uses a single-camera (eliminating alignment needs) for multi-parametric imaging (4 excitation wavelengths) and Figure 4 shows simultaneous optical mapping of intracellular calcium and membrane voltage during sustained reentry in a neonatal rat ventricular myocyte monolayer, also using a single-camera system.
Figure 4.
Simultaneous optical mapping of intracellular calcium and membrane voltage in neonatal rat ventricular myocyte monolayers. Using precisely timed alternating LED illumination and a single camera it is now possible to simultaneously record multiple parameters in a quantitative way. Unpublished data.
Some recent applications of optical mapping at varying levels of integration
Optical mapping has contributed immensely over the last 20 years to the understanding of the dynamics of electrical wave propagation in the most complex cardiac arrhythmias, including atrial and ventricular fibrillation; it has also advanced knowledge on the mechanisms of action of antiarrhythmic agents, and the mechanisms of cardiac defibrillation, all of which will ultimately contribute to better care of patients. In the following sections we briefly discuss some of the recent scientific highlights in this field of research.
Single Cell Optical Mapping
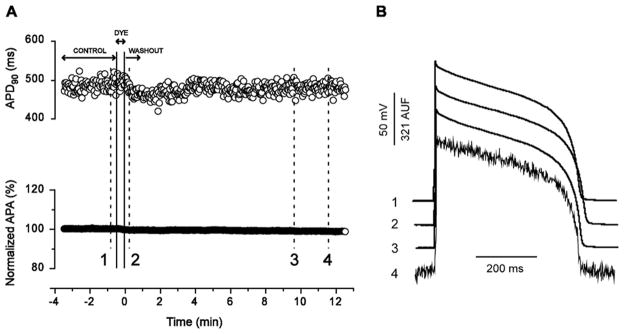
Single cell optical mapping offers the potential for higher throughput testing of treatment effects on the action potential than traditional patch electrode techniques. Additionally, voltage-sensitive dyes allow monitoring of cell excitation heterogeneities, providing spatial information not possible with electrode recordings. Windisch et al89 developed the first optical micromapping system in which single cell membrane potential was monitored optically using di-4-ANEPPS. However, single cells isolated from rodent hearts are only 5–10μm thick and in larger animals thickness may be just >10μm, thus making light levels from potentiometric and calcium indicators challenging to detect. In addition, di-4-ANEPPS suffers from being cytotoxic, to the extent that it has not been widely used for single cell electrophysiological mapping. More recently Sharma et al. used di-8-ANEPPS to study how cardiac cells respond to applied electrical fields.90,91 Since then new technologies have emerged, including a new red-shifted voltage dye, di-4-ANBDQBS and EMCCD cameras for optical AP recording in single cardiomyocytes.92 Importantly, as shown in Figure 5, loading di-4-ANBDQBS did not alter single cell APs recorded with a micropipette. Di-4-ANBDQBS yielded fluorescent signals with very high ΔF/F (19.2±4.1%) and signal-to-noise ratios (40±13.2), representing a major advancement in ANEP styryl dyes.92 Thus the presented technique provides a unique opportunity for high-throughput noninvasive AP recording in isolated cardiomyocytes.
Figure 5.
Use of the potentiometric dye, di-4-ANBDQBS, for single cell optical mapping. A, APD90 (top) and AP amplitude (APA; bottom) over time, obtained from the same continuously paced myocyte during perfusion with control solution, dye-containing solution (18.4 μM), and washout. B, Individual electrical APs (1–3) and an optical AP (4) recorded from the same myocyte in A at the time points 1–4 (indicated in A). This new dye can be utilized for single cell action potential mapping with reduced toxic effects. From Warren et al. 92 by permission.
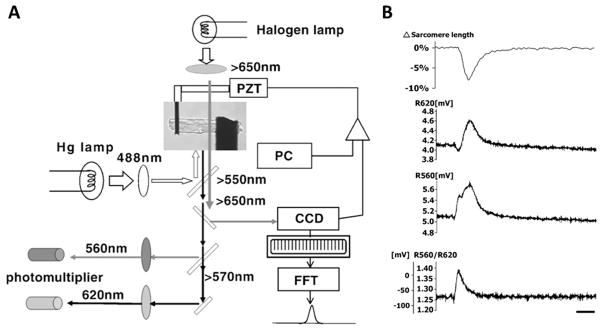
Optical mapping can be combined with other single-cell methods such a stretching to elucidate the interdependence between the mechanical state of the myocardium and its electrical activity.93 However, the information to date has been limited by the technical difficulties associated with stretching single myocytes and recording electrophysiological parameters optically, especially Vm. Recently Nishimura et al.94 combined the carbon-fiber cell-stretching technique and ratiometric measurement of di-8-ANEPPS. A schematic and example of their system are depicted in Figure 6. They found that during systole, stretching caused depolarization that prolonged the action potential duration without affecting the peak amplitude. The effect, however, was only significant in the late phase of the AP. Stretching quiescent myocytes depolarized the membrane potential in amplitude and speed dependent ways and was suppressed by cytochalasin-D treatment, suggesting participation of the cytoskeleton in cardiac mechanotransduction. Finally, ion replacement experiments revealed that although Na+ was the dominant charge carrier for large amplitude stretches, Ca2+ permeation was involved in small amplitude stretches, suggesting stretch amplitude ionic selectivity.
Figure 6.
Action potential optical mapping and stretching of single cells. A, Experimental setup for simultaneous contraction (with/without stretching) and action potential measurements. Cardiomyocytes can be stretched by carbon fibers (black rods) with simultaneous action potential recording, thus allowing direct optical measurement of electromechanical interactions. B, Recordings of simultaneous contraction (sarcomere length, top) and ratiometric optical recording of di-8-ANEPPS. From Nishimura et al. 94 by permission.
Monolayer Optical Mapping
The structural complexity of the heart has prompted the use of simplified cell culture systems to study excitation wave dynamics in cardiac tissues to precisely define cellular heterogeneity on impulse propagation.41,95,96 To understand cell network behavior, macroscopic imaging had to be employed. However, cell culture systems present more challenges than the whole heart because fluorescence signals are depth integrated.71 Whole heart fluorescence signals are recorded from myocardial tissue that can be millimeters in thickness, whereas cardiomyocyte culture systems (e.g. neonatal rat ventricular myocyte monolayers) are only 5–10μm thick.97 This is an order of magnitude thinner than the lower limit for the depth-of-field of useful macroscopic imaging lenses, thus making monolayer macro-mapping technically challenging. The first systematic application of optical mapping to record impulse propagation in strands of cardiomyocytes was in 1993 using PDAs.98 Bub et al. then used a CCD-based system to map calcium in large areas of cardiac monolayers.99 For action potential measurements, styryl dyes (di-4-ANEPPS, di-8-ANEPPS and RH-237) are most widely used for optical mapping in myocyte monolayers.100–102
Multi-parametric imaging of Vm and intracellular Ca2+ is also possible in monolayers (see Figure 4). Dye pairs successfully used in simultaneous measurements of APs and intracellular calcium include: di-2-ANEPEQ and calcium green,103 di-4-ANEPPS and indo-1,104 di-4-ANEPPS and fluo-4,105 RH-237 and Rhod-2,87 and RH-237 and fluo-3/4.106,107 In some of these experiments, the dye spectra and their overlap were of primary interest; thus, measurements were not co-localized in space and/or time.105,107 Recently, small aggregates of human induced pluripotent stem cell derived cardiomyocyte (iPS-CMs) have been optically mapped to study Vm and intracellular Ca2+ (non-ratiometrically) separately.108
Atrial Optical Mapping
The sinoatrial node (SAN) is the site of impulse initiation and represents a regionally heterogeneous structure109,110 in which thousands of pacemakers cells synchronize their activity by mutually entraining each other to generate each beat.111 Recently, the idea of mutual entrainment has been applied to also explain the mechanism of pacemaking at the level of the single SAN cell through the concept of beat-to-beat Ca2+-dependent regulation of rate and rhythm.112 Although controversial,113–115 the fact that membrane depolarization triggers intracellular Ca2+ transients and changes of intracellular Ca2+ can affect membrane potential by modulating L-type Ca2+, chloride and sodium-calcium exchanger currents,73–75 provides credence to the hypothesis that intracellular Ca2+ may be involved in the beat-to-beat regulation of SAN activity.116
The cardiac impulse can originate from the SAN or from extranodal sites within the right atrium117 and discrete sinoatrial pathways have been hypothesized118,119 to explain the complex impulse conduction within the SAN and the observation that surface activation can occur from multiple sites at the same time. This hypothesis was not directly tested until 2009 when high resolution optical mapping of the SAN was first performed. In this study, the canine SAN was optically mapped120 and structural and functional evidence suggested discrete exit pathways that connect the SAN and atria. The canine SAN is histologically similar to the human SAN and shares the 3D structural complexity that smaller animal models lack.121 More recently optical mapping was conducted in coronary-perfused preparations from non-failing human hearts using di-4-ANBDQBS and blebbistatin to block contraction. It was shown that the human SAN is functionally insulated from the surrounding atrial myocardium save for exit pathways that serve as electrical bridges between the nodal tissue and the atrial myocardium.122
Whole Heart Optical Mapping
Understanding how electrical waves spread through the heterogeneous anatomy of the heart is critical to understanding normal impulse propagation and cardiac arrhythmia mechanisms. Optical mapping of the whole heart has led to important discoveries about such phenomena as alternans,123 bidirectional tachycardia,124 atrial125,126 and ventricular fibrillation.127,128 Below we describe recent advances in whole heart optical mapping, including a review of advances in human heart mapping.
Endoscopic Mapping of Atrial Fibrillation (AF)
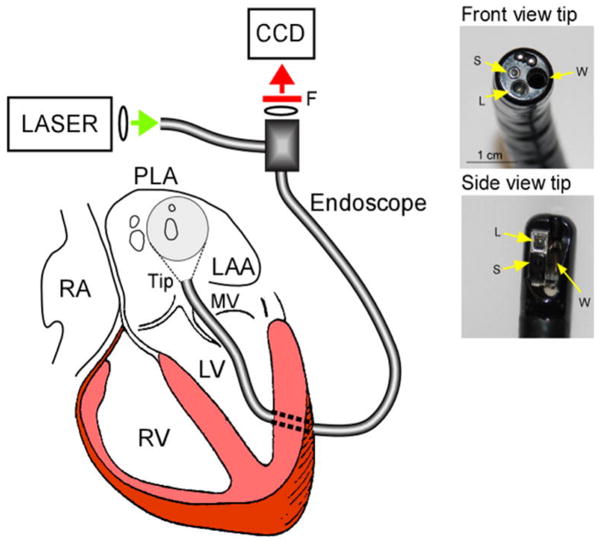
AF is the most common sustained arrhythmia seen by clinicians and is the most important cause of embolic stroke.129 While high temporal resolution can be achieved by conventional catheter-based electrical mapping, which is used in clinical electrophysiology laboratories as an aid to ablation, the limited number of intra-atrial electrodes that can be used simultaneously limits the spatial resolution and precludes any detailed tracking of electrical waves during AF. During the last two decades, the ability to use optical mapping in the research laboratory has been instrumental in providing numerous insight into AF mechanisms.130 Many such insights have found their way to clinical practice, including the identification of sources that maintain fibrillatory activity.131 Importantly, the use of optical mapping has been accompanied with the development of software that permits combined time- and frequency-domain analyses of optical signals, which led to the discovery that AF can be driven by discrete sources of high frequency periodic activity (rotors). The waves emanating from such rotors interact with either functional or anatomic obstacles in their path, resulting in the phenomenon of fibrillatory conduction.132 In addition we now recognize important frequency gradients between the left and right atrium in AF.133 In most cases the region with fastest activity is the posterior left atrium (PLA), which harbors the rotors that drive the overall arrhythmia.134 Of note, catheter-based AF ablation procedures in patients are most successful in terminating AF when targeting the PLA. In recent experimental studies, epicardial optical mapping on the PLA has been complemented with simultaneous endocardial mapping of the PLA using a dual-channel rigid borescope coupled to a CCD camera (Figure 7).135 To date, this has provided the best approach to visualize the patterns of activation in the most relevant region for AF maintenance, and the demonstration that, at least in some episodes, the rotors that maintain AF correspond to three-dimensional scroll waves that span from the endocardium to epicardium.
Figure 7.
Left, Experimental set-up showing the dual-channel cardio-endoscope inserted in the LA though a minimal left ventricular opening and across the mitral valve (MV). Right, Deflectable direct-view and side-view tips and the corresponding working (W), light delivery (L) and acquisition (S) channels. From Kalifa et al.135 by permission.
Scroll waves in the human ventricles
VF, the most important cause of sudden cardiac death, has been defined as turbulent ventricular electrical activity which precludes pumping of blood.14 The idea that 3-dimensional scroll waves underlie the mechanism of turbulent electrical wave propagation in human VF has been around for many years. A recent study combining optical mapping, mathematical modeling and analysis of VF frequency in 11 species, from the mouse to the horse, indicates that the inter-beat interval of VF scales as the fourth power of body mass.136 This suggests that there might be a strong similarity in the underlying mechanisms of VF in most, if not all, mammalian species, which may be of considerable fundamental and practical significance when analyzing the mechanisms of human VF. Recently, Nanthakumar et al. 137 provided the first direct demonstration of wavebreaks and rotor formation in severely diseased, explanted human hearts. Wavefronts as large as the vertical length of the optical field were also found, which suggested a high degree of organization. New findings from simultaneous epicardial and endocardial multi-electrode mapping, complemented by optical mapping, in patients with dilated cardiomyopathy138 suggested that during induced VF episodes, stable reentrant wavefronts occur in the endocardium and the epicardium. The same authors demonstrated a stable source in the endocardium, with a highly organized pattern in the local electrogram and a simultaneous and disorganized pattern in the epicardium (breakthroughs), consistent with the idea of 3-dimensional scroll waves.
Transmural optical mapping using optrodes
One limitation of optical mapping is the lack of methodology for making three-dimensional measurements. Multiple intramural electrical recordings can be obtained using plunge needle electrode arrays.139,140 Optical mapping through the wall of the ventricles is technically challenging. Thin optical–fibers offer a solution to this bioengineering problem. Optical fiber systems called “optrodes” have been developed for intramural measurements of Vm using voltage-sensitive dyes. In 2001 Hooks et al.141 designed an optrode that consisted of a bundle of seven optical fibers enclosed in a glass pipette that was inserted into heart muscle. Laser excitation light was used and fluorescence emission (di-4-ANEPPS) was measured using PDAs. In 2003, Byars et al.142 designed an improved optrode system that utilized a more stable light source (100-W mercury arc lamp) and the RH-237 dye, which has its absorption maximum close to very bright lines of the mercury lamp. The new optrode system also utilized better light-collecting optical fibers (i.e., higher numerical aperture). Care must be taken because exposed fiber ends can form secondary sources of polarization when electrical fields are applied. A major innovation in design the Fast laboratory143 ensures optimal light delivery into the tissue by polishing fiber ends at 45° and coating with reflective mirrors.143 This newest optrode design combines high signal quality (~30% better signal-to-noise ratios) with increased durability for repeated use and reduced stimulus artifact. As optrode technology advances so will our understanding of intramural impulse propagation in whole hearts, where data is limited.
Human Heart Failure
Recent studies have utilized optical mapping techniques in an attempt to uncover important features of the electrophysiological substrate of failing human hearts. In 2010 Glukhov et al.144 used di-4-ANEPPS to optically map action potentials in non-failing and cardiomyopathic human left-ventricular wedge preparations. The authors reported strong evidence of a transmural APD gradient in the human heart. Furthermore, it was concluded that heart failure results in the heterogeneous prolongation of APD, which reduces the normal transmural APD gradients. Immunostaining and fluorescent mapping of connexin43 (Cx43) expression revealed that downregulation of subepicardial Cx43 expression may underlie the electrophysiological remodeling evident in failing hearts. The first report of dual voltage and Ca2+ mapping of failing human hearts was published in 2011. Lou et al.145 utilized multi-parametric optical mapping to simultaneously map action potentials (RH-237) and calcium transients (rhod-2) in coronary-perfused left-ventricular wedge preparations. Despite inherent limitations of the preparation, this study demonstrated transmural heterogeneity of EC-coupling and calcium handling in normal hearts and revealed the transmurally heterogenous remodeling of these properties in heart failure.
Panoramic Optical Mapping
During VF, activation waves are being conducted transmurally in 3-D. One major limitation of traditional optical mapping is the 2-D, single-side field of view. The anterior surface of the heart is typically mapped, which may complicate matters because the electrical wave that emanates as part of arrhythmic activity may originate, or be organized, outside this field of view. And although epicardial conduction velocities can be quantified, caution needs to be taken to interpret these values because of the curved surface of the heart. Apparent epicardial conduction velocity is an accurate representation of actual conduction velocity only when the wavefront propagates nearly parallel to the epicardial surface and field of view. Significantly more information regarding impulse propagation can be gleaned from panoramic imaging. Panoramic imaging involves the arrangement of detectors and/or mirrors around a preparation to allow acquisition of signal from the entire 3D surface of the heart. Panoramic mapping data will enhance our ability to investigate the electrophysiology and non-linear dynamics of the heart. Panoramic imaging provides an improved global perspective from which to investigate cardiac conduction in normal and diseased conditions. Panoramic systems have been described that provide one front view and two back mirror views of isolated hearts, thus extending single-camera optical imaging capabilities.146 Multiple PDAs have also been employed to provide a panoramic view of impulse propagation in rabbit hearts.147
Future Directions for Cardiac Optical Mapping
Bioengineering
The advent of iPS cell technology has generated much excitement in the cardiac research community, ushering in the era of regenerative and personalized medicine. The use of iPS cells for therapies obviates many of the boundaries imposed by using human embryonic stem (ES) cells. Though some researchers proceed into this field with caution,148 the excitement and possibilities of iPS cell technology has increased the urgency to improve bioengineering and optical mapping methodologies to enable the mechanistic study of human cardiac preparations manifesting disease phenotypes, drug testing in-vitro and novel cellular therapies.149,150 For investigation of inherited cardiomyopathy and arrhythmia mechanisms, human iPSC derived cardiomyocytes (iPSC-CMs) offer unique advantages over commonly utilized transgenic animal model systems. First, animal model systems do not precisely recapitulate EC-coupling mechanisms of human cardiac cells, thus making the use of iPSC-CMs more attractive. Second, the use of patient specific iPSC-CMs permits direct study of specific mutations without the need for site directed mutagenesis and complex transgenesis techniques that may result in unexpected compensatory mechanisms that mask the true cardiac arrhythmia phenotype. For example, in the case of CPVT, caused by mutations of the ryanodine receptor, the production of patient specific iPSC-CMs will enable isolation of the mutant channel, opening the door to detailed biophysical analysis of single channel function and response to pharmacological agents. Third, the generation of iPSC-CM monolayers will allow for the creation of patient specific monolayers to study arrhythmia mechanisms and to determine the effect of potential therapeutics on impulse propagation. This offers a clear advantage over the commonly used neonatal rat ventricular myocyte monolayer system. Fourth, iPSC-CMs provide a novel system for the study of cardiogenesis and may reveal important genetic and electrophysiological mechanisms of cardiac development in patients with congenital heart defects. Major advances, however, must be made in cardiac directed differentiation techniques, especially for the maturation of the de novo myocytes, to significantly advance the field.
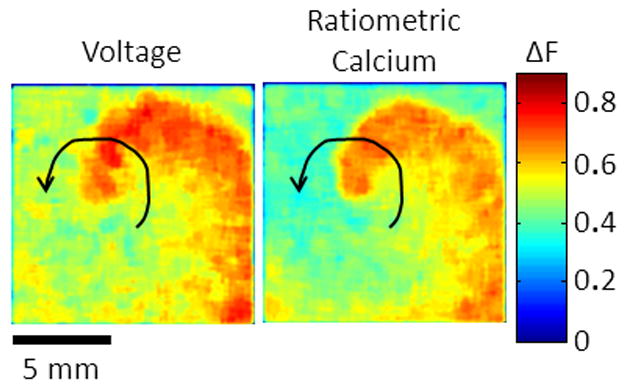
To date, most reports using iPSC-CMs to study electrophysiology have used single-cell patch-clamp techniques. However, the laborious nature of these techniques precludes efficient screening of electrical wave propagation through a functional syncytium of cardiac tissue.150 Micro-electrode arrays and optical mapping have been employed on small human iPSC-CM aggregates, but observed conduction velocities have been reportedly very low (< 2.5cm s−1).108,151 As innovative bioengineering approaches are developed152 to generate cardiac patches, hoped to repair infarcted hearts, optical mapping of these bioengineered constructs will be vital to assess function and electrical connectivity. Using a single-camera/LED-illumination system, we recently imaged action potential (di-8-ANEPPS) and ratiometric calcium (fura-4F) transients and wave propagation in relatively large (diameter ≥ 1cm) human iPSC-CM monolayers (unpublished work). The conduction velocity of wave propagation in these iPSC-CM monolayers was similar to that recorded in neonatal rat ventricular myocyte monolayers: ~22cm/s. Figure 8 shows an example of a spontaneous activation spreading through the entire iPSC-CM monolayer. Thus, iPSC-CM monolayers offer an attractive in-vitro human system for study of arrhythmia mechanisms and drug screening.
Figure 8.
Vm and ratiometric Ca2+ (3 parameter) optical mapping in human iPSC-CM monolayer. Using a triple LED illumination system and a single high-speed EMCCD camera, Vm was recorded using di-8-ANEPPS and Ca2+ was recorded ratiometrically using fura-4F, a low-affinity dye. Unpublished data.
Optogenetics
Channelrhodopsins are protein channels that transport cations across the cell membrane in response to light stimulation.153 Four distinct channelrhodopsins have been discovered: ChR1 & ChR2 from Chlamydomonas reinhardtii and VChR1 & VChR2 from Volvox carteri. These channelrhodopsins function in Nature as sensory photoreceptors that microalgae use for phototaxis (movement towards or away from light), optimizing light conditions for photosynthesis. In 2005 the light sensitive gene was first delivered to cells using genetically engineered viruses and pulses of light can now be used to precisely stimulate transfected excitable cells.154,155 This technique has gained widespread popularity in the neuroscience and cardiac fields and is now known as optogenetics.156,157
The cardiac pacemaker initiates every cardiac contraction and in pathological conditions can be substituted by surgically-implantable battery-operated devices.158 Recently, optogenetics has been used to optically control cardiac function in zebrafish hearts.159 This year Jia et al.160 reported the first combination of optical excitation and optical imaging to capture light-triggered cardiac muscle contractions and high-resolution electrical waves in mammalian cardiac cells and tissue.160 More recently, Abilez et al.161 demonstrated the potential of optogenetic control in human cardiac cells using a combined experimental/computational approach. It was demonstrated that channelrhodopsin-2 (ChR2) can be stably expressed in human pluripotent embryonic stem cells (hESC), which can then be differentiated into functional human cardiomyocytes using defined protocols.162 This was the first creation of ChR2-expressing human cardiomyocytes that could successfully be triggered using light. In computational work, they also showed the utility of optogenetics to generate propagated cardiac impulses. This technology may lead to a light-triggered biological pacemaker created from a patient’s own iPSC-CMs. However, this is a long way away and as optogenetic technology advances, it will be critical to utilize optical mapping techniques to provide detailed insight into the electrophysiological function of optogenetic manipulations.
Conclusion
Cardiac optical mapping has evolved to a powerful and essential technology for studying cardiovascular function and disease. Over the last two to three decades the technological advances in cardiac optical mapping have provided new mechanistic insights into electro-mechanical function, arrhythmias and disease mechanisms from the single cell to the whole organ. Its application continues to expand, even penetrating the fields of bioengineering and optogenetics. It was only in 2007 that sub-millimeter spatial resolution (0.65–0.85 mm) optical mapping demonstrated that VF in human hearts is associated with wave breaks and singularity point formation and is maintained by high frequency rotors and fibrillatory conduction.4 With the development of new infrared voltage-sensitive dyes, optical mapping may one day be used to provide in-vivo examination of AP propagation using sophisticated catheter-based mapping protocols. Last, but not least, optical mapping will also prove to be a pivotal tool in the development of regenerative cardiac research and therapies, including molecular genetic advances.
Acknowledgments
We thank Cellular Dynamics International for the generous donation of the iPSC-CM used in the experiment shown in Figure 8, and Dr. Tim Kamp for his mentorship in iPSC technology.
Sources of Funding:
This work was supported by NIH grants P01-HL039707 and P01-HL087226 (J.J.), the Leducq Foundation (J.J.), and the Clarendon Fund Scholarship (P.L.).
Non-Standard Abbreviations
- LED
Light Emitting Diode
- SAN
Sinoatrial Node
- EMCCD
Electron Multiplying Charge-Coupled Device
- PDA
Photodiode Array
- CMOS
Complementary Metal-Oxide Semiconductor
Footnotes
Disclosures: None
References
- 1.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: A mechanism of atrial fibrillation. Cardiovascular Research. 2002;54:204–216. doi: 10.1016/s0008-6363(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 2.Trayanova NA. Whole-heart modeling. Circulation Research. 2011;108:113–128. doi: 10.1161/CIRCRESAHA.110.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 4.Nanthakumar K, Jalife J, Massé S, Downar E, Pop M, Asta J, Ross H, Rao V, Mironov S, Sevaptsidis E, Rogers J, Wright G, Dhopeshwarkar R. Optical mapping of langendorff-perfused human hearts: Establishing a model for the study of ventricular fibrillation in humans. American Journal of Physiology -Heart and Circulatory Physiology. 2007;293:H875–H880. doi: 10.1152/ajpheart.01415.2006. [DOI] [PubMed] [Google Scholar]
- 5.Fabiato A, Fabiato F. Use of chlorotetracycline fluorescence to demonstrate ca2+-induced release of ca2+ from the sarcoplasmic reticulum of skinned cardiac cells. Nature. 1979;281:146–148. doi: 10.1038/281146a0. [DOI] [PubMed] [Google Scholar]
- 6.Song L-S, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 8.Salzberg BM, Davila HV, Cohen LB. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973;246:508–509. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- 9.Efimov IR, Fedorov VV, Joung B, Lin S-F. Mapping cardiac pacemaker circuits. Circulation Research. 2010;106:255–271. doi: 10.1161/CIRCRESAHA.109.209841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Entcheva E, Bien H. Macroscopic optical mapping of excitation in cardiac cell networks with ultra-high spatiotemporal resolution. Progress in Biophysics and Molecular Biology. 2006;92:232–257. doi: 10.1016/j.pbiomolbio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Entcheva E, Kostov Y, Tchernev E, Tung L. Fluorescence imaging of electrical activity in cardiac cells using an all-solid-state system. IEEE Trans Biomed Eng. 2004;51:333–341. doi: 10.1109/TBME.2003.820376. [DOI] [PubMed] [Google Scholar]
- 12.Berenfeld O, Mandapati R, Dixit S, Skanes AC, Chen JAY, Mansour M, Jalife J. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the langendorff-perfused sheep heart. Journal of Cardiovascular Electrophysiology. 2000;11:869–879. doi: 10.1111/j.1540-8167.2000.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 13.Mironov S, Jalife J, Tolkacheva EG. Role of conduction velocity restitution and short-term memory in the development of action potential duration alternans in isolated rabbit hearts. Circulation. 2008;118:17–25. doi: 10.1161/CIRCULATIONAHA.107.737254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–879. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omichi C, Lamp ST, Lin S-F, Yang J, Baher A, Zhou S, Attin M, Lee M-H, Karagueuzian HS, Kogan B, Qu Z, Garfinkel A, Chen P-S, Weiss JN. Intracellular ca dynamics in ventricular fibrillation. American Journal of Physiology -Heart and Circulatory Physiology. 2004;286:H1836–H1844. doi: 10.1152/ajpheart.00123.2003. [DOI] [PubMed] [Google Scholar]
- 16.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, Pozzan T. Beat-to-beat oscillations of mitochondrial [ca2+] in cardiac cells. EMBO J. 2001;20:4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaniv Y, Juhaszova M, Wang S, Fishbein KW, Zorov DB, Sollott SJ. Analysis of mitochondrial 3d-deformation in cardiomyocytes during active contraction reveals passive structural anisotropy of orthogonal short axes. PLoS ONE. 2011;6:e21985. doi: 10.1371/journal.pone.0021985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zorov DB, Kobrinsky E, Juhaszova M, Sollott SJ. Examining intracellular organelle function using fluorescent probes. Circulation Research. 2004;95:239–252. doi: 10.1161/01.RES.0000137875.42385.8e. [DOI] [PubMed] [Google Scholar]
- 19.Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circ Res. 2004;95:21–33. doi: 10.1161/01.RES.0000130529.18016.35. [DOI] [PubMed] [Google Scholar]
- 20.Efimov I, Huang D, Rendt J, Salama G. Optical mapping of repolarization and refractoriness from intact hearts. Circulation. 1994;90:1469–1480. doi: 10.1161/01.cir.90.3.1469. [DOI] [PubMed] [Google Scholar]
- 21.Banville I, Gray RA, Ideker RE, Smith WM. Shock-induced figure<$downlink>-of-eight reentry in the isolated rabbit heart. Circulation Research. 1999;85:742–752. doi: 10.1161/01.res.85.8.742. [DOI] [PubMed] [Google Scholar]
- 22.Zaitsev AV, Guha PK, Sarmast F, Kolli A, Berenfeld O, Pertsov AM, de Groot JR, Coronel R, Jalife J. Wavebreak formation during ventricular fibrillation in the isolated, regionally ischemic pig heart. Circulation Research. 2003;92:546–553. doi: 10.1161/01.RES.0000061917.23107.F7. [DOI] [PubMed] [Google Scholar]
- 23.Robbins MS, Hadwen BJ. The noise performance of electron multiplying charge-coupled devices. IEEE Transactions on Electron Devices. 2003;50:1227–1232. [Google Scholar]
- 24.Madan SK, Bhaumik B, Vasi JM. Experimental observation of avalanche multiplication in charge-coupled devices. IEEE Transactions on Electron Devices. 1983;ED-30:694–699. [Google Scholar]
- 25.Coates CG, Denvir DJ, McHale NG, Thornbury KD, Hollywood MA. Optimizing low-light microscopy with back-illuminated electron multiplying charge-coupled device: Enhanced sensitivity, speed, and resolution. J Biomed Opt. 2004;9:1244–1252. doi: 10.1117/1.1805559. [DOI] [PubMed] [Google Scholar]
- 26.Rogers KL, Martin JR, Renaud O, Karplus E, Nicola MA, Nguyen M, Picaud S, Shorte SL, Brulet P. Electron-multiplying charge-coupled detector-based bioluminescence recording of single-cell ca2+ J Biomed Opt. 2008;13:031211. doi: 10.1117/1.2937236. [DOI] [PubMed] [Google Scholar]
- 27.Albeanu DF, Soucy E, Sato TF, Meister M, Murthy VN. Led arrays as cost effective and efficient light sources for widefield microscopy. PLoS ONE. 2008;3:e2146. doi: 10.1371/journal.pone.0002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee P, Bollensdorff C, Quinn TA, Wuskell JP, Loew LM, Kohl P. Single-sensor system for spatially-resolved, continuous and multi-parametric optical mapping of cardiac tissue. Heart Rhythm. 2011 doi: 10.1016/j.hrthm.2011.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grynkiewicz G, Poenie M, Tsien RY. A new generation of ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 30.Davila HV. A large change in axon fluorescence that provides a promising method for measuring membrane potential. Nature: New biology. 1973;241:159–160. doi: 10.1038/newbio241159a0. [DOI] [PubMed] [Google Scholar]
- 31.Salama G, Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 1976;191:485–487. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- 32.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. The Journal of Physiology. 1995;486:1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou L, Deo M, Furspan P, Pandit SV, Mironov S, Auerbach DS, Gong Q, Zhou Z, Berenfeld O, Jalife J. A major role for herg in determining frequency of reentry in neonatal rat ventricular myocyte monolayer. Circ Res. 2010;107:1503–1511. doi: 10.1161/CIRCRESAHA.110.232470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz V, Grzeda KR, Desplantez T, Pandit SV, Mironov S, Taffet SM, Rohr S, Kleber AG, Jalife J. Adenoviral expression of iks contributes to wavebreak and fibrillatory conduction in neonatal rat ventricular cardiomyocyte monolayers. Circ Res. 2007;101:475–483. doi: 10.1161/CIRCRESAHA.107.149617. [DOI] [PubMed] [Google Scholar]
- 35.Laurita KR, Girouard SD, Rosenbaum DS. Modulation of ventricular repolarization by a premature stimulus: Role of epicardial dispersion of repolarization kinetics demonstrated by optical mapping of the intact guinea pig heart. Circulation Research. 1996;79:493–503. doi: 10.1161/01.res.79.3.493. [DOI] [PubMed] [Google Scholar]
- 36.Morad M, Salama G. Optical probes of membrane potential in heart muscle. The Journal of Physiology. 1979;292:267–295. doi: 10.1113/jphysiol.1979.sp012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen LB. Optical measurement of membrane potential. Reviews of physiology, biochemistry and pharmacology. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- 38.Lev-Ram V, Grinvald A. Ca2+- and k+-dependent communication between central nervous system myelinated axons and oligodendrocytes revealed by voltage-sensitive dyes. Proceedings of the National Academy of Sciences. 1986;83:6651–6655. doi: 10.1073/pnas.83.17.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fluhler E, Burnham VG, Loew LM. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry. 1985;24:5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- 40.Schaffer P. Di-4-anepps causes photodynamic damage to isolated cardiomyocytes. Pflügers Archiv. 1994;426:548–551. doi: 10.1007/BF00378533. [DOI] [PubMed] [Google Scholar]
- 41.Rohr S, Salzberg BM. Multiple site optical recording of transmembrane voltage (msortv) in patterned growth heart cell cultures: Assessing electrical behavior, with microsecond resolution, on a cellular and subcellular scale. Biophysical Journal. 1994;67:1301–1315. doi: 10.1016/S0006-3495(94)80602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sill B, Hammer PE, Cowan DB. Optical mapping of langendorff-perfused rat hearts. J Vis Exp. 2009:e1138. doi: 10.3791/1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salama G, Choi B-R. Images of action potential propagation in heart. Physiology. 2000;15:33–41. doi: 10.1152/physiologyonline.2000.15.1.33. [DOI] [PubMed] [Google Scholar]
- 44.Bachtel AD, Gray RA, Stohlman JM, Bourgeois EB, Pollard AE, Rogers JM. A novel approach to dual excitation ratiometric optical mapping of cardiac action potentials with di-4-anepps using pulsed led excitation. Biomedical Engineering, IEEE Transactions on. 2011;58:2120–2126. doi: 10.1109/TBME.2011.2148719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salama G, Choi BR, Azour G, Lavasani M, Tumbev V, Salzberg BM, Patrick MJ, Ernst LA, Waggoner AS. Properties of new, long-wavelength, voltage-sensitive dyes in the heart. Journal of Membrane Biology. 2005;208:125–140. doi: 10.1007/s00232-005-0826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matiukas A, Mitrea BG, Pertsov AM, Wuskell JP, Wei M-d, Watras J, Millard AC, Loew LM. New near-infrared optical probes of cardiac electrical activity. American Journal of Physiology -Heart and Circulatory Physiology. 2006;290:H2633–H2643. doi: 10.1152/ajpheart.00884.2005. [DOI] [PubMed] [Google Scholar]
- 47.Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei M-d, Watras J, Loew LM. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm. 2007;4:1441–1451. doi: 10.1016/j.hrthm.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circulation Research. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 49.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annual Review of Physiology. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 50.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 51.Laflamme MA, Becker PL. Ca2+-induced current oscillations in rabbit ventricular myocytes. Circulation Research. 1996;78:707–716. doi: 10.1161/01.res.78.4.707. [DOI] [PubMed] [Google Scholar]
- 52.Bers DM, Despa S, Bossuyt J. Regulation of ca2+ and na+ in normal and failing cardiac myocytes. Annals of the New York Academy of Sciences. 2006;1080:165–177. doi: 10.1196/annals.1380.015. [DOI] [PubMed] [Google Scholar]
- 53.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure : Roles of sodium-calcium exchange, inward rectifier potassium current, and residual {beta}-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 54.Priori SG, Chen SRW. Inherited dysfunction of sarcoplasmic reticulum ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashimura T, Briston SJ, Trafford AW, Napolitano C, Priori SG, Eisner DA, Venetucci LA. In the ryr2r4496c mouse model of cpvt, β-adrenergic stimulation induces ca waves by increasing sr ca content and not by decreasing the threshold for ca waves / novelty and significance. Circulation Research. 2010;107:1483–1489. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 56.Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff J-M, Da Costa A, Sebillon P, Mannens MMAM, Wilde AAM, Guicheney P. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circulation Research. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 57.Meli AC, Refaat MM, Dura M, Reiken S, Wronska A, Wojciak J, Carroll J, Scheinman MM, Marks AR. A novel ryanodine receptor mutation linked to sudden death increases sensitivity to cytosolic calcium / novelty and significance. Circulation Research. 2011;109:281–290. doi: 10.1161/CIRCRESAHA.111.244970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollingworth S, Gee KR, Baylor SM. Low-affinity ca2+ indicators compared in measurements of skeletal muscle ca2+ transients. Biophys J. 2009;97:1864–1872. doi: 10.1016/j.bpj.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsien RY. Fluorescent probes of cell signaling. Annual Review of Neuroscience. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- 60.Vladimir GF. Simultaneous optical imaging of membrane potential and intracellular calcium. Journal of Electrocardiology. 2005;38:107–112. doi: 10.1016/j.jelectrocard.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 61.Wokosin DL, Loughrey CM, Smith GL. Characterization of a range of fura dyes with two-photon excitation. Biophysical Journal. 2004;86:1726–1738. doi: 10.1016/S0006-3495(04)74241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rochefort NL, Konnerth A. Genetically encoded ca2+ sensors come of age. Nat Meth. 2008;5:761–762. doi: 10.1038/nmeth0908-761. [DOI] [PubMed] [Google Scholar]
- 63.Kohl P, Noble D. Life and mechanosensitivity. Progress in Biophysics and Molecular Biology. 97:159–162. doi: 10.1016/j.pbiomolbio.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 64.Dillon S. Optical recordings in the rabbit heart show that defibrillation strength shocks prolong the duration of depolarization and the refractory period. Circulation Research. 1991;69:842–856. doi: 10.1161/01.res.69.3.842. [DOI] [PubMed] [Google Scholar]
- 65.Li T, Sperelakis N, Teneick RE, Solaro RJ. Effects of diacetyl monoxime on cardiac excitation-contraction coupling. Journal of Pharmacology and Experimental Therapeutics. 1985;232:688–695. [PubMed] [Google Scholar]
- 66.Efimov IR, Mazgalev TN. High-resolution, three-dimensional fluorescent imaging reveals multilayer conduction pattern in the atrioventricular node. Circulation. 1998;98:54–57. doi: 10.1161/01.cir.98.1.54. [DOI] [PubMed] [Google Scholar]
- 67.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation–contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 68.Huke S, Knollmann BC. Increased myofilament ca2+-sensitivity and arrhythmia susceptibility. Journal of Molecular and Cellular Cardiology. 2010;48:824–833. doi: 10.1016/j.yjmcc.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. The Journal of Clinical Investigation. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rohde GK, Dawant BM, Shien-Fong L. Correction of motion artifact in cardiac optical mapping using image registration. Biomedical Engineering, IEEE Transactions on. 2005;52:338–341. doi: 10.1109/TBME.2004.840464. [DOI] [PubMed] [Google Scholar]
- 71.Baxter WT, Mironov SF, Zaitsev AV, Jalife J, Pertsov AM. Visualizing excitation waves inside cardiac muscle using transillumination. Biophysical Journal. 2001;80:516–530. doi: 10.1016/S0006-3495(01)76034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bishop MJ, Rodriguez B, Qu F, Efimov IR, Gavaghan DJ, Trayanova NA. The role of photon scattering in optical signal distortion during arrhythmia and defibrillation. Biophysical Journal. 2007;93:3714–3726. doi: 10.1529/biophysj.107.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyatt CJ, Mironov SF, Vetter FJ, Zemlin CW, Pertsov AM. Optical action potential upstroke morphology reveals near-surface transmural propagation direction. Circulation Research. 2005;97:277–284. doi: 10.1161/01.RES.0000176022.74579.47. [DOI] [PubMed] [Google Scholar]
- 74.Janks DL, Roth BJ. Averaging over depth during optical mapping of unipolar stimulation. Biomedical Engineering, IEEE Transactions on. 2002;49:1051–1054. doi: 10.1109/TBME.2002.802057. [DOI] [PubMed] [Google Scholar]
- 75.Ramshesh V. Spatial localization of cardiac optical mapping with multiphoton excitation. J Biomed Opt. 2003;8:253. doi: 10.1117/1.1559831. [DOI] [PubMed] [Google Scholar]
- 76.Hyatt CJ, Mironov SF, Wellner M, Berenfeld O, Popp AK, Weitz DA, Jalife J, Pertsov AM. Synthesis of voltage-sensitive fluorescence signals from three-dimensional myocardial activation patterns. Biophysical Journal. 2003;85:2673–2683. doi: 10.1016/s0006-3495(03)74690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bray M-A, Wikswo JP. Examination of optical depth effects on fluorescence imaging of cardiac propagation. Biophysical Journal. 2003;85:4134–4145. doi: 10.1016/S0006-3495(03)74825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bishop MJ, Rodriguez B, Eason J, Whiteley JP, Trayanova N, Gavaghan DJ. Synthesis of voltage-sensitive optical signals: Application to panoramic optical mapping. Biophysical Journal. 2006;90:2938–2945. doi: 10.1529/biophysj.105.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hillman EMC, Bernus O, Pease E, Bouchard MB, Pertsov A. Depth-resolved optical imaging of transmural electrical propagation in perfused heart. Opt Express. 2007;15:17827–17841. doi: 10.1364/oe.15.017827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walton RD, Mitrea BG, Pertsov AM, Bernus O. A novel near-infrared voltage-sensitive dye reveals the action potential wavefront orientation at increased depths of cardiac tissue. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4523–4526. doi: 10.1109/IEMBS.2009.5334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lakatta EG, Guarnieri T. Spontaneous myocardial calcium oscillations. Journal of Cardiovascular Electrophysiology. 1993;4:473–489. doi: 10.1111/j.1540-8167.1993.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 82.Tweedie D, Harding SE, MacLeod KT. Sarcoplasmic reticulum ca content, sarcolemmal ca influx and the genesis of arrhythmias in isolated guinea-pig cardiomyocytes. Journal of Molecular and Cellular Cardiology. 2000;32:261–272. doi: 10.1006/jmcc.1999.1070. [DOI] [PubMed] [Google Scholar]
- 83.Fedida D, Noble D, Rankin AC, Spindler AJ. The arrhythmogenic transient inward current i(ti) and related contraction in isolated guinea-pig ventricular myocytes. Journal of Physiology. 1987;392:523–542. doi: 10.1113/jphysiol.1987.sp016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circulation Research. 1989;65:115–126. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- 85.Hiraoka M, Kawano S, Hirano Y, Furukawa T. Role of cardiac chloride currents in changes in action potential characteristics and arrhythmias. Cardiovascular Research. 1998;40:23–33. doi: 10.1016/s0008-6363(98)00173-4. [DOI] [PubMed] [Google Scholar]
- 86.Gray RA, Iyer A, Bray M-A, Wikswo JP. Voltage-calcium state-space dynamics during initiation of reentry. Heart Rhythm. 2006;3:247–248. doi: 10.1016/j.hrthm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 87.Choi B-R, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: Mechanisms underlying concordant alternans. The Journal of Physiology. 2000;529:171–188. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holcomb MR, Woods MC, Uzelac I, Wikswo JP, Gilligan JM, Sidorov VY. The potential of dual camera systems for multimodal imaging of cardiac electrophysiology and metabolism. Experimental Biology and Medicine. 2009;234:1355–1373. doi: 10.3181/0902-RM-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Windisch H, Ahammer H, Schaffer P, Müller W, Platzer D. Optical multisite monitoring of cell excitation phenomena in isolated cardiomyocytes. Pflügers Archiv European Journal of Physiology. 1995;430:508–518. doi: 10.1007/BF00373887. [DOI] [PubMed] [Google Scholar]
- 90.Sharma V, Susil RC, Tung L. Paradoxical loss of excitation with high intensity pulses during electric field stimulation of single cardiac cells. Biophysical Journal. 2005;88:3038–3049. doi: 10.1529/biophysj.104.047142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma V, Tung L. Ionic currents involved in shock-induced nonlinear changes in transmembrane potential responses of single cardiac cells. Pflügers Archiv European Journal of Physiology. 2004;449:248–256. doi: 10.1007/s00424-004-1335-9. [DOI] [PubMed] [Google Scholar]
- 92.Warren M, Spitzer KW, Steadman BW, Rees TD, Venable P, Taylor T, Shibayama J, Yan P, Wuskell JP, Loew LM, Zaitsev AV. High-precision recording of the action potential in isolated cardiomyocytes using the near-infrared fluorescent dye di-4-anbdqbs. American Journal of Physiology -Heartand Circulatory Physiology. 2010;299:H1271–H1281. doi: 10.1152/ajpheart.00248.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RAB, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in ca2+ spark rate. Circulation Research. 2009;104:787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nishimura S, Kawai Y, Nakajima T, Hosoya Y, Fujita H, Katoh M, Yamashita H, Nagai R, Sugiura S. Membrane potential of rat ventricular myocytes responds to axial stretch in phase, amplitude and speed-dependent manners. Cardiovascular Research. 2006;72:403–411. doi: 10.1016/j.cardiores.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 95.Rohr S, Scholly D, Kleber A. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circulation Research. 1991;68:114–130. doi: 10.1161/01.res.68.1.114. [DOI] [PubMed] [Google Scholar]
- 96.Auerbach DS, Grzda KR, Furspan PB, Sato PY, Mironov S, Jalife J. Structural heterogeneity promotes triggered activity, reflection and arrhythmogenesis in cardiomyocyte monolayers. J Physiol. 2011;589:2363–2381. doi: 10.1113/jphysiol.2010.200576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Domke J, Parak WJ, George M, Gaub HE, Radmacher M. Mapping the mechanical pulse of single cardiomyocytes with the atomic force microscope. European Biophysics Journal. 1999;28:179–186. doi: 10.1007/s002490050198. [DOI] [PubMed] [Google Scholar]
- 98.Fast V, Kleber A. Microscopic conduction in cultured strands of neonatal rat heart cells measured with voltage-sensitive dyes. Circulation Research. 1993;73:914–925. doi: 10.1161/01.res.73.5.914. [DOI] [PubMed] [Google Scholar]
- 99.Bub G, Glass L, Publicover NG, Shrier A. Bursting calcium rotors in cultured cardiac myocyte monolayers. Proceedings of the National Academy of Sciences. 1998;95:10283–10287. doi: 10.1073/pnas.95.17.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cysyk J, Tung L. Electric field perturbations of spiral waves attached to millimeter-size obstacles. Biophys J. 2008;94:1533–1541. doi: 10.1529/biophysj.107.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Diego C, Pai RK, Chen F, Xie LH, De Leeuw J, Weiss JN, Valderrabano M. Electrophysiological consequences of acute regional ischemia/reperfusion in neonatal rat ventricular myocyte monolayers. Circulation. 2008;118:2330–2337. doi: 10.1161/CIRCULATIONAHA.108.789149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zlochiver S, Muñoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophysical Journal. 2008;95:4469–4480. doi: 10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bullen A, Saggau P. Indicators and optical configuration for simultaneous high-resolution recording of membrane potential and intracellular calcium using laser scanning microscopy. Pflügers Archiv European Journal of Physiology. 1998;436:788–796. doi: 10.1007/s004240050703. [DOI] [PubMed] [Google Scholar]
- 104.Laurita KR, Singal A. Mapping action potentials and calcium transients simultaneously from the intact heart. American Journal of Physiology - Heart and Circulatory Physiology. 2001;280:H2053–H2060. doi: 10.1152/ajpheart.2001.280.5.H2053. [DOI] [PubMed] [Google Scholar]
- 105.Johnson PL, Smith W, Baynham TC, Knisley SB. Errors caused by combination of di-4 anepps and fluo3/4 for simultaneous measurements of transmembrane potentials and intracellular calcium. Annals of Biomedical Engineering. 1999;27:563–571. doi: 10.1114/1.198. [DOI] [PubMed] [Google Scholar]
- 106.Fast VG, Ideker RE. Simultaneous optical mapping of transmembrane potential and intracellular calcium in myocyte cultures. Journal of Cardiovascular Electrophysiology. 2000;11:547–556. doi: 10.1111/j.1540-8167.2000.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 107.Kong WEI, Walcott GP, Smith WM, Johnson PL, Knisley SB. Emission ratiometry for simultaneous calcium and action potential measurements with coloaded dyes in rabbit hearts: Reduction of motion and drift. Journal of Cardiovascular Electrophysiology. 2003;14:76–82. doi: 10.1046/j.1540-8167.2003.02077.x. [DOI] [PubMed] [Google Scholar]
- 108.Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L, Zambidis ET. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovascular Research. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 110.Boyett MR, Dobrzynski H. The sinoatrial node is still setting the pace 100 years after its discovery. Circulation Research. 2007;100:1543–1545. doi: 10.1161/CIRCRESAHA.107.101101. [DOI] [PubMed] [Google Scholar]
- 111.Michaels D, Matyas E, Jalife J. Mechanisms of sinoatrial pacemaker synchronization: A new hypothesis. Circulation Research. 1987;61:704–714. doi: 10.1161/01.res.61.5.704. [DOI] [PubMed] [Google Scholar]
- 112.Yaniv Y, Maltsev VA, Escobar AL, Spurgeon HA, Ziman BD, Stern MD, Lakatta EG. Beat-to-beat ca2+-dependent regulation of sinoatrial nodal pacemaker cell rate and rhythm. Journal of Molecular and Cellular Cardiology. doi: 10.1016/j.yjmcc.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DiFrancesco D, Noble D. The funny current has a major pacemaking role in the sinus node. Heart Rhythm. doi: 10.1016/j.hrthm.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 114.DiFrancesco D, Noble D. Rebuttal to “the funny current in the context of the coupled clock pacemaker cell system” by victor a. Maltsev and edward g. Lakatta. Heart Rhythm. doi: 10.1016/j.hrthm.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lakatta EG, Maltsev VA. What if the shoe doesn’t fit? Rebuttal to difrancesco and noble “the funny current has a major pacemaking role in the sinus node”. Heart Rhythm. doi: 10.1016/j.hrthm.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ju Y-K, Allen DG. Intracellular calcium and na+-ca2+ exchange current in isolated toad pacemaker cells. The Journal of Physiology. 1998;508:153–166. doi: 10.1111/j.1469-7793.1998.153br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bromberg BI, Hand DE, Schuessler RB, Boineau JP. Primary negativity does not predict dominant pacemaker location: Implications for sinoatrial conduction. American Journal of Physiology -Heart and Circulatory Physiology. 1995;269:H877–H887. doi: 10.1152/ajpheart.1995.269.3.H877. [DOI] [PubMed] [Google Scholar]
- 118.Schuessler RB. Abnormal sinus nodefunction in clinical arrhythmias. Journal of Cardiovascular Electrophysiology. 2003;14:215–217. doi: 10.1046/j.1540-8167.2003.02229.x. [DOI] [PubMed] [Google Scholar]
- 119.Boineau J, Schuessler R, Mooney C, Wylds A, Miller C, Hudson R, Borremans J, Brockus C. Multicentric origin of the atrial depolarization wave: The pacemaker complex. Relation to dynamics of atrial conduction, p-wave changes and heart rate control. Circulation. 1978;58:1036–1048. doi: 10.1161/01.cir.58.6.1036. [DOI] [PubMed] [Google Scholar]
- 120.Fedorov VV, Schuessler RB, Hemphill M, Ambrosi CM, Chang R, Voloshina AS, Brown K, Hucker WJ, Efimov IR. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circulation Research. 2009;104:915–923. doi: 10.1161/CIRCRESAHA.108.193193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.James TN. Anatomy of the human sinus node. The Anatomical Record. 1961;141:109–139. doi: 10.1002/ar.1091410205. [DOI] [PubMed] [Google Scholar]
- 122.Fedorov VV, Glukhov AV, Chang R, Kostecki G, Aferol H, Hucker WJ, Wuskell JP, Loew LM, Schuessler RB, Moazami N, Efimov IR. Optical mapping of the isolated coronary-perfused human sinus node. Journal of the American College of Cardiology. 2010;56:1386–1394. doi: 10.1016/j.jacc.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laurita KR, Rosenbaum DS. Cellular mechanisms of arrhythmogenic cardiac alternans. Prog Biophys Mol Biol. 2008;97:332–347. doi: 10.1016/j.pbiomolbio.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cerrone M, Noujaim SF, Tolkacheva EG, Talkachou A, O’Connell R, Berenfeld O, Anumonwo J, Pandit SV, Vikstrom K, Napolitano C, Priori SG, Jalife J. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007;101:1039–1048. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gray RA, Ayers G, Jalife J. Video imaging of atrial defibrillation in the sheep heart. Circulation. 1997;95:1038–1047. doi: 10.1161/01.cir.95.4.1038. [DOI] [PubMed] [Google Scholar]
- 126.Yamazaki M, Vaquero LM, Hou L, Campbell K, Zlochiver S, Klos M, Mironov S, Berenfeld O, Honjo H, Kodama I, Jalife J, Kalifa J. Mechanisms of stretch-induced atrial fibrillation in the presence and the absence of adrenocholinergic stimulation: Interplay between rotors and focal discharges. Heart Rhythm. 2009;6:1009–1017. doi: 10.1016/j.hrthm.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Girouard SD, Pastore JM, Laurita KR, Gregory KW, Rosenbaum DS. Optical mapping in a new guinea pig model of ventricular tachycardia reveals mechanisms for multiple wavelengths in a single reentrant circuit. Circulation. 1996;93:603–613. doi: 10.1161/01.cir.93.3.603. [DOI] [PubMed] [Google Scholar]
- 128.Efimov IR, Sidorov V, Cheng Y, Wollenzier B. Evidence of three-dimensional scroll waves with ribbon-shaped filament as a mechanism of ventricular tachycardia in the isolated rabbit heart. Journal of Cardiovascular Electrophysiology. 1999;10:1452–1462. doi: 10.1111/j.1540-8167.1999.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 129.Seet RCS, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation. 2011;124:477–486. doi: 10.1161/CIRCULATIONAHA.111.029801. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, Zaitsev AV, Vaidyanathan R, Auerbach DS, Landas S, Guiraudon G, Jalife J, Berenfeld O, Kalifa J. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circulation Research. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 131.Jalife J. Experimental and clinical af mechanisms: Bridging the divide. Journal of Interventional Cardiac Electrophysiology. 2003;9:85–92. doi: 10.1023/a:1026251517004. [DOI] [PubMed] [Google Scholar]
- 132.Filgueiras-Rama D. High-resolution endocardial and epicardial optical mapping in a sheep model of stretch-induced atrial fibrillation. Journal of visualized experiments. 2011 doi: 10.3791/3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001;103:2631–2636. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- 134.Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–633. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 135.Kalifa J, Klos M, Zlochiver S, Mironov S, Tanaka K, Ulahannan N, Yamazaki M, Jalife J, Berenfeld O. Endoscopic fluorescence mapping of the left atrium: A novel experimental approach for high resolution endocardial mapping in the intact heart. Heart Rhythm. 2007;4:916–924. doi: 10.1016/j.hrthm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Noujaim SF, Berenfeld O, Kalifa J, Cerrone M, Nanthakumar K, Atienza F, Moreno J, Mironov S, Jalife J. Universal scaling law of electrical turbulence in the mammalian heart. Proc Natl Acad Sci U S A. 2007;104:20985–20989. doi: 10.1073/pnas.0709758104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nanthakumar K, Jalife J, Masse S, Downar E, Pop M, Asta J, Ross H, Rao V, Mironov S, Sevaptsidis E, Rogers J, Wright G, Dhopeshwarkar R. Optical mapping of langendorff-perfused human hearts: Establishing a model for the study of ventricular fibrillation in humans. Am J Physiol Heart Circ Physiol. 2007;293:H875–880. doi: 10.1152/ajpheart.01415.2006. [DOI] [PubMed] [Google Scholar]
- 138.Nair K, Umapathy K, Farid T, Masse S, Mueller E, Sivanandan RV, Poku K, Rao V, Nair V, Butany J, Ideker RE, Nanthakumar K. Intramural activation during early human ventricular fibrillation. Circ Arrhythm Electrophysiol. 2011;4:692–703. doi: 10.1161/CIRCEP.110.961037. [DOI] [PubMed] [Google Scholar]
- 139.El-Sherif N, Chinushi M, Caref EB, Restivo M. Electrophysiological mechanism of the characteristic electrocardiographic morphology of torsade de pointes tachyarrhythmias in the long-qt syndrome : Detailed analysis of ventricular tridimensional activation patterns. Circulation. 1997;96:4392–4399. doi: 10.1161/01.cir.96.12.4392. [DOI] [PubMed] [Google Scholar]
- 140.Frazier D, Krassowska W, Chen P, Wolf P, Danieley N, Smith W, Ideker R. Transmural activations and stimulus potentials in three-dimensional anisotropic canine myocardium. Circulation Research. 1988;63:135–146. doi: 10.1161/01.res.63.1.135. [DOI] [PubMed] [Google Scholar]
- 141.Hooks DA, LeGrice IJ, Harvey JD, Smaill BH. Intramural multisite recording of transmembrane potential in the heart. Biophysical Journal. 2001;81:2671–2680. doi: 10.1016/S0006-3495(01)75910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Byars JL, Smith WM, Ideker RE, Fast VG. Development of an optrode for intramural multisite optical recordings of vm in the heart. Journal of Cardiovascular Electrophysiology. 2003;14:1196–1202. doi: 10.1046/j.1540-8167.2003.03203.x. [DOI] [PubMed] [Google Scholar]
- 143.Wei K, Pollard AE, Fast VG. A new optrode design for intramural optical recordings. Biomedical Engineering, IEEE Transactions on. 2011;58:3130–3134. doi: 10.1109/TBME.2011.2167623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circulation Research. 2010;106:981–991. doi: 10.1161/CIRCRESAHA.109.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lou Q, Fedorov VV, Glukhov AV, Moazami N, Fast VG, Efimov IR. Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure / clinical perspective. Circulation. 2011;123:1881–1890. doi: 10.1161/CIRCULATIONAHA.110.989707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin S. Panoramic optical imaging of electrical propagation in isolated heart. J Biomed Opt. 1999;4:200. doi: 10.1117/1.429910. [DOI] [PubMed] [Google Scholar]
- 147.Qu F, Ripplinger CM, Nikolski VP, Grimm C, Efimov IR. Three-dimensional panoramic imaging of cardiac arrhythmias in rabbit heart. Journal of Biomedical Optics. 2007;12:044019. doi: 10.1117/1.2753748. [DOI] [PubMed] [Google Scholar]
- 148.Priori SG. Induced pluripotent stem cell-derived cardiomyocytes and long qt syndrome: Is personalized medicine ready for prime time? Circulation Research. 2011;109:822–824. doi: 10.1161/CIRCRESAHA.111.253724. [DOI] [PubMed] [Google Scholar]
- 149.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long qt syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 150.Tiscornia G, Monserrat N, Belmonte JC. Modelling long qt syndrome with ips cells: Be still, my beating heart. Circ Res. 2011;108:648–649. doi: 10.1161/RES.0b013e318216f0db. [DOI] [PubMed] [Google Scholar]
- 151.Mehta A, Chung YY, Ng A, Iskandar F, Atan S, Wei H, Dusting G, Sun W, Wong P, Shim W. Pharmacological response of human cardiomyocytes derived from virus-free induced pluripotent stem cells. Cardiovascular Research. 2011 doi: 10.1093/cvr/cvr132. [DOI] [PubMed] [Google Scholar]
- 152.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation Research. 2011 doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 154.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 155.Zhang F, Wang L-P, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Meth. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 156.Deisseroth K. Optogenetics. Nat Meth. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hegemann P, Moglich A. Channelrhodopsin engineering and exploration of new optogenetic tools. Nat Meth. 2011;8:39–42. doi: 10.1038/nmeth.f.327. [DOI] [PubMed] [Google Scholar]
- 158.Wood MA, Ellenbogen KA. Cardiac pacemakers from the patient’s perspective. Circulation. 2002;105:2136–2138. doi: 10.1161/01.cir.0000016183.07898.90. [DOI] [PubMed] [Google Scholar]
- 159.Arrenberg AB, Stainier DYR, Baier H, Huisken J. Optogenetic control of cardiac function. Science. 2010;330:971–974. doi: 10.1126/science.1195929. [DOI] [PubMed] [Google Scholar]
- 160.Jia Z, Valiunas V, Lu Z, Bien H, Liu H, Wang H-Z, Rosati B, Brink PR, Cohen IS, Entcheva E. Stimulating cardiac muscle by light: Cardiac optogenetics by cell delivery. Circulation: Arrhythmia and Electrophysiology. 2011 doi: 10.1161/CIRCEP.111.964247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abilez Oscar J, Wong J, Prakash R, Deisseroth K, Zarins Christopher K, Kuhl E. Multiscale computational models for optogenetic control of cardiac function. Biophysical Journal. 2011;101:1326–1334. doi: 10.1016/j.bpj.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotech. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]