Abstract
The characteristics of the matrix (composition, structure, mechanical properties) and external culture environment (pulsatile perfusion, physical stimulation) are critically important for engineering functional myocardial tissue. We report the development of chitosan-collagen scaffolds with micro-pores and an array of parallel channels (~200 μm in diameter) that were specifically designed for cardiac tissue engineering with mechanical stimulation. The scaffolds were designed to have the structural and mechanical properties similar to those of the native human heart matrix. Scaffolds were seeded with neonatal rat heart cells and subjected to dynamic tensile stretch using a custom-designed bioreactor. The channels enhanced oxygen transport and facilitated the establishment of cell connections within the construct. The myocardial patches (14 mm in diameter, 1–2 mm thick) consisted of metabolically active cells and started to contract synchronously after 3 days of culture. Mechanical stimulation with high tensile stresses promoted cell alignment, elongation, and the expression of connexin-43 (Cx-43). This study confirms the importance of scaffold design and mechanical stimulation for the formation of contractile cardiac constructs.
Keywords: cardiac, tissue engineering, scaffold, mechanical stimulation, perfusion, contractile function
1. Introduction
As cardiac myocytes are terminally differentiated, they do not proliferate, and the human heart cannot regenerate itself following myocardial infaction (Leor et al., 2005). Cardiac tissue engineering strategies for heart regeneration use cardiogenic and vasculogenic cells, biomaterial scaffolds and bioreactors to regenerate myocardial tissue with functional and morphological properties similar to those of native myocardium (Eschenhagen and Zimmermann 2005; Zammaretti and Jaconi 2004). Engineered tissues can also serve as models of high biological fidelity for studies of cardiac development and disease (Bursac et al., 1999).
The contractile behavior and electromechanical coupling between the cells, and the oriented and anisotropic structure of the heart matrix present unique challenges to engineering of myocardial tissue in vitro (Akins 2002; Zandonella 2003; Radisic et al., 2007; Cohen and Leor 2004; Zimmermann et al., 2006). The most critical requirements are thought to be those related to the establishment of blood flow and electromechanical coupling with the host cells (Radisic et al., 2008; Tandon et al., 2009).
Advanced approaches to the in vitro engineering of clinically sized cardiac muscle utilize mechanical (Zimmermann et al., 2006) and electrical stimuli (Radisic et al., 2008) to enhance functional coupling of the cells, and the generation of mechanical force), and the convective-diffusive supply of oxygen (to support cell viability in thick constructs.
In one approach, neonatal rat heart cells were cultured in collagen gel and Matrigel with the application of mechanical stretch (Eschenhagen and Zimmermann 2005; Zimmermann et al., 2002). Cells formed highly differentiated cardiac muscle syncytium that exhibited contractile and electrophysiological properties of working myocardium. Implantation in a rat model of myocardial infarction showed graft survival and vascularization (Zimmermann et al., 2002). When placed over the infarct bed, these tissue constructs delayed the thinning of the heart wall and induced functional improvement (Zimmermann et al., 2002).
In another approach, a “biomimetic” approach was utilized to engineer synchronously contracting cardiac tissue constructs (Radisic et al., 2007). To enhance oxygen supply to the cells, culture medium was perfused through a channeled scaffold seeded with cells (to mimic a capillary network) and supplemented with an oxygen carrier (to mimic hemoglobin), resulting in physiologic density of viable, differentiated cells (Radisic et al., 2008). To promote cell differentiation and assembly, cultured constructs were subjected to electrical signals designed to mimic those in native heart (Radisic et al., 2004). Electrical stimulation resulted in cell alignment and coupling, increased amplitude of synchronous contractions, and a remarkable level of ultrastructural organization of engineered myocardium (Radisic et al., 2004).
Scaffolds are critically important for cardiac tissue engineering, as they serve as structural and biochemical templates for cell growth and tissue development (Davis et al., 2005). The scaffold material should be biocompatible, contain cell attachment sites, have an interconnected porous structure enabling high density seeding of cells (Chen et al., 2008), mechanically strong and highly elastic to support cell contractions. The high density and high metabolic activity of cardiac myocytes call for high rates of oxygen supply (Radisic et al., 2006) while the diffusional penetration depth of oxygen is < 100 μm (Brown et al., 2007). Channeled scaffolds, oxygen carriers (Radisic et al., 2005) and perfusion (Radisic et al., 2004) were used to augment oxygen transport by enhancing convection and reducing diffusional distances, allowing an increase in the thickness of viable tissue.
Methods explored to further improve myocardial tissue development included the use of bioreactors that provide the necessary environmental factors, such as pulsatile perfusion (Radisic et al., 2008), mechanical stimulation (Akhyari et al., 2002), and electrical stimulation (Tandon et al., 2009). The phasic stretch was found particularly effective in enhancing cell alignment and differentiation (Zimmermann et al., 2002; Fink et al., 2000). However, the engineering of thick, compact and functional cardiac constructs, and the understanding of the underlying mechanisms of force generation and electromechanical coupling remain a challenge.
In the present study, we developed a novel scaffold for cardiac tissue engineering that was designed to mimic the structural and mechanical properties of native heart matrix, to incorporate an array of channels to facilitate mass transport and to serve as blood vessels in future studies, and to enable mechanical stimulation during culture. A chitosan-collagen composite was chosen to achieve the necessary mechanical, structural and compositional features. Cell behavior and tissue development were investigated in combined experimental and modeling studies, toward engineering a functional myocardial tissue patch.
2. Materials and Methods
2.1. Scaffold Fabrication
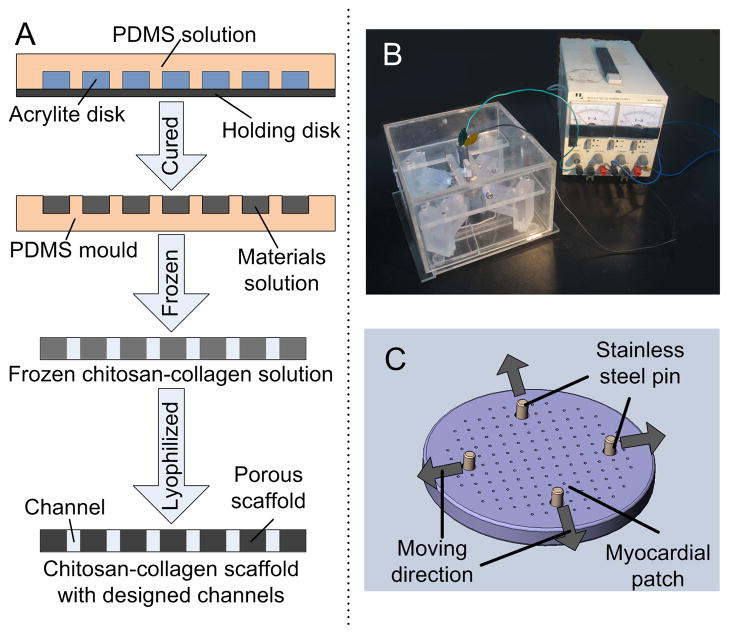
Chitosan-collagen scaffolds with an array of parallel channels were fabricated by a custom-designed molding process (Fig. 1A). First, acrylite disks (14 mm in diameter, 2 mm thick) with distributed vertical channels (200 μm in diameter, 1 mm center to center spacing) were fabricated using a computerized carbon dioxide laser machine (Epilog, Pololu Robotics and Electronics, Las Vegas, NV). Polydimethylsiloxane (PDMS) (Essex Chemical, Sylgard184, Corning, NY) molds were cast using a silicone elastomer base and a curing agent mixture at 10:1 ratio, and cured at 60 °C for 4 h. The PDMS molds were gently peeled off the acrylite surfaces and washed with 100% ethanol.
Figure 1. Scaffold and bioreactor design.
A Fabrication of chitosan-collagen channeled scaffold. PDMS (polydimethylsiloxane) solution was poured onto a laser-cut acrylite disk with vertical channels. After curing at 60 °C for 4 h, PDMS molds were peeled off the acrylite surface and a chitosan-collagen solution was poured into the mold. After slow freezing from 4 °C to −20 °C, the sample was removed out of the mold and lyophilized to form a porous chitosan-collagen scaffold with an array of channels. B Mechanical stimulation device consisted of an adjustable electric source and a chamber. Four sliding blocks were situated symmetrically inside the chamber, to execute back and forth movement by a motor controlled cam. C The myocardial patches were installed in the device by four stainless steel pins that were placed into the four large holes, and fixed to each sliding block. The displacement frequency was regulated by cam geometry and the rotation speed of the motor. Arrows indicate the directions of motion of each stainless steel pin.
Chitosan-collagen solution was prepared by first dissolving chitosan (medium molecular weight, Sigma, St. Louis, MO) in 1% acetic acid (weight/volume, w/v) and collagen type I (derived from rat tail, Sigma, St. Louis, MO) in distilled water, and then mixing the two solutions to the final concentration of 0.5% chitosan (w/v) and 0.1% collagen (w/v). The solution was poured into the PDMS molds, and the air bubbles were removed by applying vacuum. After slow freezing of the solution (from 4 °C to −20 °C over 24 h), the mixture was stored overnight, taken out of the molds, and lyophilized for additional 48 h.
Before each experiment, the freeze-dried scaffolds were cross-linked with 0.5% sodium tripoly-phosphate solution (TPP, Yili Fine Chemicals, Beijing, China) for 10 min, washed with phosphate buffered saline (PBS, Invitrogen, Carlsbad, CA) and neutralized with 75% ethanol. Before cell seeding, crosslinked scaffolds were sterilized with 100% ethanol, coated with 10 μg/mL fibronectin (Sigma) at room temperature for 40 min, and air dried before cell seeding.
2.2. Cell Preparation, Scaffold Seeding and Cultivation
Cells were obtained and used in accordance with the ethical guidelines for research at Columbia University. Cardiac myocytes were isolated from 2-day-old neonatal Sprague-Dawley rats as previously reported (Radisic et al., 2008). Briefly, rat ventricles were quartered, washed, and incubated at 4 °C in Hank’s balanced salt solution (HBSS, Invitrogen) with 0.06% trypsin (w/v), and subjected to a series of digestion (4 min, 37 °C, 50 rpm) in 0.1% collagenase type II (w/v) in HBSS. The cell suspensions from the first 4–5 digestions were collected and centrifuged at 121 g for 4 min. The supernatant was removed and cells were resuspended in culture medium (high glucose Dulbecco’s Modified Eagle Medium, DMEM with 10% fetal bovine serum, FBS). Resuspended cells were plated into a T75 flask in the incubator for 75 min to enrich cardiomyocytes by collecting the nonadherent cells.
The cell number and viability were determined using a hemocytometer and trypan blue exclusion. Isolated cardiac myocytes were seeded into air-dried fibronectin-coated scaffolds hydrated with cell culture medium. To achieve uniform cell seeding, both circular sides of the scaffolds were seeded, 15 min apart, with 5×106 cells/scaffold (corresponding to a cell density of 1.6–3.2×107 cells/cm3). Cell-seeded scaffolds were cultured statically for one day, to allow the cells to attach, and randomly divided into the bioreactor group and static control group, to be cultured as described below in a 37 °C/5% CO2 incubator.
2.3. Bioreactor Cultivation with Mechanical Stimulation
A two-dimensional (2D) stretch device was designed to apply prescribed nonuniform stress fields to myocardial patches (Fig. 1B) through the control of the movement of the pins in the four large holes (1 mm in diameter) near the edge of the scaffold (Fig. 1C). The complete bioreactor device was autoclaved before each experiment, and the displacement and frequency of the movement were pre-set to the desired levels by varying the geometry of the cam and the rotating speed of the motor. The myocardial patches were mounted into the device, culture medium was added (100 mL per bioreactor chamber, and half of the medium was changed after 3 days of culture) that enabled the cultivation of n = 3 patches per device that were stimulated simultaneously under the same mechanical regime. Driven by a motor-controlled cam system, the four stainless steel pins moved back and forth, to subject the myocardial patches to cyclic strain. The dynamic stretch was applied continuously for 6 days, with an amplitude of 1 mm at each pin at a frequency of 1 Hz. Statically cultured patches were used as controls. As the pins in the diametric direction were 10 mm apart and moved in the opposite direction, the applied nominal strain was around 20%.
2.4. Scaffold characterization
2.4.1. Mechanical testing
Rectangular samples of the scaffolds with an array of 200 μm diameter channels with 1 mm center-to-center spacing and measuring 8 mm × 40 mm × 2.2 mm were prepared for mechanical testing. Such simple geometry facilitates mounting onto the testing device and enables direct calculation of the modulus from measured force and applied strain. The tensile properties were measured with a standardized tension test, using a mechanical tester (Instron, Norwood, MA). The samples were stretched at a rate of 0.2% strain/second till rupture (n = 3). The strain and stress values were recorded at 200 ms intervals, and the strain-stress curve was plotted. The elastic modulus was calculated from the slope of the linear region of the strain-stress curve in the region of strain between 20% and 60%. The tensile strength and percentage elongation were obtained from the values of scaffold stress and strain, respectively, at rupture. These determined properties were independent of the shape of the scaffold and used for the further fine element analysis.
2.4.2. Cell proliferation and viability
To confirm the scaffold biocompatibility, mouse skeletal myoblast C2C12 cells were seeded into the fibronectin-coated scaffolds (n = 3) at a density of 5×105 cells/scaffold (corresponding to 1.6–3.2×106 cells/cm3), and cultured in DMEM (high glucose, with 10% FBS). A lower cell density was used here because the C2C12 cells proliferate much faster than the neonatal cardomyocytes during the time of culture. Cellular metabolism was evaluated using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Invitrogen) (n = 3–6). Statistical tests were performed with the Student t test for cells in the scaffold and on the monolayer at each time point. The confidence level was set to be 0.05. The optical density (OD) was calculated as the difference between the measured value of the cell-seeded group and the negative cell-free control. To determine cell viability and distribution, the cell-seeded scaffolds were stained with Live/Dead Viability/Cytotoxicity Kit (Invitrogen) after 3 days of culture, using cell monolayers on tissue culture treated well plates as controls.
2.4.3. Finite element simulation
The displacement and stress of the myocardial patch at the maximum deformation during one cycle of mechanical stimulation were modeled by finite element analysis (ANSYS). A computer-aided design (CAD) model with the planar geometry of the fabricated myocardial patch was created and imported into the ANSYS program. Four symmetrical forces were applied at each of the four pins in the 1-mm holes, by applying a displacement of 1 mm. The myocardial patch was considered as an incompressible hyperelastic material, and the nonlinear strain-stress relationship obtained from our tensile tests was used for numerical simulation with ANSYS. Finite element solutions were obtained using a standard 8-node hexahedral element with a fine mesh of >160,000 elements in total. The distributions of displacement and the equivalent stress (von Mises stress) were calculated.
2.4.4. Histological staining
After 6 days, myocardial patches were fixed with 10% neutral buffered formalin, embedded in paraffin, bisected (en-face and in cross-section), and sectioned to 10 μm. Hematoxylin and eosin (H&E) staining was performed for general evaluation. For immunohistochemistry, the slices were deparaffinized, blocked, and incubated first with rabbit anti-connexin-43 (1:50, Chemicon, Temecula, CA), and then with fluorescein-conjugated goat anti-rabbit IgG (1:200, Chemicon). The cell nuclei were stained with DAPI-containing mounting medium (Vector Laboratories, Burlingame, CA) and imaged using inverted fluorescence microscopy (Olympus American, Center Valley, PA).
2.4.5. Scanning electron microscopy
Scanning electron microscopy (SEM) was used for evaluating the scaffold microstructure and cell morphology. The scaffolds (in their dry state) were coated with gold and examined directly with SEM (KYKY 2800, KYKY Technology Development. China). Myocardial patches were fixed with formalin, rinsed with PBS, frozen, lyophilized for 48 h, coated with gold and observed by SEM.
3. Results
3.1. Structure and Mechanical Properties of Channeled Scaffolds
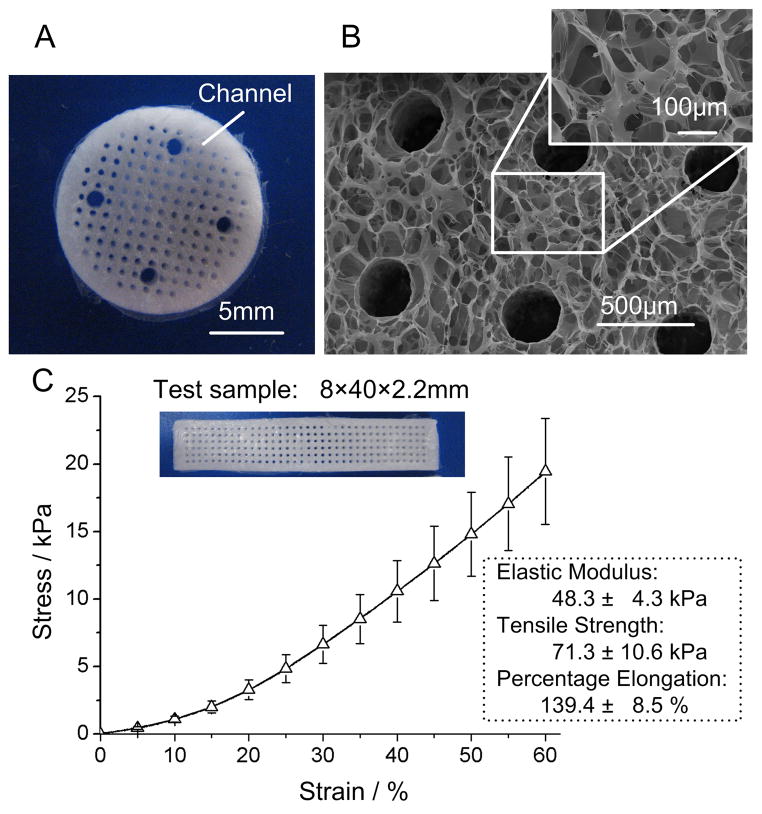
As shown in Fig. 2A & 2B, the chitosan-collagen scaffolds had a diameter of 14 mm, a thickness of about 2 mm, and an array of uniformly distributed parallel channels 200 μm in diameter and 1 mm center-to-center spacing (corresponding to 19% of volume occupied by the channels). The bulk phase of the scaffold had a microporous structure with interconnected pores, 100 μm in diameter (with approximately 60% of the volume occupied by micro-pores).
Figure 2. Scaffold properties.
A Macroscopic image and B scanning electron micrograph of a chitosan-collagen scaffold. The scaffold had a diameter of 14 mm, and was fabricated with an array of parallel vertical channels (~200 μm in diameter, 1 mm center-to-center spacing, with 19% void volume of channels), within a porous bulk polymer phase (100 μm pores, with about 60% void volume of micro-pores). Four large holes (1 mm in diameter) were situated symmetrically near the edge of the scaffold for connection to the mechanical stimulation device. C Mechanical properties of the scaffolds (n = 3; mean± SD) were determined in standardized tension tests. Rectangular test samples with channels (200 μm in diameter with 1 mm central spacing, the same as the chitosan-collagen scaffolds) had a size of 8 mm × 40 mm × 2.2 mm.
After cross-linking with TPP, the hydrated scaffolds exhibited excellent elastic properties, with an elastic modulus of ~48.3 kPa, tensile strength of ~71.3 kPa, and maximum elongation of ~140%.
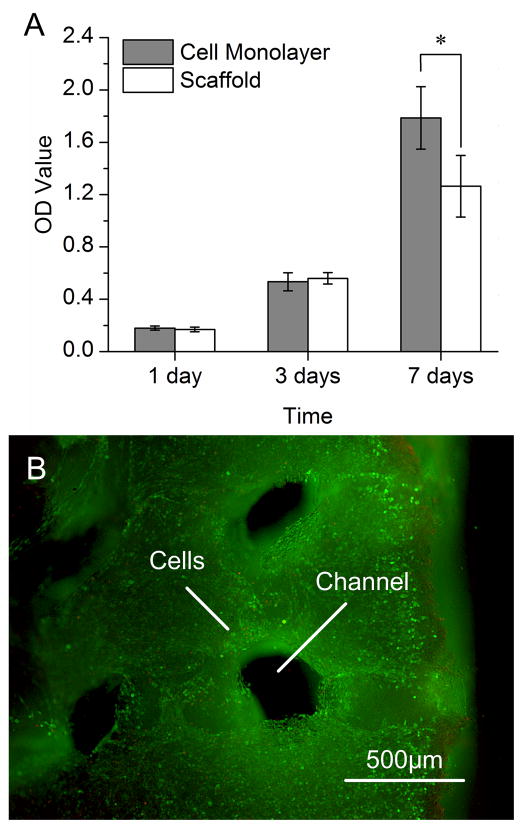
The metabolic activity of the cells within fibronectin-coated scaffolds was similar to that of the cells cultured in a monolayer. Over 7 days of cultivation, the metabolic activity increased in both culture settings (Fig. 3A). The cells proliferated and maintained their viability during cultivation in channeled scaffolds, as evidenced by the dense distribution of viable cells (green) throughout the construct with rather small numbers of dead cells (red) (Fig. 3B).
Figure 3. Cell metabolism and viability.
Scaffolds were cross-linked with 0.5% TPP (sodium tripolyphosphate), coated with 10 μg/ml fibronectin and seeded with C2C12 myoblasts at a density of 5×105 cells/scaffold. A Optical density values from MTT assay (n = 3–6; mean± SD) after 1, 3 and 7 days of culture. Cell monolayers served as controls. *Significant difference between cells in scaffolds and cell monolayer. B Scaffold biocompatibility was confirmed by Live/Dead assay after 3 days of culture. Green: live cells. Red: dead cells.
3.2. Myocardial Patch in Static Culture
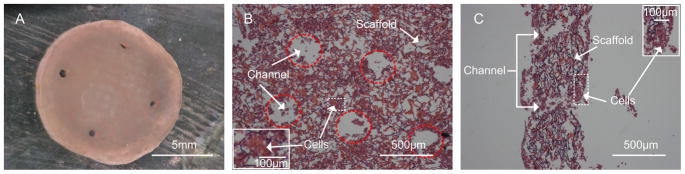
After seeded with cardiac myocytes, a compact patch formed and started to beat spontaneously from day 3 of static culture (Fig. 4A; Suppl. Video 1). The contractions were highly synchronized, at an amplitude of ~1% and with a frequency of ~0.6 Hz. The H&E staining showed high density of cells attached to the scaffolds (Fig. 4B), in the bulk phase between the scaffold channels (Fig. 4C). The cells on both sides of the scaffolds and inside the channels appeared interconnected with each other. As the patch compacted during the formalin fixation, the thickness and channel diameter on the H&E images appeared smaller than the actual size.
Figure 4. Histomorphology of myocardial patch.
A Gross appearance of the myocardial patch formed by seeding cardiac myocytes into fibronectin-coated chitosan-collagen scaffold at a density of 5×106 cells/scaffold, and statically cultured in vitro for 6 days. H&E stained sections are shown en-face (B) and in cross-section (C). Dotted red lines indicate the positions of channels.
3.3. Mechanical Stimulation and Stress Simulation
As shown in the displacement map (Fig. 5A), the myocardial patch that was mechanically stimulated during the culture deformed along the direction of motion. As expected, the largest strains occurred around the large holes (force point) and along the direction of the patch edge. The stress map (Fig. 5B) shows that the stress also concentrated around the large holes and the adjacent channels. In response to stress, the large holes deformed from a circular shape into elliptic shape, with the stress regions (the equivalent von Mises stress) arranged along the edge of the hole and the edge of the patch.
Figure 5. Distribution of stress and displacement.
Displacement map (A) and stress map (B) of the myocardial patch at the maximum deformation. C Stress distribution from the edge of the patch, along the big holes and parallel channels to the center (red arrows).
The stress value distribution curves (Fig. 5C) showed that the stress concentrated along the edge of the hole and the adjacent channels, with the maximum stress value of 30 kPa. The stress attenuated rapidly to about 5–15 kPa at a distance of about 500 μm away from the hole. Stress concentration was also observed around the channels, but at the lower levels of stress (3–13 kPa at the peaks) than along the holes.
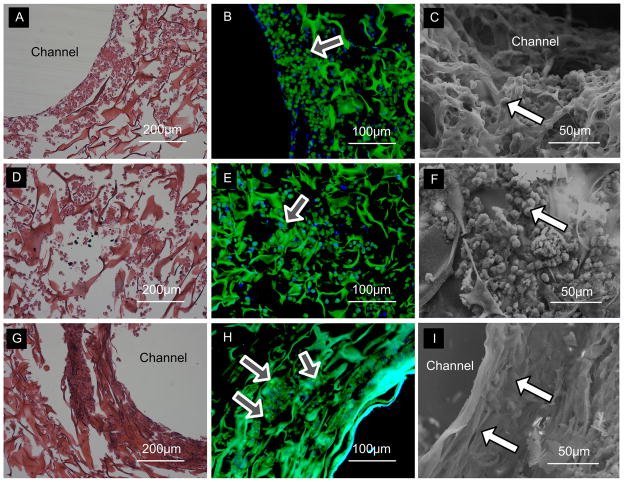
Cells stayed alive after the constructs were stretched for 6 days. Cardiac constructs cultured with the application of mechanical stimulation exhibited a number of morphological changes relative to the statically cultured constructs (Fig. 6). In the mechanical stimulation group, the cells around the holes aligned in the direction of the stress field lines (Fig. 6G), while the cells at the center of the scaffold were not elongated (Fig. 6D), and appeared similar to those in the static culture (Fig. 6A). These differences in cell shape and morphology could be clearly seen in SEM images (Figs. 6C, 6F & 6I). In static culture, cells were rounded after 7 days (Fig. 6C). The alignment and elongation of cells around the holes became more significant after mechanical stimulation, and compact cells interconnected with each other (Fig. 6I). Mechanical stimulation also appeared to result in high levels of gap junctions (Cx-43; shown as bright green spots) around the big holes (Fig. 6H). In contrast, the cells in nonstimulated patches and at the centers of simulated patches were round (Fig. 6C & 6F) and expressed low levels of Cx-43 (Fig. 6B & 6E).
Figure 6. Cell morphology.
A, D, G H&E staining. B, E, H Expression of Cx-43 (Green: Cx-43, Blue: DAPI, nucle; arrows indicating bright green dots for gap junctions). C, F, I Scanning electron micrographs (arrows pointing to individual cells). A–C Regions around the holes after 7 days of static culture of the myocardial patch. D–F The central region of the myocardial patch after 1 day of static culture and 6 days of mechanical stimulation, where the cells were exposed to low stress. G–I Regions around the holes of the myocardial patch with 1 day of static culture and 6 days of mechanical stimulation, where the cells were exposed to high stress.
4. Discussion
This study investigated a new approach to engineering myocardial tissue in vitro, by mechanical stimulation of channeled scaffolds seeded with cardiac cells. The scaffold was designed to provide biocompatibility, native-like composition, structure and biomechanics, and to degrade at a rate similar to that of cardiac tissue regeneration. We selected collagen (Vakonakis and Campbell 2007), as the widely used fully biocompatible natural biomaterial which is a major component of cardiac extracellular matrix. Because collagen scaffold degradated rapidly and had very low mechanical stiffness, we mixed collagen with chitosan (Madihally and Matthew 1999), a deacetylaeted product of chitin that is structurally similar to glycosaminoglycans and has excellent mechanical properties. Compared to synthetic materials, a scaffold made of two naturally derived biomaterials had enhanced biocompatibility and biological recognition. The scaffold also provided more robust mechanical properties (Young’s modulus of ~ 15kPa at 5% tensile strain and ~50kPa at 30% strain) while still supporting the contractile function of attached cardiomyocytes. Compared to native human heart matrix, such a scaffold has comparable elastic modulus, highly porous interconnected pores, ability to sustain large deformation, and channel structures providing precursors for vascular conduits.
Chitosan-collagen scaffolds with uniformly distributed channels and microporous structure were fabricated using a custom-designed molding process (Fig. 2A & 2B). The size of channels could be adjusted by changing the geometry of the acrylite disk. In the present study, the channel diameter was set to 200 μm, and the microporous structure of the scaffold was obtained by controlling the freezing temperature and rate. When slowly frozen from 4 °C to −20 °C, the crystallization of acetic acid and water was uniform and the interconnected homogeneous micro-pores stayed intact when freeze-dried. Mechanical testing indicated that the crosslinked channeled scaffolds were highly elastic and highly extensible, with an elastic modulus close to that of the native heart tissue (Ott et al., 2008) (Fig. 2C).
Fibronectin was used to coat the collagen-chitosan scaffold, to serve as a link between cardiac myocytes and collagen, as in the native heart. Its effectiveness was demonstrated by excellent cell attachment and viability (Fig 3A & 3B). In addition, fibronectin coating also allows cells to attach quickly onto the scaffolds. Cell monolayer grew faster than cell in scaffolds (Fig 3A) (At day 7, the cell number is 2×105 vs 1.5×105 for monolayer and constructs, respectively), which we speculate was due to the better access to oxygen and nutrients than in the interior of statically cultured constructs.
Cardiac myocytes seeded at a high density (physiologic for native myocardium) formed a compact beating myocardial patch. The scaffold channels improved tissue function in at least three different ways: (i) by providing spaces for cells adhesion and cell aggregation, (ii) by enhancing cell viability through oxygen supply into the interior of cell-seeded scaffolds, and (iii) by promoting the interconnectiveness of the cells. Overall, channeled scaffolds enabled the engineering of thick and compact cardiac constructs that were spontaneously beating from the third day of culture.
The mechanical stretching device enabled the application of a planar nonuniform stress field to the myocardial patch over 6 days of cultivation. At the region near the holes where cells experienced high stress, cardiac myocytes were particularly well aligned and elongated in the direction of the applied stress, and expressed increased levels of Cx-43. In contrast, the cells located far from the holes and at the center of the patch which experienced much lower stresses did not exhibit elongated morphologies and had low expression levels of Cx-43, similar to the cells in statically cultured patches. This study suggests that mechanical stimulation improved tissue development at a relatively high local stress.
Future work is necessary to further elucidate the effects of mechanical stimulation on tissue growth and maturation, and to understand the mechanisms by which the cells sense and respond to mechanical forces. In addition, the pharmacological studies of engineered constructs and the measurements of force generation are the subjects of our ongoing studies. The design of bioreactors capable of simultaneously providing multiparametric stimulation, such as pulsatile perfusion, mechanical stimulation, and electrical stimulation, that mimic the complex native environment, is expected to largely enhance these efforts.
5. Conclusion
We report a new approach to cardiac tissue engineering that is based on cyclic mechanical stretch of cell-seeded channeled scaffolds designed to mimic the composition, structure and mechanical properties of the native matrix. Chitosan-collagen scaffolds with an array of channels were fabricated using a custom-designed process providing the necessary microporous structure and mechanical properties for engineering a myocardial patch. Mechanical stimulation enhanced cell elongation, alignment and the expression of cardiac markers Cx-43 at regions of high local stresses. This study confirmed the critical roles of scaffold design and mechanical stimulation for the formation of functional engineered cardiac tissue.
Supplementary Material
Contractile behavior of neonatal cardiomyocytes seeded collagen-chitosan scaffold at day 3 without mechanical stimulation or electrical pacing. The frame rate is 30 fps, and the total time is around 5 second.
Acknowledgments
We gratefully acknowledge support of the NIH (EB002520 and HL076485 to GVN) and China Scholarship Council (Fellowship 2008621053 to TZ).
References
- Akhyari P, Fedak P, Weisel RD, et al. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 2002;106:I137–I142. [PubMed] [Google Scholar]
- Akins RE. Can tissue engineering mend broken hearts? Circ Res. 2002;90:120–122. [PubMed] [Google Scholar]
- Brown DA, MacLellan WR, Laks H, et al. Analysis of oxygen transport in a diffusion-limited model of engineered heart tissue. Biotechnol Bioeng. 2007;97:962–975. doi: 10.1002/bit.21295. [DOI] [PubMed] [Google Scholar]
- Bursac N, Papadaki M, Cohen RJ, et al. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol-Heart C. 1999;277:H433–H444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- Chen QZ, Harding SE, Ali NN, et al. Biomaterials in Cardiac Tissue Engineering: Ten Years of Research Survey. Mat Sci Eng R. 2008;59:1–37. [Google Scholar]
- Cohen S, Leor J. Rebuilding broken hearts. Sci Am. 2004;291:44–51. [PubMed] [Google Scholar]
- Davis ME, Hsieh PC, Grodzinsky AJ, et al. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97:8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T, Zimmermann WH. Engineering myocardial tissue. Circ Res. 2005;97:1220–1231. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- Fink C, Ergun S, Kralisch D, et al. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. Faseb J. 2000;14:669–679. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Therapeut. 2005;105:151–163. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Madihally SV, Matthew H. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–1142. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Radisic M, Deen W, Langer R, et al. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol-Heart C. 2005;288:H1278–H1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- Radisic M, Malda J, Epping E, et al. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- Radisic M, Marsano A, Maidhof R, et al. Cardiac tissue engineering using perfusion bioreactor systems. Nature Protocols. 2008;3:719–738. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Park H, Gerecht S, et al. Biomimetic approach to cardiac tissue engineering. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362:1357–1368. doi: 10.1098/rstb.2007.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. P Natl Acad Sci Usa. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Yang LM, Boublik J, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol-Heart C. 2004;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- Tandon N, Cannizzaro C, Chao PG, et al. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakonakis L, Campbell LD. Extracellular matrix: from atomic resolution to ultrastructure. Curr Opin Cell Biol. 2007;19:578–583. doi: 10.1016/j.ceb.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammaretti P, Jaconi M. Cardiac tissue engineering: regeneration of the wounded heart. Curr Opin Biotech. 2004;15:430–434. doi: 10.1016/j.copbio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Zandonella C. Tissue engineering: The beat goes on. Nature. 2003;421:884–886. doi: 10.1038/421884a. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Schneiderbanger K, Schubert P, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contractile behavior of neonatal cardiomyocytes seeded collagen-chitosan scaffold at day 3 without mechanical stimulation or electrical pacing. The frame rate is 30 fps, and the total time is around 5 second.