Abstract
Context
Calcific Aortic Valve Disease (CAVD) is an active pathological process leading to biomineralization of the aortic cusps. We characterized circulating and tissue Osteopontin (OPN) as a biomarker for CAVD.
Objectives
Here we investigate the post-translational modifications of circulating OPN and correlate the phosphorylation status with the ability to prevent calcification.
Methods
Circulating OPN levels were estimated in CAVD patients (n=51) and controls (n=56). In a subgroup of 27 subjects, OPN was purified and the phosphorylation status analyzed.
Results
Plasma OPN levels were significantly elevated in CAVD patients as compared to the controls and correlates with the aortic valve calcium score. Our study demonstrates that phospho-threonine levels of OPN purified from controls were higher when compared to CAVD subjects, while phosphoserine and phospho-tyrosine levels were comparable between the two groups.
Conclusion
The dephosphorylation of circulating OPN correlates with severe valvular calcification in patients with CAVD.
Introduction
Calcific Aortic Valve Disease (CAVD) is a slow, but progressive, pathological condition of the aortic valve characterized, in its final stage, by dystrophic calcification of the valve leaflets (Freeman and Otto 2005, Goldbarg et al 2007). It is the most frequent valvular disease, with a prevalence of 3–9%, and the main cause for valve replacement in the adult population (Bach et al 2007). Despite the high prevalence and mortality associated with aortic valve calcification little is known about its pathological mechanisms. For many decades, the disease has been considered a result of normal aging resulting from prolonged “wear and tear” of the aortic valve with concomitant passive calcium deposition on the valve leaflets (Cowell et al 2004). However, recent data does not support this simplistic concept. The degeneration of aortic valve starts with a normal trileaflet aortic valve; initial phases of the disease include mild thickening of the leaflets (aortic valve sclerosis, AVSc) while more advanced stages are associated with impaired leaflet motion and resistance to forward blood flow (aortic valve stenosis, AVS). The current understanding of the pathophysiological mechanisms underlying CAVD is still not fully elucidated. It has been suggested that mechanical stress, in addition to atherosclerotic risk factors, leads to valvular endothelial dysfunction/leakage followed by neo-angiogenesis, deposition of lipids and other compounds. This triggers inflammation, thereby activating valvular interstitial cell leading to their osteoblastic transdifferentiation, extracellular matrix remodeling which ultimately leads to active calcification (Freeman and Otto 2005, Goldbarg et al 2007, O’Brien 2006 and Beckmann et al 2010)
Clinical examination, echocardiography and cardiac catheterization are the major methods to diagnose CAVD and the treatment of choice for symptomatic AVS is aortic valve replacement (AVR) (Cowell et al 2004). Other treatment options, such as percutaneous valve replacement or aortic valvuloplasty, offer some benefits in terms of lower invasiveness and hospitalization time, but are not applicable to all patients (Balmer et al 2004, Perin et al 2009). Balloon aortic valvuloplasty is a well-established and well-studied procedure with nontrivial complication rates, very high rates of recurrent stenosis and moderately high rates of aortic insufficiency (Balmer et al. 2004, Wang et al 1997). Recently completed PARTNER trial on percutaneous aortic valve implantation in inoperable patients with severe aortic stenosis shows significantly reduced death rates in patients and significant improvements in health-related quality of life that were maintained for at least 1 year (Leon et al 2010, Reynolds et al 2011). However long term performance of these prostheses remains unknown at the present time. Mineralization of bioprostheses is also a major contributor to failure (Siddiqui, Abraham and Butany 2009). The mechanisms involved in dystrophic calcification of these valves are believed to resemble closely the bio-mineralization process in native aortic valves (Freeman and Otto 2005, Speer and Giachelli 2004). Notably, surgical valve replacement in any of its forms leaves the underlying mechanism that caused the original valvular degeneration, untreated. Acceleration of valve failure of either native or bioprosthetic valves is attributed to active calcium deposition and degeneration of the leaflets. The calcification of aortic bio-prostheses suggests that circulating molecules implicated in the regulation of bio-mineralization must be involved in the calcification process.
Osteopontin (OPN) is a multifunctional glycol-phospho-protein that plays an important role in bone remodeling via differentiation and stimulation of osteoclasts. Besides its function in bone tissue, OPN is also implicated in a variety of acute, as well as, chronic inflammatory processes, including wound healing, fibrosis and atherosclerosis (Cho et al 2009). Furthermore, OPN is involved in the biomineralization of dystrophic and ectopic sites, including the aortic valve According to available reports, phosphorylation status is important in regulating OPN interaction with macrophages, its ability to modulate inflammatory pathways and inhibition of calcification in vitro (Ashkar et al 2000, Jono, Peinado and Giachelli. 2000).
We have previously reported that high plasma OPN levels are associated with the presence of CAVD, suggesting OPN could serve as a novel biomarker to monitor the calcification process (Yu et al 2009). We observed that individuals with no signs of valve calcification had lower OPN levels in comparison to patients suffering from moderate to severe aortic valve calcification suggesting a correlation between plasma OPN levels and the severity of CAVD. However, the biological activities of OPN are controlled by multiple mechanism(s) including post-translational modifications of the protein. In the present study we report the plasma purification and immuno-phosphorylation analysis of circulating OPN protein to establish its role as a biomarker for CAVD.
Methods
Patient Population
The present study has been conducted in accordance to the code of ethical standards of the University of Pennsylvania School of Medicine Institutional Review Board (IRB) guidelines. We enrolled subjects who were undergoing routine echocardiography at the echocardiographic laboratory and patients with any degree of CAVD undergoing aortic valve surgery. IRB approval and informed consent were obtained for the subject enrollment. Clinical information of the subjects was obtained by personal interview and chart review. Exclusion criteria for the study included: presence of bicuspid aortic valve, premature menopause and/or osteoporosis, prior aortic valve surgery, rheumatic heart disease, endocarditis, active malignancy, chronic liver failure, calcium regulation disorders (hyperparathyroidism, hyperthyroidism, and hypothyroidism), serum creatinine ≥ 1.5mg/dl, and chronic or acute inflammatory states (sepsis, autoimmune disease, and inflammatory bowel disease). Control tissues were obtained through collaborations with the Valley-Columbia Heart and Vascular Institute, the heart transplant research program of the University of Pennsylvania School of Medicine and The Gift of Life Program.
Echocardiographic and Doppler data
All patients underwent a comprehensive echocardiographic assessment including, M-mode, two-dimensional and Color Doppler and were conducted by a certified echo-cardiographer using commercially available ultrasound systems. All measurements were performed according to the American Society of Echocardiography recommendations. Patients with echocardiographically normal aortic valve with calcium scores 1 (no calcium) were included as controls. Presence of aortic stenosis was defined as an aortic valve area (AVA) <2.0 cm2. Aortic valve calcification was assessed, and a calcium score of 1 to 4 was assigned by a single cardiologist based on the method described as: 1- no calcification; 2 - mildly calcified (small isolated spots); 3- moderately calcified (multiple larger spots); 4 - severely calcified (extensive thickening and calcification of all cusps) (Rosenhek et al 2000).
Plasma OPN analysis
Blood samples were collected in EDTA vials centrifuged, separated plasma and stored at −80°C until analysis. Plasma OPN levels were measured in Human Osteopontin ELISA Kit (R&D Systems, Minneapolis, MN), following the manufacturer's instructions.
Cells, antibodies and reagents
Human coronary artery smooth muscle cells, alpha medium, L-glutamine, fetal bovine serum and Penicillin streptomycin solutions were purchased from Invitrogen (Carlsbad, CA). Removal of IgG and human serum albumin was carried out using the Proteoprep® Blue Albumin and IgG depletion kit, Sigma. Recombinant human OPN and anti human OPN goat polyclonal antibody were purchased from R&D systems. Phospho-threonine and phospho-tyrosine from Cell Signaling (Danvers, MA), Anti-OPN Rabbit polyclonal (Santacruz Biotechnology, CA) and phosphoserine antibody was purchased from Invitrogen (Carlsbad, CA). Phosphorylation of recombinant OPN was carried out using Casein kinase II (New England Biolabs, Ipswich, MA).
Circulating OPN purification
A two-step purification protocol was designed to partially purify OPN from plasma of CAVD patients and controls. Albumin and IgG were removed from 250µl plasma sample using Cibracon blue/Protein A gel (Proteoprep® Blue Albumin and IgG depletion kit, Sigma). This step removes human serum albumin (HSA) and the major subclasses of gamma globulin (IgG) from serum and plasma. After HSA/IgG removal, plasma samples were incubated with anti-human OPN Rabbit IgG (Santacruz Biotechnology, CA) overnight at 4° C. The immunoprecipitated product was incubated with protein A Agarose beads and washed extensively to minimize non specific binding of antibody. The product was then detected by western blotting. Recombinant OPN and non-immune IgG were used as controls.
Characterization of OPN phosphorylation status
Post-translational phosphorylation status of plasma OPN was analyzed using phospho specific antibodies. HSA/IgG depleted plasma samples were immunoprecipitated with the anti-OPN polyclonal antibody as described above, partially purified OPN was then analyzed by Western blotting and probed with different antibodies. Blots were developed and scanned using densitometry.
Calcification assay
In vitro calcification assay was performed using Human coronary artery smooth muscle cells. Cells were cultured in Alpha MEM containing Penicillin streptomycin, L-glutamine and 10% FBS, until 70–80% confluent. At this point, the growth medium was replaced by calcification media (α-MEM containing 2mM phosphate buffer) in all wells except the control. 50ng of recombinant OPN (R&D Systems) was used directly along with Casein Kinase II phosphorylated recombinant OPN in separate wells. Media was replaced every two days in similar way. At day 14, media was aspirated from each well; cells were washed twice with PBS and incubated overnight in 0.6N HCL at 4°C for calcium extraction. Calcium was estimation from the supernatant using the Calcium assay reagent (Biovision, Mountain View, CA). The remaining cells were washed and harvested using 1N NaOH + 3%SDS. Total proteins were estimated and amount of calcium deposited was expressed as Calcium conc. mg /ml of protein.
Statistical Analysis
Statistical analysis was carried out using SPSS software (Version 15). Continuous variables were expressed as Mean ± Standard Error. Comparisons of continuous variables between groups were performed with the Student’s t test. Non-parametric (Mann-Whitney U test) tests were carried out for all categorical variables. A value of p < 0.05 was considered significant.
Results
Baseline Patients Characteristics
On the total of 51 patients, 56.9 % were male. Their age was 76.16±8.26 years. The initial aortic valve area was 0.82±0.05, 38 patients had systemic hypertension, 15 had diabetes and 28 had cholesterol level above 200 mg. All patients underwent comprehensive echocardiographic assessment including, M-mode, two-dimensional and color Doppler echocardiography. Table-I further characterizes the study groups. Plasma OPN levels were estimated in 51 CAVD patients and 56 controls. Individuals with CAVD exhibited higher plasma levels of OPN compared to controls (64.38 vs 30.32ng/ml; p<0.001) [Figure 1A]. Aortic valve calcification score was 3.28±0.08 in CAVD patients and 1.0 ± 0.0 in controls (p < 0.001) [Figure 1B]. Plasma OPN levels in CAVD patients with calcium score 3 and 4 were comparable but significantly lower as compared to the controls [Figure 1C].
Table 1.
Patients Baseline Characteristics.
| Demographics | Controls N-56 |
Aortic stenosis N=51 |
Significance |
|---|---|---|---|
| Age (in years) | 62.8±2.2 | 76.16±8.26 | <0.001 |
| Male | 29 (51.7%) | 29 (56.9%) | NS |
| Smokers | 19 (33.9%) | 20 (39.2%) | NS |
| Diabetes | 15 (26.8%) | 15 (29.4%) | NS |
| Hypertension | 39 (69.6%) | 38 (74.5%) | NS |
| Cerebral Vascular Accident | 1 (1.6%)) | 01 (2.0%) | NS |
| Peripheral vascular disease | 4 (7.1%) | 04 (8.0%) | NS |
| Hyperlipidemia | 29 (51.8%) | 28 (54.9%) | NS |
| Coronary artery disease | 17 (35.6%) | 17 (29.4%) | NS |
| Adjusted calcium (mg/dl) | 9.2 ± 0.5 | 9.3 ± 0.6 | NS |
| AVC score | 1.0±0.0 | 3.28±0.08 | <0.001 |
| Plasma Osteopontin (ng/ml) | 30.32±6.17 | 64.38±5.17 | <0.001 |
CVA: Cerebral vascular accident, PVD: peripheral vascular disease, AVC: aortic valve calcium, NS: Non significant.
Figure 1. Circulating OPN purification.
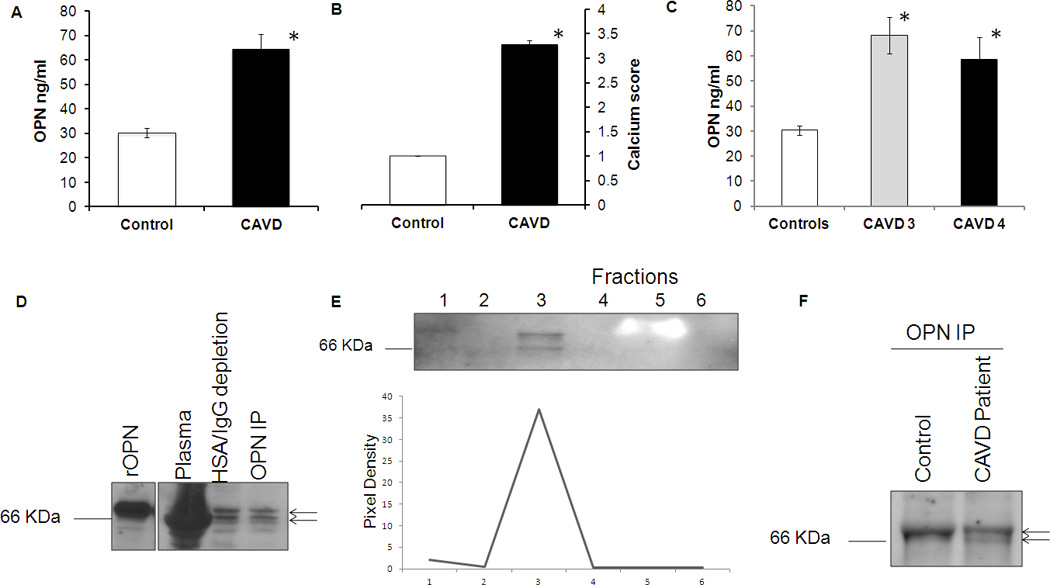
(A) Plasma OPN level in control and CAVD patients. Graph bar representing circulating OPN level in CAVD patients and healthy controls. OPN levels were measured in triplicates using ELISA assay. Value represents mean ±SE. * denotes p- value <0.05. (B) Bar graph representation of aortic valve calcium score in controls and CAVD patients. * denotes p-value <0.05. Controls had calcium score of 1 while CAVD patients represented 3 and 4. (C) Bar graph representation of plasma OPN levels in controls and CAVD patients with calcium score 3 and 4. Values represent mean±SE. * denotes p-value<0.05 (D) Representative pattern for osteopontin purification from CAVD patients and healthy controls. 50 ng of recombinant OPN was used as a positive control (Labe 1). Total plasma (2nd lane) was depleted of HSA and IgG using ProteoSeek Albumin/IgG Removal Kit (3rd lane) and then used for OPN Immunoprecipitation with a specific OPN antibody (Lane 4). The experiments were repeated for the subjects enrolled in the study as reported in table I. (E) Western blot showing different fractions collected during sample elution. Fraction 3rd had the highest amount of purified OPN. (F) Western blotting of purified OPN from CAVD patients and controls. Arrows indicates double bands.
Plasma purification of circulating human OPN
To the best of our knowledge, there are no currently available protocols to purify OPN from human plasma. To analyze potential qualitative differences in circulating OPN from CAVD patients and controls, OPN was analyzed in the plasma samples from 27 individuals by Western blotting with antibody specific to OPN [Figure 1D, IE and 1F]. Because of the high protein concentration in plasma, the detection of OPN in plasma by Western blotting was not possible as the large excess of albumin (Human Serum Albumin – HSA) masks the specific OPN bands [Fig. 1D – Lane: Plasma]. We therefore performed two-step purification: First we removed albumin and IgG from plasma using a cibracon blue/protein A gel (ProteoSeek Albumin/IgG Removal Kit, Thermo Scientific). This step removes human serum albumin (HSA) and the major subclasses of gamma globulin (IgG). Notably, plasma OPN is detectable in plasma deprived of HSA and IgG [Figure 1D – Lane: HSA/IgG depletion]. As a second purification step, we partially purified OPN from plasma by immunoprecipitation as describe in the methods section. Briefly, the flow through of the HSA/IgG purification step was immunoprecipitated using an OPN specific antibody over night at 4 C. Western blotting analysis of partially purified OPN from plasma were performed [Figure 1D – Lane: OPN IP]. The immunopurified protein was fractionated using acid elution buffer. Fractions were analyzed for the presence of OPN [Figure 1E]. These results demonstrate that circulating OPN can be partially purified from plasma of patients with CAVD.
The same purification protocol was then performed on plasma of healthy controls enrolled in the protocol after echocardiographic analysis confirms the absence of calcium (Calcium score 1) in the leaflets. To maintain the same immunopurification protocol’s conditions, the amount of plasma used from CAVD and controls was normalized based on the total OPN levels detected by ELISA [Figure 1A]. Partially purified OPN from patients and controls was analyzed by Western blotting as show in figure 1E. Interestingly Western blotting analysis of partially purified OPN from plasma of CAVD patients shows two bands with different electrophoretic mobility when compared to controls [arrows Figure 1D and 1F). Differences in electrophoretic mobility could be explained by a differential post-translational modification of OPN protein. OPN is synthesized as a 34 kDa protein that undergoes a variety of post-translational modifications, Native human OPN contains up to 34 phospho-serine, 2 phospho-threonine, 5 O-glycosylated threonine and no N-glycosylation sites [Figure 3A]. These modifications generate different forms of OPN with varying apparent molecular masses of 44- to 75-kDa.
Figure 3. Differential Post-translational Modification of human circulating Osteopontin in patients with CAVD and healthy controls.
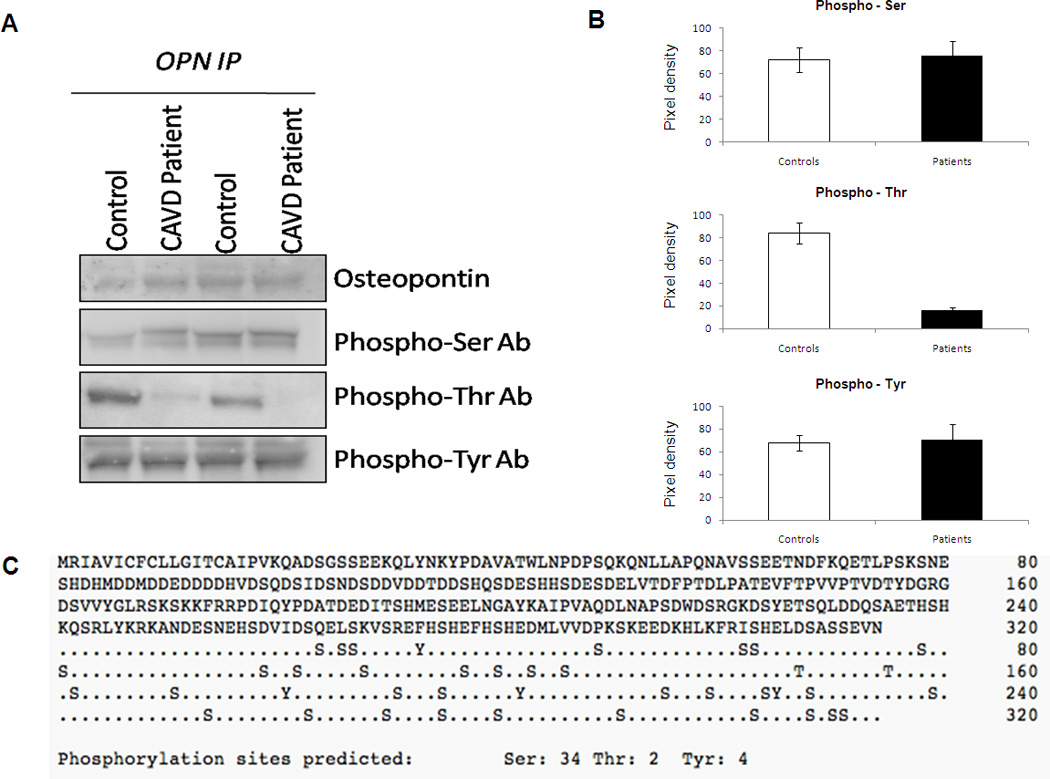
(A) Representative western blot for the purified OPN from CAVD and Control subjects. OPN were purified as described in the methods and subject to Western blotting analysis with phospho-serine, phospho-threonine and phospho-tyrosine specific antibodies. (B) Densitometry analysis of level of phospho-serine, phospho-tyrosine, and phospho-threonine of circulation purified OPN from plasma of CAVD patients and control. * p- value <0.05. (C) Analysis of osteopontin protein using NetPhos 2.0 Server to predict serine, threonine and tyrosine phosphorylation sites.
In vitro calcification assay
OPN phosphorylation on specific sites plays an important role in determining the physiological functions. It is reported that phosphorylated OPN inhibits calcification in vitro (Jono, Peinado & Giachelli 2000). To test this model we phosphorylated recombinant OPN and tested the ability to control calcium deposition in a smooth muscle cell based in vitro calcification assay. Representative western blot for the OPN and phospho-threonine expression in the phosphorylated and non phosphorylated OPN recombinant protein are shown in Figure 2A indicating equal amounts of the recombinant protein. As shown in Figure 2B and 2C, bacteria-derived recombinant OPN, containing no post-translational modification did not inhibit human smooth muscle cell bio-mineralization, while phosphorylated recombinant OPN inhibits calcium deposition in vitro. These experiments confirm that the phosphorylation status controls the ability of OPN to inhibit biomineralization. We then analyzed post-translational modifications on purified circulating OPN from CAVD and healthy controls to understand why elevated levels of OPN, a protein that inhibits calcification, are associated with calcified valves.
Figure 2. In vitro calcification assay.

(A) Representative western blot for OPN and phospho-threonine expression in the phosphorylated and non-phosphorylated OPN recombinant used for the in vitro analysis. Ponceau staining shows equal loading of the samples. (B) Human aortic smooth muscle cells treated with 3mM phosphate buffer (Pi) in presence or absence of phosphorylated/dephosphorylated OPN. Cells treated with Pi + rOPN showed calcium deposits while Pi+ phosphorylated OPN completely prevents calcium deposition as indicated in the figure. (C) Bar graph representation of calcium levels in the treated smooth muscle cells. Amount of total calcium was expressed as calcium mg/ml of protein.
Differential post-translational modification of human circulating OPN in patients with CAVD and healthy controls
Since there are no phospho-osteopontin antibodies commercially available we performed OPN immunoprecipitation experiments followed by Western blotting using phospho-specific antibodies to identify phosphorylated residues. We purified circulating OPN from CAVD and healthy subjects as reported above. Figures 3A and 3B show that purified circulating OPN in healthy controls is highly phosphorylated at the threonine residues when compared to purified OPN from CAVD patients, while phospho-serine and phosphor-tyrosine levels were comparable between the two groups of subjects. Figure 3C provides the analysis of OPN protein using Netphos 2.0 Software to predict the serine threonine and tyrosine phosphorylation sites in the OPN protein.
Discussion
In vitro and in vivo studies have shown that valvular calcification is an active process controlled by inflammatory mediators and the balance between osteoblast-like differentiation and osteoclast recruitment at the tissue level, as well as the levels of bone matrix proteins, such as OPN. The hemodynamic severity and progression of aortic stenosis is well correlated with both the presence and degree of aortic valve calcification (Davies, Gershlick and Balcon 1991).
High levels of OPN in native human calcified aortic valves from patients undergoing valve replacements have been shown by immunohistochemistry, reverse transcription–polymerase chain reaction, Western blotting, and in situ hybridization analyses (Mohler et al 1997, O’Brien et al 1995, Rajamannan et al 2003). In addition to native valves, increased OPN levels were also found by immunohistochemistry in calcified valve allografts (Shetty et al 2006) and in areas of calcification in glutaraldehyde-pretreated bioprosthetic porcine valves (Shen et al 1997). Elevated circulating OPN levels are also reported in other cardiovascular diseases, such as atherosclerosis, ischemic heart disease, heart failure, and rheumatic mitral stenosis (Minoretti et al 2006, Soejima et al 2007, Soejima et al 2006) An association between elevated levels of plasma OPN and both aortic valve calcification and the degree of aortic stenosis was also described in a recent report (Yu et al 2009). Also other plasma and serum markers such pro-BNP (Weber et al 2005), BNP (Berger-Klein J et al 2004), and ADMA (Ngo et al 2007) have previously been shown to be associated with aortic valve calcification and stenosis. However, OPN appears to be the only biomarker implicated in both inflammation and biomineralization stages during aortic valve calcification and subsequent stenosis.
In this study, we begin the characterization of the post-translational modifications between OPN purified from CAVD patients and healthy controls. Based on the reported results, the phosphorylation of OPN threonine residues is detected in healthy controls and absent in the circulating OPN isolated from CAVD patients. A phosphorylation site prediction analysis of OPN protein, based on commercially available software, reveals presence of threonine at 147 and 155 position, which could be the possible sites for threonine phosphorylation. Interestingly, OPN is variably phosphorylated on both Serine and Threonine residues, which appears to control its biological activity. Furthermore the characterization of human circulating OPN correlates with the biological activities of phosphorylated (control) and dephosphorylated recombinant OPN (CAVD patient) suggesting that the dephosphorylation of circulating human OPN correlates with severe valvular calcification in patients with Calcific Aortic Valve Disease [Figure 4].
Figure 4. Schematic representation of correlation of OPN Dephosphorylation with valvular calcification.
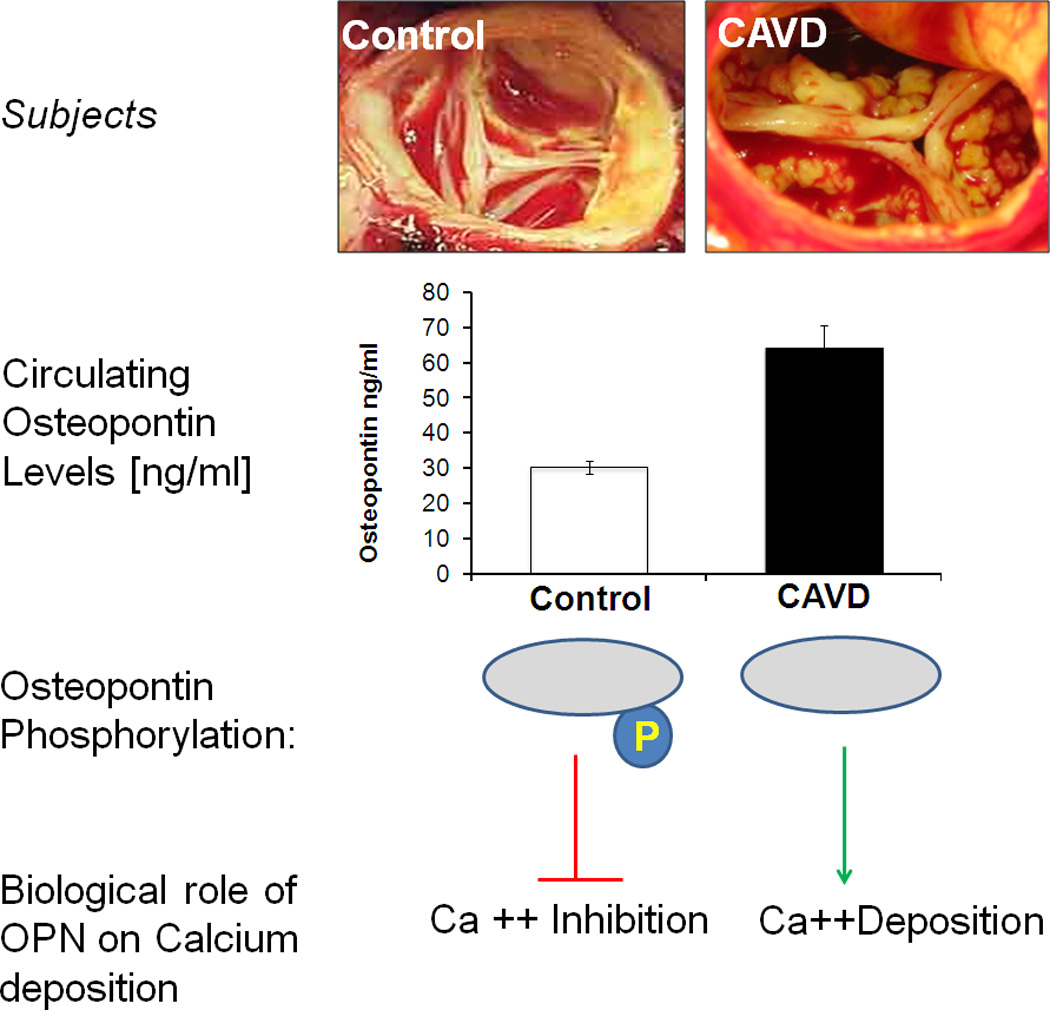
Plasma osteopontin levels were higher in CAVD patients as compared to controls. We observed increased phospho-threonine levels of purified OPN from healthy controls than CAVD patients, while the levels of phospho-serine and phospho-tyrosine were consistent between the two groups. Further, in vitro calcification assay confirms that phosphorylated recombinant OPN, which resembles the circulating OPN from healthy controls, prevents calcium deposition whereas the dephosphorylated protein which mimics patient’s plasma OPN loses its protective role thereby allowing calcium deposition on the cellular surface.
Conclusion
The general purpose of biomarkers includes disease identification, grading disease severity, providing pathophysiological clues, prognostic information, and assessing the effects of different therapeutic interventions. Due to the multiple biological pathways leading to CAVD, several potential biomarkers have been described which can be helpful in identifying the presence, severity, progression and prognosis of CAVD (Beckmann et al 2010). The association of these biomarkers with CAVD so far has been studied only quantitatively, but the biological activities of these molecules are often controlled by different mechanism(s) like protein-protein interactions and post-translational modifications. Here we present the first post-translational characterization of OPN as a biomarker for CAVD demonstrating the importance of its phosphorylation status as a key factor in maintaining the protective effects to prevent ectopic calcification. Future studies will be directed towards the identification of a better timing of this process with the goal of developing therapeutic measures before calcific deposits irreversibly damage the aortic valve.
Acknowledgments
This project is currently supported by Award Number RC1HL100035 from the National Heart, Lung, And Blood Institute, NIH (GF), by the “Harrison Memorial Fund” of the University of Pennsylvania School Of Medicine (GF) and by the Victor Musso Family (JBG).
References
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- Bach DS, Radeva JI, Birnbaum HG, Fournier AA, Tuttle EG. Prevalence, referral patterns, testing, and surgery in aortic valve disease: leaving women and elderly patients behind? J Heart Valve Dis. 2007;16:362–369. [PubMed] [Google Scholar]
- Balmer C, Beghetti M, Fasnacht M, Friedli B, Arbenz U. Balloon aortic valvuloplasty in pediatrics patients: progressive aortic regurgitation is common. Heart. 2004;90:77–81. doi: 10.1136/heart.90.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann E, Grau JB, Sainger R, Poggio P, Ferrari G. Insights into the Use of Biomarkers in Calcific Aortic Valve Disease. J. Heart Valve Dis. 2010;19:441–452. [PMC free article] [PubMed] [Google Scholar]
- Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109:2302–2308. doi: 10.1161/01.CIR.0000126825.50903.18. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Cho HJ, Kim HS. Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep. 2009;11:206–213. doi: 10.1007/s11883-009-0032-8. [DOI] [PubMed] [Google Scholar]
- Cowell SJ, Newby DE, Boon NA, Elder AT. Calcific aortic stenosis: same old story? Age Ageing. 2004;33:538–544. doi: 10.1093/ageing/afh175. [DOI] [PubMed] [Google Scholar]
- Davies SW, Gershlick AH, Balcon R. Progression of valvar aortic stenosis: a long-term retrospective study. Eur Heart J. 1991;12:10–14. doi: 10.1093/oxfordjournals.eurheartj.a059815. [DOI] [PubMed] [Google Scholar]
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- Goldbarg SH, Elmariah S, Miller MA, Fuster V. Insights into degenerative aortic valve disease. J Am Coll Cardiol. 2007;50:1205–1213. doi: 10.1016/j.jacc.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley M. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:2533–2539. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- Koos R, Kühl HP, Mühlenbruch G, Wildberger JE, Günther RW, Mahnken AH. Prevalence and clinical importance of aortic valve calcification detected incidentally on CT scans: comparison with echocardiography. Radiology. 2006;241:76–82. doi: 10.1148/radiol.2411051163. [DOI] [PubMed] [Google Scholar]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- Minoretti P, Falcone C, Calcagnino M, Emanuele E, Buzzi MP, Coen E, Geroldi D. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J. 2006;27:802–807. doi: 10.1093/eurheartj/ehi730. [DOI] [PubMed] [Google Scholar]
- Mohler ER, 3rd, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–552. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- Ngo DT, Heresztyn T, Mishra K, Marwick TH, Horowitz JD. Aortic stenosis is associated with elevated plasma levels of asymmetric dimethylarginine (ADMA) Nitric Oxide. 2007;16:197–201. doi: 10.1016/j.niox.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Novaro GM, Aronow HD, Mayer-Sabik E, Griffin BP. Plasma homocysteine and calcific aortic valve disease. Heart. 2004;90:802–803. doi: 10.1136/hrt.2003.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- O'Brien KD. Pathogenesis of Calcific Aortic Valve Disease: a disease process comes of age (and a good deal more) Arterioscler Thromb Vasc Biol. 2006;26:1721–1728. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- Perin MA, Brito FS, Jr, Almeida BO, Pereira MA, Abizaid A, Tarasoutchi F, Grube F. Percutaneous aortic valve replacement for the treatment of aortic stenosis: early experience in Brazil. Arq Bras Cardiol. 2009;93:299–306. doi: 10.1590/s0066-782x2009000900015. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ, for the Placement of Aortic Transcatheter Valves (PARTNER) Investigators Health-Related Quality of Life After Transcatheter Aortic Valve Replacement in Inoperable Patients With Severe Aortic Stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- Shen M, Marie P, Farge D, Carpentier S, De Pollack C, Hott MC, Chen L, Martinet B, Carpentier A. Osteopontin is associated with bioprosthetic heart valve calcification in humans. C R Acad Sci III. 1997;320:49–57. doi: 10.1016/s0764-4469(99)80086-9. [DOI] [PubMed] [Google Scholar]
- Shetty R, Pepin A, Charest A, Perron J, Doyle D, Viosine P, et al. Expression of bone-regulatory proteins in human valve allografts. Heart. 2006;92:1303–1308. doi: 10.1136/hrt.2005.075903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]
- Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55:135–144. doi: 10.1111/j.1365-2559.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- Soejima H, Irie A, Fukunaga T, Oe Y, Kojima S, Kaikita K, Kawano H, Sugiyama S, Yoshimura M, Kishikawa H, Nishimura Y, Ogawa H. Osteopontin expression of circulating T cells and plasma osteopontin levels are increased in relation to severity of heart failure. Circ J. 2007;71:1879–1884. doi: 10.1253/circj.71.1879. [DOI] [PubMed] [Google Scholar]
- Soejima H, Irie A, Fukunaga T, Sugamura K, Kojima S, Sakamoto T, Yoshimura M, Nishimura Y, Ogawa H. Elevated plasma osteopontin levels were associated with osteopontin expression of CD4+ T cells in patients with unstable angina. Circ J. 2006;70:851–856. doi: 10.1253/circj.70.851. [DOI] [PubMed] [Google Scholar]
- Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63–70. doi: 10.1016/S1054-8807(03)00130-3. [DOI] [PubMed] [Google Scholar]
- Wang A, Harrison JK, Bashore TM. Balloon aortic valvuloplasty. Prog Cardiovasc Dis. 1997;40:27–36. doi: 10.1016/s0033-0620(97)80020-5. [DOI] [PubMed] [Google Scholar]
- Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–850. doi: 10.1161/CIRCULATIONAHA.105.582882. [DOI] [PubMed] [Google Scholar]
- Weber M, Arnold R, Rau M, Elsaesser A, Brandt R, Mitrovic V, Hamm C. Relation of N-terminal pro B-type natriuretic peptide to progression of aortic valve disease. Eur Heart J. 2005;26:1023–1030. doi: 10.1093/eurheartj/ehi236. [DOI] [PubMed] [Google Scholar]
- Yu JP, Skolnick A, Ferrari G, Heretis K, Mignatti P, Pintucci G, Rosenzweig B, Diaz-Cartelle J, Kronzon I, Perk G, Pass HI, Galloway AC, Grossi EA, Grau JB. Correlation between Plasma Osteopontin Levels and Aortic Valve Calcification: Potential Insights into the Pathogenesis of Aortic Valve Calcification and Stenosis. J Thorac Cardiovasc Surg. 2009;138:196–199. doi: 10.1016/j.jtcvs.2008.10.045. [DOI] [PubMed] [Google Scholar]


