Abstract
Maternal food restriction during pregnancy results in adverse consequences for offspring, including obesity and cardiovascular disease. Early pregnancy is a critical period for this programming effect. Leptin is a regulator of energy homeostasis that also affects placental and fetal development. As food restriction results in decreased serum leptin levels, at least in non-pregnant animals, leptin depletion may be one mechanism by which food restriction affects development. The goal of this study was to test whether moderate food restriction affects serum leptin concentrations during the first half of pregnancy. We found that restriction to 50% of ad libitum consumption levels resulted in a significant decrease in serum leptin concentrations in both pregnant and non-pregnant female mice. There was no significant difference in serum leptin concentrations between non-pregnant females and at pregnancy day 11.5 when fed ad libitum. However, there was a difference in the source of leptin during pregnancy, with greater production in visceral fat in pregnant mice, and greater production in subcutaneous fat in non-pregnant mice. Leptin concentrations were dependent on time of day and time of sampling relative to feeding, particularly in restricted mice. There was a significant difference in serum leptin concentrations between fed and restricted mice when they were fed and sampled in afternoon, but not when they were fed and sampled in morning. We conclude that food restriction results in a significant decrease in leptin concentration during the first half of pregnancy in mice, but that detection of this relationship is subject to experimental design considerations.
Keywords: leptin, food restriction, pregnancy
Introduction
There is a large and growing body of evidence, known as the Developmental Origins of Health and Disease (DOHaD) hypothesis, that maternal food restriction during pregnancy causes permanent developmental changes that may not become apparent until offspring reach adulthood. Furthermore, early pregnancy is a critical time for these programming effects. In the Dutch Hunger Winter Study, it was found that the offspring of women who were exposed to famine only during the first trimester of pregnancy grew up to have a higher body mass index, LDL/HDL ratio, and rate of cardiovascular disease than infants who developed before or after the famine [1] [2]. Similarly, offspring of sheep or rats that underwent nutrient restriction during early-mid pregnancy exhibited increased adiposity as adults [3] [4] [5]. Although deficiencies in individual dietary components such as methyl donors and protein have been implicated in this process, overall energy restriction likely plays a role [6] [7].
We hypothesize that depletion of leptin, a hormone produced by adipose tissue, is a potential mediator of these effects of food restriction during early pregnancy on embryo development. Leptin levels generally fall in response to food restriction, and leptin affects reproductive physiology and embryonic development. For example, replacement of leptin restores ovarian cycles in food-restricted mice and in women with low body fat [8] [9], indicating that depletion of leptin is the immediate cause of reproductive failure in cases of low energy storage. Leptin is required for puberty, ovulation, lactation, and likely for embryo implantation [10] [11] [12]. Within the embryo, it is required for normal muscle and skeletal development [13,14]. Thus, loss of leptin likely has developmental consequences. However, it is not known whether leptin concentrations decline in response to food restriction during pregnancy, as they do in non-pregnant animals.
In non-pregnant animals, obesity from various causes is associated with elevated serum leptin concentrations whereas weight loss is associated with declining leptin concentrations [15,16,17]. Leptin concentrations also fall in response to fasting in both mice and humans, even after periods too brief to cause weight loss ([10,18,19,20]. This short term change is most likely mediated by changes in the concentration of insulin, which directly stimulates leptin production [17,21]. In addition to regulation by adiposity and food restriction, leptin concentrations are influenced by time of day, with a peak occurring during the night in both rodents and humans [22,23].
There are significant changes in the regulation of leptin production during pregnancy. Serum leptin concentrations increase dramatically during pregnancy in every mammalian species studied thus far [3,24,25,26,27]. This is due in part to high concentrations of estrogen, which stimulates leptin production. [25,28]. In addition, there is an increase in the binding protein, LEPRE, which extends the half-life of circulating leptin [29]. The placenta contributes significantly to leptin production in the pregnant human [26,28] little brown bat [24], and rat [27]. In contrast, although one group has found evidence of placental leptin production in the mouse [30,31], others have not [32,33,34], and increased secretion from abdominal fat accounts for at least some of the increase in serum leptin in the pregnant mouse [25,34].
The effect of food restriction on serum leptin concentrations has not been examined during early pregnancy, a critical period for developmental programming [1,2,4,5]. During mid-pregnancy in sheep, nutrient restriction to 60% of metabolizable energy requirements (~40% ad libitum consumption) from days 28-80 resulted in significantly lower leptin concentrations than in pregnant controls [3]. In late pregnancy, rats restricted to 50% of ad libitum intake from days 10-20 showed a slight decrease in leptin concentrations at day 16, but a significant increase at day 20 [35]. We hypothesize that in the first half of pregnancy, food restriction significantly decreases serum leptin concentrations and leptin gene expression, just as it does in non-pregnant mice. In addition, we examine relative differences in leptin production from subcutaneous and visceral fat between pregnant and non-pregnant mice. Finally, we show that timing of feeding and sampling significantly affects measurement of serum leptin concentrations.
Materials and Methods
Ethics statement
All animal procedures were approved by the University of Missouri institutional animal care and use committee (protocol #4123) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal procedures
Female Swiss Webster mice were obtained from Harlan (http://www.harlan.com) and individually housed at the University of Missouri. They were maintained on a 12:12 light: dark cycle, with lights on at 0600. Mice were 8-12 weeks of age at the start of each experiment. All mice were fed AIN-93G diet for pregnancy and lactation (Research Diets, New Brunswick, NJ) in a powdered, blue-dyed form in specialized feeding dishes so that average daily individual food consumption levels could be determined by food weighing [36]. Mice were euthanized by means of a carbon dioxide filled chamber and cervical dislocation. Terminal blood samples were collected by cardiac puncture. Serum was obtained by centrifugation of blood samples at 1000 × g for 12 minutes. All animal procedures were approved by the University of Missouri Animal Care and Use Committee.
Daily pattern of serum leptin concentrations following 10-day food restriction
Average daily food consumption was determined in 36 female mice fed ad libitum over a 10 day adjustment period. They were then divided into two groups: one fed ad libitum, and one fed to 50% of the food consumed per day during the adjustment period. This is the same degree of maternal food restriction demonstrated to cause programming of adult obesity in rats[5], and that was associated with increased serum leptin in rats during late gestation [35]. All mice were fed and weighed daily at 17:00-18:00 for 10 days. On the 10th day, 3 mice from each treatment group were sacrificed each at 10:00, 14:00, 18:00, 22:00, 02:00 and 06:00. Mice sacrificed at 18:00 had not been fed since the previous day. Blood was collected by cardiac puncture for measurement of serum leptin and insulin levels and blood glucose.
10-day food restriction in pregnant vs. non-pregnant female mice
Food consumption was determined as above in 32 female mice that were then divided into four treatment groups of 8 mice each: non-pregnant, ad libitum fed; non-pregnant, 50% food restricted; pregnant, ad libitum fed; pregnant, 50% food restricted. Seven of the mice assigned to the pregnant ad libitum group and six of the mice assigned to the pregnant restricted group were judged to be plug-positive (day 0.5) and respective treatments were begun on pregnancy day 1.5. Two mice in the pregnant restricted group were not pregnant at the time of sacrifice and were excluded from analysis. Mice were fed each day at 17:00. All mice were sacrificed at 16:00-17:00 on the 10th day of treatment (pregnancy day 11.5) and blood collected by cardiac puncture for analysis of serum leptin, insulin and blood glucose. Subcutaneous and visceral fat were dissected and frozen in Trireagent (Sigma, St. Louis, MO) at −80 °C for analysis of leptin gene expression.
Effect of morning feeding
An additional 15 females were divided into the same four treatment groups, non-pregnant, ad libitum fed; non-pregnant, 50% food restricted; pregnant, ad libitum fed; pregnant, 50% food restricted for days 1.5-11.5 of pregnancy or the equivalent time period. However, these mice were fed in the morning, at 09:30 and sacrificed at 12:00 for blood collection by cardiac puncture.
Statistics
GraphPad Prism software (GraphPad, La Jolla, CA) was used for statistical analyses. Serum leptin levels were compared among groups by two-way ANOVA, with diet and pregnancy status as factors, followed by Bonferroni post-tests. The same analysis method was used to compare insulin and glucose levels among diet and reproductive status groups.
Measurement of Blood Glucose
Blood glucose was measured using a One-Touch® Ultra Glucose Meter and One-Touch® test strips (LifeScan Inc., Milpitas, CA). Prior to conducting the blood glucose readings, the meter was calibrated using a control solution provided by the manufacturer. For each measurement, a test strip was inserted into the meter, and following the instructions on the meter’s screen, one drop of fresh whole blood was expelled from a syringe onto the end of the test strip.
Measurement of Serum Leptin and Insulin
Serum Leptin was measured in duplicate using a Mouse Leptin ELISA (Millipore, Billerica, MA; formerly Linco). The inter-assay CV was 8.0%. Manufacturer-determined sensitivity is 0.05 ng/ml. Serum insulin was measured in duplicate using a Rat/Mouse Insulin ELISA (Millipore). The inter-assay CV was 9.3%. Manufacturer-determined sensitivity is 0.2 ng/ml.
Real time RT-PCR
Adipose tissue samples were lysed in Trireagent (Sigma) using an Omni GLH homogenizer. Samples were centrifuged at 10,000 × g to remove debris (pellet) and excess lipids (top layer) before the addition of chloroform After phase separation, the aqueous phase was removed and mixed with an equal volume of buffer RLT (RNEasy Mini Kit, Qiagen, Valencia, CA), then ethanol. This mixture was applied to an RNEasy column and RNA purified according to manufacturer’s instructions. RNA quality and quantity were assessed spectrophotometrically. Equal amounts of total RNA were reversed transcribed by using Superscript III Reverse Transcriptase and random hexamers according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). Sequences for LEP specific primers: CAGCCTGCCTTCCCAAAAT and ATGGAGGAGGTCTCGGAGAT and probe: 5′FAM TGCTGCAGATAGCCAATGACCTGG, were designed using Primer Express (Applied Biosystems, Foster City, CA). LEP was amplified by using Taqman Universal PCR Master Mix (Applied Biosystems). Results were normalized to an 18s RNA control reaction (forward-TTCGGAACTGAGGCCATGAT; reverse-TTTCGCTCTGGTCCG TCTTG) performed with RT2 SYBR Green/ROX qPCR Master Mix (SABiosciences/Qiagen, Frederick MD), in which cDNA was first diluted 1:10. 18s has been shown to be stably expressed in adipose tissue [37]. All reactions were performed in triplicate on an Applied Biosystems 7500 Real-time PCR System. Within each tissue type (subcutaneous or visceral), Ct were analyzed by three-way ANOVA with diet, fat depot pregnancy status as factors, followed by Holm-Sidak post-tests, by using SigmaPlot software.
Results
Daily pattern of serum leptin concentrations following 10-day food restriction
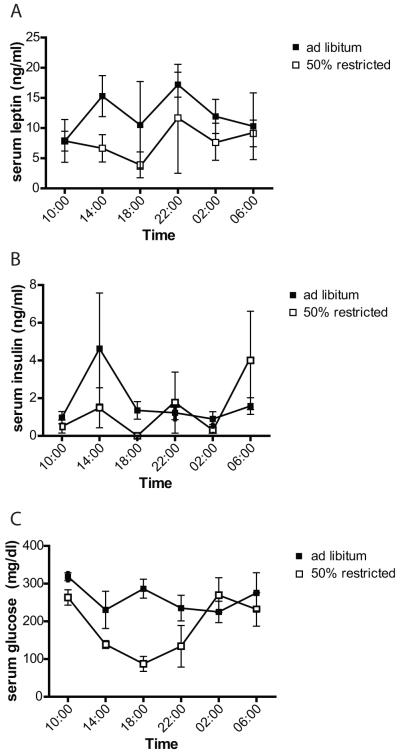
Leptin concentrations are affected by time of day. Therefore, we first conducted a pilot experiment to examine whether differences in serum leptin concentration caused by food restriction are also affected by time of day. Daily patterns of serum leptin concentrations were similar in mice fed ad libitum, and those fed 50% of ad libitum levels (restricted), with a peak in the night at 22:00, and a decrease by morning (Figure 1a). In both diet groups, there was a nadir at 18:00, the time point furthest from the last feeding, although food was continuously available in the ad libitum group (Figure 1a). Over a 24 hour period, leptin concentrations were higher in the ad libitum fed group than in the restricted group, although at some time points, particularly in the morning, there was no apparent difference in leptin concentrations between the two groups (Figure 1a).
Figure 1. Interaction between circadian pattern of leptin release and food restriction.
Thirty six female mice were fed ad libitum, or given 50% of their average consumption (“restricted”) for a period of 10 days. Three mice in each group were sacrificed every 4h and leptin (A) and insulin (B) measured by ELISA, and blood glucose (C) with a blood glucose meter. Bars represent SEM.
In the restricted group, serum insulin was undetectable, and mean blood glucose concentration lowest, at 18:00, the time at which leptin concentrations were also lowest (Figure 1). As with leptin, insulin concentrations were lower in the restricted group than the ad libitum group over the 24 h period (Figure 1b). In the ad libitum group, neither mean blood glucose nor insulin concentrations varied greatly over time, suggesting that food consumption was fairly constant, and that a peak in food consumption was probably not responsible for the night time peak in leptin (Figure 1).
10-day food restriction in pregnant vs. non-pregnant female mice
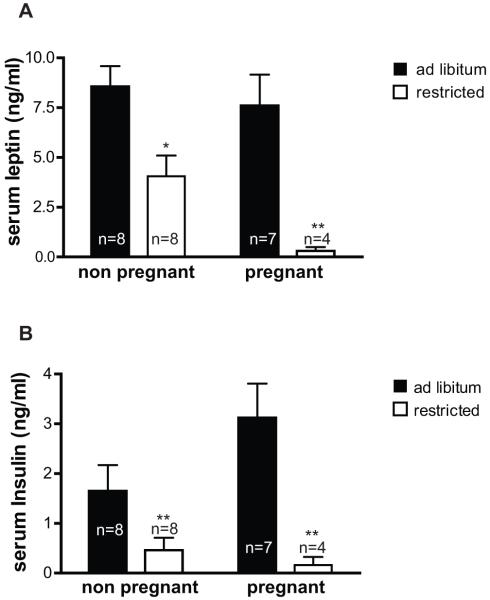
We then compared the effects of moderate food restriction on leptin concentrations during the first half of pregnancy and in non-pregnant females. Mice were again randomized into ad libitum and restricted groups for 10 days, with all mice fed in the afternoon and sacrificed at 16:00, the time at which the largest difference in leptin concentrations was expected based on the time-course experiment (Figure 1a). Serum leptin concentrations were similar in pregnant and non-pregnant animals fed ad libitum (Figure 2a). Leptin significantly decreased in response to food restriction in both non-pregnant and pregnant animals, but the decrease was more dramatic in the pregnant mice. Differences in leptin concentrations were mirrored by differences in insulin concentrations, which were also significantly decreased by food restriction in both reproductive states, but more so in pregnant than in non-pregnant mice (Figure 2b).
Figure 2. Effect of food restriction in pregnant vs. non-pregnant mice.
Mice were fed either ad libitum or to 50% of their average ad libitum consumption levels (“restricted”) from days 1.5 −11.5 of pregnancy, or for the same length of time in non-pregnant females. Mice were fed daily at 16:00 and were sacrificed at 16:00 on the 10th day for blood collection. Serum leptin (A) and insulin (B) were measured by ELISA. Effects of diet and pregnancy status were compared by two-way ANOVA and Bonferroni post-tests. By ANOVA, diet had a significant effect on both leptin (p< 0.0001) and insulin (p=0.0006) concentrations, whereas pregnancy did not have a significant effect. Bonferroni * p<0.05, **p<0.01 vs. ad libitum.
In addition to insulin, adiposity regulates leptin concentrations, at least in non-pregnant animals. Indeed, the decrease in leptin concentrations mirrored changes in body weight. Both pregnant and non-pregnant mice lost 14% of their body weight after 10 days food restriction (Table 1). Among the ad libitum fed mice, those in the pregnant group gained weight by pregnancy day 11.5, but there was no change in weight over the same time period in the non-pregnant group.
Table 1.
Weight changes during 10 day diet experiment
| Pregnant | Non-pregnant | |||
|---|---|---|---|---|
| Diet | Initial Weight (g) | Final Weight (g) | Initial Weight (g) | Final Weight (g) |
| ad libitum | 26.0 ± 0.7 a | 30.1 ± 0.6 (+4.1) b | 25.0 ± 0.8 a | 25.6 ± 0.7 (−0.3) a |
| 50% restricted | 25.4 ± 0.4 a | 21.8 ± 1.2 (−3.6) c | 25.9 ± 0.7 a | 21.3 ± 0.6 (−3.6) c |
Data are mean ± SEM. Weights with different superscript letters are significantly different, p<0.01; Two-way ANOVA with Bonferroni posttests.
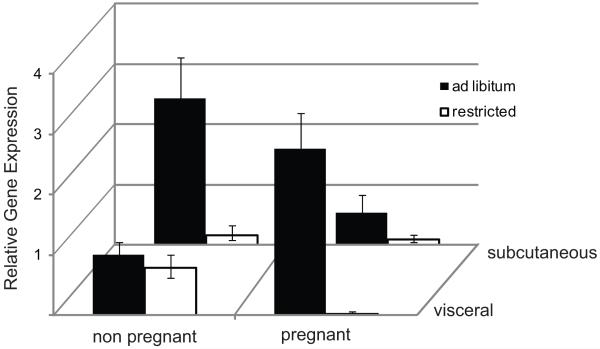
At the end of the 10 day diet period, subcutaneous and visceral adipose tissue samples were taken for analysis of leptin mRNA content by real-time RT-PCR (Figure 3). Reflecting the changes in serum leptin concentrations, LEP gene expression was significantly lower in restricted animals than in ad libitum fed animals (p <0.001). Within the restricted group, leptin expression was significantly different between fat types (p<0.001) when reproductive states were averaged and was significantly lower within pregnant mice when fat depots were averaged (p<0.001). There was also an overall difference in LEP expression between pregnant and non-pregnant mice (p<0.001), and a difference in the expression pattern across the two depots between pregnant and non-pregnant mice. Among the ad libitum fed mice, in subcutaneous fat, LEP expression was higher in non-pregnant mice than in pregnant mice (p<0.001). In the ad libitum fed, pregnant mice, LEP expression was significantly higher in visceral fat than in subcutaneous fat (p<0.001).,
Figure 3. Leptin gene expression in subcutaneous and visceral fat.
At the conclusion of the 10 day diet experiment, subcutaneous and visceral fat were collected and leptin gene expression assessed by real-time RT-PCR. Data were normalized to 18s. All expression is shown as fold-change relative to that in visceral fat of ad libitum fed, non-pregnant mice. Error bars represent 2ΔΔCtCt ±SEM. Comparisons among treatment groups were made by three-way ANOVA and Holm-Sidak post-tests. Diet (restricted vs ad libitum) and reproductive state (pregnant vs non-pregnant) but not fat depot had significant overall effects on LEP expression.
Effect of morning feeding and sampling
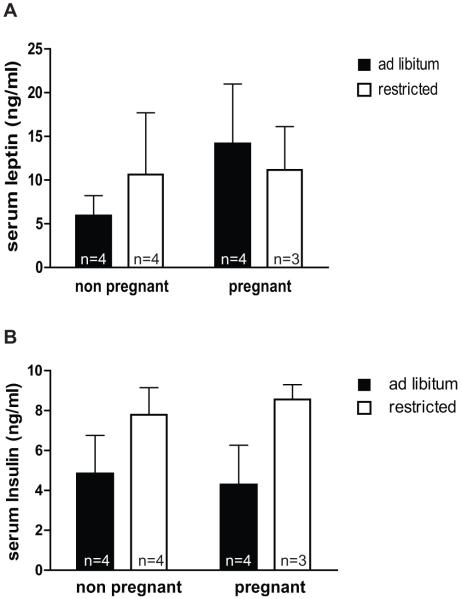
In the time-course experiment above, leptin concentrations declined in the morning regardless of diet, and plasma concentrations of leptin did not appear to be different between feeding groups in the morning (Figure 1a). In addition, we observed that when the mice on the restricted diet were presented with food each afternoon, they rapidly consumed all of it. This may cause a spike in glucose and insulin and briefly increases leptin secretion in the restricted mice. We hypothesized that failure to control for timing of sampling and feeding could lead to a failure to detect the overall effect of food restriction on leptin concentrations. To test this, we fed pregnant and non-pregnant mice ad libitum or restricted diets each morning at 09:30 for 10 days and all mice were sacrificed on the 10th day at 12:00 for blood collection. With this feeding schedule, we observed that the ad libitum mice ate throughout the day and night, but consumed most food during the dark period (18:00 to 06:00) whereas mice in the restricted group ate all of their food within a few hours after it was provided each morning. Accordingly, there was a trend towards higher insulin concentrations in the restricted vs. ad libitum fed mice (p=0.057) at noon, though this was not significant due to large variation in the ad libitum group (Figure 4b). There was no difference in leptin concentrations between the ad libitum and restricted groups, though if anything, leptin was slightly higher in the restricted than the ad libitum group among the non-pregnant mice (Figure 4a). Leptin concentrations in the restricted group under this morning feeding/sampling routine (Figure 4a) were considerably higher than they had been when animals were fed at night and sampled in the afternoon (Figure 2a), reflecting the recent food consumption and high insulin concentrations.
Figure 4. Effect of morning feeding and sampling on detection of food restriction effects.
Mice were fed either ad libitum or to 50% of their average ad libitum consumption levels (“restricted”) from days 1.5 −11.5 of pregnancy, or for the same length of time in non-pregnant females. Mice were fed daily at 09:30 and were sacrificed at 12:00 on the 10th day for blood collection. Serum leptin (A) and insulin (B) were measured by ELISA. Effects of diet and pregnancy status were compared by two-way ANOVA and Bonferroni post-tests. There were no significant differences in leptin or insulin levels between diets or between pregnant and non-pregnant mice.
Discussion
Consistently with previous experiments in multiple species, we found that LEP expression in non-pregnant mice was higher in subcutaneous fat than in visceral fat [38,39,40,41]. Although it has previously been shown that secretion of leptin by abdominal visceral fat increases at mid-pregnancy in mice [25], this is the first study to examine the relative effect of pregnancy on subcutaneous and visceral fat leptin. We found that there is a switch from higher LEP expression in subcutaneous fat of non-pregnant mice to higher expression in visceral fat at mid-pregnancy. The reason for this switch is unknown, but may be due to changes in fat distribution, or to differential responsiveness of the depots to hormones associated with pregnancy. Fat mass increases by the addition of stored fat to existing adipocytes. Leptin release per adipocyte is roughly proportional to the amount of stored fat in that cell while 18s, the control gene in this study, remains constant per cell as the amount of stored lipids increases. In non-pregnant mice, the difference between subcutaneous and visceral fat LEP production can be mostly accounted for by differences in adipocyte size, which is greater in subcutaneous fat [41]. Pregnancy fat gain occurs primarily in visceral fat [42], and the resulting increase in visceral adipocyte size may promote higher LEP expression per cell or per 18s RNA, thereby explaining the switch that we observed.
Direct hormone stimulation of LEP expression during pregnancy may also promote increased visceral vs. subcutaneous leptin expression. Progesterone, though associated with both pregnancy and weight gain, does not directly influence leptin production [43]. In vivo, but not in vitro, estrogen treatment stimulates leptin secretion in visceral fat from pregnant mice [25]. However, estrogen stimulates LEP gene expression equally in adipocytes isolated from subcutaneous and visceral fat of ovariectomized rats [44], which does not support a role for estrogen in differential leptin expression between subcutaneous and visceral fat during pregnancy. A more likely candidate for preferential stimulation of visceral fat LEP expression is glucocorticoids, which potently stimulate leptin secretion from pregnant mouse visceral fat [25]. Glucocorticoid receptor expression is higher in epididymal (visceral) than inguinal (subcutaneous) and retroperitoneal (visceral) fat in male mice [41]. The synthetic glucocorticoid dexamethasone does not affect LEP gene expression differently in human visceral and subcutaneous fat, but does have a greater effect on leptin secretion in visceral fat [45,46]
Surprisingly, despite significant weight gain during the first 11.5 days of pregnancy, pregnant mice did not have higher concentrations of serum leptin than their non-pregnant counterparts on the ad libitum diet. This is consistent with previous studies, which show a sharp increase in serum leptin beginning just after this time in pregnancy [25,29,34]. Kronfeld-Schor et al. [25] and others suggested that a simultaneous increase in the leptin binding protein, Lepre, may be absorbing the increased leptin secretion from visceral fat, but Marakami et al. [47] showed that Lepre injections actually increased leptin measurements. The switch in leptin production that we have identified, with significant loss of subcutaneous leptin expression, may explain why serum leptin is unchanged despite the increases in body weight and visceral leptin secretion.
Leptin concentrations declined in response to 50% food restriction in non-pregnant females in our study. This finding is consistent with some studies [15,22], but differs from the results of one study (Shi et al.) that specifically examined sex differences in the leptin response to moderate food restriction [48], and found no decrease in leptin in females. Our study employed a longer (10 days vs 6) and more severe (50% vs 60% of ad libitum) food restriction, which may be required for a significant decrease in leptin concentrations, although mice in both studies showed significant weight loss. The most likely explanation for the differing results is higher initial adiposity of the mice in our study vs. that of Shi et al. Our mice were approximately 20% heavier at the start of the feeding experiment, and serum leptin concentrations were considerably higher in the ad libitum fed mice at the end of the present experiment vs that of Shi et al (8.56 vs 2.42 ng/ml). Thus, our mice may have simply had more fat, and more leptin, to lose by food restriction. We conclude that, with a large enough initial leptin concentration, leptin does fall in response to food restriction in female mice.
Leptin also fell significantly in response to moderate food restriction during the first half of pregnancy. This period is of particular interest because of evidence that it is critical for the developmental programming of adult disease (DOHaD). In addition, we reasoned that in the period of pregnancy before the dramatic rise in leptin concentrations, regulation of leptin concentrations was likely to be similar to that in non-pregnant animals. In rats, Jelks et al. (2009) [35], found an increase in leptin concentrations following food restriction in on day 20 (but not day 16) of gestation. Thus, it is likely that the hormonal milieu of pregnancy predominates over adiposity and food intake as key regulators of leptin in late pregnancy. The differing results may also reflect a species difference; it was placental leptin production that increased in restricted rats in the Jelks et al. study, and adipose tissue appears to be the more significant source of leptin in the pregnant mouse [25,32,33,34]. Like adipose tissue, the placenta (in humans) is stimulated to produce leptin by insulin [49] and estrogen [28], but may differ in regulation by cellular lipid content or other factors.
Finally, we found that detection of the effects of food restriction on leptin concentrations was strongly dependent on the timing of both feeding and sampling and their interaction. It is already well-established that circadian rhythms of leptin release result in a decline in leptin concentrations in the morning. We found that this resulted in an inability to detect differences between the ad libitum and restricted groups during the morning. Thus, it is critical to carefully select the time of sampling in order to determine the effect of any treatment on leptin concentrations. It is even more challenging to control for the timing of food intake in food restriction studies. We observed that, whereas ad libitum mice tended to “graze” throughout the day and night, restricted mice tended to “binge” when presented with food each day. Therefore, postprandial effects, such as insulin release, are amplified and synchronized in the restricted mice. As a result, we hypothesized that if mice were fed and sampled in the morning, leptin concentrations would be at their lowest for the day in the ad libitum mice, due to circadian rhythms, and at their highest for the day in restricted mice, due to recent food consumption. Indeed, we were unable to detect a significant difference in leptin concentrations between treatment groups, and there were tendencies towards higher leptin and higher insulin in the restricted group at the time of sampling with this experimental design. In contrast, when we sampled in the afternoon, and not postprandially, leptin and insulin concentrations were significantly higher in the ad libitum fed mice. These findings are a reminder that a consideration of postprandial effects is critical for design of any food restriction experiment.
We conclude that there are changes in leptin gene expression during early pregnancy, with a switch to predominately visceral fat leptin expression from the predominately subcutaneous leptin expression in non-pregnant animals. However, food restriction results in leptin depletion during the first half of pregnancy in mice, just as in non-pregnant animals. Adult health has been shown to be particularly vulnerable to programming by food restriction during early pregnancy. Because leptin influences such variables as embryo implantation [12], placental cell differentiation [50] and placental amino acid transport [51], as well as fetal muscle and skeletal development [13,14] the shortage of leptin following food restriction that we have demonstrated here is worthy of future study as one route by which by which food restriction exerts its effects.
Ethical Standards
The authors declare that the reported experiments comply with all applicable laws of the United States
Acknowledgements
The authors thank Anchal Sethi for technical assistance. This work was supported by grant number HD055231 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 2.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, et al. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 3.Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- 4.Jones AP, Assimon SA, Friedman MI. The effect of diet on food intake and adiposity in rats made obese by gestational undernutrition. Physiol Behav. 1986;37:381–386. doi: 10.1016/0031-9384(86)90194-0. [DOI] [PubMed] [Google Scholar]
- 5.Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science. 1982;215:1518–1519. doi: 10.1126/science.7063860. [DOI] [PubMed] [Google Scholar]
- 6.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- 7.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007;104:19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 9.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 10.Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, et al. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 11.Malik NM, Carter ND, Murray JF, Scaramuzzi RJ, Wilson CA, et al. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology. 2001;142:5198–5202. doi: 10.1210/endo.142.12.8535. [DOI] [PubMed] [Google Scholar]
- 12.Ramos MP, Rueda BR, Leavis PC, Gonzalez RR. Leptin serves as an upstream activator of an obligatory signaling cascade in the embryo-implantation process. Endocrinology. 2005;146:694–701. doi: 10.1210/en.2004-1186. [DOI] [PubMed] [Google Scholar]
- 13.Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides. 2007;28:1012–1019. doi: 10.1016/j.peptides.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sainz N, Rodriguez A, Catalan V, Becerril S, Ramirez B, et al. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1alpha in ob/ob mice. PLoS One. 2009;4:e6808. doi: 10.1371/journal.pone.0006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 16.Niskanen LK, Haffner S, Karhunen LJ, Turpeinen AK, Miettinen H, et al. Serum leptin in obesity is related to gender and body fat topography but does not predict successful weight loss. Eur J Endocrinol. 1997;137:61–67. doi: 10.1530/eje.0.1370061. [DOI] [PubMed] [Google Scholar]
- 17.Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, et al. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 18.MacDougald OA, Hwang CS, Fan H, Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1995;92:9034–9037. doi: 10.1073/pnas.92.20.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, et al. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab. 1997;82:561–565. doi: 10.1210/jcem.82.2.3757. [DOI] [PubMed] [Google Scholar]
- 20.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 21.Block SS, Rhoads RP, Bauman DE, Ehrhardt RA, McGuire MA, et al. Demonstration of a role for insulin in the regulation of leptin in lactating dairy cows. J Dairy Sci. 2003;86:3508–3515. doi: 10.3168/jds.S0022-0302(03)73955-1. [DOI] [PubMed] [Google Scholar]
- 22.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha MK, Sturis J, Ohannesian J, Magosin S, Stephens T, et al. Ultradian oscillations of leptin secretion in humans. Biochem Biophys Res Commun. 1996;228:733–738. doi: 10.1006/bbrc.1996.1724. [DOI] [PubMed] [Google Scholar]
- 24.Kronfeld-Schor N, Zhao J, Silvia BA, Mathews PT, Zimmerman S, et al. Hyperleptinemia in pregnant bats is characterized by increased placental leptin secretion in vitro. Endocrine. 2001;14:225–233. doi: 10.1385/ENDO:14:2:225. [DOI] [PubMed] [Google Scholar]
- 25.Kronfeld-Schor N, Zhao J, Silvia BA, Bicer E, Mathews PT, et al. Steroid-dependent up-regulation of adipose leptin secretion in vitro during pregnancy in mice. Biol Reprod. 2000;63:274–280. doi: 10.1095/biolreprod63.1.274. [DOI] [PubMed] [Google Scholar]
- 26.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 27.Amico JA, Thomas A, Crowley RS, Burmeister LA. Concentrations of leptin in the serum of pregnant, lactating, and cycling rats and of leptin messenger ribonucleic acid in rat placental tissue. Life Sci. 1998;63:1387–1395. doi: 10.1016/s0024-3205(98)00405-6. [DOI] [PubMed] [Google Scholar]
- 28.Gambino YP, Maymo JL, Perez-Perez A, Duenas JL, Sanchez-Margalet V, et al. 17Beta-estradiol enhances leptin expression in human placental cells through genomic and nongenomic actions. Biol Reprod. 2010;83:42–51. doi: 10.1095/biolreprod.110.083535. [DOI] [PubMed] [Google Scholar]
- 29.Gavrilova O, Barr V, Marcus-Samuels B, Reitman M. Hyperleptinemia of pregnancy associated with the appearance of a circulating form of the leptin receptor. J Biol Chem. 1997;272:30546–30551. doi: 10.1074/jbc.272.48.30546. [DOI] [PubMed] [Google Scholar]
- 30.Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, et al. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci U S A. 1997;94:11073–11078. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoggard N, Hunter L, Lea RG, Trayhurn P, Mercer JG. Ontogeny of the expression of leptin and its receptor in the murine fetus and placenta. Br J Nutr. 2000;83:317–326. doi: 10.1017/s0007114500000398. [DOI] [PubMed] [Google Scholar]
- 32.Zhao J, Kunz TH, Tumba N, Schulz LC, Li C, et al. Comparative analysis of expression and secretion of placental leptin in mammals. Am J Physiol Regul Integr Comp Physiol. 2003;285:R438–446. doi: 10.1152/ajpregu.00776.2002. [DOI] [PubMed] [Google Scholar]
- 33.Malik NM, Carter ND, Wilson CA, Scaramuzzi RJ, Stock MJ, et al. Leptin expression in the fetus and placenta during mouse pregnancy. Placenta. 2005;26:47–52. doi: 10.1016/j.placenta.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Tomimatsu T, Yamaguchi M, Murakami T, Ogura K, Sakata M, et al. Increase of mouse leptin production by adipose tissue after midpregnancy: gestational profile of serum leptin concentration. Biochem Biophys Res Commun. 1997;240:213–215. doi: 10.1006/bbrc.1997.7638. [DOI] [PubMed] [Google Scholar]
- 35.Jelks A, Belkacemi L, Han G, Chong WL, Ross MG, et al. Paradoxical increase in maternal plasma leptin levels in food-restricted gestation: contribution by placental and adipose tissue. Reprod Sci. 2009;16:665–675. doi: 10.1177/1933719109334257. [DOI] [PubMed] [Google Scholar]
- 36.Townsend KL, Lorenzi MM, Widmaier EP. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocrine. 2008;33:176–188. doi: 10.1007/s12020-008-9070-1. [DOI] [PubMed] [Google Scholar]
- 37.Catalan V, Gomez-Ambrosi J, Rotellar F, Silva C, Rodriguez A, et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res. 2007;39:495–500. doi: 10.1055/s-2007-982502. [DOI] [PubMed] [Google Scholar]
- 38.Montague CT, Prins JB, Sanders L, Digby JE, O’Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46:342–347. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- 39.van Harmelen V, Dicker A, Ryden M, Hauner H, Lonnqvist F, et al. Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes. 2002;51:2029–2036. doi: 10.2337/diabetes.51.7.2029. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa Y, Masuzaki H, Isse N, Okazaki T, Mori K, et al. Molecular cloning of rat obese cDNA and augmented gene expression in genetically obese Zucker fatty (fa/fa) rats. J Clin Invest. 1995;96:1647–1652. doi: 10.1172/JCI118204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Guo KY, Diaz PA, Heo M, Leibel RL. Determinants of leptin gene expression in fat depots of lean mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R226–234. doi: 10.1152/ajpregu.00392.2001. [DOI] [PubMed] [Google Scholar]
- 42.Gunderson EP, Sternfeld B, Wellons MF, Whitmer RA, Chiang V, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 2008;16:1078–1084. doi: 10.1038/oby.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casabiell X, Pineiro V, Peino R, Lage M, Camina J, et al. Gender differences in both spontaneous and stimulated leptin secretion by human omental adipose tissue in vitro: dexamethasone and estradiol stimulate leptin release in women, but not in men. J Clin Endocrinol Metab. 1998;83:2149–2155. doi: 10.1210/jcem.83.6.4849. [DOI] [PubMed] [Google Scholar]
- 44.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140:1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 45.Halleux CM, Servais I, Reul BA, Detry R, Brichard SM. Multihormonal control of ob gene expression and leptin secretion from cultured human visceral adipose tissue: increased responsiveness to glucocorticoids in obesity. J Clin Endocrinol Metab. 1998;83:902–910. doi: 10.1210/jcem.83.3.4644. [DOI] [PubMed] [Google Scholar]
- 46.Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, et al. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol. 1998;275:E507–515. doi: 10.1152/ajpendo.1998.275.3.E507. [DOI] [PubMed] [Google Scholar]
- 47.Murakami T, Otani S, Honjoh T, Doi T, Shima K. Influence of the presence of OB-Re on leptin radioimmunoassay. J Endocrinol. 2001;168:79–86. doi: 10.1677/joe.0.1680079. [DOI] [PubMed] [Google Scholar]
- 48.Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab. 2007;293:E316–326. doi: 10.1152/ajpendo.00710.2006. [DOI] [PubMed] [Google Scholar]
- 49.Meissner U, Ostreicher I, Allabauer I, Rascher W, Dotsch J. Synergistic effects of hypoxia and insulin are regulated by different transcriptional elements of the human leptin promoter. Biochem Biophys Res Commun. 2003;303:707–712. doi: 10.1016/s0006-291x(03)00401-7. [DOI] [PubMed] [Google Scholar]
- 50.Schulz LC, Widmaier EP, Qiu J, Roberts RM. Effect of leptin on mouse trophoblast giant cells. Biol Reprod. 2009;80:415–424. doi: 10.1095/biolreprod.108.073130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88:1205–1211. doi: 10.1210/jc.2002-021332. [DOI] [PubMed] [Google Scholar]