Abstract
Fragile X syndrome (FXS) is the most common form of inherited intellectual disability in humans. Individuals affected with the disorder exhibit a deficiency of the fragile X mental retardation protein (FMRP), due to transcriptional silencing of the Fmr1 gene. It is widely accepted that learning deficits in FXS result from impaired synaptic function and/or plasticity in the brain. Interestingly, recent evidence suggests that conditional knockout of Fmr1 in neural progenitor cells in mice impairs hippocampal neurogenesis, which in turn contributes to learning impairments. To examine the nature of the neurogenic impairments and determine whether they impact the morphology of the dentate gyrus, we assessed the extent of neural progenitor cell proliferation, survival, and differentiation in older adult Fmr1 knockout mice. Here we show that the number of fast- proliferating cells in the subgranule layer of the dentate gyrus, as well as the subsequent survival of these cells, are dramatically reduced in Fmr1 knockout mice. In addition, the number of mature neurons in the granule layer of the dentate gyrus of these mice is significantly smaller than in WT littermate controls, suggesting that impaired proliferation and survival of neural progenitor cells compromises the structure of the dentate gyrus. Impaired adult neurogenesis may underlie, at least in part, the learning deficits that characterize fragile X syndrome.
Keywords: Fragile X Syndrome, Fmr1, neurogenesis, learning and memory, cognition
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability (mental retardation) in humans (O’Donnell and Warren, 2002). FXS is caused by excessive methylation of a CGG repeat expansion in the promoter region of the fragile X mental retardation 1 (FMR1) gene, leading to transcriptional silencing and loss of production of the fragile X mental retardation protein [FMRP, (Bassell and Warren, 2008)]. FMRP is highly expressed in neurons and appears to regulate protein translation by binding to specific mRNA species. Existing mouse models of the disorder have either a complete or conditional ablation of Fmr1 (Guo et al., 2011; Kooy et al., 1996). Fmr1 knockout (Fmr1-KO) mice exhibit impairments in various learning and memory tasks as well as defects in synaptic plasticity (LTP and LTD) in several brain structures, suggesting that the cognitive impairments in FXS are due to synaptic dysfunction (Bagni and Greenough, 2005; Brennan et al., 2006; Krueger and Bear, 2011; Krueger et al., 2011). Additionally, increasing evidence suggests a major role for neurogenesis in the neuropathology of FXS (Guo et al., 2011; Luo et al., 2010). Neurogenesis takes place throughout postnatal life at two sites in the brain (Li et al., 2009; Mu et al., 2010). New neurons born in the subgranule layer of the dentate gyrus (DG) are added to the dentate granule layer, while new neurons originating in the subventricular zone replace dying neurons in the olfactory bulb (Imayoshi et al., 2009). Increasing evidence suggests that hippocampal neurogenesis plays a role in learning and memory, and in DG-dependent learning in particular (Deng et al., 2010). A recent study examined the role of FMRP in adult neurogenesis and hippocampus-dependent learning and memory using an inducible conditional Fmr1 deletion and restoration mouse. Selective deletion of Fmr1 from neural stem cells led to impaired performance on two hippocampus-dependent learning tasks, while restoration of Fmr1 in these cells rescued the learning deficits. The deletion of Fmr1 from neural stem cells led to an increase in the number of glial fibrillary acidic protein (GFAP+) and S100β+ or Ki67+DCX− (doublecortin) neural stem cells and a reduction in Ki67+DCX+ neuroblasts, Ki67-DCX+ immature neurons, and NeuN+ mature neurons (Guo et al., 2011). However, it is not clear whether the reduction in the extent of the neuronal lineage is a result of compromised neuronal differentiation or poor survival of neural progenitor cells. Importantly, these experiments used relatively young mice (2–3 months of age), and a relatively short survival time (56 days post Fmr1 knockout); hence the long-term impact of reduced numbers of newly- generated neurons in the DG is not clear.
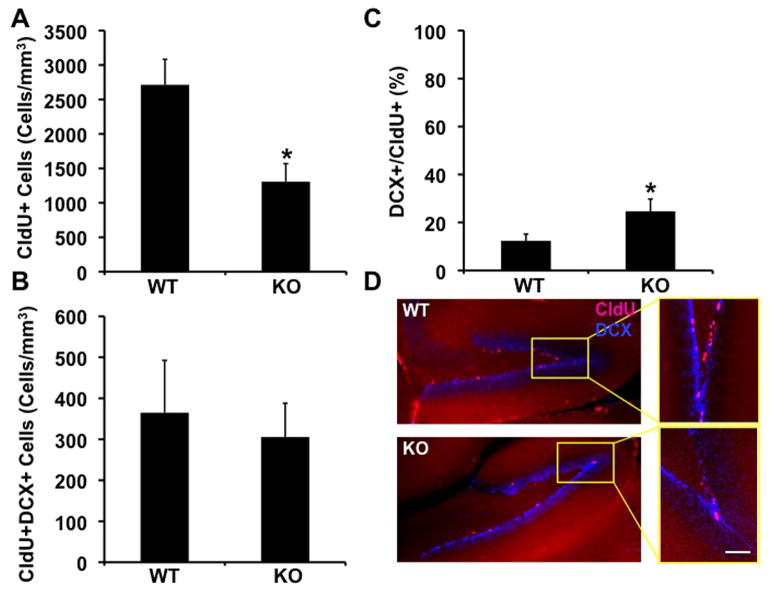
To determine the effect of lack of FMRP on the number of fast proliferating cells in the subgranular layer of the dentate gyrus, 9–12 month old littermate Fmr1-KO and wild type (WT) mice (C57Bl/6J congenic background) were injected with Chlorodeoxyuridine [CldU, (Vega and Peterson, 2005)] twice a day, 3 days before sacrifice (N=5 Fmr1-KO, N=7 WT). The extent of proliferation and survival of neural progenitor cells in brain sections of these mice was examined by immunohistochemistry and quantified by unbiased stereology as previously described (Demars et al., 2010). We observed that the number of fast proliferating cells was dramatically lower (by about 50%) in the brains of Fmr1-KO mice compared to wild type controls (Figure 1A, D). To examine the populations of newly- generated immature neurons, the number of cells coexpressing CldU and DCX was quantified. We observed a reduction in the number of CldU+DCX+ cells, but this reduction was not statistically significant (Figure 1B, D), suggesting that this reduction results from reduced proliferation rather than impaired neuronal maturation. To confirm this, we calculated the percentage of fast proliferating (CldU+) cells also expressing DCX (i.e., neuroblasts), relative to the number of proliferating cells. Evidently, the percentage of neuroblasts in the population of fast-proliferating progenitors was higher in the Fmr1-KO mice, suggesting that absence of FMRP affects extent of neural progenitor cell proliferation, but does not specifically alter the neuronal differentiation of these cells (Figure 1C).
Figure 1.
Reduced number of fast-proliferating neural progenitor cells in the dentate gyrus of Fmr1-KO mice. A. Quantitative analysis of the number of CldU+, fast-proliferating neural progenior cells in the brains of Fmr1-KO and WT mice by unbiased stereology (*= p 0.0173, students t-test). B. An insignificant reduction in the number of neuroblasts in the dentate gyrus of Fmr1-KO mice compared to WT littermates, as determined by unbiased stereology. C. The number of neuroblasts as a percentage of the number of fast proliferating neural progenitor cells is higher in the dentate gyrus of Fmr1-KO mice (*= p 0.0468, student’s t-test). D. Brain sections of Fmr1-KO and WT mice doubly-stained with antibodies raised against CldU and DCX. Selected areas are shown in high power in inserts. Scale bar= 150μ Inserts= 75μ.
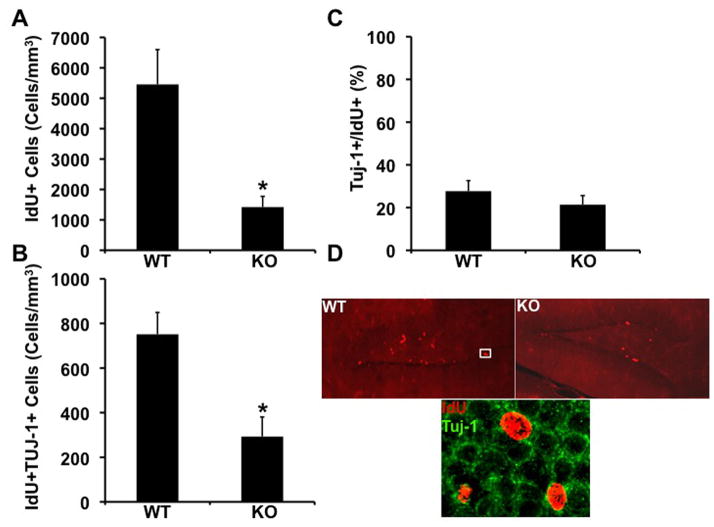
To examine the effect of lack of FMRP on the survival of neural progenitor cells, Fmr1-KO and WT littermate mice were injected with the thymidine analog iododeoxyuridine (IdU) for 3 consecutive days and sacrificed 12 days after the last dose (N=5 KO and N=6 WT). Quantification of the number of IdU+ cells in brain sections of these mice revealed a dramatic (about 75%) reduction in the number of surviving IdU+ cells in the Fmr1-KO dentate gyrus (Figure 2A, D). To determine whether the survival of new neurons is impaired as well, the number of IdU+ cells expressing β-tubulin (IdU+Tuj1+) cells was quantified. We observed a significant reduction in the number of new neurons surviving in the dentate gyrus of Fmr1-KO mice (Figure 2B). To determine whether this reduction results from impaired survival of neural progenitor cells or, more specifically, from impaired survival of new neurons, we calculated the number of new neurons as a percentage of the number of surviving cells. We found no significant difference between the ratio of new neurons and surviving neural progenitor cells in the dentate gyrus of Fmr1-KO compared to WT mice (Figure 1C), suggesting that deficiency of new neurons results from compromised survival of the neural progenitor cells.
Figure 2.
Reduced rate of survival of neural progenitor cells in the dentate gyrus of Fmr1-KO mice. A. A dramatic reduction in the number of IdU+, surviving neural progenitor cells in the dentate gyrus of Fmr1-KO mice (*= p 0.0130, student’s t-test). B. A significant reduction in the number of surviving neuroblasts (IdU+Tuj1+) in the brains of Fmr1-KO mice (*= p 0.0077, student’s t-test). C. The number of surviving neuroblasts (IdU+Tuj1+) as a percentage of the number of surviving neural progenitor cells (IdU+) cells is unchanged in the dentate gyrus of Fmr1-KO compared to WT mice. D. IdU+ cells immunolabeled in brain sections of Fmr1-KO and WT mice. Lower panel: High power image of an IdU+Tuj1+ neuroblast in the dentate gyrus of WT mice. Scale bar= 50μ.
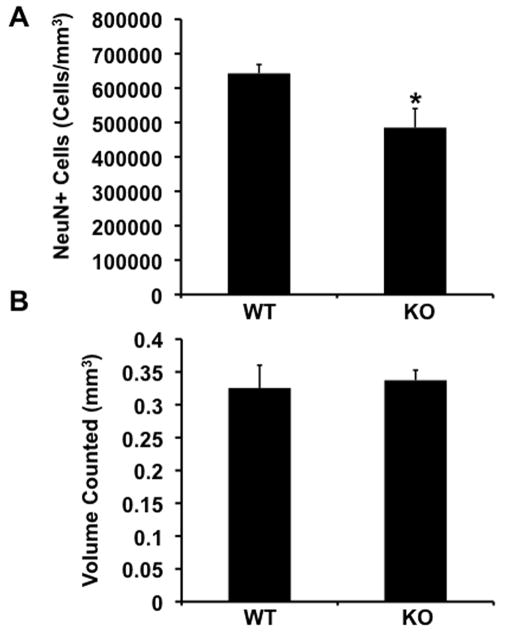
To determine whether a deficiency in the survival of neural progenitor cells in the subgranule layer of the dentate gyrus of Fmr1-KO mice results in a reduced number of mature neurons in the granule layer of the dentate gyrus, we quantified the number of NeuN+ neurons in this layer (N=5 KO and N=6 WT). Unbiased stereological analysis revealed that the granule layer of the dentate gyrus of Fmr1-KO mice contained 25% fewer neurons than the same layer of wild-type mice (Figure 3A). This reduction did not affect the volume of the layer (Figure 3B). These results suggest that there are fewer mature neurons in the dentate gyrus of adult Fmr1-KO mice.
Figure 3.
Fewer mature neurons in the dentate gyrus of Fmr1-KO mice. A. Unbiased stereological analysis of the number of NeuN+ neurons in the dentate gyrus of Fmr1-KO and WT mice (*= p 0.0228, student’s t-test). B. Volume of the dentate gyrus is similar in Fmr1-KO and WT mice.
The present experiments examined neurogenesis in the DG of a mouse model for Fragile X syndrome in middle age (9–12 month old-mice). There were three main findings: First, the numbers of rapidly-proliferating cells in the subgranule layer of the DG were substantially reduced in mice lacking FMRP (Fmr1-KO) compared to WT littermate control mice. Second, the survival of newly- generated cells was greatly reduced in mice lacking FMRP. Impairments in both processes affected the number of newly- committed neurons, but this effect did not seem to be the result of a defect in neuronal maturation. Third, estimates of the numbers of mature granule cells in the DG were significantly lower in Fmr1-KO mice than in WT mice, suggesting that decreases in proliferation and survival of adult-derived neurons have a measurable impact on the total neuronal population in this region.
Short-term labeling with CldU showed a 50% decrease in cell proliferation in Fmr1-KO mice, with a significant increase in the proportion of cells double-labeled with DCX as a marker of neuronal differentiation. This finding is in contrast to a previous study that reported increased cell proliferation in Fmr1-KO mice (Luo et al., 2010). Aside from technical issues related to the labeling method and injection schedule, the main difference appears to be the age of the animals tested: we examined mice at 9–12 month of age whereas Luo et al. (2010) studied mice at two months of age. There are now a number of studies showing that various neurobiological phenotypes change dramatically with postnatal age in Fmr1-KO mice (Grossman et al., 2010; Nimchinsky et al., 2001; Pilpel et al., 2009; Qin et al., 2005), even into adulthood (Larson et al., 2005). Therefore, it is possible that rates of neurogenesis fall substantially with age in mice lacking FMRP. Fmr1-KO mice lack FMRP production in all cells throughout the brain. Recently, Guo, et al. (2011) reported a conditional knockout of Fmr1 restricted to nestin-expressing neural stem and progenitor cells and their subsequent progeny. Interestingly, this study suggests that a conditional deletion of Fmr1 enhances neural stem cell proliferation and decreases the number of neuroblasts and immature neurons (Guo et al., 2011). While in our study the likelihood of pulsing neural stem cells is relatively low, we do find that the number of fast- proliferating cells is reduced in the Fmr1-KO mice, compared to WT controls. Nevertheless, while this reduction in the number of fast- proliferating cells is manifested by a trend toward reduced numbers of rapidly- proliferating neuroblasts, this reduction appears insignificant, and in fact, the relative number of new neurons as a percentage of fast-proliferating cells is higher in the Fmr1-KO mice, suggesting that lack of FMRP affects extent of proliferation of neural progenitor cells rather than early neuronal differentiation.
The present study also shows that lack of FMRP compromises the survival of neural progenitor cells and neuroblasts, since the number of surviving cells labeled with IdU two weeks after injection were greatly reduced (75%) in Fmr1-KO mice, compared to WT controls. Previous studies suggested that lack of FMRP enhances a fate switch, increasing the number of new astrocytes while decreasing the number of new neurons (Guo et al., 2011; Luo et al., 2010). Here we show that in addition, the reduced number of neural progenitor cells and new neurons is the result of severely compromised survival of neural progenitor cells and neuroblasts in the dentate gyrus of Fmr1-KO mice, suggesting that lack of FMRP may affect levels of growth factors and neurotrophic factors in the neurogenic niche.
Lastly, the combination of decreased proliferation and compromised survival appears to have a cumulative effect on the total population of granule neurons in the dentate, with one year-old Fmr1-KO mice having significantly fewer mature granule cells than WT controls. In agreement with Guo et al. (2011), we find that the number of neurons is significantly reduced in the granule layer of one year-old Fmr1-KO mice while the volume of the layer remains comparable to WT controls. In that regard, other studies reporting a change in the number of neurons in the granule layer of the DG as a result of modified neurogenesis, show no effect on the volume of the granule layer (Sahay et al., 2011).
Numerous recent studies suggest a role for neurogenesis in hippocampus- and DG-specific behavioral tasks (Clelland et al., 2009; Creer et al., 2010; Sahay et al., 2011). Genetic ablation of newly formed neurons in adult mice leads to an inhibition of increase in the granule cell number in the DG and impairment in contextual and spatial memory (Imayoshi et al., 2008), suggesting that lack of addition of new neurons is sufficient to induce learning impairments. Thus, our observation that the number of neurons in the DG of Fmr1-KO mice is reduced might be of great relevance to learning impairments exhibited by these mice, and to similar impairments in the human disorder.
In summary, our study provides evidence that impaired neurogenesis in mice lacking FMRP results in fewer neurons in the DG of these mice due to impaired survival of new progenitors and new neurons. Stimulation of neurogenesis may therefore represent an important therapeutic target in Fragile X syndrome.
Acknowledgments
This research was supported by grants from the National Institutes of Health (DC5793 to JL), NIH/NIA AG033570 (OL), the Brain Research Foundation (OL) and the FRAXA Research Foundation (JL).
References
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6(5):376–87. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–14. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FX, Albeck DS, Paylor R. Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 2006;5(6):467–71. doi: 10.1111/j.1601-183X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107(5):2367–72. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. Journal of neuroscience research. 2010;88(10):2103–17. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–50. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Lee KJ, Zeman MK, Jun CS, Azam HS, Arii T, Imoto K, Greenough WT, Rhyu IJ. Developmental characteristics of dendritic spines in the dentate gyrus of Fmr1 knockout mice. Brain Res. 2010;1355:221–7. doi: 10.1016/j.brainres.2010.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, Murthy AC, Goggin SL, Eisch AJ, Oostra BA, et al. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nature medicine. 2011;17(5):559–65. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Kageyama R. Continuous neurogenesis in the adult brain. Dev Growth Differ. 2009;51(3):379–86. doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Kooy RF, D’Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, Storm K, Clincke G, De Deyn PP, Oostra BA, et al. Transgenic mouse model for the fragile X syndrome. Am J Med Genet. 1996;64(2):241–5. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–29. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2011;108(6):2587–92. doi: 10.1073/pnas.1013855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci. 2005;25(41):9460–9. doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mu Y, Gage FH. Development of neural circuits in the adult hippocampus. Curr Top Dev Biol. 2009;87:149–74. doi: 10.1016/S0070-2153(09)01205-8. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shan G, Guo W, Smrt RD, Johnson EB, Li X, Pfeiffer RL, Szulwach KE, Duan R, Barkho BZ, et al. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6(4):e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Current opinion in neurobiology. 2010;20(4):416–23. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21(14):5139–46. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–38. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- Pilpel Y, Kolleker A, Berberich S, Ginger M, Frick A, Mientjes E, Oostra BA, Seeburg PH. Synaptic ionotropic glutamate receptors and plasticity are developmentally altered in the CA1 field of Fmr1 knockout mice. J Physiol. 2009;587(Pt 4):787–804. doi: 10.1113/jphysiol.2008.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Smith CB. A null mutation for Fmr1 in female mice: effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience. 2005;135(3):999–1009. doi: 10.1016/j.neuroscience.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega CJ, Peterson DA. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat Methods. 2005;2(3):167–9. doi: 10.1038/nmeth741. [DOI] [PubMed] [Google Scholar]