Abstract
Purpose
The effects of systemic metal ion exposure in patients with implants made of common prosthetic alloys continue to be a matter of concern. The aim of the study was to determine the measurement values of cobalt (Co), chromium (Cr) and molybdenum (Mo) in serum following rotating-hinge knee arthroplasty.
Methods
Blood was taken from 25 patients [mean follow-up 35 (range nine to 67) months] treated with megaprostheses (n=17) or standard rotating-hinge devices (n=8) and analysed using electrothermal graphite furnace atomic absorption spectrometry (ET-ASS).
Results
Determining the concentrations of metal ions following rotating-hinge knee arthroplasty revealed increments for Co and Cr but not Mo. Metal ion release was significantly higher in patients with megaprostheses compared to a standard rotating-hinge knee device (Co p=0,024; Cr p=0.025).
Conclusion
The authors believe there might be an additional metal ion release from the surface of the prosthesis and not only from the articulating surfaces because, in cases of rotating-hinge knee prosthesis, there is a metal-on-polyethylene articulation and not a direct metal-on-metal junction. Nevertheless, long-term studies are required to determine adverse effects of Co, Cr and Mo following total hip replacement and total knee arthroplasty.
Introduction
The potential harmful effects of elevated systemic exposure to cobalt (Co), chromium (Cr) and molybdenum (Mo) are of concern, especially following metal-on-metal hip resurfacing or standard total hip arthroplasty (THA); increased levels of metal ions in the blood and/or plasma after implantation of metal devices represents systemic exposure. High concentrations of Co and Cr are toxic and are known to interfere with biological functions [1]. Ladon et al. [2] reported chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. Case et al. [3] discovered the possibility of DNA damage due to Co and Cr following metal-on-polyethylene hip arthroplasty. Furthermore, there are concerns about the hazard of delayed-type hypersensitivity reactions, nephrotoxicity and teratogenicity [2, 4, 5].
It is well known that metal-on-metal resurfacing prostheses are associated with elevated concentrations of metal ions within the hip joint, the periarticular soft tissues and systemically due to ionization effects from the bearing surface [5–8]. Imanishi et al. [9], Back et al. [6], Daniel et al. [5], Engh et al. [10], Antoniou et al. [11], Witzleb et al. [12] and Savarino et al. [7, 13] demonstrated that the levels of Co and Cr ions increase within the first three months to two years following metal-on-metal THA. In the study of Imanishi et al. [9], there was no significant additional increase of serum metal ion concentration at the one year follow-up, whereas Back et al. [6] and Daniel et al. [5, 14] observed a decreasing trend of serum concentration between one and six years. Beyond a 30-year follow-up, Sauvè et al. [15] observed five times and three times higher serum levels of Co and Cr, respectively, following metal-on-metal THA compared with a control group. On the other hand, the measured concentrations were comparable with those from patients with modern metal-on-metal THAs. It is also known that metal ion levels in serum depend on different tribological pairings (metal-on-metal > metal-on-polyethylene) [10]. Jacobs et al. [8] suggested that acetabular and femoral components also are sources of metal ion dissemination systemically.
To the authors’ knowledge, there is little data published concerning metal ion concentrations following total knee arthroplasty (TKA). Garrett et al. [16] reported data determining differences in metal ion release following Co-Cr and oxidised zirconium TKA, but the differences between the groups compared were not statistically significant due to different prosthetic alloys. The aim of this study was to determine measurement values of serum metal ion levels in patients following rotating-hinge knee arthroplasty. Furthermore, we investigated whether patients with megaprostheses would have higher concentrations of serum metal ions than patients with standard rotating-hinge devices.
Patients and methods
From January 2003 to December 2008, 55 patients (26 men, 29 women) underwent total knee replacement using the Limb Preservation System (LPSTM/M.B.T., DePuy, Warsaw, IN, USA) or the S-ROM®NoilesTM (DePuy) rotating-hinge prosthesis in our department. There were 44 distal femoral, eight proximal tibial and three total femoral replacements. All prostheses were manufactured from Co-Cr-molybdenum (Mo) alloy according to International Standards Organisation (ISO) 5832–4. At the time of evaluation, 11 of these 55 patients had died due to an underlying disease or an unrelated cause. Additionally, five were lost to follow-up, and 14 did not want to participate in our investigation. Blood was thus taken from 25 patients [mean follow-up 35 (range 9–67) months] (Table 1) treated with megaprostheses (LPSTM/MBT, n = 17) or standard rotating-hinge devices (S-ROM®NoilesTM, n = 8) using stainless-steel needles attached to no-additive plastic vacuum tubes (VACUETTE®). All needles and tubes were from the same batch. None of the patients had a history of renal impairment. All blood samples were collected by one observer (JF) on equal setting conditions. All specimens were centrifuged at 4,000 rpm within two hours and stored at −10°C until analysis. Concentrations of Co, Cr and Mo were determined using electrothermal graphite furnace atomic absorption spectrometry (ET-ASS) in the same laboratory. The levels of metal ions in serum were recorded in concentrations expressed as micrograms per decilitre (μg/dl).
Table 1.
Demographic data of the study population with respect to sex, age, definite diagnosis, localisation and side, type of prosthesis, time of follow-up and evidence of disease
| Patient no.. | Age at operation (years) + sex | Diagnosis | Localisation + side | Device | Follow-up (months) | Status |
|---|---|---|---|---|---|---|
| 1 | 70, M | Infection TKA | Femur distal, R | LPS | 11 | n.a. |
| 2 | 60, M | Infection TKA | Femur distal, R | S-ROM Noiles | 52 | n.a. |
| 3 | 58, M | Pseudarthrosis | Tibia prox., R | LPS | 23 | n.a. |
| 4 | 81, W | Infection TKA | Femur distal, R | S-ROM Noiles | 15 | n.a. |
| 5 | 70, M | Chondrosarcoma G2 | Femur distal, R | LPS | 43 | NED |
| 6 | 83, F | Periprosthetic fracture | Femur distal, R | LPS | 19 | n.a. |
| 7 | 79, F | Infection TKA | Femur distal, L | S-ROM Noiles | 62 | n.a. |
| 8 | 54, F | Follicular lymphoma G3 | Femur distal, L | LPS | 51 | NED |
| 9 | 62, F | Infection TKA | Femur distal, L | S-ROM Noiles | 36 | n.a. |
| 10 | 29, M | Osteosarcoma G3 | Tibia prox., R | LPS | 23 | NED |
| 11 | 17, M | Osteosarcoma G3 | Femur distal, R | LPS | 39 | NED |
| 12 | 48, M | Infection TKA | Femur distal, L | LPS | 31 | n.a. |
| 13 | 19, M | Osteosarcoma G3 | Tibia prox., L | LPS | 58 | NED |
| 14 | 73, M | Infection TKA | Femur distal, L | S-ROM Noiles | 48 | n.a. |
| 15 | 76, F | Periprosthetic fracture | Femur distal, R | LPS | 44 | n.a. |
| 16 | 72, F | Osteoarthritis | Femur distal, R | S-ROM Noiles | 17 | n.a. |
| 17 | 15, M | Osteosarcoma G3 | Femur distal, L | LPS | 67 | NED |
| 18 | 46, M | Osteosarcoma G3 | Tibia prox., L | LPS | 43 | NED |
| 19 | 38, F | Myxoid liposarcoma G2 | femur distal, L | LPS | 19 | NED |
| 20 | 28, M | Chondrosarcoma G2 | femur distal, L | LPS | 9 | NED |
| 21 | 79, F | Infection TKA | femur distal, L | LPS | 10 | n.a. |
| 22 | 40, M | Osteosarcoma G3 | femur distal, R | LPS | 44 | NED |
| 23 | 79, M | Infection TKA | femur distal, R | S-ROM Noiles | 49 | n.a. |
| 24 | 69, M | Infection TKA | femur distal, L | LPS | 49 | n.a. |
| 25 | 75, M | Infection TKA | femur distal, R | S-ROM Noiles | 19 | n.a. |
TKA total knee arthroplasty, NED no evidence of disease, n.a. not applicable, LPS Limb Preservation System
Results were analysed to calculate the mean level of each ion in plasma. Additionally, the correlation between metal ions was determined (Pearson correlation coefficient). Furthermore, two subgroups were organised—patients with megaprostheses and patients with a standard rotating-hinge device—in order to perform an intergroup comparison. Due to the asymmetric distribution of metal ion levels, a nonparametric test (Mann–Whitney U test) was used. A p value of <0.05 was considered statistically significant. For statistic analysis, the PASW Statistics 16.0 programme (SPSS Inc., Chicago, IL, USA) was used.
Results
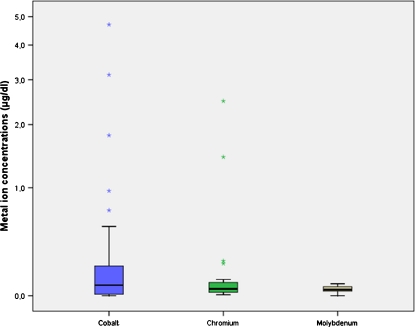
Mean results for Co, Cr and Mo in the serum of all patients with rotating-hinge arthroplasty were 0.52 μg/dl (range 0–4.70 μg/dl), 0.213 μg/dl (range 0.06–2.49 μg/dl) and 0.05 μg/dl (range 0–0.08 μg/dl; Fig. 1). Compared with reference values from the laboratory, values for Co (normal range 0–0.05 μg/dl) and Cr (normal range 0–0.19 μg/dl) increased tenfold and onefold, respectively, whereas Mo (normal range 0–0,10 μg/dl) was within physiological limits. Testing the correlation between Co and Cr concentration revealed a correlation coefficient of 0.946, which was highly significant (p < 0,001).
Fig. 1.
Results of cobalt (Co), chromium (Cr) and molybdenum (Mo) measured in patients with megaprostheses and standard rotating-hinge devices at a mean follow-up of 35 (range 9–67) months. Statistical analysis revealed a highly significant correlation between Co and Cr concentrations (Pearson 0.946; p < 0.001)
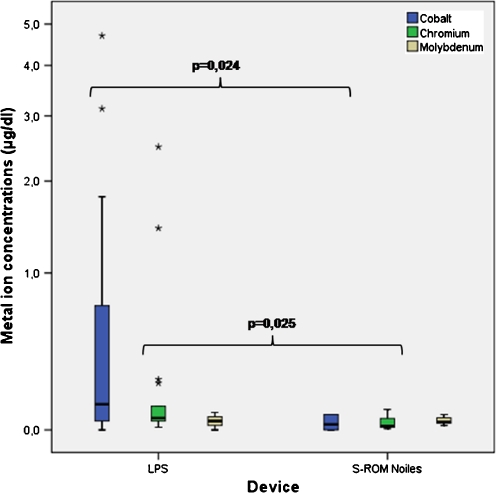
Considering the similar length of follow-up of both groups, mean results for Co from patients treated with megaprostheses (mean 0.75 μg/dl, range 0–4.70 μg/dl) were 25 times higher compared to patients with a standard rotating-hinge knee (mean: 0.03 μg/dl, range: 0–0.07 μg/dl; Fig. 2). The mean concentrations for Cr in patients with megaprostheses (Cr: 0.298 μg/dl, range 0.012-2.490 μg/dl) were nine times higher compared to those in patients with a standard rotating-hinge device (mean 0.033 μg/dl, range 0.006–0.095 μg/dl; Fig. 2). The different serum concentrations between both implant groups were statistically significant (Co p = 0.024; Cr p = 0.025; Table 2). In the megaprostheses group, there was a high correlation between the concentration of Co and Cr, with a Pearson correlation coefficient of 0.945 (p < 0.001). The concentration of Co also correlated with Cr in the standard rotating-hinge group (Pearson 0.764, p = 0.027) but not as much as in the Mo group. A possible explanation for the differences between implant groups could be prostheses size or implant surfaces.
Fig. 2.
Concentrations of cobalt (Co), chromium (Cr) and molybdenum (Mo) divided by implant groups. Patients with megaprostheses had higher concentrations of Co and Cr in plasma compared with patients with a standard rotating-hinge knee. These differences were statistically significant
Table 2.
Results of the different plasma concentrations of metal ions in patients treated with megaprostheses and standard rotating-hinge devices
| Megaprostheses | Standard rotating-hinge devices | Significance | |
|---|---|---|---|
| Serum metal ion levels (range) | |||
| Co (μg/dl) | 0.75 (0–4.70) | 0.03 (0–0.07) | 0.024 |
| Cr (μg/dl) | 0.298 (0.012–2.490) | 0.033 (0.006–0.095) | 0.025 |
| Mo (μg/dl) | 0.04 (0–0.08) | 0.04 (0.02–0.07) | 0.746 |
Co cobalt, Cr chromium, Mo molybdenum
Discussion
Our study revealed increased and strongly correlating serum levels for Co and Cr (p < 0.001) in patients following rotating-hinge TKA. As expected, referring to previous published data, Mo values were within limits. In contrast, determining serum concentrations of metal ions in patients with megaprostheses showed 25 times and nine times higher levels for Co and Cr, respectively, compared to patients with standard rotating-hinge devices. Statistical analysis revealed significant differences between the implant groups (Co p = 0.024; Cr p = 0.025). Although there was increased Co and Cr concentration in serum, there was no evidence of systemic Co and/or Cr intoxication, but continued long-term follow-up is needed to assess the possibility of such a complication.
Metal ion toxicity, metal hypersensitivity and metal carcinogenicity are of concern following metal-on-metal THR or hip-resurfacing arthroplasty. Case et al. [17] demonstrated the dissemination of metal debris to lymph nodes, liver, spleen and bone marrow. Hart et al. [18] proved adverse effects of metal ions on the T-cell count (only CD8+ lymphocytes) in patients following metal-on-metal hip resurfacing. The authors interpreted their findings as suggesting a negative influence on the immune system. Savarino et al. [13] reported haematological and immunological alterations in patients with metal-on-metal or metal-on-polyethylene arthroplasty, whereas these effects could not be observed in patients with ceramic-on-ceramic prostheses. These findings led on to the hypothesis that metal ions, especially Co and Cr, might have a toxic effect on myelopoesis and the immune system. The in vitro study of Allen et al. [19] demonstrated the reduction of osteoblastic activity due to metal ions. The authors suggested that this finding could be an explanation for a weakened bone–implant interface of metal-on-metal implants and therefore an important mechanism of aseptic loosening in vivo. Dustan et al. [20] found higher mean levels of Co and Cr in whole blood and urine in patients with loosened metal-on-metal implants compared with a group with stable implants. Therefore, these authors recommend determination of Co in whole blood and urine to identify patients with loosened implants. Apart from the different implantation side, there were three cases of implant loosening in our series, but all cases were related to earlier deep prosthetic infections (secondary aseptic loosening). On the other hand, Antoniou et al. [11] suggested that increased metal ion levels had no effects on oxidative stress makers (total antioxidants, peroxides and nitrated proteins), and the study of Grübl et al. [21] showed that there was no increase in cancer incidence at a minimum ten year follow-up following metal-on-metal THR. Nevertheless, all these assumptions are still of concern and have to be verified in further long-term studies.
Daniel et al. [5, 14] reported a significant increase of Co and Cr in the blood at one year follow-up to metal-on-metal hip resurfacing, with a decreasing trend at four and six years after surgery. Within metal-on-metal hip resurfacing, higher levels of metal ions are observed in patients with smaller device diameter [1, 11, 12, 14, 22, 23]. On the other hand, Engh et al. [10] found no difference in ion levels between two metal-on-metal head sizes at two years of follow-up. Furthermore, several other factors lead to a variability in serum levels of metal ions, such as different implant size, incorrect adjustment of components (inclination, anteversion, arc of cover), differences in manufacture and metallurgy and metal corrosion.
De Haan et al. [1], Imanishi et al. [9] and Langton et al. [22, 23] found significantly higher levels of metal ions in patients with steeply-inclined resurfacing components. In contrast to that, the level of activity had no influence on the metal ion concentrations. Langton et al. [23] also reported the influence of the position of the acetabular component on the concentrations of Co and Cr following hip resurfacing while Witzleb et al. [12] showed inverse results. Savarino et al. [7], Witzleb et al. [12] and Antoniou et al. [11] reported the significant increase of Co and Cr following metal-on-metal hip arthroplasty and according to our findings Mo was unaltered. An explanation for this finding is the low percentage of Mo used for the manufacture of the cobalt-chromium-molybdenum alloy.
Nevertheless, there are several reports concerning serum metal ion concentrations following metal-on-metal hip arthroplasty or resurfacing arthroplasty but there is little data published on systemic metal ion concentrations following TKA or other orthopaedic implants.
Recently, Garrett et al. [16] reported a study determining differences in metal ion release following Co–Cr and oxidised zirconium TKA. Despite the lack of Co and Cr used in prostheses in the oxidised zirconium control group, no statistically significant differences in serum Co and Cr ion levels were found between groups. On the basis of these results, the authors concluded that there is no significant increase of serum metal ion levels following TKA several years after implantation. Conversely, our study showed that there is an increase of metal ion levels in serum following rotating-hinge TKA. Furthermore, there are significant differences between megaprostheses and standard rotating-hinge devices regarding Co and Cr serum concentrations (Table 2). Zeh et al. [24] reported the release of metal ions after metal-on-metal total disc arthroplasty. After an average follow-up of 15 months, the mean serum concentration for Co and Cr were 4.8 μg/L (=0.48 μg/dl) and 1.93 μg/L (=0.193 μg/dl). In our study, mean levels of Co (0.52 μg/dl) and Cr (0.213 μg/dl) of all patients were similar compared with the results of Zeh et al. [24], although different implantation sides. An explanation for the elevated levels of Co and Cr might be corrosion of the implants (proportional to the surface area of the components) and abrasive wear of the soft tissues. Our data also show that the size, particularly of the surface, of the implant might also play an important role, because patients with megaprostheses showed higher concentrations of Co and Cr than patients with standard rotating-hinge devices. Nevertheless, we are unable to provide a safe range of Co and Cr serum concentrations because of the wide variation levels encountered. In addition, possible toxic and teratological risks of elevated metal ion concentrations are unknown, but it can be stated that there is a long-term ion release after rotating-hinge knee arthroplasty.
One possible limitation of our study is the absence of any preoperative Co and Cr plasma concentrations because the preoperative concentrations correlated positively with postoperative values. We observed a relatively small number of patients, and those patients were split into two subpopulations. Therefore, further patients need to be added to the series, along with preoperative Co and Cr values. Furthermore, the mean follow-up of clinical examination and blood levels was only 35 months. It should be noted that this study evaluated the increase of serum metal ion levels following rotating-hinge knee arthroplasty using two devices of different sizes but made of the same prosthetic alloy.
Conclusion
Determining serum concentrations of metal ions in patients following rotating-hinge knee arthroplasty showed increased increments for Co and Cr but not for Mo. Furthermore, patients treated with megaprostheses showed 25 times and nine times, respectively, higher Co and Cr levels compared with patients with standard rotating-hinge devices. The effects of systemic metal ion exposure in patients with implants made of common prosthetic alloy (Co, Cr, Mo) continue to be a matter of concern. Exposure should be carefully monitored to clarify the biological effects of ion dissemination and identify risks concerning long-term toxicity of metals. Therefore, long-term studies are required to determine adverse effects of Co, Cr and Mo following THR and TKA.
Acknowledgments
Conflict of interest The authors declare that they have no conflict of interest.
Footnotes
The study was approved by the Ethics Committee, and informed consent was obtained from all patients.
Contributor Information
Joerg Friesenbichler, Email: joerg.friesenbichler@medunigraz.at.
Werner Maurer-Ertl, Phone: +43-316-38581198, FAX: +43-316-38514806, Email: maeck2000@gmx.at.
Patrick Sadoghi, Email: patrick.sadoghi@klinikum-graz.at.
Thomas Lovse, Email: thomas.lovse@klinikum-graz.at.
Reinhard Windhager, Email: reinhard.windhager@meduniwien.ac.at.
Andreas Leithner, Email: andreas.leithner@medunigraz.at.
References
- 1.Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90(10):1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 2.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19(8 Suppl 3):78–83. doi: 10.1016/j.arth.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Case CP. Chromosomal changes after surgery for joint replacement. J Bone Joint Surg Br. 2001;83(8):1093–1095. doi: 10.1302/0301-620X.83B8.12755. [DOI] [PubMed] [Google Scholar]
- 4.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87(1):28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 5.Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study. J Bone Joint Surg Br. 2007;89(2):169–173. doi: 10.1302/0301-620X.89B2.18519. [DOI] [PubMed] [Google Scholar]
- 6.Back DL, Young DA, Shimmin AJ. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin Orthop Relat Res. 2005;438:177–181. doi: 10.1097/01.blo.0000166901.84323.5d. [DOI] [PubMed] [Google Scholar]
- 7.Savarino L, Granchi D, Ciapetti G, Cenni E, Nardi Pantoli A, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in patients with metal-on-metal hip bearings in total joint replacement: a comparison with metal-on-polyethylene bearings. JBiomed Mat Res. 2002;63(5):467–474. doi: 10.1002/jbm.10299. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs JJ, Skipor AK, Patterson LM, Hallab NJ, Paprosky WG, Black J, Galante JO. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80(10):1447–1458. doi: 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Imanishi T, Hasegawa M, Sudo A. Serum metal ion levels after second-generation metal-on-metal total hip arthroplasty. Arch Orthop Trauma Surg. 2010;130(12):1447–1450. doi: 10.1007/s00402-010-1056-9. [DOI] [PubMed] [Google Scholar]
- 10.Engh CA, Jr, MacDonald SJ, Sritulanondha S, Thompson A, Naudie D, Engh CA. 2008 John Charnley award: metal ion levels after metal-on-metal total hip arthroplasty: a randomized trial. Clin Orthop Relat Res. 2009;467(1):101–111. doi: 10.1007/s11999-008-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoniou J, Zukor DJ, Mwale F, Minarik W, Petit A, Huk OL. Metal ion levels in the blood of patients after hip resurfacing: a comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J Bone Joint Surg Am. 2008;90(Suppl 3):142–148. doi: 10.2106/JBJS.H.00442. [DOI] [PubMed] [Google Scholar]
- 12.Witzleb WC, Ziegler J, Krummenauer F, Neumeister V, Guenther KP. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta Orthop. 2006;77(5):697–705. doi: 10.1080/17453670610012863. [DOI] [PubMed] [Google Scholar]
- 13.Savarino L, Granchi D, Ciapetti G, Cenni E, Greco M, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in stable hip arthroplasties using metal-on-metal articulating surfaces: a comparison between short- and medium-term results. J Biomed Mater Res A. 2003;66(3):450–456. doi: 10.1002/jbm.a.10595. [DOI] [PubMed] [Google Scholar]
- 14.Daniel J, Ziaee H, Pradhan C, McMinn DJ. Six-year results of a prospective study of metal ion levels in young patients with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2009;91(2):176–179. doi: 10.1302/0301-620X.91B2.21654. [DOI] [PubMed] [Google Scholar]
- 15.Sauve P, Mountney J, Khan T, Beer J, Higgins B, Grover M. Metal ion levels after metal-on-metal Ring total hip replacement: a 30-year follow-up study. J Bone Joint Surg Br. 2007;89(5):586–590. doi: 10.1302/0301-620X.89B5.18457. [DOI] [PubMed] [Google Scholar]
- 16.Garrett S, Jacobs N, Yates P, Smith A, Wood D. Differences in metal ion release following cobalt-chromium and oxidized zirconium total knee arthroplasty. Acta Orthop Bel. 2010;76(4):513–520. [PubMed] [Google Scholar]
- 17.Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, Solomon L. Widespread dissemination of metal debris from implants. J Bone Joint Surg Br. 1994;76(5):701–712. [PubMed] [Google Scholar]
- 18.Hart AJ, Hester T, Sinclair K, Powell JJ, Goodship AE, Pele L, Fersht NL, Skinner J. The association between metal ions from hip resurfacing and reduced T-cell counts. J Bone Joint Surg Br. 2006;88(4):449–454. doi: 10.1302/0301-620X.88B4.17216. [DOI] [PubMed] [Google Scholar]
- 19.Allen MJ, Myer BJ, Millett PJ, Rushton N. The effects of particulate cobalt, chromium and cobalt-chromium alloy on human osteoblast-like cells in vitro. J Bone Joint Surg Br. 1997;79(3):475–482. doi: 10.1302/0301-620X.79B3.7415. [DOI] [PubMed] [Google Scholar]
- 20.Dunstan E, Sanghrajka AP, Tilley S, Unwin P, Blunn G, Cannon SR, Briggs TW. Metal ion levels after metal-on-metal proximal femoral replacements: a 30-year follow-up. J Bone Joint Surg Br. 2005;87(5):628–631. doi: 10.1302/0301-620X.87B5.15384. [DOI] [PubMed] [Google Scholar]
- 21.Grubl A, Marker M, Brodner W, Giurea A, Heinze G, Meisinger V, Zehetgruber H, Kotz R. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25(7):841–848. doi: 10.1002/jor.20381. [DOI] [PubMed] [Google Scholar]
- 22.Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90(9):1143–1151. doi: 10.1302/0301-620X.90B9.20785. [DOI] [PubMed] [Google Scholar]
- 23.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, Nargol AV. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91(10):1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 24.Zeh A, Becker C, Planert M, Lattke P, Wohlrab D. Time-dependent release of cobalt and chromium ions into the serum following implantation of the metal-on-metal Maverick type artificial lumbar disc (Medtronic Sofamor Danek) Arch Orthop Trauma Surg. 2009;129(6):741–746. doi: 10.1007/s00402-008-0677-8. [DOI] [PubMed] [Google Scholar]