Abstract
Purpose
Wear is a major contributor to osteolysis and aseptic loosening of total hip replacements (THR). Both alumina (Al2O3) and cobalt-chrome (CoCr) femoral heads are commonly used. We investigated wear comparing alumina heads to cobalt-chrome heads against conventional cemented polyethylene (PE) cups for up to ten years.
Methods
Linear wear was measured with radiostereometry (RSA). Our material was derived from two prospective randomised trials that investigated fixation of femoral stems, not wear, and was evaluated retrospectively (Level III).
Results
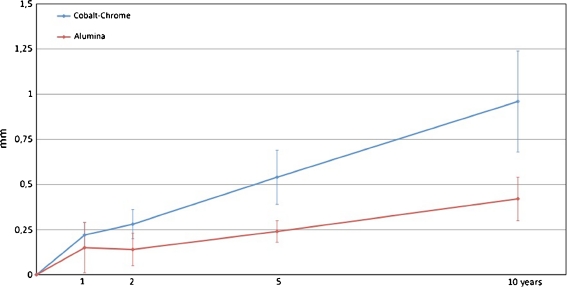
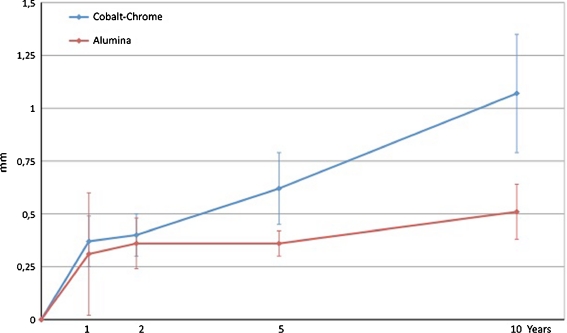
The mean (95% CI) proximal head penetration was 0.96 mm (0.68–1.23) in the cobalt-chrome group and 0.42 mm (0.30–0.53) in the alumina group at ten years (P = 0.001). The mean (95% CI) 3D penetration was 1.07 mm (0.79–1.35) and 0.53 mm (0.38–0.63), respectively, at ten years (P = 0.001).
Conclusion
Alumina heads performed better than cobalt-chrome heads in this study after ten-year follow-up.
Introduction
Reduction of wear is of great importance in hip replacement surgery since wear particles induce osteolysis [1, 2]. This problem has been addressed in several ways. Smaller heads reduce the area of articulation and thus wear [3], but are more vulnerable to dislocation [4]. Different metal alloys are considered to be the standard material in femoral heads in hip replacement surgery and other materials should be compared to these in clinical trials. They are safe, durable and well proven against PE [5].
Alternative bearings to metal on PE, such as different types of ceramics on PE, ceramics on ceramics, and metal on metal, have other known complications including chipping and breakage of ceramics [6, 7], squeaking [8], and release of metal ions [9]. Several types of bearings such as metal on metal and ceramic on metal have been marketed without sufficient documentation in clinical trials. This may have led to an increased revision burden and inferior outcomes for individual patients [10].
New components and concepts should be introduced stepwise to avoid clinical failures [11]. Routine use of different femoral heads requires good documentation. The material should be equally safe to use and exhibit superior wear performance compared to metal heads.
Ceramic heads have been gaining popularity and are widely used [12]. However, the main concern with ceramic heads has historically been breakage. Reports of incidences of breakage vary from 0.004% to 13.4% [13–15]. Breakage of a ceramic head will inevitably lead to revision surgery. These revisions are challenging surgically and limit the choice of revision implants [16]. A well functioning ceramic head is believed to produce less wear than metal heads on PE because it has a smoother surface and better wettability [6].
In contrast to simulator studies [17], clinical studies have been less consistent as both superior and inferior wear properties for ceramic heads compared to metal heads on PE have been reported [18, 19]. These results are also difficult to interpret as different measurement methods have been used, such as Livermore, Polyware, and Dorr and Wan. Another issue is that both linear wear (mm/year) and volumetric wear (mm3/year) are reported in the literature and thus results are difficult to compare [20]. Register studies have shown better prosthesis survival with ceramic compared to metal heads [21]. Large cohorts with long-term clinical follow-up, or smaller studies with high precision measurements are necessary to evaluate the wear performance of different implants. Some authors argue that 0.1 mm/year is the threshold of tolerable linear PE wear. Osteolysis is rarely seen in patients with wear below this level [2]. Other authors stress the fact that the nature of periprosthetic osteolysis is multifactorial [22]. The aim of this study was to investigate whether alumina heads reduce wear and osteolysis compared to cobalt-chrome heads against conventional PE in a long-term follow-up with RSA.
Patients and methods
Eighty-seven hips (84 patients) were operated up on in two randomised trials (RCTs) conducted at the same centre, in the same time-period (1992–94) and by the same surgeon (BN) (Table 1). The first RCT [23] included 40 hips (40 patients) comparing the Scientific hip prosthesis (SHP) (Biomet, Bridgend, UK) with the Lubinus SP2 prosthesis (Waldemar Link, Hamburg, Germany). Thirty-eight of these hips had cobalt-chrome heads and two had alumina heads (both with SP2 stems). Both SHP and Lubinus cups were cemented all-polyethylene made of conventional PE (UHMWPE) (gamma sterilised in inert atmosphere). The second RCT [24] included 47 hips (44 patients) and compared bone cement with reduced proportion of monomer (Cemex RX, Tecres, Verona, Italy) with standard bone cement (Palacos, Schering Plough, Labo N.V., Belgium). All hips received a cemented Lubinus SP2 stem and a Lubinus cup made of conventional PE gamma sterilised (3 Mrad) in inert atmosphere. All cases received a 28-mm head (Table 1).
Table 1.
Description of patients, groups and follow-up
| Characteristic | Head material: cobalt-chrome | Head material: alumina | |||
|---|---|---|---|---|---|
| Prosthesis | SHP | SP2 | SP2 | ||
| Cement | Palacos | Palacos | Cemex | ||
| Included (hips) | 40 | 44 (47) | |||
| Age at operation (range) | 67 (55–78) | 67 (52–78) | 65 (51–76) | 70 (51–81) | |
| Gender (male / female) | 8/12 | 8/12 | 10/14 | 9/14 | |
| Weight at operation (range) | 70 (48–100) | 71 (53–92) | 68 (53–96) | 70 (53–95) | |
| Preoperative HHS (range) | 39 (15–57) | 42 (23–61) | 47 (29–71) | 48 (17–64) | |
| Randomized | 20 | 20 | 24 | 23 | |
| Excluded | 1 | 3 | 1 | 0 | |
| Index RSA | 19 | 17 | 23 | 23 | |
| Drop-outs at ten years | Dead | 7 | 5 | 6 | 4 |
| Revised | 1 | 0 | 1 | 1 | |
| Did not meet | 0 | 3 | 1 | 3 | |
| Ten-year RSA | 11 | 9 | 15 | 16 | |
RSA radiostereometry
RSA
In both groups tantalum beads were introduced as markers in the periacetabular bone, cup and stem. Three to nine 0.8 mm markers were used in each segment. Radiostereometry (RSA Biomedical, Umea, Sweden) was used to measure migration and wear [25]. Stereo-radiographs were taken postoperatively, after three and six months, and at one, two, five and ten years. Proximal wear was measured as translation of the femoral head into the cup along the y-axis. 3D wear was expressed as a resultant of all translations along x-y-z axes of the head into the cup [25]. When we calculated the change in annual wear all patients were included, not only those who were followed up for ten years. Cup stability was measured as translations and rotation of the cup in relation to the peri-acetabular markers. In the cobalt-chrome group 20 hips were accessible for ten-year wear measurements. In the Cemex RX vs. Palacos study, wear could be measured in 31 hips at ten years. Precision was defined as the closeness of agreement between repeat measurements. The precision (SD) for proximal wear was determined from 139 repeat measurements and was 0.08 (0.1) mm. Precision for cup stability was calculated by the same method (Table 2).
Table 2.
RSA results: head penetration (linear wear) at ten years in mm (95% CI), cup movements as translations in mm (95% CI) and rotations in degrees (95% CI). Precisions as mean difference between double examinations and level of significance
| Wear results | Cobalt-chrome | Alumina | Precision (SD) | Significance | ||
|---|---|---|---|---|---|---|
| Proximal (y-axis) | 0.96 (0.68–1.23) | 0.42 (0.30–0.53) | 0.08 (0.1) | P < 0.001 | ||
| 3D (x + y + z-axis) | 1.07 (0.79–1.35) | 0.53 (0.38–0.63) | P < 0.001 | |||
| Subgroup | SHP | SP2 | Cemex | Palacos | ||
| Proximal (y-axis) | 1.10 (0.74–1.47) | 0.78 (0.31–1.25) | 0.40 (0.23–0.57) | 0.43 (0.25–0.62) | ||
| 3D (x + y + z-axis) | 1.17 (0.80–1.53) | 0.96 (0.44–1.47) | 0.49 (0.32–0.65) | 0.52 (0.33–0.72) | ||
| Cup movements | ||||||
| Translation x-axis | 0.43 (0.17–0.69) | 0.28 (0.17–0.40) | 0.04 (0.05) | P = 0.19 | ||
| Translation y-axis | 0.71 (0.27–1.14) | 0.33 (0.22–0.44) | 0.03 (0.06) | P = 0.63 | ||
| Translation z-axis | 0.35 (0.20–0.51) | 0.27 (0.16–0.39) | 0.08 (0.13) | P = 0.32 | ||
| Rotation x-axis | 0.82 (0.36–1.29) | 0.46 (0.27–0.64) | 0.28 (0.74) | P = 0.05 | ||
| Rotation y-axis | 0.83 (0.34–1.32) | 0.47 (0.24–0.70) | 0.28 (0.46) | P = 0.06 | ||
| Rotation z-axis | 1.43 (0.18–2.68) | 0.66 (0.41–0.92) | 0.15 (0.40) | P = 0.36 | ||
RSA radiostereometry, CI confidence interval, SD standard deviation
Radiology
Conventional radiographs were obtained ten years postoperatively. Cup position, radiolucent lines, and osteolysis were evaluated with Mdesk® (Mdesk, RSA Biomedical, Umea, Sweden), which is a software for measurements of implant positioning and the size of osteolytic lesions. We used a modification of the DeLee and Charnley zones [26], dividing the hemisphere into three equal zones.
Osteolysis was defined as a lytic lesion of more than 1 cm2 in the AP view. A radiolucent line (RLL) in the cement-bone interface was defined as a line wider than one millimetre in the AP view. RLL was evaluated in each Charnley/DeLee zone. We used the same grading as DeLee and Charnley: no RLL = 1, <50% = 2, 50–99% = 3 and 100% = 4. All radiographs were evaluated by two of the authors (JD, SMR). The mean of both measurements was used as the final grading.
Clinical outcome
All patients were scored using Harris hip score (HHS) preoperatively. A telephone interview using the HHS questionnaire was conducted for all patients after ten years. We excluded the tests for range of motion (ROM) in both groups because it was measured in only one group at ten years [27].
Statistics
The difference in wear was compared using a two-sided independent samples t-test with significance level of p = 0.05 since the values were normally distributed. Simple linear regression models were used both to evaluate whether differences between the subgroups could affect our results and to compute the slopes for annual wear. For comparisons of cup movements a nonparametric test (Mann-Whitney U) was performed. For differences in radiolucency we used the Pearson’s chi-square test. Statistical analyses were conducted using PASW statistics version 18 (SPSS Inc., Chicago, IL, USA). Inter-observer reliability was expressed by Kappa value [28].
Results
RSA
At ten years the mean (95% CI) proximal wear was 0.96 mm (0.68–1.23) for the cobalt-chrome heads and 0.42 (0.30–0.53) mm for the alumina heads. The mean difference in proximal wear was 0.54 (0.28–0.79) mm (p = 0.001). Mean 3D penetration was 1.07 mm (0.79–1.35) and 0.53 mm (0.38–0.63), respectively. The mean difference in 3D penetration was 0.54 (0.30–0.82) mm (p = 0.001) (Figs. 1 and 2). The difference in annual wear was calculated from one to ten years and showed a 0.048-mm lower wear per year in the alumina group for proximal wear (95% CI, 0.018–0.078; p = 0.003). For 3D wear the reduction was 0.047 mm (95% CI, −0 77 to 0.016; p = 0.002). Linear and 3D wear for each cohort as well as each subgroup at ten years was measured (Table 2). A tendency towards increased anterior tilt (forward rotation around the x-axis) (p = 0.053), and retroversion (positive rotation around the y-axis) (p = 0.059) was found in the cobalt-chrome group. Cup movement was measured for rotations and translations in both groups (Table 2).
Fig. 1.
Proximal wear (95% CI) up to ten years for articulations with alumina and cobalt-chrome heads
Fig. 2.
3D wear (95%CI) up to ten years for articulations with alumina and cobalt-chrome heads
Conventional radiography
We found nine subjects with RLL in Delee/Charnley zone 3 in the cobalt-chrome group and four in the alumina group (Table 3) (p < 0.05). In the other zones there was a tendency towards the same, but the difference was not statistically significant. There were no differences in osteolytic lesions (Table 3). Kappa for inter-observer reliability was 0.6 in zone 1, 0.7 in zone 2 and 0.4 in zone 3.
Table 3.
Radiological results: cup inclination, radiolucencies (RLL) and osteolysis between the groups. RLL’s are classified 1–4 (1 = 0% / 2 = 1–49% / 3 = 50–99% / 4 = 100%) within each modified Charnley and Delee zone in AP radiograph. Osteolysis >1 cm2
| Measure | Cobalt-chrome, n = 19a | Alumina, n = 27b | Significance |
|---|---|---|---|
| Cup inclination (range) | 50 (29–60) | 47 (27–64) | P = 0.81 |
| Charnley/Delee 1 | 11/8/1/0 | 18/4/5/0 | P = 0.09 |
| Charnley/Delee 2 | 15/5/0/0 | 24/3/0/0 | P = 0.87 |
| Charnley/Delee 3 | 11/7/2/0 | 23/1/2/1 | P = 0.07 |
| Osteolysis | 1 | 0 | Not significant |
aOne patient did not have conventional radiographs at ten years
bFour patients did not have conventional radiographs at ten years
Clinical outcome
After ten years, ROM tests were excluded from the HHS questionnaire. This gave us a maximum score of 95 points. The median (range) HHS was 84.5 (65–91) points in the alumina group and 83.5 (11–91) points in the cobalt-chrome group (p = 0.61).
Discussion
The results of our study show a reduction in wear of about 50% when using aluminium-oxide heads versus cobalt-chrome heads with conventional PE cups after ten years. This is important as a reduction in wear can reduce osteolysis and hence aseptic loosening [29]. Some studies have found favourable wear rates for alumina on PE [19, 30] compared to metal on PE, but some report the opposite [18]. Different trials have used various head sizes, different modes of implant fixation, different polyethylene materials, and a number of methods of measurement, all of which are factors making comparisons of different trials difficult or impossible. There are also indications that modern ceramics produce less wear than older ceramics [31].
Wear-reduction did not influence clinical outcome (HHS) and revision rate in this trial. However, we found a trend towards increased radiolucency around the cups in the cobalt-chrome group in zones 1 and 3 (p = 0.09 and p = 0.07). We also found a trend towards increased movement of cups in the cobalt-chrome group in all directions and rotations. Ten years may be a too short follow-up to show differences in osteolysis and cup loosening [32, 33].
There are several limitations to this study. First, in the cobalt-chrome group, both SHP and Lubinus SP2 cups were used. Both acetabular components were made of partly cross-linked polyethylene (gamma irradiated with 3 MRad in inert atmosphere), but were made of different resins (GUR 1020 and GUR 1050). These polyethylenes have shown similar wear patterns and clinical performance in other studies. We found indications for higher wear rate in the group with SHP cups than SP2 cups (Table 2). This difference was not significant in our material; p = 0.23 for proximal wear and p = 0.45 for 3D wear. We also conducted a linear regression analysis that showed that cup-type did not explain the difference between the groups (R square 0.029; 95% CI, −0.26 to 1.01). Second, femoral heads from different manufacturers were used. Both Link and Biomet produced their cobalt-chrome heads to the ISO 7206-2 standard. The surface roughness of the heads was below 0.05 mm in both groups, so this should not be a significant confounder. Third, subjects in the alumina group were randomised to different bone-cements (Palacos R and Cemex Rx). We found no difference between these groups for proximal wear (p = 0.78) or 3D wear (p = 0.74). Fourth, our study is an observational evaluation of two RCTs from the same centre and the same period (Umea, Sweden 1992–94), and thus not a true randomised trial. The inclusion criteria were the same and the subjects were recruited from the same population, statistically there were no differences in patient demographics and therefore we consider the groups to be comparable. Last, this study was performed using conventional polyethylene. Most orthopaedic surgeons have acknowledged that modern cross-linked polyethylene has superior wear properties to conventional polyethylene and have changed their practice accordingly. It is not certain that our findings are transferable to cross-linked polyethylene, as results from recent studies vary [34, 35]. One can speculate that the differences in wear would be lower with more wear-resistant polyethylene.
We report a long-term follow-up using a high precision measuring method (RSA) [25]. Differences in wear between the groups are highly significant. The patient groups are recruited from the same population and operated by the same surgeon (BN). Differences in wear between the groups are correlated with indications of accelerated osteolysis and increased cup movement that may be an early sign of cup loosening. The subjects in our study were on average 67 years old and thus not a population that offers the most kinematic challenge to the articulation. Younger and more active patients will wear their prosthesis more. It is therefore likely that the difference we found would be even larger in younger and more demanding patients as wear is a function of activity, not time [36]. Our findings support the use of alumina heads to reduce PE wear in THR.
References
- 1.Wilkinson JM, Hamer AJ, Stockley I, Eastell R. Polyethylene wear rate and osteolysis: critical threshold versus continuous dose-response relationship. J Orthop Res. 2005;23:520–525. doi: 10.1016/j.orthres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 3.Kesteris U, Ilchmann T, Wingstrand H, Onnerfalt R. Polyethylene wear in Scanhip arthroplasty with a 22 or 32 mm head: 62 matched patients followed for 7–9 years. Acta Orthop Scand. 1996;67:125–127. doi: 10.3109/17453679608994655. [DOI] [PubMed] [Google Scholar]
- 4.Bystrom S, Espehaug B, Furnes O, Havelin LI. Femoral head size is a risk factor for total hip luxation: a study of 42,987 primary hip arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74:514–524. doi: 10.1080/00016470310017893. [DOI] [PubMed] [Google Scholar]
- 5.Furnes OHL, Espehaug B, Steindal K, Sørås TE (2007) The Norwegian Arthropasty Register. Report 2007. University of Bergen ISBN 978-82-91847-12-2, Bergen, Norway
- 6.Lang JE, Whiddon DR, Smith EL, Salyapongse AK. Use of ceramics in total hip replacement. J Surg Orthop Adv. 2008;17:51–57. [PubMed] [Google Scholar]
- 7.Hannouche D, Hamadouche M, Nizard R, Bizot P, Meunier A, Sedel L. Ceramics in total hip replacement. Clin Orthop Relat Res. 2005;430:62–71. doi: 10.1097/01.blo.0000149996.91974.83. [DOI] [PubMed] [Google Scholar]
- 8.Keurentjes JC, Kuipers RM, Wever DJ, Schreurs BW. High incidence of squeaking in THAs with alumina ceramic-on-ceramic bearings. Clin Orthop Relat Res. 2008;466:1438–1443. doi: 10.1007/s11999-008-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauve P, Mountney J, Khan T, Beer J, Higgins B, Grover M. Metal ion levels after metal-on-metal ring total hip replacement: a 30-year follow-up study. J Bone Joint Surg Br. 2007;89:586–590. doi: 10.1302/0301-620X.89B5.18457. [DOI] [PubMed] [Google Scholar]
- 10.Johanson PE, Fenstad AM, Furnes O, Garellick G, Havelin LI, Overgaard S, Pedersen AB, Karrholm J. Inferior outcome after hip resurfacing arthroplasty than after conventional arthroplasty. Evidence from the Nordic Arthroplasty Register Association (NARA) database, 1995 to 2007. Acta Orthop. 1995;81:535–541. doi: 10.3109/17453674.2010.525193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malchau H (1995) On the importance of stepwise introduction of new hip implant technology. PhD thesis, Gotenburg University, Sweden
- 12.Hannouche D, Zaoui A, Zadegan F, Sedel L, Nizard R. Thirty years of experience with alumina-on-alumina bearings in total hip arthroplasty. Int Orthop. 2011;35:207–213. doi: 10.1007/s00264-010-1187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritsch E. Ceramic head fractures in THR. Clin Orthop Relat Res. 1996;328:129–136. doi: 10.1097/00003086-199607000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Willmann G. Ceramic femoral head retrieval data. Clin Orthop Relat Res. 2000;379:22–28. doi: 10.1097/00003086-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Fritsch EW, Gleitz M. Ceramic femoral head fractures in total hip arthroplasty. Clin Orthop Relat Res. 1996;328:129–136. doi: 10.1097/00003086-199607000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Allain J, Roudot-Thoraval F, Delecrin J, Anract P, Migaud H, Goutallier D. Revision total hip arthroplasty performed after fracture of a ceramic femoral head. A multicenter survivorship study. J Bone Joint Surg Am. 2003;85-A:825–830. doi: 10.2106/00004623-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Clarke IC, Gustafson A. Clinical and hip simulator comparisons of ceramic-on-polyethylene and metal-on-polyethylene wear. Clin Orthop Relat Res. 2000;379:34–40. doi: 10.1097/00003086-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Sychterz CJ, Engh CA, Jr, Young AM, Hopper RH, Jr, Engh CA. Comparison of in vivo wear between polyethylene liners articulating with ceramic and cobalt-chrome femoral heads. J Bone Joint Surg Br. 2000;82:948–951. doi: 10.1302/0301-620X.82B7.9885. [DOI] [PubMed] [Google Scholar]
- 19.Schuller HM, Marti RK. Ten-year socket wear in 66 hip arthroplasties. Ceramic versus metal heads. Acta Orthop Scand. 1990;61:240–243. doi: 10.3109/17453679008993508. [DOI] [PubMed] [Google Scholar]
- 20.Ebramzadeh E, Sangiorgio SN, Lattuada F, Kang JS, Chiesa R, McKellop HA, Dorr LD. Accuracy of measurement of polyethylene wear with use of radiographs of total hip replacements. J Bone Joint Surg Am. 2003;85-A:2378–2384. doi: 10.2106/00004623-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Hallan G, Dybvik E, Furnes O, Havelin LI. Metal-backed acetabular components with conventional polyethylene: a review of 9113 primary components with a follow-up of 20 years. J Bone Joint Surg Br. 2010;92:196–201. doi: 10.1302/0301-620X.92B2.22179. [DOI] [PubMed] [Google Scholar]
- 22.Callaghan JJ, Cuckler JM, Huddleston JI, Galante JO. How have alternative bearings (such as metal-on-metal, highly cross-linked polyethylene, and ceramic-on-ceramic) affected the prevention and treatment of osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl 1):S33–S38. doi: 10.5435/00124635-200800001-00008. [DOI] [PubMed] [Google Scholar]
- 23.Nivbrant B, Karrholm J, Soderlund P. Increased migration of the SHP prosthesis: radiostereometric comparison with the Lubinus SP2 design in 40 cases. Acta Orthop Scand. 1999;70:569–577. doi: 10.3109/17453679908997844. [DOI] [PubMed] [Google Scholar]
- 24.Nivbrant B, Karrholm J, Rohrl S, Hassander H, Wesslen B. Bone cement with reduced proportion of monomer in total hip arthroplasty: preclinical evaluation and randomized study of 47 cases with 5 years’ follow-up. Acta Orthop Scand. 2001;72:572–584. doi: 10.1080/000164701317268987. [DOI] [PubMed] [Google Scholar]
- 25.Karrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J. Radiostereometry of hip prostheses. Review of methodology and clinical results. Clin Orthop Relat Res. 1997;344:94–110. [PubMed] [Google Scholar]
- 26.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 27.Sanjeev Sharma RS, Draviraj KP, Bhamra MS. Use of telephone interviews to follow up patients after total hip replacement. J Telemed Telecare. 2005;11(4):211–214. doi: 10.1258/1357633054068883. [DOI] [PubMed] [Google Scholar]
- 28.Altmann D (1999) Practical statistics for medical research. Chapman & Hall/CRC
- 29.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2:102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusaba A, Kuroki Y, Kondo S, Hirose I, Ito Y, Shirasaki Y, Tateishi T, Scholz J. Frictional torque and wear of retrieved hip prostheses: a comparison between alumina/PE and Co-Cr/PE prostheses. J Long Term Eff Med Implants. 2002;12:53–62. [PubMed] [Google Scholar]
- 31.Kawanabe K, Tanaka K, Tamura J, Shimizu M, Onishi E, Iida H, Nakamura T. Effect of alumina femoral head on clinical results in cemented total hip arthroplasty: old versus current alumina. J Orthop Sci. 2005;10:378–384. doi: 10.1007/s00776-005-0911-y. [DOI] [PubMed] [Google Scholar]
- 32.Tarasevicius S, Kesteris U, Robertsson O, Wingstrand H. Femoral head diameter affects the revision rate in total hip arthroplasty: an analysis of 1,720 hip replacements with 9–21 years of follow-up. Acta Orthop. 2006;77:706–709. doi: 10.1080/17453670610012872. [DOI] [PubMed] [Google Scholar]
- 33.Oparaugo PC, Clarke IC, Malchau H, Herberts P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature. Acta Orthop Scand. 2001;72:22–28. doi: 10.1080/000164701753606644. [DOI] [PubMed] [Google Scholar]
- 34.Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop Relat Res. 2009;467:3290–3296. doi: 10.1007/s11999-009-1038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22:125–129. doi: 10.1016/j.arth.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Schmalzried TP. Wear is a function of use, not time. Clin Orthop Relat Res. 2000;381:36–46. doi: 10.1097/00003086-200012000-00005. [DOI] [PubMed] [Google Scholar]