Abstract
Introduction
Cancer is a devastating disease; however, several therapeutic advances have recently been made, wherein EGFR and its family members have emerged as useful biomarkers and therapeutic targets. EGFR, a transmembrane glycoprotein is a member of the ERBB receptor tyrosine kinase superfamily. EGFR binds to its cognate ligand EGF, which further induces tyrosine phosphorylation and receptor dimerization with other family members leading to enhanced uncontrolled proliferation. Several anti-EGFR therapies such as monoclonal antibodies and tyrosine kinase inhibitors have been developed, which has enabled clinicians to identify and treat specific patient cohorts.
Areas covered
In this review, the basic mechanism of EGFR activation and the role of EGFR signaling in cancer progression, has been covered. Furthermore, current developments made towards targeting the EGFR signaling pathway for the treatment of epithelial cancers and a summary of the various anti-EGFR therapeutic agents that are currently in use, has also been made.
Expert opinion
EGFR signaling is a part of a complex network that has been the target of effective cancer therapies. However, further understanding of the system is required to develop an effective anticancer regiment. A combination therapy comprising of an anti-EGFR and a chemotherapeutic/chemopreventive agent will exhibit a multi-pronged approach that can be developed into a highly attractive and specific molecular oriented remedy.
1. Introduction
1.1 Epidermal Growth Factor Receptor (EGFR)
Cancer is a complex, multifactorial and devastating disease that has baffled researchers over the years. Recently, many investigators have demonstrated that over-expression of receptors and growth factors, oncogene activations and tumor suppressor gene inactivation are the root causes for the development of an aggressive and resistant cancer phenotype. Dysfunctions in intracellular signaling pathways have also been implicated in the development and progression of cancer. It thus becomes imperative to understand the functional roles of altered signaling pathways during neoplastic transformation as it may provide new clues towards identifying aberrant events that lead to this disease and enable us to develop strategies to prevent and treat cancer at an earlier stage.
Stanley Cohen, Nobel Prize Laureate in Physiology/Medicine, discovered epidermal growth factor (EGF) 25 years ago and elucidated its role in cell growth. This furthered our knowledge on signaling events in cancer biology and enabled us to face challenges posed by the abnormal cellular events resulting in cancer. Epidermal growth factor receptors (EGFRs) are a large family of receptor tyrosine kinases (TK) expressed in several types of cancer, including breast, lung, esophageal, and head and neck. EGFR and its family members are the major contributors of a complex signaling cascade that modulates growth, signaling, differentiation, adhesion, migration and survival of cancer cells. Due to their multi-dimensional role in the progression of cancer, EGFR and its family members have emerged as attractive candidates for anti-cancer therapy [1]. Specifically the aberrant activity of EGFR has shown to play a key role in the development and growth of tumor cells, where it is involved in numerous cellular responses including proliferation and apoptosis [2]. This review encompasses the complexity of this highly conserved EGFR signaling module and the central role it plays in a diverse array of biological processes.
1.2 Ligand binding and structural elucidation of EGFR
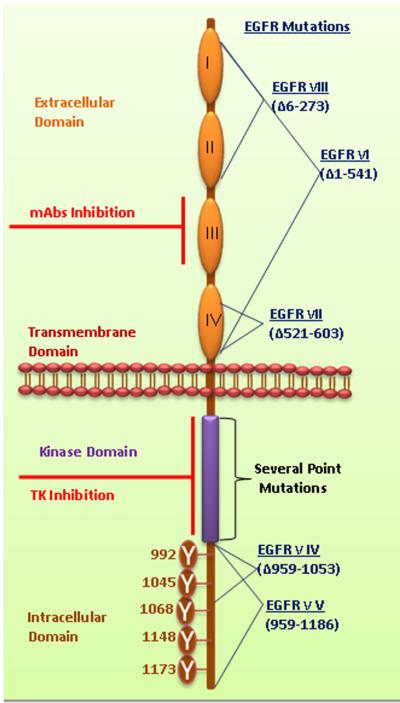
The ErbB family of receptors comprises of four known members namely ErbB1/EGFR/HER1 (in humans), ErbB2/HER2/Neu, ErbB3/HER3 and ErbB4/HER4. Throughout this review, the receptors of the EGFR family will be represented as EGFR, HER2, HER3 and HER4. They are transmembrane glycoproteins with molecular weights ranging from 170 to 185 KDa [3]. Structurally, the ErbB family members consist of (i) a cysteine-rich, extracellular N-terminal ligand binding domain and a dimerization arm, (ii) a hydrophobic transmembrane domain, and (iii) an intracellular, highly conserved, cytoplasmic C-terminal tyrosine kinase domain with several phosphorylation sites. Since the intracellular tyrosine kinase domain is highly conserved, the variable extracellular ligand binding domain enables binding to different ligands. Further, the extracellular region of EGFR is subdivided into four domains (I, II, III and IV) (Fig. 1a) [4, 5]. The crystal structure of the ectodomains of EGFR, HER3 and HER4 revealed two distinct conformations: (i) a closed, inactive conformation and (ii) an open, active conformation. In the closed conformation, domains II and IV interact with each other at the intermolecular level, thus preventing domains I and III from interacting with their cognate ligand [6, 7]. Both the open and closed conformations remain in equilibrium with each other [8, 9]. The open conformation is facilitated by the moving away of domains II and IV, thereby enabling domains I and III to expose their ligand-binding pocket and interact with their corresponding ligand. As a result, the dimerization arm in domain II then interacts with an identical dimerization arm of another receptor molecule to form a homodimer [4, 5]. The closed conformation is favored in the absence of a ligand. However, binding of a ligand shifts the equilibrium and stabilizes the open conformation, further enabling the accumulation of active homodimers and maintaining active receptor signaling [8, 9]. In addition EGFR promotes heterodimerization with other members of the HER family, including HER2, HER3 and HER4. Thus, EGFR may initiate cellular signaling cascades by itself through homodimerzation or through transactivation with other HER family members (heterodimerization). It has been previously shown that various ligands can induce specific heterodimerization, for example EGF can induce heterodimerization of EGFR with HER2, HER3 or HER4. Similarly NRG4 induces heterodimerization of HER4 with HER1, HER2 and HER3 [10]. Thus homo and heterodimerization of EGF receptors facilitates complex signaling cascades.
Figure 1a. Structural elucidation of EGFR.
EGFR family members share a common domain arrangement comprised of a cysteine-rich extracellular domain, a transmembrane domain and an intracellular tyrosine kinase domain with several phosphorylation sites. The extracellular domains are subdivided and numbered as I-IV. Numerous phosphorylation sites were mentioned in cytoplasmic domain of EGFR. Monoclonal antibodies target the extracellular domain III. The small molecule tyrosine kinase inhibitors bind with the ATP binding pockets in the tyrosine kinase domain. Several deletion mutations occur in the EGFR such as EGFRvI (AAΔ1–541), EGFRvIII (AAΔ6–273), EGFRvII (AAΔ521–603), EGFRvIV (AAΔ959–1053) and EGFRvV (AAΔ959–1186). Additionally, several point mutations occur in TK domain of EGFR. Furthermore, the details of EGFR mutations were discussed by Kuan et al.2001 [116]. (AAΔ amino acid deletion).
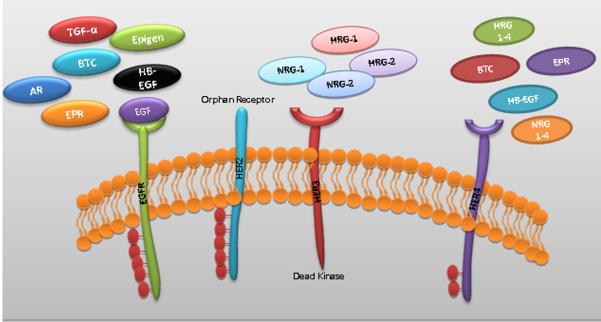
ErbB family members can be activated by 13 known ligands, which include EGF, transforming growth factor alpha (TGF-α), amphiregulin (AR), betacellulin (BTC), heparin-binding EGF-like growth factor (HB-EGF), epiregulin (EPR), epigen (EPG) and neuregulins 1–6 (NRG) [10, 11]. Among these ligands, EGF, TGF-α, AR, BTC and EPR bind specifically to HER1. A characteristic feature of HER2 is its inability to bind to any of the known ligands of the EGFR family. However, recent studies from our lab have shown that HER2 is regulated and stabilized by MUC4 mucin further potentiating HER2 and its downstream signaling in pancreatic and ovarian cancer [12, 13]. Absence of ligand binding property of HER2 mandates that its activation occurs only as a consequence of a heterodimer formation with EGFR, HER3 or HER4 to generate high-affinity complexes for different ligands. On the other hand, HER3 acts as a receptor for growth factors of the Herregulin-1(HRG-1) and HRG-2 groups. BTC, HB-EGF, EPR and proteins from the HRG groups also bind specifically to HER4. Since HER3 has no intrinsic kinase activity and signals only when dimerized with another ErbB receptor, blocking the TK activity of EGFR, HER2, and HER4 precludes HER3 signaling [14] (Fig1b).
Figure 1b. Structures of all four human EGF receptors' extracellular regions with their respective ligands.
EGFR can specifically bind with EGF, TGF-α, AR, BTC and EPR ligands. HER2 is an orphan receptor with no known ligand. HER3 lacks intrinsic tyrosine kinase domain and binds to Herregulin-1, 2 (HRG-1&2). HER4 binds to HRG1-4, NRG1-4, HB-EGF, BTC and EPR.
1.3 General mechanism of EGFR signaling activation and initiation of a diverse array of cellular pathways
Activation of EGFR signaling is triggered by ligand-induced receptor dimerization following which the tyrosine residues present in the intrinsic kinase domain of one receptor cross phosphorylates specific residues in the C-terminal tail of the partnering receptor, thus providing a scaffold for the recruitment of effector proteins [15, 16]. This occurs via the Src homology 2 (SH2) and phosphotyrosine binding (PTB) domains on the effector proteins and the phosphotyrosine motif present on the intracellular tyrosine kinase domain of the receptor. On subsequent dissociation, the activated adaptor and effector proteins will further stimulate their corresponding signaling cascades, which include the KRAS-BRAF-MEK-ERK pathway, phosphoinositide 3-kinase (PI3K), phospholipase C gamma protein pathway, the anti-apoptotic AKT kinase pathway and the STAT signaling pathway, which leads to cell proliferation, angiogenesis, migration, survival, and adhesion [10, 17]. These cellular processes are often deregulated in malignant cells due to the several mutations harbored in various genes involved in these pathways.
1.4 Internalization and endosomal sorting of EGFR
EGFR signaling is primarily diffused through the process of internalization, ubiquitination and degradation of the receptor–ligand complex, resulting in the transient down-regulation of EGFR [11]. Upon signal termination, the receptor-ligand complex (EGF-EGFR) is internalized through clathrin coated pits (CCP) from the plasma membrane. These CCPs pinch off from the membrane to give rise to endocytic vesicles that fuse with the early endosomes (EE), thereby releasing the receptor complex in the EEs. After its translocation in to the EEs, the receptors can undergo either one of the two distinct processes that will determine their fate: they can either be recycled back to the cell surface or be taken up into the intraluminal vesicles (ILVs), a pathway eventually leading to the lysosomal degradation of EGFR. In the EEs, EGFR interacts with the clathrin-binding protein AP-2 showing similarity with constitutively endocytosed receptors, such as transferrin and low-density lipoprotein receptor for sorting in the CCP [18, 19]. However, direct interaction of EGFR and AP-2 is not necessary for EGFR internalization [20]. Hence other interacting partners are required for EGFR in the CCPs to facilitate sorting into the plasma membrane microdomains. Additionally, EGFR kinase activity has been shown to be important for the CCP sorting mechanism [21]. Hence the various interacting partners should bind to the EGFR modifications present in its activated state (i.e. the residues are either phosphorylated or ubiquitinated) to enable sorting.
The docking sites created as a result of the cross phosphroylation between the two dimerizing receptors, allow the receptors to interact with adapter molecules such as Grb2 and Casitas B-lineage lymphoma proto-oncogene (Cbl), an ubiquitin ligase. Cbl is responsible for promoting EGFR ubiquitination, which adds mono or polyubiquitins to EGFR [22]. Cbl can bind directly to EGFR by binding to the single phosphorylated tyrosine residue on EGFR (Y1045) or bind indirectly to phosphorylated Y1068 and Y1086 of EGFR via its interacting partner Grb2 [23, 24]. The binding of Cbl with phosphorylated Y1045 is not necessary for EGFR endocytosis as it has been shown that the Y1045F mutant of EGFR cannot bind with Cbl directly but still efficiently internalized as the wild-type EGFR [22, 25]. On the other hand, the binding of Cbl to EGFR via Grb2 is important for receptor internalization [26]. It has long been assumed that the key signal for the EGFR translocation to CCPs is the Cbl- mediated ubiquitination (multiple monoubiquitination) of EGFR. EEs containing internalized EGFR will mature in to late endosomes (LEs), and ubiquitinate EGFR in ILVs, which will eventually be degraded by lysosomes [27].
1.5 Ligand receptor stabilization in the endosomes
The stability of the activated receptor-ligand complex in the EEs is highly regulated by the pH (mild acidic) of the environment. Activated homodimers are comparatively stable and remain harbored with Cbl, which ultimately leads to its endocytic sorting to lysosomes for receptor degradation. In contrast, heterodimers, such as EGFR-HER2 are less stable and undergo uncoupling in EE, causing Cbl to dissociate from the receptor-ligand complex and result in the receptor recycling back to the cell surface [28]. In addition, different EGFR ligands undergo different fates during endosomal trafficking. For example, the EGF-EGFR complex undergoes the degradation pathway, whereas the TGF-α-receptor complex follows the recycling pathway [29]. This difference in the stability of the ligand-receptor complex and the ligand dependent choice of degradative/ recycling pathway is largely dependent on the low pH in the endosomes [30].
1.6 Nuclear accumulation of ErbB family members and transactivation of signaling
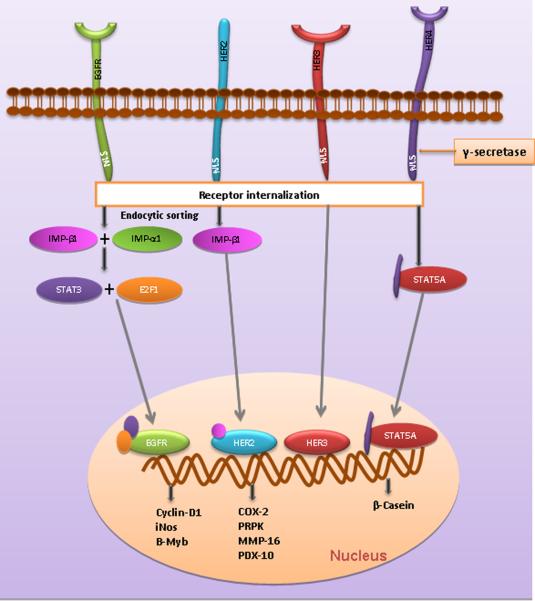
EGFR family members initiate signaling from the cell membrane and the signal is transmitted to the nucleus via cytoplasmic intermediates [31]. Several tyrosine kinase receptors like the VEGF receptor, FGF receptor and NGF receptor or their fragments have been known to enter the nucleus from the plasma membrane by various mechanisms and may act as a kinase or a transcription factor in the nucleus. EGFR and its family members such as HER2, rat p185neu, HER3, and truncated C-terminal HER4 have been consistently detected in the nucleus of tumor specimens of various organs such as breast, oral cavity, esophagus, ovary, cervix, skin and prostate with poor clinical prognosis [32–41]. Several lines of evidence suggest that receptor internalization is the initial step for nuclear translocation and involves interaction with nuclear import proteins such as importin beta1 for HER2 and importin beta1 and importin alpha1 for EGFR [42, 43]. EGFR and HER3 have been shown to translocate to the nucleus as intact full-length receptors. In contrast only the intracellular C-terminal fragment of HER4 translocates into the nucleus after undergoing gamma secretase mediated cleavage [44]. Though the full-length HER2 was found in the nucleus, it is unclear whether the intracellular domain (ICD) of HER2 can alone translocate into the nucleus like HER4. This nuclear accumulation of HER family members has been attributed to the existence of nuclear localization signals (NLSs) within the C-terminal region of HER3, HER4 and a putative juxtamembrane region of EGFR. Although the nuclear translocation of HER2 has been reported, the molecular mechanism underlying the nuclear targeting of HER2 remains unclear [37, 39, 45].
In addition, recent reports pointed out that EGFR receptors lack a putative DNA binding domain, so it is presumed that following nuclear entry these EGFR receptors will interact with DNA binding transcription factors such as signal transducer and activator of transcription 3 (STAT3), STAT5A, E2F1, DNA-dependent protein kinase (DNA-PK), and proliferating cell nuclear antigen (PCNA) [46, 47] which also accounts for the EGFR family members nuclear accumulation event. It is also proposed that the nuclear import of EGFR regulates gene expression by binding to an AT-rich sequence (ATRS) of the target gene's promoter [31, 39]. Recent reports also indicate the RNA helicase A (RHA) is a DNA-binding partner of EGFR and regulates the transcription of its target genes in the nucleus of cancer cells [48]. To this end, frequent overexpression of inducible nitric oxide synthase (iNOS), cyclin D1, and B-Myb are associated with the promoter binding ability of the nuclear accumulated EGFR with STAT3 and E2F1 cofactors [31, 46, 47] (Fig. 2).
Figure 2. The nuclear accumulation and receptor internalization of EGFR family members.
Upon signal activation, EGFR accumulates in the nucleus facilitated by binding with transcription factors E2F1 and STAT3 through importins, leading to up-regulation of B-Myb, iNOS and Cyclin D1. HER2 interacts with importin 2 and is internalized, leading to the expression of Cox-2, PRPK, MMP-16 and PDX-10. HER4 interacts with STAT5a and the C-terminal fragment alone accumulates in the nucleus after undergoing gama secreatase mediated cleavage.
2. EGFR targeted therapies
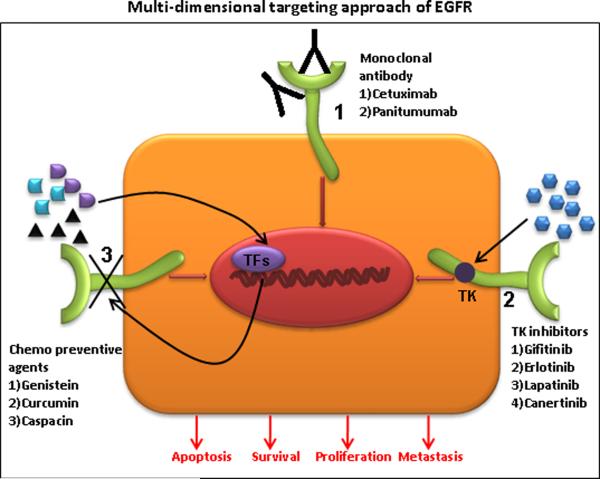
Given the functional involvement of EGFR in diverse cellular processes, several approaches have been developed that target and interfere with EGFR mediated effects. Two distinct therapeutic approaches currently employed for targeting EGFR in various human malignancies are the use of monoclonal antibodies and small molecule tyrosine kinase inhibitors (Table 1). Each of these approaches have distinct mechanism of action; while anti-EGFR antibodies bind to extracellular domains and TK inhibitor target the intra cellular TK domain. Recent studies have indicated the use of various chemopreventive agents in downregulating EGFR at gene level (Fig. 3). Furthermore, several studies have substantiated and conferred significant benefits of anti-EGFR agents in several types of solid tumors including colorectal, head and neck cancer, NSCLC and pancreatic cancer in terms of overall survival, progression free survival and overall response rate [49–53].
Table 1.
Comparison between EGFR specific monoclonal antibodies vs. Tyrosine Kinase Inhibitors
| Characteristic features | Anti-EGFR monoclonal antibodies | Anti EGFR TKI |
|---|---|---|
| Nature of the molecule and Size | Biological, recombinant immunoglobulin either of type IgG2 (Panitumumab) or IgG1 (Cetuximab) and large proteins approximately 150 kDa. | Synthetic chemicals, Small molecules and approximately 500 Da. |
| Half life | 3.1 to 7.8 days. | Less as compared to mAbs (Gefitinib 48 hrs and erlonitinib 36 hrs). |
| Specificity | Binds to ectodomain with high specificity. | Lesser as compared to mAbs, either selective to specific nucleotide binding site or dual or multi selective. |
| Mode of action | Therapy suited for extracellular targets, activates endocytosis and apoptosis. | Effective against both intracelluar and extracellular targets, inhibits phosphorylation and downstream proliferative signal and induces of apoptosis. |
| Inhibition | Achieved at lower concentrations. | Higher concentration is required and dependent on the cell type. |
| Toxicity, mode of administration and dosage | Less toxic, intravenous and weekly once or bi weekly. | Mild or highly toxic, oral and daily. |
| Success rate in clinical studies | Higher (18% chimeric and 24% humanized). | Lesser as compared to mAbs(5%). |
| Immune response to the therapy | Immune-antibody response which will render antibody therapy ineffective. | No such reaction. |
| Response to therapy | Less or ineffective against EGFRvIII. Fails to recognize the extracellular ligand binding domain. | EGFR independent constitutively activated K-RAS signaling will impair inhibitor response to the therapy. |
| Cost and advantage | Expensive and in therapeutics it is highly specific and selective. | Inexpensive and Specific to tyrosine kinase domain. |
| Adverse effect | Severe acne like rash, pruritis rash, fevers, chills, rigors, urticaria, dyspnea, wheezing, angioedema, dizziness, hypotension, anaphylaxsis reaction, bronchospasm, cardiac arrest, photosensitivity, hypomagnesemia, cardiac toxicity, nausea, weight loss and abdominal pain. | Acneiform skin rash, serum transaminase elevations, stomatitis,bone pain, dyspnea, alveolitis, pneumonitis and interstitial pneumonia. |
Figure 3. Schematic representation of EGFR inhibition in multidimensional approaches.
(1) Therapeutic invention site for EGFR specific monoclonal antibodies (Cetuximab and Panitumumab), (2) Potential target for small molecule tyrosine kinase inhibitors (Gifitinib, Erlotinib, Lapatinib and Canertinib), (3) Synergistic role of chemopreventive agents (Genistein, Curcumin and Caspacin).
2.1. Anti-EGFR monoclonal antibodies
The monoclonal antibodies against EGFR were specifically designed to be directed against the extracellular region of EGFR which creates a ligand competitive inhibition, thus preventing receptor dimerization, auto-phosphorylation and downstream signaling [54]. Apart from inhibiting EGFR signaling, these monoclonal antibodies will induce receptor internalization, ubiqutinization, degradation and prolonged downregulation [55]. Interestingly, there are two other proposed mechanisms of action, where the binding of the monoclonal antibody may lead to the induction of antibody dependent cell mediated cytotoxicity resulting in the induction of endocytosis and, to a lesser extent, complement mediated cytotoxicity [56].
2.1.1 Cetuximab
(IMC-C225 / Erbitux) is an FDA approved human–murine chimeric anti-EGFR monoclonal antibody. [57]. Cetuximab binds to the second (L2) domain of EGFR thereby blocking its downstream signaling by prompting receptor internalization and encumbering ligand-receptor interaction. Increasing evidence shows that 60% of the patients expressing a KRAS wild-type tumor respond effectively to cetuximab treatment. This supported the use of KRAS as a biomarker in predicting tumor response in patients with EGFR positive, KRAS wild-type advanced colorectal cancer [58]. Cetuximab is used as a monotherapy in patients who have failed with irinotecan and oxaliplatin based chemotherapy. In patients using cetuximab as monotherapy, the tumor regression rate was 10.8% and tumor growth was delayed by 1.5 months [59–61]. However a recent study that combined cetuximab with oxaliplatin and capecitabine did not show any benefit even in patients with wild-type KRAS tumors [62]. In 2008, the Committee for Medicinal Products for Human Use (CHMP) approved Cetuximab for patients with advanced colorectal cancer who had 75% EGFR positive expression and wild-type KRAS in their tissues and had failed oxaliplatin- or irinotecan-based chemotherapy. Cetuximab was approved by FDA in 2004 and by CHMP in 2008 in combination with platinum-based therapy for the treatment of patients with squamous cell carcinoma of the head and neck with metastatic disease and in combination with radiation therapy for locally advanced cancer. Raben et al investigated the antitumor activity of cetuximab on four non-small cell lung cancer (NSCLC) cell lines, with H157(high), A549, H226 (moderate) and H322 (low) EGFR expression, in monotherapy, and in combination therapy with ionizing radiation where cetuximab reduced the rate of cellular proliferation in vitro [63]. The available clinical data on cetuximab in advanced NSCLC are contradictory and cetuximab is currently undergoing phase III trials in advanced NSCLC. Apart from EGFR inhibition, cetuximab also down regulated VEGF, IL-2 and bFGF [64, 65].
2.1.2 Panitumumab
(formerly ABX-EGF) is the first FDA (2006) approved human monoclonal antibody used for the treatment of EGFR-expressing metastatic colorectal cancer. It is a human monoclonal antibody specific to human EGFR, developed by immunizing transgenic mice (XenoMouse) that are capable of producing light and heavy chains of human immunoglobulin [66]. Preclinical studies with ABX-EGF have shown that 50% inhibitory concentration (approximately 3 nmol/L) is required to eliminate large tumors of human origin in xenografts in nude mice and to prevent solid tumor formation [67]. Previous findings have shown that activating mutations in the KRAS gene resulted in EGFR-independent activation of MAPK signaling in colorectal cancer. Hence, colorectal cancer patients carrying the wild-type KRAS showed a promising response to panitumumab treatment [68]. Recently, in a meta-analysis study Petrelli et al demonstrated the effectiveness of two USFDA approved monoclonal antibodies (cetuximab and panitumab) used independently or in conjunction with chemotherapy in KRAS wild type patients with metastatic colorectal cancer. Addition of the targeted agent improved response rates (RR – 1.69; p = 0.003), progression free survival (HR – 0.65; p = 0.0006) and overall survival (HR – 0.84; p = 0.03)[51].
2.2. EGFR targeted tyrosine kinase inhibitors
Tyrosine kinase inhibitors (TKI) are small molecules that are either reversible or irreversible by nature. They exist as adenosine triphosphate (ATP) analogues and inhibit EGFR signaling by competing and binding with ATP binding pockets on the intracellular catalytic kinase domain of receptor tyrosine kinases (RTKs), thereby preventing autophosphorylation and activation of several downstream signaling pathways [69]. Type I and II reversible inhibitors compete with ATP molecules that recognize the kinase active conformation. Irreversible inhibitors bind to the kinase active site covalently by specifically reacting with a nucleophilic cysteine residue. In addition, irreversible inhibitors have the advantage of prolonged clinical effects and a decreased need for frequent dosing, although it may compromise specificity and tolerability [70]. However, a safety profile of these targeted strategies should also be considered as the use of EGFR inhibitors (as a single agent or in combination therapy) results in cutaneous skin toxicities (rashes) such as acneiform eruptions, hyperpigmentation, xerosis, trichomegaly, paronychia etc. The immediate side effect associated with HER1/EGFR inhibitor is mostly mild to moderate, but in some patients it can even lead to a reduction in the dosage level of that particular therapeutic agent or even cessation of the treatment but most of the side effects will be temporary reactions against the inhibitor treatments and will be resolved after a few weeks of discontinuing the therapy [71–73]. Further the side effects of the approved TKI's (Erlotinib and Gifitinib) and monoclonal antibodies (Cetuximab and Panitumumab) are discussed in table.1.
2.2.1 Gefitinib
(ZD1839/Iressa): Gefitinib is an anilinoquinazoline derived EGFR tryrosine kinase inhibitor and was first characterized in the year 1996 [74, 75]. It is an orally active low-molecular-weight EGFR inhibitor with selective tyrosine kinase activity but does not inhibit serine-threonine kinase activity. Gefitinib has a 200-fold greater affinity for EGFR relative to the other ErbB family members [76]. It demonstrates good non-competitive interference with EGFR-specific ligand signaling and prevents autophosphorylation of EGFR in various EGFR-positive tumor cell lines and xenografts [74]. Due to the overexpression of EGFR, gefitinib is approved for the treatment of patients with NSCLC after failure of both platinum-based or docetaxel chemotherapies. Clinical studies with gefitinib have revealed that dosages of 250 or 500 mg daily is the estimated measurement in the IDEAL (Iressa Dose Evaluation in Advanced Lung cancer) 1 trial. Also, similar results were obtained in the IDEAL-2 trial, where the inhibition of EGFR resulted in a 70% increase in patient responses. The maximal tolerated dosage, assessed in phase I trials, is 700 mg/day; however, the dosage of 250 mg per day was fixed for subsequent studies as the biological half-life of gefitinib is 28 hours and peak plasma concentration after it gets absorbed is 3–7 hours. The preliminary results of gefitinib in the treatment of lung cancer are encouraging as the results from two randomized phase II trials show that gefitinib is the first anti-EGFR agent that exhibited clinically proven anti-tumor effect in patients of NSCLC [77, 78].
Gefitinib showed significant anti-proliferative effects when orally administered in A549 NSCLC, MCF-7 breast carcinoma, HX62 ovarian, prostrate and colorectal carcinoma human xenograft models at a low nanomolar concentration [74]. However, it is estimated that higher concentrations may be required to block in vivo EGFR activity due to the high intracellular concentration of ATP [79]. It has also been observed that at concentrations more than 100-fold, gefitinib inhibits other tyrosine kinase receptors including HER2 [79, 80]. Though the specific mechanism of anti-proliferative activity of gefitinib is not clear, it is contemplated that it up-regulates cyclin-dependent kinase (CDK) inhibitor p27 and down-regulates transcription factor c-fos, resulting in the inhibition of CDK activity and G1 phase cell-cycle arrest [79]. Earlier studies have shown that gefitinib can also inhibit cell growth in HER2-overexpressing breast cancer cells [80, 81].
Combination therapy of gefitinib with standard cytotoxic drugs (topoisomerase I inhibitors, platinum and taxanes,) has produced a dose-dependent supra-additive increase in growth inhibition in vivo without an increase in severity of co-administrated cytotoxic drugs [69, 82]. Also, a synergistic anti-tumor and pro-apoptotic effect was obtained when cancer cells from the human colon, ovarian, lung, and breast were treated with ionizing radiation. A significant expression of several genes like STAT1, STAT3, STAT5A, STAT5B, and beta-catenin were correlated and considered as candidate markers for predicting a response to gefitinib in a larger patients cohort study [83]. However these strategies have not yet been proven to be efficacious in the clinical setting.
2.2.2 Erlotinib
(OSI-774; Tarceva): Erlotinib hydrochloride is another FDA-approved low molecular weight molecule similar to gefitinib, available in the form of an orally potent and selectively reversible inhibitor of EGFR tyrosine kinase. Like gefitinib, erlotinib functions as an ATP analogue by competing with ATP binding pockets within the RTKs. Also, it exerts anti-proliferative effects, cell-cycle arrest and apoptosis [84, 85]. Studies in human cancer cells found that it inhibits EGF-dependent cell proliferation at nanomolar concentrations and blocks cell-cycle progression in the G1 phase [84]. Erlotinib is currently approved in patients with relapsed NSCLC and for maintenance therapy in advanced NSCLC patients whose disease had not progressed after four cycles of platinum-based first-line chemotherapy. It is also approved for use in locally advanced, unresectable or metastatic pancreatic cancer patients in combination with a gemcitabine [49, 53].
A randomized study in patients having advanced NSCLC and who had failed standard cytotoxic chemotherapy, showed improved clinical symptoms and a better survival rate with erlotinib [84, 86, 87]. Similarly, in another clinical study with advanced stage or recurrent metastatic NSCLC patients who were positive for EGFR and exposed to platinum-based therapy, erlotinib showed improved clinical symptoms [88]. Erlotinib, when combined with carboplatin/gemcitabine or cisplatin/paclitaxel in two separate randomized phase III studies did not produce a survival advantage over chemotherapy alone [89–91]. Retrospective analyses did not exhibit any clinical significance between EGFR expression and EGFR TKI activity [92].
2.2.3 Lapatinib
(GW-572016): Lapatinib is an orally active, reversible and specific RTK inhibitor of both EGFR and HER2 as well it was also found to exhibit activity against an found to have activity against AKT overexpressing human tumor xenografts [93]. Due to its nonselective nature of EGFR inhibition, it accounts for a broader spectrum of anti-tumor activity with improved efficacy. Similar to that of gefitinib and erlotinib, this molecule also binds to the ATP binding pocket of both recombinant EGFR and HER2 protein kinase by 50% (IC50) at concentrations of 10.8 and 9.3 nmol/L, respectively [94], thus preventing auto phosphorylation and subsequent inhibition of downstream signaling. Preclinical studies showed that lapatinib inhibits the proliferative effect of HER2-overexpressing BT474 breast cancer at low concentrations (100nmol/L). On the other hand, lapatinib inhibits MCF-7 and T47D human breast cancer cells at 30- to 40-fold higher concentrations. A series of preclinical studies have shown significant inhibitory effects when lapatinib is used as a single agent or when combined with trastuzumab in HER2-positive breast cancer cells [94].
Clinical trials conducted for lapatinib in breast cancer patients revealed that it is effective in patients expressing HER2-positive breast cancer based on which the USFDA approved lapatinib in 2007 for the treatment of metastatic breast cancer [95]. But lapatinib was also found to additionally affect EGFR as well. A phase III study with advanced breast cancer has demonstrated that the combination of lapatinib and capecitabine was superior to capecitabine alone in HER2-positive breast cancer patients [96]. A double-blinded, multi-center study consisting of 1286 patients with advanced breast cancer, of which 219 had HER-2 positive tumors, revealed that the combination of lapatinib and letrozole had improved the progression-free survival when compared to monotherapy with letrozole alone [97–99]. Phase II studies with lapatinib in recurrent or metastatic EGFR or HER2 positive adenoid cystic carcinoma and nonadenoid cystic carcinoma of the salivary gland showed that lapatinib was a well-tolerated cytostatic drug with prolonged tumor stabilization [100].
2.2.4. Canertinib
(CI-1033): Canertinib is a 3-chloro 4-fluoro 4-anilinoquinazoline compound. It is an orally active low-molecular-weight irreversible pan-EGFR family TKI [101]. This is a new generation TKI, designed to alkylate a cysteine residue specific to ErbB family receptors, resulting in irreversible inhibition of these receptors and their downstream mitogenic signaling pathways. CI-1033 binds to the ATP binding pocket and the acrylamide side chain at carbon 6, and closely associates with cysteine residue 773 of EGFR and residues 784 and 778 of HER2 and HER4, respectively resulting in their permanent inactivation. On the other hand, it also effectively inhibits HER3-dependent signaling due to the unavailability of partner receptors for heterodimerization of EGFR family members [102, 103]. Due to this characteristic feature, CI-1033 exhibits greater efficacy and a broader spectrum of anti-proliferative activity. In a previous in vitro study in colorectal carcinoma and head-and-neck carcinoma that co-express activated forms of EGFR and HER2, canertinib was able to block cell growth and further down-regulate targeted specific genes which were postulated to be important for in vivo neoplastic cell transformation and also, a recent study evaluated that the critical role of ERK1/2 and its phospho form [104]. They showed that the in vitro and in vivo inhibitory effects of CI-1033 in combination with radiation therapy which inhibited proliferation and ERK1/2 phosphorylation [104]. A study by Ako et al 2007 has shown that canertinib inhibits cancer cell growth in a dose-dependent manner in esophageal cancer cell lines (TT, TE2, TE6 and TE10) which co-express EGFR and HER2 with simultaneous inhibition of MAPK and AKT [105]. It has been recently demonstrated that canertinib could potentially induce apoptosis in HL 60 and U 937 human leukemia cell lines in an EGFR independent manner, which further proves that this drug is a potential candidate for the treatment of patients with acute myeloid leukemia (AML) [106]. Additionally, CI-1033 holds promise as a novel anti-cancer agent as it can act synergistically with several other anti-cancer agents such as gemcitabine, resulting in the suppression of mitogen-activated protein kinase (MAPK) and AKT in HER2 overexpressing breast cancer cell lines MDA-MB-453 and BT474 [107]. In breast cancer cell lines overexpressing EGFR, CI-1033 synergistically inhibited growth with both ionizing radiation and cisplatin [108, 109]. CI-1033 can also inhibit the constitutively active mutant EGFRvIII.
Additionally, it not only inhibits tyrosine phosphorylation but it also induces ubiqutination and endocytosis of the receptors. Growth inhibition and apoptosis can be achieved in 1 micromolar or nano molar range and this selectivity explains the minimal toxicity observed in multi-dose animal studies [110]. Similar inhibitory effects were also reported against HER3, further leading to intracellular degradation of HER2. In EGFR and HER2 dependent preclinical models, canertinib produces rapid, irreversible inhibition of receptor phosphorylation, without blocking tyrosine kinase activity of other receptors such as platelet-derived growth factor receptor (PDGF), fibroblast growth factor receptor (FGFR) and insulin receptor [76]. Canertinib has been shown to have activity against a variety of human breast carcinomas in both in vitro and in vivo tumor xenograft models [111].
2.3. EGFR mutations and its therapeutic implications
EGFR mutations play a critical role in imparting oncogenic potential to the cells, which in turn, results in various malignancies. The most frequently activated mutations of EGFR were found to be point mutations and in-frame deletion mutations. The latter are termed as EGFR variants, named in the order of their discovery (EGFRvI to EGFRvV), and have been found to be associated with oncogenesis. The most common among them is the constitutively active, oncogenic mutant EGFR type III (EGFRvIII) resulting from deletions of exon 2–7. EGFRvIII has been shown to induce oncogenic transformation of mouse fibroblasts and is associated with poor prognosis in NSCLC, breast and glioblastoma patients [112, 113]. EGFR somatic mutations are inimitable and are a more common trait for a subset of NSCLC patients (Women, East Asian, non-smokers), although infrequent, it was also found in squamous cell carcinomas of the lung and other epithelial malignancies [114, 115]. Inframe deletion mutation (exon 19 deletion) of EGFR accounts for 45% of mutations and point mutation (L858R in exon 21) accounts for another 40% of mutations while the remaining 10–20% accounts for exon 18 and exon 20 deletion (Fig. 1a). Details of EGFR mutations were elaborately discussed in a review written by Kuan et al. 2001 [116]. All of these are activating mutations due to which downstream proliferative, prosurvival and antiapoptotic signals are turned on [115]. KRAS and EGFR mutations have been found to be overlapping in NSCLC patients. This could explain their primary resistance mechanism to erlotinib and gefitinib [117]. EGFR T790M recurrent mutation accounts for 50% EGFR mutation cases, which display a resistance mechanism to TKI's such as gefitinib and erlotinib [118, 119]. In addition the mechanisms of primary and secondary resistance were reviewed by Nguyen et al. and several others [120, 121]. Further it has been shown that by using EGFR specific irreversible inhibitors in vitro, these resistances can be overcome.
It has been reported that the presence of somatic mutations in the tyrosine kinase domain of the EGFR gene predicted response to gefitinib and were associated with an improved outcome [122]. It is now apparent that NSCLC with mutations in the EGFR are a distinct subgroup of NSCLC that is particularly responsive to EGFR tyrosine-kinase inhibitors (TKIs). Biomarker analyses in multiple studies have indicated that patients with EGFR mutations have a pronounced response to EGFR TKIs [123–125]. Recent studies evaluating EGFR TKIs in patients with these activating EGFR gene mutations demonstrated a consistent improvement in progression-free survival, but none of these trials have demonstrated an improvement in overall survival[126–128].
2.4 Other novel therapeutic approaches
Recently, cancer chemoprevention has gained considerable attention, due to its use of dietary bioactive compounds, either alone or in combination with other anti-cancer drugs, to prevent cancer progression. It has been reported that capsaicin inhibits invasion and migration of human HT 1080 fibrosarcoma cell lines in EGF-induced phosphorylation of FAK, PKC, Akt, Raf, ERK1/2,p38 and MAPK and down regulation of MMP-9, indicating capsaicin as a novel anti-cancer and anti-metastatic agent [129]. Similarly, another molecule that has gained a lot of attention in chemoprevention is curcumin. In vitro studies show that pancreatic cancer cells treated with curcumin enhances apoptosis by suppressing the activity of ERK, as well as by down regulating EGFR signaling in pancreatic cancer [130]. Apart from the above mentioned dietary bioactive compounds, a combination therapy of erlotinib and genistein (a naturally occurring isoflavone present in soybean) potentiates growth inhibition and enhanced apoptosis in pancreatic cancer and NSCLC by down regulating EGFR, survivin and Bcl-xL [131]. It has been hypothesized that genistein enhances the activity of EGFR-TKIs in NSCLCs via the inhibition of NF-κB. Additionally the anti-tumor activity of EGFR-TKIs is found to correlate with the down-regulated levels of Akt. It is well established that Akt activates NF-κB, which in turn transcribes genes that are important for cell survival, invasion and metastasis. Thus, it is apparent that the addition of genistein to EGFR-TKIs in the NSCLC cell lines elevates the anti-tumor effect through down-regulation of NF-κB [132].
3. Emerging role of EGFR signaling in the tumor microenvironment
In addition to neoplastic cells, the tumor microenvironment (TME) contains many cell types including endothelial cells and their precursors, inflammatory cells, pericytes, neutrophils, fibroblasts, T and B lymphocytes, natural killer cells (NK), antigen presenting cells (APC) and components of the extracellular matrix (ECM). There is growing consensus that the components of TME play an active role in tumor initiation and progression. Recent findings show that EGFR is expressed in almost all non-neoplastic cell types, except in the mature cells of the lymphohemopoietic system [14]. It is believed that active EGFR signaling in the non-neoplastic cells of the TME plays a supportive role in tumor cell proliferation, angiogenesis and metastasis [133]. EGFR signaling activation can stimulate the synthesis and secretion of a number of angiogenic regulating factors, such as vascular endothelial growth factor (VEGF), Interleukin-8 (IL-8) and basic fibroblast growth factor (bFGF) [134]. Tumor stimulated angiogenesis is regulated by potent pro-angiogenic factors such as EGF and TGF-α [135]. The main signaling pathway activated by EGFR is the Ras/PI3K pathway where VEGF mRNA expression was observed in glioblastoma cells [136]. Experimental stimulation of glioma cells by EGF resulted in the overexpression of VEGF, which was found to be associated with PTEN, a negative regulator of PI3K/AKT signaling [137, 138]. It has been shown that mutated PTEN synergizes with EGFR activation to elevate VEGF mRNA expression in glioblastoma cells [138]. Similarly, gefitinib significantly inhibited both the basal and EGF-induced production of VEGF in prostate cancer cells [139]. In addition, it was shown that gefitinib transcriptionally down regulated VEGF expression by decreasing the binding ability of the transcription factor Sp1 to the VEGF promoter in squamous cell carcinoma cells [138]. When nude mice bearing orthotopically implanted human pancreatic carcinomas were treated with a TKI PKI 166, a significant reduction in VEGF and IL-8 secretions by the tumor cells was observed [140, 141]. Parallel observations were obtained with the anti-EGFR C225 (cetuximab) antibody where a significant reduction of VEGF, both at the transcriptional and translational levels in A431 vulvar squamous cancer cell lines, was observed [142]. In head and neck squamous carcinoma cells (HNSCC), exogenous EGF resulted in a significant reduction of both VEGF and IL-8 levels when treated with C225 or with EGFR tyrosine kinase inhibitor PD15035 [143]. Perrotte et al observed a marked reduction in the expression of three angiogenic factors, VEGF, IL-8 and bFGF, in bladder cancer and transitional cell carcinoma (TCC) when treated with C225 antibody. While these in vitro studies demonstrated the potential involvement of EGFR signaling tumorigenic events in the TME specifically angiogenesis no reduction in the levels of the angiogenic factors were observed in vivo in following geftinib treatment for prostate cancer [65].
4 Expert opinions
Exploring the EGFR family members and their corresponding signaling pathways have provided us with an unfathomable knowledge in understanding the molecular basis of epithelial malignancies. This has enabled us to develop EGFR–targeted therapies, many of which have been approved for human use. Since EGFR plays an integral role in malignant cell growth, proliferation, motility and survival of cancer cells and is widely observed in several malignancies, it was one of the first molecules to be selected for the treatment of cancer. Although this signaling cascade has been studied for more than 25 years, several aspects still remain elusive to us. For instance, it is unclear how the mutational status, gene copy number and EGFR overexpression impacts various intracellular signaling pathways in cancer. This remains as a major obstacle owing to the cross talk between EGFR family members and other signaling pathways, leading to therapeutic resistance. Research has to be focused in other directions, such as identifying a biomarker that can predict anti-EGFR therapy response. One good example is the KRAS gene mutation, which is a part of the downstream signaling pathway of the EGFR family members. Thus mutational status can predict the therapeutic response for the USFDA-approved EGFR monoclonal antibody cetuximab.
Currently, monoclonal antibodies and small molecule inhibitors of tyrosine kinase are the most preferred therapeutic strategies used, either alone or in combination with radiation or chemotherapy. Previous studies suggest that chemotherapy has the potential to affect normal cells in addition to neoplastic cells and thus therapies using compounds that specifically inhibit a target molecule (monoclonal antibodies or TKI) in a more specific subpopulation of neoplastic cells will have a direct implication on disease progression. Further, specific inhibition of receptor tyrosine kinases may be a lucid strategy to inhibit cancer cell function that strongly relies on its downstream signaling pathways. Therefore specific inhibitors which are currently under preclinical and clinical evaluation are a better strategy. One major advantage of using TKI's over monoclonal antibodies is their ability to inhibit cells that either do not over express or carry the mutant receptor or carry the truncated forms of the receptor that are constitutively activated.
Among the available therapies, irreversible TKIs appear to be the better choice when compared to monoclonal antibodies and other reversible inhibitors as they exhibit sustained action. However their major drawback is their non-specificity and TK point mutation that can lead to resistance. In addition, the molecule should 1) be orally active, 2) perform well when used alone, 3) exhibit differential cytotoxicity, 4) have high efficacy, 5) not cross react, 6) have a sustained drug effect, and 7) do not develop resistance when used in combination with radiation and other therapies. Additionally the combined use of a chemotherapeutic tyrosine kinase inhibitor with a chemopreventive agent can potentially have an adjuvant action by inhibiting transcriptional factors and intercepting the autocrine loops generated by the activation of EGFR. It can also be critical in reversing chemoresistance and radioresistance of chemopreventive agents improving their efficacy and by reducing any compensatory mechanism and toxicity.
ARTICLE HIGHLIGHT BOX
The aberrant activity of EGFR has shown to play a key role in the development and growth of tumor cells, where it is involved in numerous cellular responses including proliferation and apoptosis.
EGFR binds to its cognate ligand EGF which further induces tyrosine phosphorylation and receptor dimerization with other family members leading to enhanced uncontrolled proliferation.
Two distinct therapeutic approaches currently employed for targeting EGFR in various human malignancies (monoclonal antibodies and small molecule tyrosine kinase inhibitors).
Monoclonal antibodies against EGFR were specifically designed to be directed against the extracellular region of EGFR which creates a ligand competitive inhibition, thus preventing receptor dimerization, auto-phosphorylation and downstream signaling.
Tyrosine kinase inhibitors (TKI) are small molecules that are either reversible or irreversible by nature.
EGFR mutations play a critical role in imparting oncogenic potential to the cells, which in turn, results in various malignancies.
A combination therapy comprising of an anti-EGFR and a chemotherapeutic or chemopreventive agent will exhibit a multi-pronged approach that can be developed into a highly attractive and specific molecular oriented remedy for this complex disease.
Acknowledgments
Declaration of interest The authors on this work are supported in part by the grants from the Department of Defense (BC074639, BC083295, and BC09742), the National Institutes of Health (RO1 CA78590, EDRN UO1 CA111294, RO1 CA133774, RO1 CA131944, SPORE P50 CA127297, RO3 CA139285, R21 CA156037), VA Career Development Award and the Susan Komen Foundation (KG070826).
Reference List
- (1).Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- (2).Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–43. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- (3).Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ferguson KM, Berger MB, Mendrola JM, et al. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11:507–17. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- *(5).Ogiso H, Ishitani R, Nureki O, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–87. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- *(6).Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–3. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- *(7).Garrett TP, McKern NM, Lou M, et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763–73. doi: 10.1016/s0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- *(8).Bouyain S, Longo PA, Li S, et al. The extracellular region of ErbB4 adopts a tethered conformation in the absence of ligand. Proc Natl Acad Sci U S A. 2005;102:15024–9. doi: 10.1073/pnas.0507591102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ref: 5–8: Demonstrates the structure of ligand bind to the extracellular domain of the receptor and further ligand mediated receptor activation.
- (9).Dawson JP, Berger MB, Lin CC, et al. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol Cell Biol. 2005;25:7734–42. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- **(11).Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]; This review extensively describes the role of Erbb family members signaling in biological system, illustrating with computational modeling.
- *(12).Chaturvedi P, Singh AP, Chakraborty S, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–70. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study demonstrating the interaction of EGF like domains in MUC4 with the orphan receptor HER-2 in human pancreatic cancer cells.
- *(13).Ponnusamy MP, Singh AP, Jain M, et al. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br J Cancer. 2008;99:520–6. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study demonstrating the interaction of EGF like domains in MUC4 with the orphan receptor HER-2 in human ovarian cancer cells.
- (14).Normanno N, De LA, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- (15).Siwak DR, Carey M, Hennessy BT, et al. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: current knowledge and future challenges. J Oncol. 2010:568938. doi: 10.1155/2010/568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–87. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Baselga J, Albanell J. Epithelial growth factor receptor interacting agents. Hematol Oncol Clin North Am. 2002;16:1041–63. doi: 10.1016/s0889-8588(02)00055-2. [DOI] [PubMed] [Google Scholar]
- *(18).Madshus IH, Stang E. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J Cell Sci. 2009;122:3433–9. doi: 10.1242/jcs.050260. [DOI] [PubMed] [Google Scholar]
- *(19).Roepstorff K, Grovdal L, Grandal M, et al. Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol. 2008;129:563–78. doi: 10.1007/s00418-008-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(20).Nesterov A, Wiley HS, Gill GN. Ligand-induced endocytosis of epidermal growth factor receptors that are defective in binding adaptor proteins. Proc Natl Acad Sci U S A. 1995;92:8719–23. doi: 10.1073/pnas.92.19.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(21).Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–80. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- *(22).Jiang X, Sorkin A. Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic. 2003;4:529–43. doi: 10.1034/j.1600-0854.2003.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- **(23).Levkowitz G, Waterman H, Ettenberg SA, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–40. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]; This paper demonstrates c-Cbl mediated EGFR degradation.
- *(24).Waterman H, Katz M, Rubin C, et al. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J. 2002;21:303–13. doi: 10.1093/emboj/21.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(25).Grovdal LM, Stang E, Sorkin A, et al. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res. 2004;300:388–95. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- *(26).Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci U S A. 2007;104:16904–9. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(27).Barriere H, Nemes C, Du K, et al. Plasticity of polyubiquitin recognition as lysosomal targeting signals by the endosomal sorting machinery. Mol Biol Cell. 2007;18:3952–65. doi: 10.1091/mbc.E07-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(28).Lenferink AE, Pinkas-Kramarski R, van de Poll ML, et al. Differential endocytic routing of homo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17:3385–97. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(29).Decker SJ. Epidermal growth factor and transforming growth factor-alpha induce differential processing of the epidermal growth factor receptor. Biochem Biophys Res Commun. 1990;166:615–21. doi: 10.1016/0006-291x(90)90853-f. [DOI] [PubMed] [Google Scholar]
- *(30).Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ref: 18–30: Illustrates the role of EGFR in endocytosis and ubiqutination process.
- **(31).Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- **(32).Edwards JG, Swinson DE, Jones JL, et al. EGFR expression: associations with outcome and clinicopathological variables in malignant pleural mesothelioma. Lung Cancer. 2006;54:399–407. doi: 10.1016/j.lungcan.2006.08.012. [DOI] [PubMed] [Google Scholar]
- **(33).Hoshino M, Fukui H, Ono Y, et al. Nuclear expression of phosphorylated EGFR is associated with poor prognosis of patients with esophageal squamous cell carcinoma. Pathobiology. 2007;74:15–21. doi: 10.1159/000101047. [DOI] [PubMed] [Google Scholar]
- **(34).Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Treat. 2006;95:211–8. doi: 10.1007/s10549-005-9011-0. [DOI] [PubMed] [Google Scholar]
- **(35).Marti U, Burwen SJ, Wells A, et al. Localization of epidermal growth factor receptor in hepatocyte nuclei. Hepatology. 1991;13:15–20. [PubMed] [Google Scholar]; Ref: 31–35: Describes the nuclear translocation of EGFR.
- **(36).Ni CY, Yuan H, Carpenter G. Role of the ErbB-4 carboxyl terminus in gamma-secretase cleavage. J Biol Chem. 2003;278:4561–5. doi: 10.1074/jbc.M210504200. [DOI] [PubMed] [Google Scholar]; This research paper describes Erbb-4 cleave through gamma secreatase action, which leads to formation of a soluble intracellular domain, which might show an independent function of the membrane associated receptor.
- (37).Offterdinger M, Schofer C, Weipoltshammer K, et al. c-erbB-3: a nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157:929–39. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Psyrri A, Yu Z, Weinberger PM, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–62. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- (39).Wang SC, Lien HC, Xia W, et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–61. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- (40).Xia W, Wei Y, Du Y, et al. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol Carcinog. 2009;48:610–7. doi: 10.1002/mc.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Xie Y, Hung MC. Nuclear localization of p185neu tyrosine kinase and its association with transcriptional transactivation. Biochem Biophys Res Commun. 1994;203:1589–98. doi: 10.1006/bbrc.1994.2368. [DOI] [PubMed] [Google Scholar]
- (42).Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–9. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- (43).Lo HW, Ali-Seyed M, Wu Y, et al. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin beta1 and CRM1. J Cell Biochem. 2006;98:1570–83. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- **(44).Ni CY, Murphy MP, Golde TE, et al. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]; Research article demonstrated that γ-secretase processing of ErbB-4 was necessary for nuclear localization of ErbB-4.
- (45).Lo HW, Xia W, Wei Y, et al. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 2005;65:338–48. [PubMed] [Google Scholar]
- (46).Hanada N, Lo HW, Day CP, et al. Co-regulation of B-Myb expression by E2F1 and EGF receptor. Mol Carcinog. 2006;45:10–7. doi: 10.1002/mc.20147. [DOI] [PubMed] [Google Scholar]
- (47).Lo HW, Hsu SC, Ali-Seyed M, et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–89. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- (48).Huo L, Wang YN, Xia W, et al. RNA helicase A is a DNA-binding partner for EGFR-mediated transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2010;107:16125–30. doi: 10.1073/pnas.1000743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Bareschino MA, Schettino C, Troiani T, et al. Erlotinib in cancer treatment. Ann Oncol. 2007;18(Suppl 6):vi35–41. vi35–vi41. doi: 10.1093/annonc/mdm222. [DOI] [PubMed] [Google Scholar]
- *(50).Giaccone G, Gonzalez-Larriba JL, van Oosterom AT, et al. Combination therapy with gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, gemcitabine and cisplatin in patients with advanced solid tumors. Ann Oncol. 2004;15:831–8. doi: 10.1093/annonc/mdh188. [DOI] [PubMed] [Google Scholar]; This research article shows that suggest that EGF-stimulated receptor phosphorylation is not required for the internalization of the ligand-receptor complex which was proved using two monoclonal antibodies against the extracellur domain of EGFR.
- (51).Petrelli F, Borgonovo K, Cabiddu M, et al. Cetuximab and panitumumab in KRAS wild-type colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2011;26:823–33. doi: 10.1007/s00384-011-1149-0. [DOI] [PubMed] [Google Scholar]
- (52).Petrelli F, Barni S. Anti-EGFR-targeting agents in recurrent or metastatic head and neck carcinoma: A meta-analysis. Head Neck. 2011;10 doi: 10.1002/hed.21858. [DOI] [PubMed] [Google Scholar]
- (53).Rocha-Lima CM, Soares HP, Raez LE, et al. EGFR targeting of solid tumors. Cancer Control. 2007;14:295–304. doi: 10.1177/107327480701400313. [DOI] [PubMed] [Google Scholar]
- (54).Burgess AW, Cho HS, Eigenbrot C, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–52. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- **(55).Sunada H, Magun BE, Mendelsohn J, et al. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc Natl Acad Sci U S A. 1986;83:3825–9. doi: 10.1073/pnas.83.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]; A proof of clinical study demonstrating the synergism between an anti EGFR antibody and a chemotherapeutic agent.
- (56).Kimura H, Sakai K, Arao T, et al. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98:1275–80. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ennis BW, Lippman ME, Dickson RB. The EGF receptor system as a target for antitumor therapy. Cancer Invest. 1991;9:553–62. doi: 10.3109/07357909109018953. [DOI] [PubMed] [Google Scholar]
- (58).van Krieken JH, Jung A, Kirchner T, et al. KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: proposal for an European quality assurance program. Virchows Arch. 2008;453:417–31. doi: 10.1007/s00428-008-0665-y. [DOI] [PubMed] [Google Scholar]
- (59).Coutinho AK, Rocha Lima CM. Metastatic colorectal cancer: systemic treatment in the new millennium. Cancer Control. 2003;10:224–38. doi: 10.1177/107327480301000306. [DOI] [PubMed] [Google Scholar]
- (60).Cohen RB. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer. 2003;2:246–51. doi: 10.3816/CCC.2003.n.006. [DOI] [PubMed] [Google Scholar]
- (61).Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- (62).Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–14. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Raben D, Helfrich B, Ciardiello F, et al. Understanding the mechanisms of action of EGFR inhibitors in NSCLC: what we know and what we do not know. Lung Cancer. 2003;41(Suppl 1):S15–22. doi: 10.1016/s0169-5002(03)00135-1. [DOI] [PubMed] [Google Scholar]
- (64).Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer. 2001;8:3–9. doi: 10.1677/erc.0.0080003. [DOI] [PubMed] [Google Scholar]
- (65).Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–65. [PubMed] [Google Scholar]
- (66).Yang XD, Jia XC, Corvalan JR, et al. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38:17–23. doi: 10.1016/s1040-8428(00)00134-7. [DOI] [PubMed] [Google Scholar]
- (67).Lynch DH, Yang XD. Therapeutic potential of ABX-EGF: a fully human anti-epidermal growth factor receptor monoclonal antibody for cancer treatment. Semin Oncol. 2002;29:47–50. doi: 10.1053/sonc.2002.31522. [DOI] [PubMed] [Google Scholar]
- (68).Hecht JR, Patnaik A, Berlin J, et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer. 2007;110:980–8. doi: 10.1002/cncr.22915. [DOI] [PubMed] [Google Scholar]
- (69).Ciardiello F. Epidermal growth factor receptor tyrosine kinase inhibitors as anticancer agents. Drugs. 2000;60(Suppl 1):25–32. doi: 10.2165/00003495-200060001-00003. [DOI] [PubMed] [Google Scholar]
- (70).Slichenmyer WJ, Fry DW. Anticancer therapy targeting the erbB family of receptor tyrosine kinases. Semin Oncol. 2001;28:67–79. doi: 10.1016/s0093-7754(01)90284-2. [DOI] [PubMed] [Google Scholar]
- (71).Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317–26. doi: 10.1016/j.jaad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- (72).Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107–19. doi: 10.1007/s11523-009-0114-0. [DOI] [PubMed] [Google Scholar]
- (73).Widakowich C, de CG, Jr., de AE, et al. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2007;12:1443–55. doi: 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- (74).Wakeling AE, Barker AJ, Davies DH, et al. Specific inhibition of epidermal growth factor receptor tyrosine kinase by 4-anilinoquinazolines. Breast Cancer Res Treat. 1996;38:67–73. doi: 10.1007/BF01803785. [DOI] [PubMed] [Google Scholar]
- (75).Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999;82:241–50. doi: 10.1016/s0163-7258(98)00045-x. [DOI] [PubMed] [Google Scholar]
- (76).Thomas SM, Grandis JR. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat Rev. 2004;30:255–68. doi: 10.1016/j.ctrv.2003.10.003. [DOI] [PubMed] [Google Scholar]
- (77).Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–46. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- (78).Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- (79).Arteaga CL, Johnson DH. Tyrosine kinase inhibitors-ZD1839 (Iressa) Curr Opin Oncol. 2001;13:491–8. doi: 10.1097/00001622-200111000-00012. [DOI] [PubMed] [Google Scholar]
- (80).Moulder SL, Yakes FM, Muthuswamy SK, et al. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–95. [PubMed] [Google Scholar]
- (81).Normanno N, Maiello MR, De LA. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs): simple drugs with a complex mechanism of action? J Cell Physiol. 2003;194:13–9. doi: 10.1002/jcp.10194. [DOI] [PubMed] [Google Scholar]
- (82).Sirotnak FM, Zakowski MF, Miller VA, et al. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885–92. [PubMed] [Google Scholar]
- (83).Natale RB. Biologically targeted treatment of non-small-cell lung cancer: focus on epidermal growth factor receptor. Clin Lung Cancer. 2003;5(Suppl 1):S11–7. doi: 10.3816/clc.2003.s.010. [DOI] [PubMed] [Google Scholar]
- (84).Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–48. [PubMed] [Google Scholar]
- (85).Ranson M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br J Cancer. 2004;90:2250–5. doi: 10.1038/sj.bjc.6601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Perez-Soler R. Phase II clinical trial data with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib (OSI-774) in non-small-cell lung cancer. Clin Lung Cancer. 2004;6(Suppl 1):S20–3. doi: 10.3816/clc.2004.s.010. [DOI] [PubMed] [Google Scholar]
- (87).Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- (88).Herbst RS, Kies MS. Gefitinib: current and future status in cancer therapy. Clin Adv Hematol Oncol. 2003;1:466–72. [PubMed] [Google Scholar]
- (89).Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- (90).Herbst RS, Fukuoka M, Baselga J. Gefitinib--a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:956–65. doi: 10.1038/nrc1506. [DOI] [PubMed] [Google Scholar]
- (91).Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- (92).Zhang XT, Li LY, Wang SL, et al. Improvements in quality of life and disease-related symptoms in patients with advanced non-small cell lung cancer treated with gefitinib. Chin Med J (Engl) 2005;118:1661–4. [PubMed] [Google Scholar]
- (93).Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- (94).Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- (95).Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30:1426–47. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- (96).Kroep JR, Ouali M, Gelderblom H, et al. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study. Ann Oncol. 2011;22:207–14. doi: 10.1093/annonc/mdq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–31. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- (98).Johnston S, Pippen J, Jr., Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–46. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- (99).Johnston SR. Are current drug development programmes realising the full potential of new agents? The scenario. Breast Cancer Res. 2009;11(Suppl 3):S21. doi: 10.1186/bcr2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Agulnik M, Cohen EW, Cohen RB, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978–84. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- (101).Smaill JB, Palmer BD, Rewcastle GW, et al. Tyrosine kinase inhibitors. 15. 4-(Phenylamino)quinazoline and 4-(phenylamino)pyrido[d]pyrimidine acrylamides as irreversible inhibitors of the ATP binding site of the epidermal growth factor receptor. J Med Chem. 1999;42:1803–15. doi: 10.1021/jm9806603. [DOI] [PubMed] [Google Scholar]
- (102).Fry DW, Bridges AJ, Denny WA, et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci U S A. 1998;95:12022–7. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Fry DW. Site-directed irreversible inhibitors of the erbB family of receptor tyrosine kinases as novel chemotherapeutic agents for cancer. Anticancer Drug Des. 2000;15:3–16. [PubMed] [Google Scholar]
- (104).Nyati MK, Maheshwari D, Hanasoge S, et al. Radiosensitization by pan ErbB inhibitor CI-1033 in vitro and in vivo. Clin Cancer Res. 2004;10:691–700. doi: 10.1158/1078-0432.ccr-1041-03. [DOI] [PubMed] [Google Scholar]
- (105).Ako E, Yamashita Y, Ohira M, et al. The pan-erbB tyrosine kinase inhibitor CI-1033 inhibits human esophageal cancer cells in vitro and in vivo. Oncol Rep. 2007;17:887–93. doi: 10.3892/or.17.4.887. [DOI] [PubMed] [Google Scholar]
- (106).Trinks C, Djerf EA, Hallbeck AL, et al. The pan-ErbB receptor tyrosine kinase inhibitor canertinib induces ErbB-independent apoptosis in human leukemia (HL-60 and U-937) cells. Biochem Biophys Res Commun. 2010;393:6–10. doi: 10.1016/j.bbrc.2010.01.055. [DOI] [PubMed] [Google Scholar]
- (107).Nelson JM, Fry DW. Akt, MAPK (Erk1/2), and p38 act in concert to promote apoptosis in response to ErbB receptor family inhibition. J Biol Chem. 2001;276:14842–7. doi: 10.1074/jbc.M008786200. [DOI] [PubMed] [Google Scholar]
- (108).Gieseg MA, de BC, Ferguson LR, et al. Evidence for epidermal growth factor receptor-enhanced chemosensitivity in combinations of cisplatin and the new irreversible tyrosine kinase inhibitor CI-1033. Anticancer Drugs. 2001;12:683–90. doi: 10.1097/00001813-200109000-00007. [DOI] [PubMed] [Google Scholar]
- (109).Rao GS, Murray S, Ethier SP. Radiosensitization of human breast cancer cells by a novel ErbB family receptor tyrosine kinase inhibitor. Int J Radiat Oncol Biol Phys. 2000;48:1519–28. doi: 10.1016/s0360-3016(00)01358-4. [DOI] [PubMed] [Google Scholar]
- (110).Slichenmyer WJ, Elliott WL, Fry DW. CI-1033, a pan-erbB tyrosine kinase inhibitor. Semin Oncol. 2001;28:80–5. doi: 10.1016/s0093-7754(01)90285-4. [DOI] [PubMed] [Google Scholar]
- (111).Allen LF, Lenehan PF, Eiseman IA, et al. Potential benefits of the irreversible pan-erbB inhibitor, CI-1033, in the treatment of breast cancer. Semin Oncol. 2002;29:11–21. doi: 10.1053/sonc.2002.34049. [DOI] [PubMed] [Google Scholar]
- (112).Batra SK, Castelino-Prabhu S, Wikstrand CJ, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ. 1995;6:1251–9. [PubMed] [Google Scholar]
- (113).Lee JC, Vivanco I, Beroukhim R, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- (115).Sequist LV, Joshi VA, Janne PA, et al. Epidermal growth factor receptor mutation testing in the care of lung cancer patients. Clin Cancer Res. 2006;12:4403s–8s. doi: 10.1158/1078-0432.CCR-06-0099. [DOI] [PubMed] [Google Scholar]
- (116).Kuan CT, Wikstrand CJ, Bigner DD. EGF mutant receptor vIII as a molecular target in cancer therapy. Endocr Relat Cancer. 2001;8:83–96. doi: 10.1677/erc.0.0080083. [DOI] [PubMed] [Google Scholar]
- (117).Adjei AA. K-ras as a target for lung cancer therapy. J Thorac Oncol. 2008;3:S160–S163. doi: 10.1097/JTO.0b013e318174dbf9. [DOI] [PubMed] [Google Scholar]
- *(118).Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- **(119).Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ref 118, 119: Demonstrates the impact of secondary mutations in EGFR kinase domain in the development of acquired resistance.
- *(120).Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–9. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(121).Rizvi NA, Rusch V, Pao W, et al. Molecular Characteristics Predict Clinical Outcomes: Prospective Trial Correlating Response to the EGFR Tyrosine Kinase Inhibitor Gefitinib with the Presence of Sensitizing Mutations in the Tyrosine Binding Domain of the EGFR Gene. Clin Cancer Res. 2011;17:3500–6. doi: 10.1158/1078-0432.CCR-10-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ref. 120 and 121. Describes identification of activating mutations in the EGFR tyrosine kinase domain and patients response to the anti EGFR therapy.
- (122).Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- (123).Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–52. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- (124).Hirsch FR, Varella-Garcia M, Bunn PA, Jr., et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–42. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- (125).Zhu CQ, da Cunha SG, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:4268–75. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- (126).Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- (127).Mitsudomi T. Advances in target therapy for lung cancer. Jpn J Clin Oncol. 2010;40:101–6. doi: 10.1093/jjco/hyp174. [DOI] [PubMed] [Google Scholar]
- (128).Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- (129).Hwang YP, Yun HJ, Choi JH, et al. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 2011;55:594–605. doi: 10.1002/mnfr.201000292. [DOI] [PubMed] [Google Scholar]
- (130).Lev-Ari S, Starr A, Vexler A, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26:4423–30. [PubMed] [Google Scholar]
- (131).El-Rayes BF, Ali S, Ali IF, et al. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-kappaB. Cancer Res. 2006;66:10553–9. doi: 10.1158/0008-5472.CAN-06-2333. [DOI] [PubMed] [Google Scholar]
- (132).Gadgeel SM, Ali S, Philip PA, et al. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer. 2009;115:2165–76. doi: 10.1002/cncr.24250. [DOI] [PubMed] [Google Scholar]
- (133).De LA, Carotenuto A, Rachiglio A, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–67. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- (134).Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- (135).Schreiber AB, Winkler ME, Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986;232:1250–3. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- (136).Maity A, Pore N, Lee J, et al. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3'-kinase and distinct from that induced by hypoxia. Cancer Res. 2000;60:5879–86. [PubMed] [Google Scholar]
- (137).Goldman CK, Kim J, Wong WL, et al. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–33. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Pore N, Liu S, Haas-Kogan DA, et al. PTEN mutation and epidermal growth factor receptor activation regulate vascular endothelial growth factor (VEGF) mRNA expression in human glioblastoma cells by transactivating the proximal VEGF promoter. Cancer Res. 2003;63:236–41. [PubMed] [Google Scholar]
- (139).Sini P, Wyder L, Schnell C, et al. The antitumor and antiangiogenic activity of vascular endothelial growth factor receptor inhibition is potentiated by ErbB1 blockade. Clin Cancer Res. 2005;11:4521–32. doi: 10.1158/1078-0432.CCR-04-1954. [DOI] [PubMed] [Google Scholar]
- (140).Bruns CJ, Solorzano CC, Harbison MT, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–35. [PubMed] [Google Scholar]
- (141).Solorzano CC, Baker CH, Tsan R, et al. Optimization for the blockade of epidermal growth factor receptor signaling for therapy of human pancreatic carcinoma. Clin Cancer Res. 2001;7:2563–72. [PubMed] [Google Scholar]
- (142).Hirata A, Ogawa S, Kometani T, et al. ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase. Cancer Res. 2002;62:2554–60. [PubMed] [Google Scholar]
- (143).Bancroft CC, Chen Z, Yeh J, et al. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappaB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer. 2002;99:538–48. doi: 10.1002/ijc.10398. [DOI] [PubMed] [Google Scholar]