Abstract
Beta-adrenergic receptor antagonists (β-blockers) are the therapy of choice for the long QT syndrome (LQTS) but their efficacy is not homogeneous: propranolol and nadolol are the most effective whereas metoprolol is associated with more treatment failures. Propranolol has a blocking effect on the sodium current (“membrane stabilizing” effect) and it has been hypothesized that the efficacy of nadolol might be due to a similar effect. Accordingly, we used whole-cell patch clamp recording to assess propranolol, nadolol, and metoprolol block of wild-type or mutant cardiac sodium channels (Nav1.5) co-expressed with β1 subunit in tsA201 cells. Nadolol had a ~ 20% non-use-dependent blocking effect on peak sodium current and no effect on the persistent current evoked by the LQT3 mutant A1330D, whereas propranolol blocked Nav1.5 in a use-dependent manner and reduced A1330D persistent current. Metoprolol had no effect on either the peak or persistent current. Analysis of the biophysical properties of the channel revealed that both nadolol and propranolol cause hyperpolarizing shifts on voltage-dependence of activation and steady state inactivation whereas metoprolol shifts only the activation curve. These results provide partial explanation for the differences between nadolol and metoprolol but do not explain the similar clinical efficacy of nadolol and propranolol.
Keywords: Long QT syndrome, β-blockers, Sodium channel blockers, Nadolol
INTRODUCTION
The congenital long QT syndrome is a genetic disorder which represents a leading cause of sudden death in the young and has a prevalence of 1:2,000 life births1. It is characterized by prolongation of the QT interval on the surface ECG and by the propensity to syncope, cardiac arrest and sudden death, especially during increases in sympathetic activity2. Thirteen LQTS genes have been identified so far but the most important ones remain the first 3 described which control two potassium (IKr and IKs) and one sodium (INa) current2. There is an impressive correlation between genotype and phenotype, particularly as it concerns the triggers for life-threatening arrhythmias3 and this knowledge provides the rationale for gene-specific management in LQTS4.
As therapy is concerned, already the first large series of patients5,6 clearly indicated that β-adrenergic blockade was the most effective way to prevent cardiac events in LQTS, and β-blockers rightly constitute the first therapy of choice. The drug most frequently used, and with great success, was propranolol. Subsequently, many cardiologists assumed that any other β-blocker would have been equally effective. This is clearly true for nadolol, another non-selective β-blocker, but is not equally true for all other drugs in the same class. Indeed, and contrary to commonly held views, β-blockers are not all “Indians from the same tribe”7.
Chatrath et al. were the first to call attention, in a small series, to the fact that some β-blocker might be less efficacious than others and found that atenolol was associated with a higher rate of treatment failures8. A large study, involving over 400 LQTS patients treated with β-blockers9, confirms the high success rate of propranolol and nadolol and identifies metoprolol as the drug with the highest failure rate. The strikingly different protective effect of propranolol and metoprolol is explained by the fact that propranolol, but not metoprolol, has a significant blocking effect on the sodium current (the so-called “membrane stabilizing effect)10. There have been no extensive studies on the effect of nadolol on this current. In the present study we have explored the possibility that also nadolol possesses a sodium current blocking effect that might explain its major antiarrhythmic efficacy.
MATERIALS AND METHODS
Cell transfection and mutagenesis
Cells (tsA201) were transiently transfected with 0.3 µg of pRcCMV-hH1 encoding NaV1.5 protein (WT) using FuGene6 (Roche Diagnostics) combined with 0.3 µg of a bicistronic plasmid (pEGFP-IRES-hβ1) encoding the enhanced green fluorescence protein and the human β1subunit. Forty-eight hours after transfection the cells were plated onto round glass cover slips and transferred to a chamber on the stage of an inverted microscope equipped with a fluorescent lamp. Only cells exhibiting green fluorescence were used for patch-clamp experiments.
Site directed mutagenesis, to obtain the hH1-A1330D plasmid (A1330D), was done on pRcCMV-hH1 using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene). A rare variant present in the original WT cDNA (R1027Q) was also reverted to the common allele (R1027). The mutated plasmid was fully sequenced to verify the presence of the mutation and to exclude any other alteration.
Electrophysiology
The sodium current was recorded at room temperature using the whole cell patch clamp technique. The bath solution contained (mM): 145 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, pH 7.35 (adjusted with NaOH). The pipette solution contained (mM): 10 NaF, 110CsF, 20CsCl, 2 EGTA, 10 HEPES, pH 7.35 (adjusted with CsOH). Data acquisition was carried out with Axopatch 200B amplifier connected to the 1440A Digidata and pClamp 10.0 software (Axon Instruments). Electrode resistance ranged from 0.9–1.3 MΩ. Pipette capacitance was corrected and whole cell capacitance and series resistance were 90% compensated. Whole cell currents were acquired at 50 kHz and filtered at 5kHz. Recordings from cells exhibiting current smaller than −0.8 nA were excluded from analysis to avoid a possible endogenous channel contamination. Leak current was subtracted using a P/4 procedure. Whole cell capacitance was assessed by integrating the capacitive transient elicited by 10 mV voltage step from −120 to −110 mV. Voltage-dependence of activation and steady-state inactivation were measured using the specific protocols depicted in the corresponding figures.
Tonic block was measured at 0.02 Hz (100ms step from −120 mV to −10 mV). Steady-state Use-dependent block was achieved in response to trains of 150 pulses to −10 mV at 1Hz. Tonic block and use-dependent block were calculated as the difference between the peak current in the presence of the drug and the current in control normalized to the current amplitude recorded in the absence of the drug. The level of sustained current was expressed as percentage of peak current calculated dividing the TTX-sensitive current (digital subtraction of the current recorded after perfusion with 30 µM TTX from the current in control) at the last 5 ms of 100 ms step by the peak current.
Drugs
All drugs: nadolol, metoprolol, propranolol, mexiletine HCl (Sigma Aldrich), were freshly prepared before experiments from 10 mM stock solutions stored at −20 C°. TTX (Tocris) was freshly prepared before experiments from 3mM stock solution stored at −20 C°.
Data Analysis
Data analysis was carried out in pClamp 10.0 (Axon Instruments), Excel (Microsoft), Origin 7.5 (Microcal Software). Data are presented as mean ± SEM. Statistical significance was tested using Student’s t test; p<0.05 was considered statistically significant.
RESULTS
The effect of three different β-blockers on Na channels expressed in tsA201 cells was investigated. Wildtype (WT) channels and β subunit (hβ1) were co-expressed and sodium current (INa) was measured using whole-cell patch-clamp. We first compared the effect of three different nadolol concentrations (10 µM, 40 µM and 200 µM) with the effect of propranolol (40 µM) and metoprolol (40 µM). Propranolol is known to reduce the peak INa and represented our positive control for drug effect while metoprolol has minimal effects on peak INa10,11 and represented our negative control.
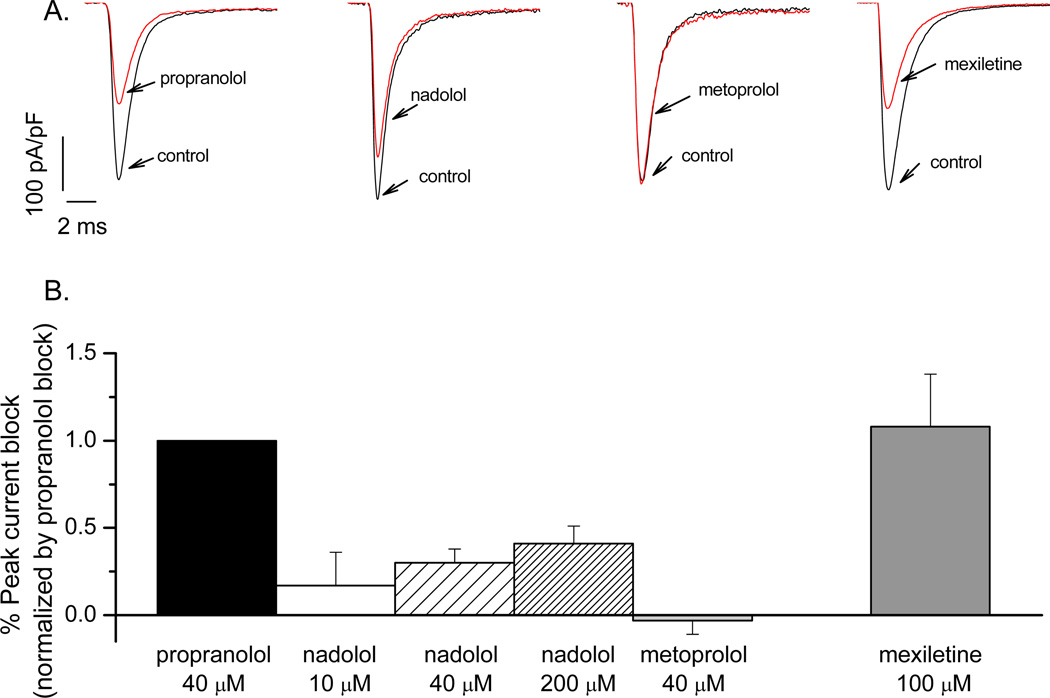
Figure 1 illustrates representative current recordings and average data for tonic block by propranolol (−43±5%, n=14), nadolol (−7±8%, −12±3%, −17±6%, n ≥ 5), metoprolol (1.31±3%, n=16), or mexiletine (−47±12 % n=6). The effect of nadolol is significantly smaller than the block induced by propranolol or mexiletine (a local anesthetic known as specific INa blocker, and effective on the late NA current12,13 with gene-specific therapeutic implications4) but is greater than that induced by metoprolol (p <0.05, Table 1).
FIGURE 1.
Representative current recordings illustrating the effect of drugs on the peak Na-current are in panel A with average data presented in panel B. Each bar graph represents the degree of inhibition exerted by the drug on the peak Na-current normalized by the inhibition exerted by propranolol. The percent was obtained by dividing the peak current at steady state in the presence of the drug by the peak current in the control (drug-free) condition and then normalized by the percent inhibition induced by propranolol. The tonic block was developed by depolarizing the cells to −10 mV from −120 mV (100 ms) at 0.02 Hz.
TABLE 1.
| Peak INa block | |
|---|---|
| Propranolol 40µM | −43 ± 5%‡ |
| Nadolol 10 µM | −7 ± 8 %* |
| Nadolol 40 µM | −12 ± 3 %*‡ |
| Nadolol 200 µM | −17 ± 6 %*‡ |
| Metoprolol 40 µM | 1.3 ± 3%* |
| Mexiletine 100 µM | −47 ± 12%‡ |
p<0.05, compared to propranolol or mexiletine;
p<0.05, compared to metoprolol
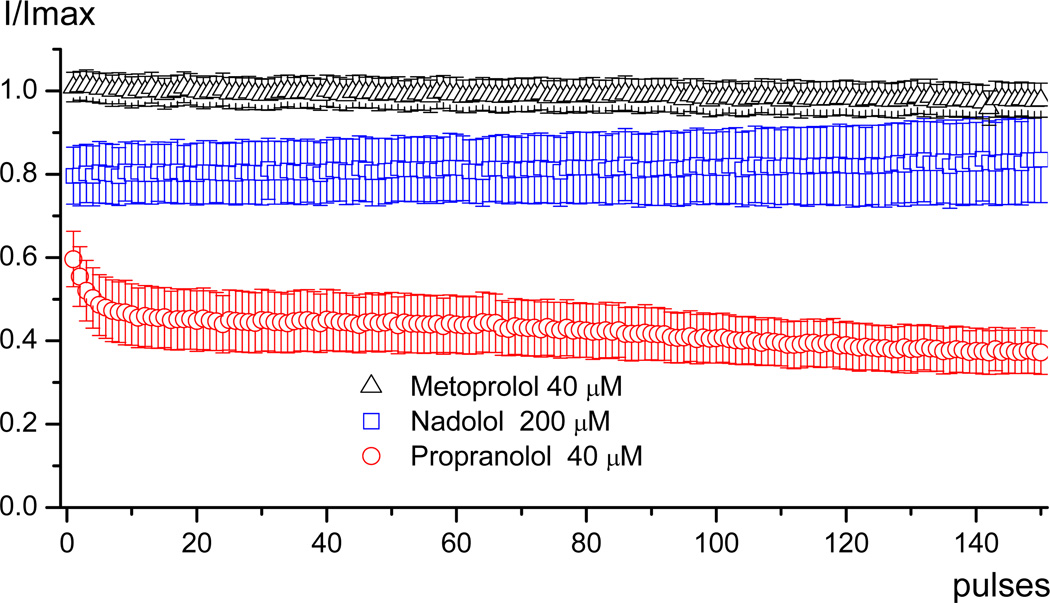
Figure 2 illustrates use-dependent block of WT channels by nadolol, propranolol and metoprolol. While propranolol has a strong use-dependent effect, nadolol and metoprolol do not. Nonetheless, nadolol exerts a notable tonic block in these experiments that does not exhibit use-dependence.
FIGURE 2.
Average data showing the use-dependent effect of the drugs. The cells were superperfused with one of the three drugs and once the steady state was reached the protocol for use-dependent block (train of 150 pulses to −10 mV from −120 mV at 1Hz) was applied. The data were obtained by dividing the peak current at each pulse during the use-dependent block protocol by the peak current in control.
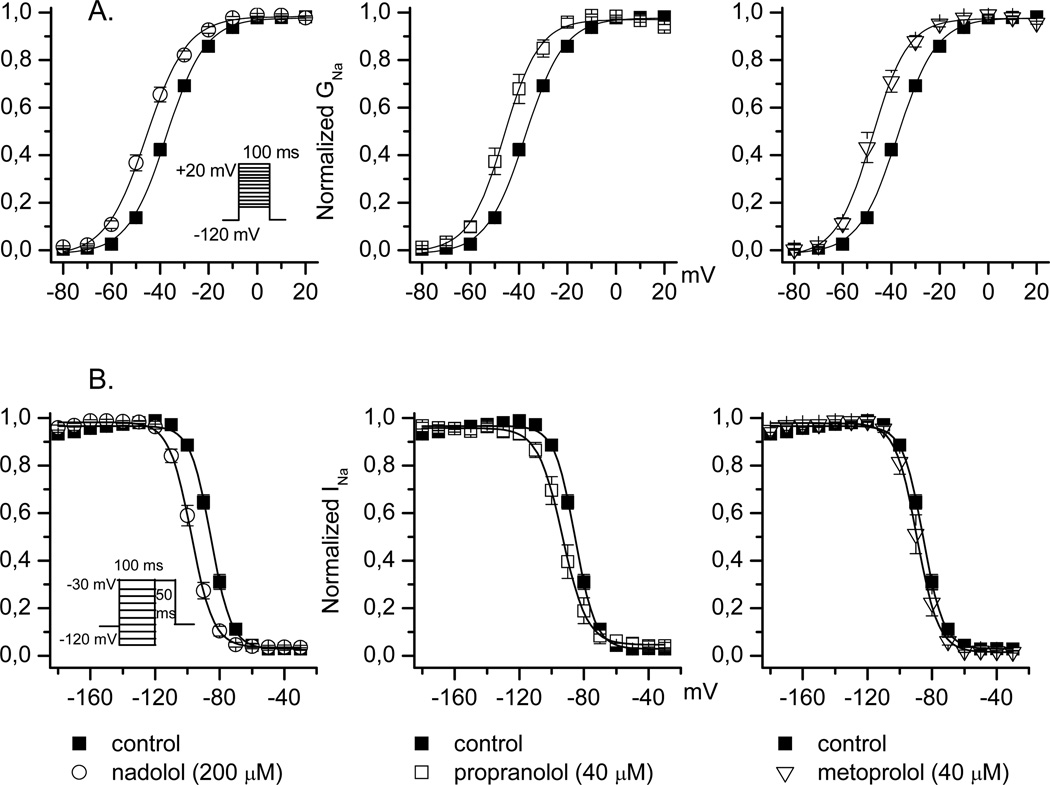
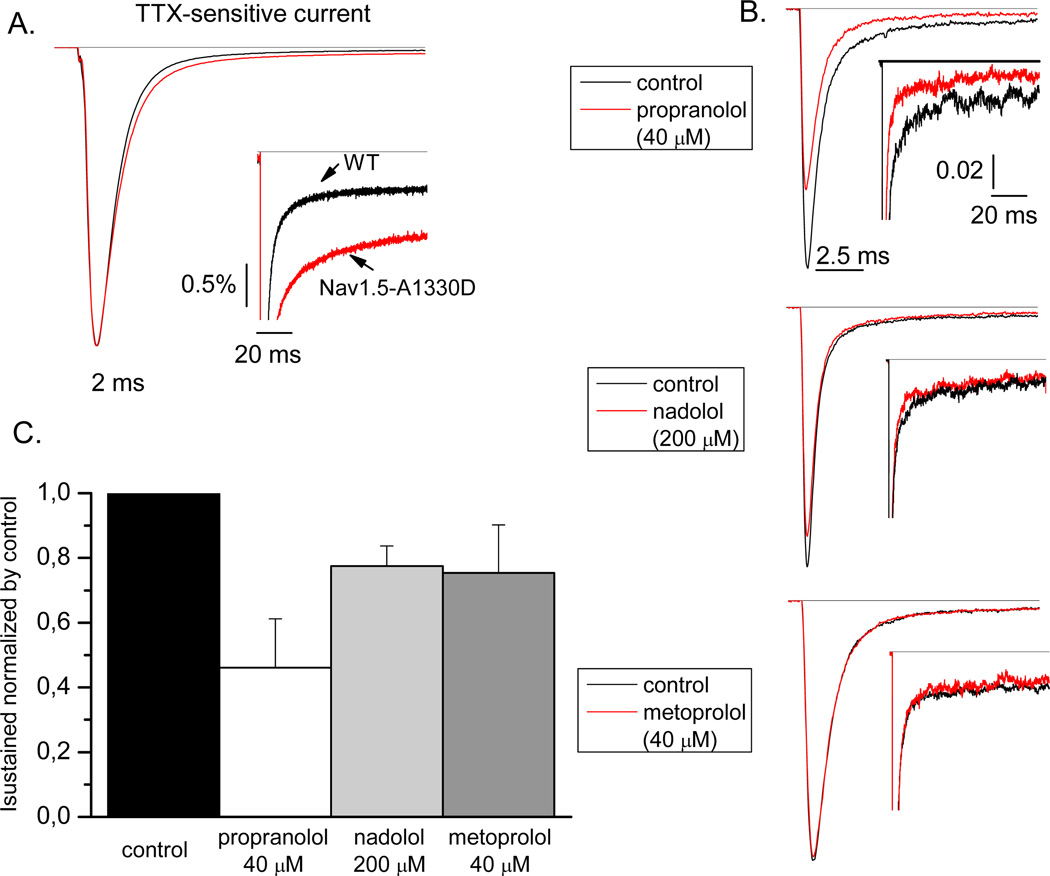
We also studied the effect of propranolol, nadolol, and metoprolol on the voltage-dependence of activation and inactivation (Figure 3). Nadolol evokes a significant hyperpolarizing shift in voltage-dependence of activation similar to that of propranolol and metoprolol (Table 2). Nadolol also causes a hyperpolarizing shift in steady-state inactivation identical to that of propranolol (Table 2). By contrast, metoprolol has no effect on steady-state inactivation. SCN5A mutations producing LQT3 generally exhibit a disruption in channel inactivation leading to an increase in persistent current, which has pathophysiological relevance during the plateau phase of the action potential. Inhibition of increased persistent current is one of the main rationale for using Na-channel blockers to treat LQT3 patients. Propranolol also inhibits persistent current conducted by certain LQT3 mutants14. We tested the effect of β-blockers on persistent current using the mutant Nav1.5-A1330D. Figure 4 (panel A) illustrates the magnitude of persistent current carried by Nav1.5-A1330D (as percentage of peak current: 1.56±0.35%, n=7) compared to wild type channel (0.45±0.07%, n=13).Propranolol was the only β-blocker that inhibits persistent current evoked by this mutant channel (0.72±0.24%, n =5 vs 1.56±0.35%, n=7 in control; Table 3).
FIGURE 3.
Conductance-voltage relationships in the presence of nadolol (200 µM), propranolol (40 µM) or metoprolol (40 µM) are shown in panel A. The protocol used is depicted in the figure, and data were fit by a Boltzmann function. Voltage dependence of Na channel availability in the presence of nadolol (200 µM), Propranolol (40 µM) or metropolol (40 µM,) are shown in panel B. The protocol used is depicted in the figure, and data were fit by a Boltzmann function
TABLE 2.
| V1/2 Activation | K | V1/2 Inactivation | K | |
|---|---|---|---|---|
| Control | −37.1± 0.8 (n=35) |
7.5 ± 0.2 | −85.7 ± 0.9 (n=25) |
5.9 ± 2.2 |
| Nadolol | −45.6 ± 1.2 * (n=7) |
8.3 ± 0.3 | −97.6 ±1.3*‡ (n=7) |
6.6 ± 0.4 |
| Propranolol | −45.2 ± 1.8 * (n=11) |
6.6 ± 0.5 | −95.4 ± 1.8*‡ (n=8) |
6.4 ± 0.4 |
| Metoprolol | −47.2 ± 1.6 * (n=14) |
6.2 ± 0.4 | −89.3 ± 2.2 (n=6) |
6.0 ± 0.3 |
p<0.05, compared to control values.
p<0.05, compared to metoprolol values.
FIGURE 4.
Typical TTX-sensitive current for WT or A1330D channels is shown in panel A. In the inset, the late current is shown. In panel B, typical recordings of INa in control and in the presence of drugs at steady state are shown. In the inset, an amplified view of the late current is shown. The current in panel A and B are normalized to the peak current recorded under control condition. In panel C, the average data showing the TTX-sensitive current in the presence of the three drugs normalized by the TTX-sensitive current in control condition (considered as 1). The TTX-sensitive current was measured as difference between current at steady state in control or in the presence of a β-blocker and current at steady state after TTX perfusion. Sustained current was measured as average current at the last 5 ms of the 100 ms step (−10 mV) and expressed as percentage of the peak TTX-sensitive current.
TABLE 3.
| INa sustained (% block) | |
|---|---|
| Control | 1.56 ± 0.3 (n=7) |
| Propranolol | 0.72 ± 0.2 (54%)* (n=5) |
| Nadolol | 1.21 ± 0.1 (22%) (n=7) |
| Metoprolol | 1.18 ± 0.2 (24%) (n=5) |
p<0.05 compared to control
DISCUSSION
The present study does not solve the mystery of the efficacy of nadolol in the management of LQTS. This might be viewed as disappointing, but only at a superficial analysis because the findings advance our knowledge. The novel data are represented by the effect of nadolol on the sodium current. The “membrane stabilizing” effect of nadolol is modest but definitely present, at variance with metoprolol which is completely devoid of it. In this regard, the data contribute to explain, to some extent, the difference observed clinically between nadolol and metoprolol.
Nadolol and the Na current
Previous studies demonstrated that β-blockers share with local anesthetics (mexiletine, ranolazine) a common site for drug block of sodium channels, that this blocking effect is dependent on the state of the channel (inactivated), and that it acts preferentially on the late Na-current than on the peak current11. Nevertheless, different β -blockers have different effects on the Na current. Propranolol as well as carvedilol are very efficient Na-channel blockers whereas metoprolol has no effect either on peak or on late Na-current. Here, we have shown that nadolol has a mild blocking effect on peak INa and no effect on late current. Furthermore, for nadolol the lack of use-dependent block is not dependent on the state of the channel as it happens for propranolol. Nadolol produces a hyperpolarizing shift of voltage-dependence of activation and steady state inactivation comparable to that of propranolol but very different compared to metoprolol, and this justifies the small inhibitory effect of nadolol on Na-current.
A possible explanation for the different effects of the β-blockers lies in their chemical structure. Propranolol has a lipophilic nature (very similar to the local anesthetics) that allows it to interact with the channel at hyperpolarized potentials by moving through the membrane, whereas nadolol has a more hydrophilic nature that makes more difficult penetrating through the membrane to reach the local anesthetic-binding site. Another difference is represented by the selectivity of the various β-blockers for the β-adrenergic receptors. Metoprolol is a selective β1-blocker while propranolol and nadolol are non-selective β- blockers, with nadolol having more affinity for β1- receptors over β2.
Nadolol and the Long QT Syndrome
Nadolol is very effective in preventing breakthrough cardiac events when used in symptomatic patients affected by LQTS and, from a practical point of view it has the same or very similar efficacy as propranolol. An extremely recent study9 on over 400 LQTS patients shows that, compared to propranolol and nadolol, metoprolol is definitely less effective and its use is associated with an unacceptably high frequency of recurrences.
Besides the fact that metoprolol at variance with propranolol and nadolol is a selective β-blocker, to explain these failures the possibility has been advanced that a differential effect on the sodium current might contribute to the clinical observations. The present study has addressed this possibility. The effect of nadolol on the sodium current, however, is a modest one and cannot be equated to that of propranolol; nonetheless, it is greater than that of metoprolol and this difference might contribute in part to their different clinical effect.
There are lessons to be learned from the present experiments and results. One is that, at the end of the day, what matters with drug therapy is the clinical outcome. It is always intellectually rewarding to find an electrophysiological explanation to the observed clinical results and label it as the “mechanism of action” but this does not happen all the time. In the present case the data contribute to understand the difference between nadolol and metoprolol but are insufficient to explain the almost identical protective efficacy of nadolol and propranolol. A second one is that the research process toward full understanding can be less than direct and may require multiple steps. The long QT syndrome is not new to reward investigators with humbling results. A good example relates to the generally accepted notion that clinical severity can be predicted by the location and functional consequences of a given mutation14, a notion that certainly does not explain the unusual clinical manifestations of the hot-spot mutation KCNQ1-A431V16. Indeed, its striking clinical severity proves that the current biophysical assessments of cellular electrophysiological effects cannot always predict the clinical phenotype16.
In conclusion, we have shown a modest but unexpected effect of nadolol on the Na peak current, of which metoprolol is devoid, which while not fully explaining its antiarrhythmic efficacy perhaps helps to understand the major difference in the protection afforded to LQTS patients by nadolol and metoprolol.-
ACKNOWLEDGEMENTS
The Authors are grateful to Pinuccia De Tomasi for expert editorial support.
Source of funding: NIH grant HL083374; Telethon grant GGP09247
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None declared.
Potential Reviewers: Michael J. Ackerman (Rochester, MN, USA); Connie R. Bezzina (Amsterdam, The Netherlands); Robert Dumaine (Sherbrooke, Qc, Canada); Eric Schulze-Bahr (Muenster, Germany); Paul Volders (Maastricht, The Netherlands).
REFERENCES
- 1.Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, Mosca F, Nespoli L, Rimini A, Rosati E, Salice P, Spazzolini C. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz PJ, Crotti L. Long QT and short QT syndromes. In: Zipes DP, Jalife J, editors. CARDIAC ELECTROPHYSIOLOGY: FROM CELL TO BEDSIDE. 5th Edition. Philadelphia: Elsevier/Saunders; 2009. pp. 731–744. [Google Scholar]
- 3.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AAM, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long QT syndrome. Gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantù F, Towbin AJ, Keating MT, Hammoude H, Brown AM, Chen LK, Colatsky TJ. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–390. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PJ. Idiopathic long QT syndrome: Progress and Questions. Am Heart J. 1985;109:399–411. doi: 10.1016/0002-8703(85)90626-x. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Crotti L, Insolia R. Long QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. doi: 10.1161/CIRCEP.111.962019. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatrath R, Bell CM, Ackerman MJ. β-blocker therapy failures in symptomatic probands with genotyped long-QT syndrome. Pediatr Cardiol. 2004;25:459–465. doi: 10.1007/s00246-003-0567-3. [DOI] [PubMed] [Google Scholar]
- 9.Chockalingam P, Girardengo G, Crotti L, Johnson JN, Harris KM, van der Heijden JF, Hauer RNW, Beckmann BM, Rydberg A, Clur SAB, Fischer M, van den Heuvel F, Kääb S, Blom NA, Ackerman MJ, Schwartz PJ, Wilde AAM. β-blockers in the treatment of congenital long QT syndrome types 1 and 2, a comparison of propranolol, metoprolol and nadolol. Submitted for publication. [Google Scholar]
- 10.Wang DW, Mistry AM, Kahlig KM, Kearney JA, Xiang J, George AL., Jr Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front Pharmacol. 2010;1:144. doi: 10.3389/fphar.2010.00144. doi: 10.3389/fphar.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bankston JR, Kass RS. Molecular determinants of local anesthetic action of β-blocking drugs: Implications for therapeutic management of long QT syndrome variant 3. J Mol Cell Cardiol. 2010;48:246–53. doi: 10.1016/j.yjmcc.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 13.Dumaine R, Wang Q, Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE. Multiple mechanisms of Na+ channel-linked long-QT syndrome. Circ Res. 1996;78:916–924. doi: 10.1161/01.res.78.5.916. [DOI] [PubMed] [Google Scholar]
- 14.Wang DW, Crotti L, Shimizu W, Pedrazzini M, Cantù F, De Filippo P, Kishiki K, Miyazaki A, Ikeda T, Schwartz PJ, George AL., Jr Malignant perinatal variant of long QT syndrome caused by a profoundly dysfunctional cardiac sodium channel. Circ Arrhythm Electrophysiol. 2008;1:370–378. doi: 10.1161/CIRCEP.108.788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss AJ, Shimizu W, Wilde AA, Towbin JA, Zareba W, Robinson JL, Qi M, Vincent GM, Ackerman MJ, Kaufman ES, Hofman N, Seth R, Kamakura S, Miyamoto Y, Goldenberg I, Andrews ML, McNitt S. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–2489. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crotti L, Spazzolini C, Schwartz PJ, Shimizu W, Denjoy I, Schulze-Bahr E, Zaklyazminskaya EV, Swan H, Ackerman MJ, Moss AJ, Wilde AA, Horie M, Brink PA, Insolia R, De Ferrari GM, Crimi G. The common Long QT Syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds-toward a mutation-specific risk stratification. Circulation. 2007;116:2366–2375. doi: 10.1161/CIRCULATIONAHA.107.726950. [DOI] [PubMed] [Google Scholar]