Abstract
Background
Methoxycarbonyl etomidate (MOC-etomidate) is a rapidly metabolized and ultra-short acting etomidate analog that does not produce prolonged adrenocortical suppression after bolus administration. Its metabolite (MOC-ECA) is a carboxylic acid whose pharmacology is undefined. We hypothesized that MOC-ECA possesses significantly lower pharmacological activity than MOC-etomidate, accounting for the latter's very brief duration of hypnotic action and inability to produce prolonged adrenocortical suppression after bolus administration. To test this hypothesis, we compared the potencies of MOC-ECA and MOC-etomidate in three biological assays.
Methods
The hypnotic potency of MOC-ECA was assessed in tadpoles using a loss-of-righting reflexes assay. The gamma-aminobutyric acid type A (GABAA) receptor modulatory potencies of MOC-ECA and MOC-etomidate were compared by defining the concentrations of each required to directly activate α1(L264T)β2γ2L GABAA receptors. The adrenocortical inhibitory potencies of MOC-ECA and MOC-etomidate were compared by defining the concentrations of each required to inhibit in vitro cortisol production by adrenocortical cells.
Results
MOC-ECA's EC50 for loss-of-righting reflexes in tadpoles was 2.8 ± 0.64 mM as compared to a previously reported value of 8 ± 2 μM for MOC-etomidate. EC50s for direct activation of GABAA receptors were 3.5 ± 0.63 mM for MOC-ECA versus 10 ± 2.5 μM for MOC-etomidate. IC50 for inhibiting in vitro cortisol production by adrenocortical cells was 30 ± 7 μM for MOC-ECA versus 0.10 ± 0.02 μM for MOC-etomidate.
Conclusions
In all three biological assays, MOC-ECA's potency was approximately 300-fold lower than that of MOC-etomidate.
Introduction
Methoxycarbonyl etomidate (MOC-etomidate) is a rapidly metabolized and ultra-short acting etomidate analog that does not produce prolonged adrenocortical suppression in rats after bolus administration.1 In common with remifentanil, esmolol, and remimazolam, it contains a metabolically-labile ester moiety that is rapidly hydrolyzed by esterases to form a carboxylic acid metabolite (Figure 1). The pharmacological activities of the carboxylic metabolites of remifentanil, esmolol, and remimazolam are orders of magnitude lower than those of their respective parent compounds (4600-fold, 300-fold to 1600-fold, and 300-fold, respectively).2-6 However, the pharmacologic activity of MOC-etomidate's carboxylic acid metabolite (MOC-ECA) has not been assessed and its potency relative to MOC-etomidate is unknown. We hypothesized that this metabolite possesses significantly lower potency than the parent compound, accounting for the latter's very brief duration of hypnotic action and inability to produce prolonged adrenocortical suppression after bolus administration. This report describes the pharmacology of MOC-ECA and compares it to that of MOC-etomidate in three biological assays.
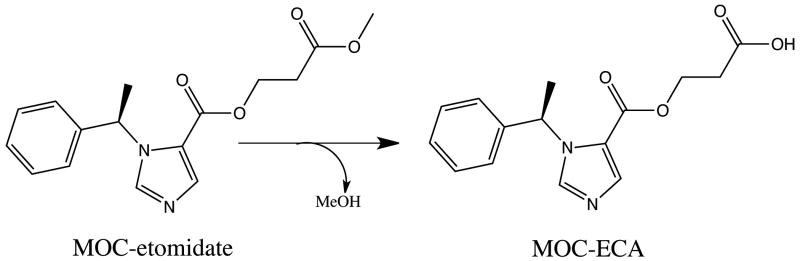
Figure 1.
Hydrolysis of methoxycarbonyl etomidate (MOC-etomidate) to its carboxylic acid metabolite (MOC-ECA).
Methods
All animal studies were conducted with the approval of the Subcommittee on Research Animal Care at the Massachusetts General Hospital, Boston, Massachusetts. Xenopus laevis tadpoles (early pre-limb stage) and adult female Xenopus laevis frogs were purchased from Xenopus One (Ann Arbor, MI) and housed in our laboratory (tadpoles) or in the Massachusetts General Hospital Center for Comparative Medicine animal care facility (frogs). MOC-etomidate was synthesized by Aberjonia Laboratories (Beverly, MA) using our previously published method.(1)MOC-ECA was synthesized in our laboratory as previously described and its highest aqueous concentration was limited in our studies to 10 mM to assure complete dissolution in aqueous solutions.7
Tadpole Loss-of-Righting Reflexes (LORR)
LORR in Xenopus laevis tadpoles was assessed as previously described.1.8 In brief, groups of 5 tadpoles were placed in water containing the desired quantity of MOC-ECA and buffered with 2.5 mM Tris HCl (pH=7.4). Tadpoles were tipped every 5 minutes with a flame-polished pipette until the response stabilized. A tadpole was determined to have LORR if it failed to right itself within 5 seconds after being turned supine. At the end of each study, tadpoles were returned to fresh water to ensure reversibility. MOC-ECA's EC50 for LORR was then determined from the concentration-dependence of LORR using the quantal method of Waud.9
GABAA Receptor Electrophysiology
Stage 4 and 5 oocytes were obtained as previously described and injected with messenger RNA encoding the α1(L264T), β2, and γ2L subunits of the human gamma-aminobutyric acid A(GABAA) receptor (∼40 ng of messenger RNA total at a subunit ratio of 1:1:2).1 We chose to use α1(L264T)β2γ2L rather than wild-type GABAA receptors to assess the relative potencies of MOC-etomidate and MOC-ECA on GABAA receptors because previous studies have shown that this mutant is very potently and effectively directly activated by anesthetics, which allows for more precise estimation of drug potency when drug solubility is limited.10 After injection, oocytes were incubated in ND-96 buffer solution (96 mM NaCl, 2 mM KCl, 1 mM CaCl2, 0.8 mM MgCl2, 10 mM HEPES, pH=7.4) containing 50 U/ml of penicillin and 50 μg/ml of streptomycin at 17°C for at least 18 hours before electrophysiological experiments.
All electrophysiological recordings were performed using the whole cell two-electrode voltage-clamp technique with oocytes voltage clamped at −50 mV using a GeneClamp 500B amplifier (Molecular Devices, Sunnyvale, CA) and perfused with ND-96 buffer with 1 mM EGTA at a rate of 4–6 ml/min. Buffer perfusion was controlled using a six-channel valve controller (Warner Instruments, Hamden, CT) interfaced with a Digidata 1322A data acquisition system (Molecular Devices) and driven by a Dell personal computer (Round Rock, TX). Current responses were recorded using Clampex 9.2 software (Molecular Devices) and processed using a Bessel (8-pole) low-pass filter with a cutoff at 50 Hz using Clampfit 9.2 software (Molecular Devices).
Currents were elicited by application of MOC-etomidate or MOC-ECA (each dissolved in ND-96 buffer with 1 mM EGTA). The recorded peak current amplitudes were normalized to control currents elicited with 1 mM GABA (also dissolved in ND-96 buffer with 1 mM EGTA)in the same oocyte. EC50s for direct activation were calculated by fitting a plot of the normalized current amplitude versus MOC-etomidate or MOC-ECA concentration to a Hill equation.
Suppression of Cortisol Synthesis
Suppression of in vitro cortisol synthesis by MOC-etomidate and MOC-ECA was quantified as previously described using an in vitro adrenocortical cell (NCI-H295R; ATCC CRL-2128) assay.8 Briefly, aliquots of cells (105 cells/well) were grown in 12-well culture plates with 2 ml of growth medium. After reaching near confluence, the growth medium was replaced with an assay medium containing 20 μM for skolin (to stimulate cortisol synthesis) and the desired concentration of either MOC-etomidate or MOC-ECA. After 48 hours, 1.2 ml of assay medium was collected from each well, centrifuged, and the cortisol concentration in the supernatant determined using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). The cortisol concentrations in the supernatant of wells containing MOC-etomidate or MOC-ECA were normalized to those in control wells without MOC-etomidate or MOC-ECA. IC50s for adrenocortical cell inhibition were then calculated by fitting a plot of the normalized cortisol concentration versus MOC-etomidate or MOC-ECA concentration to a Hill equation.
Statistical Analysis
All data are reported as mean ± SD unless otherwise noted. Curve fitting was performed using Igor Pro 6.1 (Wavemetrics, Lake Oswego, OR).
Results
The fraction of tadpoles that had LORR increased with MOC-ECA concentration and at the highest concentration studied (10 mM); 18 of 20 tadpoles had LORR (Figure 2). This LORR was reversible because all 52 tadpoles that had LORR in our studies recovered their righting reflexes when returned to water without MOC-ECA. From the MOC-ECA concentration-dependence of LORR, the EC50 for LORR was determined to be 2.8 ± 0.64 mM.
Figure 2.
Methoxycarbonyl etomidate metabolite (MOC-ECA) concentration-response curves for loss-of-righting reflexes (LORR) in tadpoles. Each symbol represents data from a single tadpole. The curve is a fit of the MOC-ECA concentration-mean response data yielding an EC50 of 2.8 ± 0.64 mM and a slope of 0.8 ± 0.16.
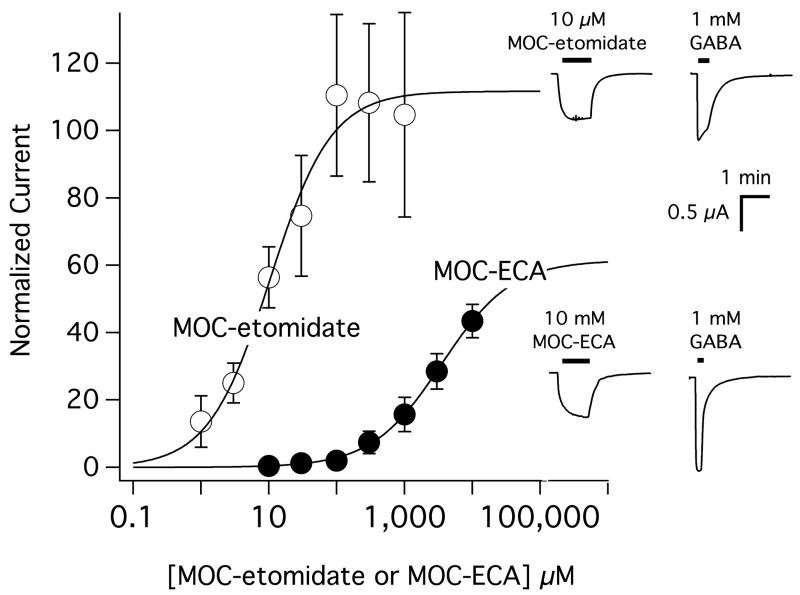
MOC-etomidate and MOC-ECA directly activated α1(L264T)β2γ2L GABAA receptors in a concentration-dependent manner (Figure 3). However, the concentration ranges over which this activation occurred differed between the two compounds by 2-3 orders of magnitude. With MOC-etomidate, this amplitude reached a plateau by 100 μM that approximated the amplitude elicited by 1 mM GABA. From the concentration-response relationship, we calculated an EC50 for direct activation of 10 ± 2.5 μM. With MOC-ECA, this amplitude failed to reach a plateau even at 10 mM, the highest concentration studied. From the MOC-ECA concentration-response relationship, we calculated an EC50 for direct activation of 3.5 ± 0.63 mM with the caveat that this value may somewhat overestimate the true potency of the metabolite because no clear plateau in the response was achieved.
Figure 3.
Direct activation of α1(L264T)β2γ2L gamma-aminobutyric acid type A (GABAA) receptors by methoxycarbonyl etomidate (MOC-etomidate) and its carboxylic acid metabolite (MOC-ECA). Current amplitudes were normalized to that elicited by 1 mM GABA in the same oocyte. Each data point is the mean normalized amplitude obtained in 4-6 oocytes. The curves are fits of the MOC-etomidate and MOC-ECA concentration-mean response data to a Hill equation yielding an EC50 of 10 ± 2.5 μM for MOC-etomidate and 3.5 ± 0.63 mM for MOC-ECA with respective maxima of 112 ± 6.2 and 61 ± 3.9 and slopes of 1.0 ± 0.19 and 0.84 ± 0.049. Upper inset shows representative current traces of activation by 10 μM MOC-etomidate and 1 mM GABA in the same oocyte. Lower inset shows representative current traces of activation by 10 mM MOC-ECA and 1 mM GABA in the same oocyte.
MOC-etomidate and MOC-ECA also suppressed in vitro cortisol synthesis by human adrenocortical cells in a concentration-dependent manner. However as with direct activation, the concentration ranges over which this action occurred differed between the two compounds by 2-3 orders of magnitude (Figure 4). A fit of the two data sets to a Hill equation yielded IC50s of 0.10 ± 0.02 μM for MOC-etomidate and 30 ± 7 μM for MOC-ECA.
Figure 4.
Inhibition of in vitro cortisol synthesis by methoxycarbonyl etomidate (MOC-etomidate) or its carboxylic acid metabolite (MOC-ECA). Cortisol concentrations were normalized to that measured in the absence of MOC-etomidate or MOC-ECA. Each data point is the mean normalized concentration obtained from 3 wells. The curves are fits of the MOC-etomidate and MOC-ECA concentration-response data to a Hill equation (slope = -1). The calculated IC50s were 0.10 ± 0.02 μM for MOC-etomidate and 30 ± 7 μM for MOC-ECA.
Conclusions
MOC-ECA possesses pharmacological activity; however, in each of the three biological assays we performed, it was approximately 300-fold less potent than its parent compound MOC-etomidate (Table 1).This finding is consistent with our hypothesis that MOC-etomidate's very brief duration of hypnotic action and inability to produce prolonged adrenocortical suppression after bolus administration results from its rapid in vivo hydrolysis to a carboxylic acid metabolite with low pharmacological activity. Forming a metabolite with low potency is particularly important if MOC-etomidate is to be used as a continuous infusion because metabolite accumulation may occur, resulting in a persistent clinical effect if the metabolite possesses significant pharmacological activity. Our studies demonstrating that MOC-ECA is 2-3 orders of magnitude less potent than MOC-etomidate in three assays suggests that recovery after continuous infusions of MOC-etomidate should occur rapidly in humans. However, clinical trials (or computer simulations) will ultimately be required to gauge the importance of any pharmacological activity of MOC-ECA in humans because the carboxylic acid metabolites of rapidly metabolized drugs may accumulate in renal failure. Under these circumstances, metabolites with even minimal activities might reach concentrations sufficient to produce significant pharmacological effects.
Table 1. Pharmacological activities of Etomidate, methoxycarbonyl (MOC)-etomidate, and MOC-ECA.
| Assay | Etomidate | MOC-etomidate | MOC-ECA | MOC-etomidate/MOC-ECA |
|---|---|---|---|---|
| Loss of righting reflexes in tadpoles (EC50) |
2.3 ± 0.13 μM a | 8 ± 2 μMc | 2,800 ± 640 μM | 1/350 |
| Direct activation of GABAA receptors (EC50) |
_ | 10 ± 2.5 μM | 3,500 ± 630 μM | 1/350 |
| Inhibition of in vitro cortisol synthesis (IC50) |
0.0013 ± 0.0002 μM b | 0.10 ± 0.02 μM | 30 ± 7 μM | 1/300 |
When assessed in a variety of assays, the carboxylic acid metabolites GI90291, ASL-8123, and CNS 7054 are similarly orders of magnitude less potent than their respective parent compounds remifentanil, esmolol, and remimazolam.2-6 All four metabolites contain an identical carboxylic acid moiety whose pKa is 4.8.12 Therefore at physiological pH, this moiety is uncharged (i.e., protonated) in 1/400 of all metabolite molecules. This value approximates the potency of MOC-ECA relative to MOC-etomidate, suggesting that MOC-ECA's pharmacological activity (and perhaps those of GI90291, ASL-8123, and CNS 7054) may arise principally from the uncharged, protonated fraction.
Acknowledgments
Funding: Supported by grants R01-GM087316 and R21-DA029253 from the National Institutes of Health, Bethesda, MD and the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Footnotes
Reprints will not be available from the authors.
Disclosures:
Name: Ri Le Ge, M.D., Ph.D.
Contribution: Dr. Ge performed the tadpole and adrenocortical cell studies.
Attestation: Dr. Ge attests to the integrity of the data and analysis.
Conflicts of Interest: This author has no conflicts of interest to declare.
Name: Ervin Pejo, B.S.
Contribution: Mr. Pejo assisted in the conduct of the tadpole and adrenocortical cell studies.
Name: Marian Haburcak, Ph.D.
Contribution: Dr. Haburcak performed the electrophysiology studies.
Conflicts of Interest: This author has no conflicts of interest to declare
Name: S. Shaukat Husain, D.Phil.
Contribution: Dr. Husain designed the synthetic route for methoxycarbonyl-etomidate's metabolite and synthesized it.
Conflict of Interest: Dr. Husain is a co-inventor on a patent application for methoxycarbonyl-etomidate submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-etomidate or related analogs.
Name: Stuart A. Forman, M.D., Ph.D.
Contribution: Dr. Forman oversaw the electrophysiology studies.
Attestation: Dr. Forman attests to the integrity of the electrophysiology studies.
Conflict of Interest: SAF is a co-inventor on a patent application for methoxycarbonyl-etomidate submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-etomidate or related analogs.
Name: Douglas E. Raines, M.D.
Contribution: Dr. Raines designed the studies, interpreted the data, and wrote the manuscript.
Attestation: Dr. Raines attests to the integrity of the data and analysis.
Conflicts: Dr. Raines is a co-inventor on a patent application for methoxycarbonyl-etomidate submitted by the Massachusetts General Hospital. He, his department, his laboratory, and his institution could receive royalties relating to the development of methoxycarbonyl-etomidate or related analogs. Dr. Raines is a consultant for and holds an equity position in Annovation BioPharma, a pharmaceutical company that seeks to develop technologies covered by that patent.
This manuscript was handled by: Tony Gin, FANZCA, FRCA, MD
Contributor Information
Ri Le Ge, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA.
Ervin Pejo, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA
Marian Haburcak, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA
S. Shaukat Husain, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA
Stuart A. Forman, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA
Douglas E. Raines, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, MA
References
- 1.Cotten JF, Husain SS, Forman SA, Miller KW, Kelly EW, Nguyen HH, Raines DE. Methoxycarbonyl-etomidate: a novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111:240–9. doi: 10.1097/ALN.0b013e3181ae63d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westmoreland CL, Hoke JF, Sebel PS, Hug CC, Jr, Muir KT. Pharmacokinetics of remifentanil (GI87084B) and its major metabolite (GI90291) in patients undergoing elective inpatient surgery. Anesthesiology. 1993;79:893–903. doi: 10.1097/00000542-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Hoke JF, Cunningham F, James MK, Muir KT, Hoffman WE. Comparative pharmacokinetics and pharmacodynamics of remifentanil, its principle metabolite (GR90291) and alfentanil in dogs. J Pharmacol Exp Ther. 1997;281:226–32. [PubMed] [Google Scholar]
- 4.Shaffer JE, Quon CY, Gorczynski RJ. Beta-adrenoreceptor antagonist potency and pharmacodynamics of ASL-8123, the primary acid metabolite of esmolol. J Cardiovasc Pharmacol. 1988;11:187–92. [PubMed] [Google Scholar]
- 5.Brosnan RJ, Pypendop BH, Siao KT, Stanley SD. Effects of remifentanil on measures of anesthetic immobility and analgesia in cats. Am J Vet Res. 2009;70:1065–71. doi: 10.2460/ajvr.70.9.1065. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbrook GS. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–6. doi: 10.1097/01.anes.0000267503.85085.c0. [DOI] [PubMed] [Google Scholar]
- 7.Cotten JF, Ge RL, Banacos N, Pejo E, Husain SS, Williams JH, Raines DE. Closed-lopp Continuous Infusions of Etomidate and Etomidate Analogs in Rats: A Comparative Study of Dosing and the Impact on Adrenocortical Function. Anesthesiology. 2011 doi: 10.1097/ALN.0b013e31821950de. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotten JF, Forman SA, Laha JK, Cuny GD, Husain SS, Miller KW, Nguyen HH, Kelly EW, Stewart D, Liu A, Raines DE. Carboetomidate: a pyrrole analog of etomidate designed not to suppress adrenocortical function. Anesthesiology. 2010;112:637–44. doi: 10.1097/ALN.0b013e3181cf40ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waud DR. On biological assays involving quantal responses. J Pharmacol Exp Ther. 1972;183:577–607. [PubMed] [Google Scholar]
- 10.Rusch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2L GABA A receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–92. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- 11.Husain SS, Ziebell MR, Ruesch D, Hong F, Arevalo E, Kosterlitz JA, Olsen RW, Forman SA, Cohen JB, Miller KW. 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: a derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J Med Chem. 2003;46:1257–65. doi: 10.1021/jm020465v. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg LS, Hostetler CK, Wagenknecht DM, Aunet DA. An accurate prediction of the pH change due to degradation: correction for a “produced” secondary buffering system. Pharm Res. 1988;5:514–7. doi: 10.1023/a:1015973425708. [DOI] [PubMed] [Google Scholar]