Factor (F)VIII, a plasma protein that is decreased or defective in individuals with hemophilia A, consists of a heavy chain (HC) comprised of A1A2B domains and a light chain (LC) comprised of A3C1C2 domains (see Ref [1] for review). FVIII is activated by thrombin or FXa to yield FVIIIa, a heterotrimer comprised of subunits designated A1, A2, and A3C1C2, that functions as a cofactor for the serine protease FIXa in the membrane-dependent conversion of zymogen FX to the serine protease, FXa (see Ref. [1] for review). FVIIIa is labile resulting from weak electrostatic interactions between the A2 subunit and the A1/A3C1C2 dimer [2,3] and its dissociation leads to dampening of factor Xase activity. Several FVIII point mutations have been shown to facilitate the rate of dissociation of A2 relative to wild type (WT) and these residues localize to the interface of the A2 domain with either A1 or A3 domains (see Ref [4] for review). Previously, we identified a number of the residues at the A1–A2 and A2–A3 domain interfaces that differentially contribute to the stabilization of FVIII and/or FVIIIa [5]. Subsequent studies identified several mutants at these regions possessing increased stability, and in particular enhanced retention of the A2 subunit in FVIIIa [6]. Based on that study we identified an Asp519Val/Glu665Val variant as the best combination of mutations to maximize FVIIIa stability [7]. Recently, we demonstrated that modifying the A1–C2 interface interaction also enhanced the stability of FVIII [8]. Arg121 at the A1 domain and Leu2302 at the C2 domain (7.73 Å separating Cα atoms; PDB#3CDZ) [9]) were chosen for the site of a nascent disulfide bridge formed by mutating these residues to Cys to yield the variant, Arg121Cys/Leu2302Cys. That study also showed that mutation of Ala108 at A1 domain to Ile improved stability by presumably increasing hydrophobic interactions. These FVIII variants possessed 3–4 fold increased FVIII thermal stability [8].
In this report we tested the hypothesis that more stable FVIII could be generated by combining the mutations at both domain interfaces. These combination FVIII variants were produced using methods as previously described [8]. FVIII thermal and chemical stability were monitored following rates of loss of FVIII activity at 57°C or in guanidinium by FXa generation assays [8], while the decay of FVIIIa activity was monitored over time at 23°C following its activation by thrombin [8]. The combination mutagenesis includes Arg121Cys/Leu2302Cys or Ala108Ile with either Asp519Ala, Asp519Val, Glu665Ala, Glu665Val, Glu1984Ala, or Glu1984Val. In addition, our earlier results from combining the mutations among the four A2 domain interface residues showed the combination of Asp519Val with Glu665Val yielded the highest overall stability parameters [7]. Therefore, we also tested the additional combination of Ala108IIle with Asp519Val/Glu665Val for the purpose of obtaining a more stable FVIII/FVIIIa.
In general, the combination mutations showed near WT like specific activity values although some exceptions were noted (Supplemental Table S1). For reasons that are not clear, combining the disulfide-bridged variant with mutations at Glu665 to Ala or Val resulted in essentially zero or ~20% the WT specific activity value, respectively, and these variants were not further evaluated.
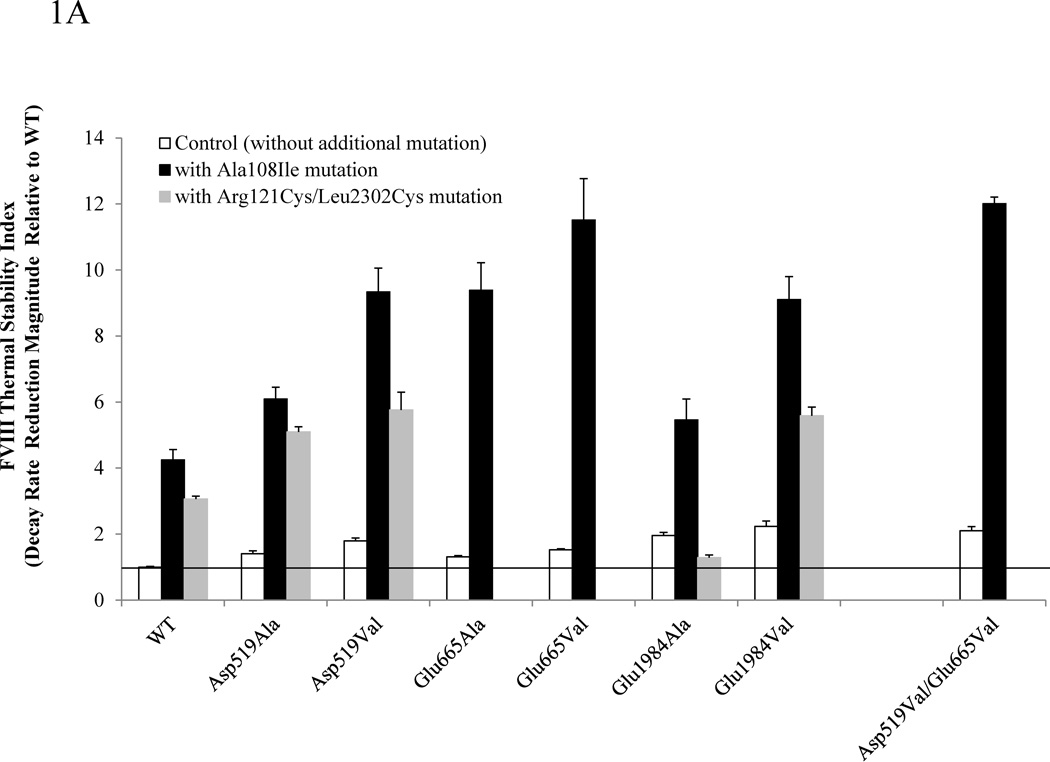
Figure 1A compares thermal stability values for the individual and combination FVIII variants. The first order rate constant for thermal decay at 57°C for WT FVIII was 0.0470 min−1 (see Supplemental Fig S-1A). The degree of stability of each mutant compared to WT was calculated by dividing a decay rate of WT by that of each mutant. Thermal stability values of single mutants (Asp519Ala, Asp519Val, Glu665Ala, Glu665Val, Glu1984Ala, and Glu1984Val) and the Asp519Val/Glu665Val double mutant remained within the range of ~1.3–2-fold increase compared with WT (blank bars, and reference [6]). Interestingly, combination mutants with Ala108Ile showed ~5–12-fold increases in the stability values over WT with Ala108Ile/Asp519Val/Glu665Val showing best stability, whereas the stability of the Ala108Ile variant alone was increased ~4-fold (filled bars). When the A2 domain interface mutations were combined with Arg121Cys/Leu2302Cys, we observed up to an ~5.5-fold increase in the stability index over the WT value, while the stability of the Arg121Cys/Leu2302Cys variant was increased ~3-fold over WT (hatched bars). In comparing increases in stability values for FVIII variants resulting from the additional mutation of Ala108Ile, we noted an ~4-fold increase in all 8 cases. A similar comparison resulting from the additional mutation of Arg121Cys/Leu2302Cys showed an ~3-fold increase observed in 4 out of 5 cases examined.
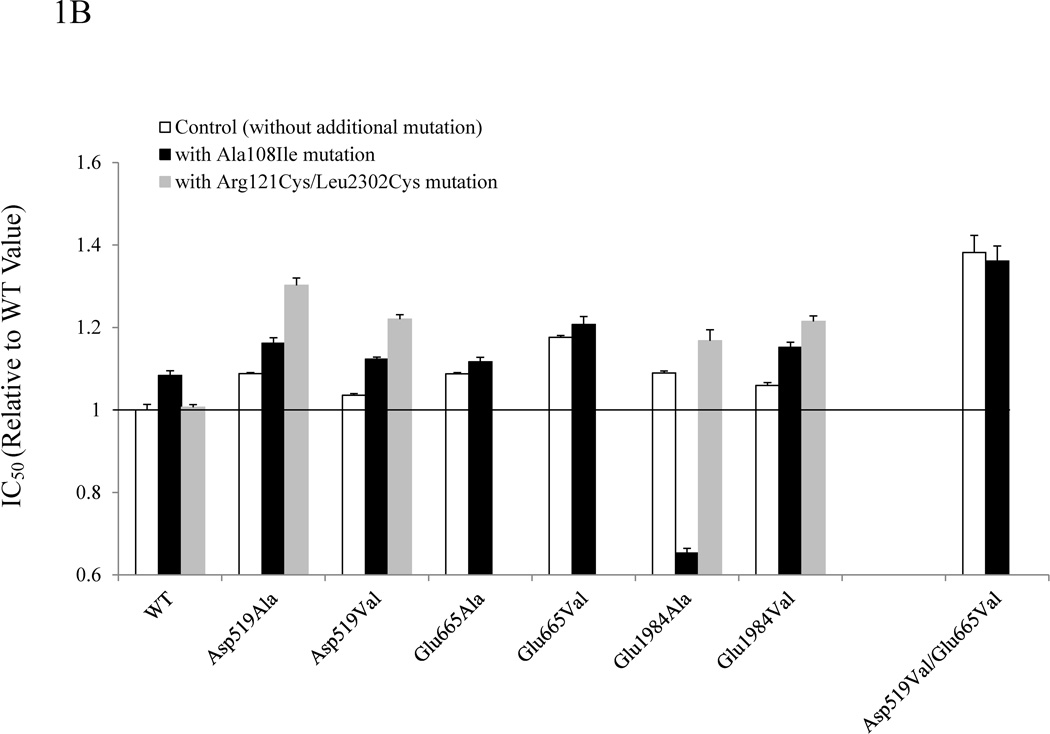
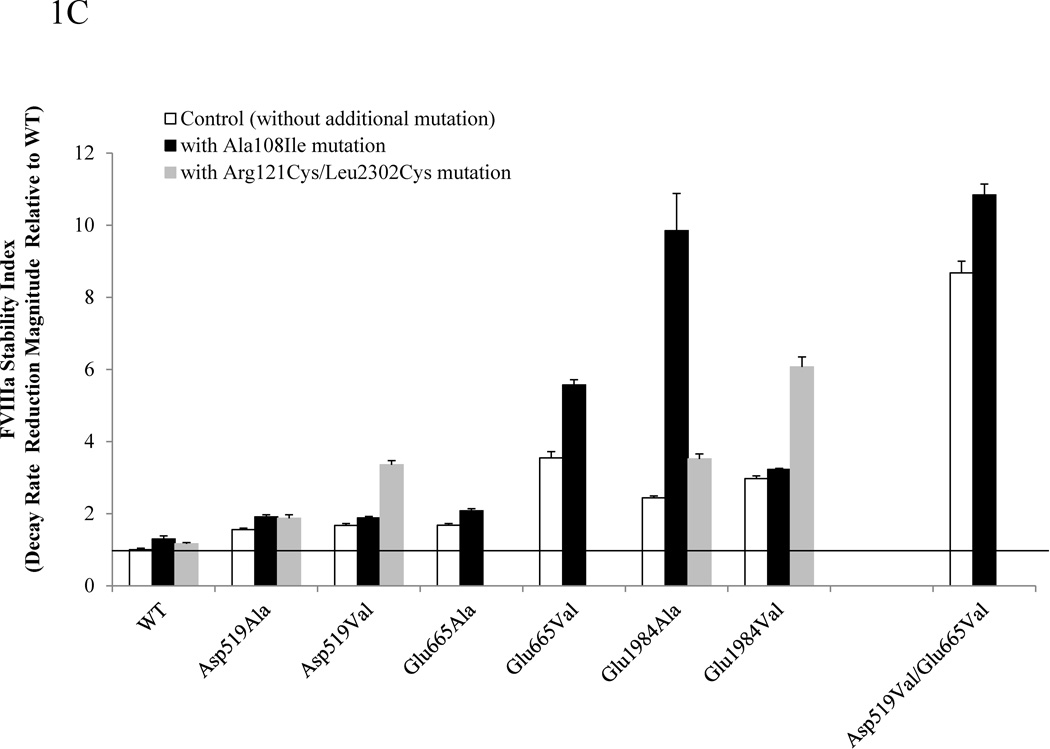
Figure 1.
Summary of stability data for FVIII Arg121Cys/Leu2302Cys or Ala108Ile in combination with A2 domain interface mutations. FVIII thermal decay (A), chemical stability (B), and FVIIIa spontaneous decay (C) for Asp519Ala, Asp519Val, Glu665Ala, Glu665Val, Glu1984Ala, Glu1984Val, or Asp519Val/Glu665Val mutants (open bars) and the variants where each of these mutants were combined with either Ala108Ile (solidbars)or Arg121Cys/Leu2302Cys (grey bars) are shown. The first order rate constants for FVIII thermal decay of WT and mutants FVIII (4 nM) at 57°C, IC50 for guanidinium, and the first order rate constants for FVIIIa decay were obtained by methods previously described [8]. For FVIII thermal decay (A) or FVIIIa decay (C) bar heights correspond to stability index values obtained by dividing the decay rate of WT by that of the variant. For guanidinium denaturation (B) results are expressed as relative IC50 values as compared with WT FVIII. Each bar represents the value averaged from three separate determinations and standard deviation values were drawn.
The increase in FVIII thermal stability appeared more pronounced by changing the A1–C2 interface than by changing the A2 domain interface [8]. In that study the binding affinity of the Ala108Ile-derived A1 subunit for purified C2 subunit showed a 3.7-fold increase compared with the affinity determined for WT-derived A1. This magnitude of increase in affinity appeared well-correlated with the increase in thermal stability (4.2-fold). Therefore, the significant increase in thermal stability of Ala108Ile/Asp519Val/Glu665Val (~12-fold) over WT may be interpreted as a result of large increases in binding energy from both interfaces.
Figure 1B compares relative IC50 values following denaturation of FVIII at increasing concentrations of guanidinium. Supplemental Figure S-1B shows a typical denaturation profile from which an IC50 value, which correlates with the degree of chemical stability, is determined. The IC50 value following guanidinium denaturation of WT FVIII was 0.82 M. Single mutants were uniformly more resistant than WT to guanidinium showing ~5–20% increases in IC50 values with the Asp519Val/Glu665Val double mutant showing an ~40% increase (open bars; and [6]). When the A2 domain interface mutations were combined with the Ala108Ile variant, IC50 values were increased by ~13 2013 22% over WT, whereas the stability of the Ala108Ile variant alone was increased ~8% over WT. Similarly, the combination of the A2 domain interface variants with Arg121Cys/Leu2302Cys yielded IC50 values that were increased by ~17 – 30% over WT with the exception of the Glu1984Ala variant, whereas the stability of Arg121Cys/Leu2302Cys alone was similar to WT. In comparing the IC50 values of WT and each A2 domain interface mutant with the Ala108Ile-containing combination mutant, we observed an increase of 5–10% in 6 out of 8 cases, while comparison with the Arg121Cys/Leu2302Cys combination mutant, resulted in ~10–25% increases in 4 out of 5 cases examined.
Enhancement of FVIIIa stability was also observed in combinations of the variants (Fig. 1C). The combination of an A2 domain interface mutation with Ala108Ile showed improved FVIIIa stability (2–11-fold increases in stability values relative to WT (WT decay value due to A2 dissociation = 0.140 min−1; see Supplemental Fig S-1C)), whereas the Ala108Ile variant was only marginally increased (~1.3-fold) relative to WT. The Asp519Val/Glu665Val/Ala108Ile triple mutant showed a maximal increase in this parameter value. Furthermore, the combination of an A2 domain interface mutation with Arg121Cys/Leu2302Cys also improved FVIIIa stability (~2–6-fold increases relative to WT). Again, Arg121Cys/Leu2302Cys alone showed marginal effects (1.2-fold increase relative to WT). Overall, FVIII/VIIIa stability experiments demonstrate that A2 domain interface mutation combined with A1–C2 interface mutation consistently yielded more stable FVIII/FVIIIa variants suggesting that both interfaces independently contribute to FVIII/FVIIIa stability. With few exceptions, combination of the Ala108Ile or Arg121Cys/Leu2302Cys in combination with the A2 domain interface variant yielded relatively small increases in FVIIIa stability. Thus the majority of the observed FVIIIa stability increase was derived from alteration of the A2 domain interface.
It was not surprising that a more pronounced effect on FVIIIa stability was seen in the A2 domain interface mutations compared with Arg121Cys/Leu2302Cys or Ala108Ile, since A2 subunit dissociation is a primary cause of FVIIIa inactivation [6]. We previously showed that mutants at the A2 domain interface, either singly or in combination, showed higher level of thrombin generation potential than WT [7]. As shown in Supplemental Fig S-2 this increase in thrombin generation potential attributed to Ala108Ile was relatively minor suggesting that FVIIIa stability is more critical for this effect than FVIII stability.
The combination of either Asp519 variant with Arg121Cys/Leu2302Cys or Ala108Ile yielded additive increases in stability. However, some exceptions were observed in combination variants containing Glu665 or Glu1984. For example, variants where Arg121Cys/Leu2302Cys were combined with a Glu665 mutation lost ~80–00% of FVIII specific activity. Furthermore, selected variants containing Glu1984Ala showed variable effects on FVIII/FVIIIa stability as well as reduced specific activity. The reason(s) for these exceptions are not known. Previously we demonstrated that hydrogen bonds from several acidic residues at the A2–A3 interface contributed to the stability of FVIIIa, but not FVIII [10]. Residues Glu665 and Glu1984 are located at this interface. Thus, the unpredicted behavior of some combination variants may reflect multiple interactions occurring at this location.
The FVIII crystal structure [9] shows the A2 domain interfaces and A1–C2 interface are separated with an estimated Cα distance of the closest approaching residues (Glu1984 and Ala108) ~30.9Å. Thus, it is unlikely that a change in one region would significantly affect the structure of the other region. For this reason we predicted combining the two classes of mutations would have additive effects on stability parameters. Indeed, most of the mutants showed additive increases in stability following mutation at both interfaces. Thus we primarily conclude that both interfaces contribute to FVIII/VIIIa stability independently.
Supplementary Material
Acknowledgements
We thank Pete Lollar and John Healey for the factor VIII cloning and expression vectors. This work was supported by NIH grants HL38199 and HL76213.
Footnotes
An account of this work was presented at the 23nd Congress of the International Society on Thrombosis and Haemostasis, July 25, 2011, Kyoto, Japan.
Disclosure of Conflict of Interest
The authors state that they have no conflict of interest.
References
- 1.Fay PJ. Activation of factor VIII and mechanisms of cofactor action. Blood Rev. 2004;18:1–15. doi: 10.1016/s0268-960x(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 2.Fay PJ, Haidaris PJ, Smudzin TM. Human factor VIIIa subunit structure. Reconstruction of factor VIIIa from the isolated A1/A3-C1-C2 dimer and A2 subunit. J Biol Chem. 1991;266:8957–8962. [PubMed] [Google Scholar]
- 3.Lollar P, Parker CG. pH-dependent denaturation of thrombin-activated porcine factor VIII. J Biol Chem. 1990;265:1688–1692. [PubMed] [Google Scholar]
- 4.Fay PJ, Jenkins PV. Mutating factor VIII: lessons from structure to function. Blood Rev. 2005;19:15–27. doi: 10.1016/j.blre.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Wakabayashi H, Fay PJ. Identification of Residues Contributing to A2 Domain-dependent Structural Stability in Factor VIII and Factor VIIIa. J Biol Chem. 2008;283:11645–11651. doi: 10.1074/jbc.M710252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakabayashi H, Varfaj F, Deangelis J, Fay PJ. Generation of enhanced stability factor VIII variants by replacement of charged residues at the A2 domain interface. Blood. 2008;112:2761–2769. doi: 10.1182/blood-2008-02-142158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakabayashi H, Griffiths AE, Fay PJ. Combining mutations of charged residues at the A2 domain interface enhances factor VIII stability over single point mutations. J Thromb Haemost. 2009;7:438–444. doi: 10.1111/j.1538-7836.2008.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakabayashi H, Griffiths AE, Fay PJ. Increasing hydrophobicity or disulfide bridging at the factor VIII A1 and C2 domain interface enhances procofactor stability. J Biol Chem. 2011;286:25748–25755. doi: 10.1074/jbc.M111.241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngo JC, Huang M, Roth DA, Furie BC, Furie B. Crystal structure of human factor VIII: implications for the formation of the factor IXa-factor VIIIa complex. Structure. 2008;16:597–606. doi: 10.1016/j.str.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Wakabayashi H, Fay PJ. Identification of Residues Contributing to A2 Domain-dependent Structural Stability in Factor VIII and Factor VIIIa. J Biol Chem. 2008;283:11645–11651. doi: 10.1074/jbc.M710252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.