Abstract
Hallmark features of type 2 diabetes mellitus include glucosuria and polyuria. Further, renal aquaporin 2 is pivotal to regulation of fluid excretion and urine osmolality. Accordingly, we tested the hypothesis that the db/db mouse displays increased glucosuria and fluid excretion but reduced urine osmolality in association with decreased renal aquaporin 2 level. In addition, we examined the effect of chromium picolinate (Cr(pic)3) which is purported to improve glycemic control. The db/db mice excreted more urine in association with marked glucose excretion but lower urine osmolality than db/m control group. Light microscopic examination of renal tissue revealed proliferation of tubular structures in db/db compared to the db/m mice, a feature validated with Ki67 immunostaining. Further, these tubules showed generally similar immunostaining intensity and pattern for aquaporin 2 indicating that proliferated tubules are of distal origin. On the other hand, renal aquaporin 2 protein level was significantly higher in the db/db than db/m group. Treatment of db/db mice with Cr(pic)3 reduced plasma glucose and hemoglobin A1c (~ 15–17%, p<0.05) and Ki67 positive cells but other parameters were similar to their untreated counterparts. Collectively, these findings suggest that proliferation of renal distal tubules and increased aquaporin 2 level likely represent an adaptive mechanism to regulate fluid excretion to prevent dehydration in the setting of marked glucosuria in the db/db mouse, features not affected by Cr(pic)3 treatment. These observations are of relevance to increasing interest in developing therapeutic agents that facilitate renal glucose elimination.
Keywords: Diabetes, kidney, Glucosuria, Aquaporin 2, Ki67, Urine osmolality, Chromium picolinate
Introduction
Water channels or aquaporins (AQPs) are membrane-associated proteins serving in the transfer of water and small molecules across cell membranes. Thirteen mammalian AQPs (AQP 0-12) have been identified, eight of which are expressed in the kidney (Nielsen et al., 2007; Schrier, 2007; Fenton and Knepper, 2007; Verbalis, 2006; Frøkiaer and Nielsen, 1997). Among them, AQP2 is well-recognized as a pivotal player in regulation of urine concentration. It is expressed in the principal cells of the renal cortical and inner medullary collecting ducts and is abundant both in the apical plasma membrane and subapical vesicles. The gene expression as well as translocation and insertion of AQP2 into plasma membrane are regulated by both vasopressin-dependent and vasopressin-independent mechanisms (Cheng et al., 2009).
Hallmark feature of type 2 diabetes mellitus include increased plasma glucose concentration and consequent increased urinary fluid and glucose excretions. Thus, it is plausible to suggest that the increased demand for fluid excretion, secondary to marked glucosuria, would be associated with adaptive changes in renal AQP2. In order to explore this aspect, we utilized a highly relevant animal model of type 2 diabetes mellitus, namely the db/db mouse and its lean db/m control; the db/db mouse is suggested to be the most relevant animal model of human type 2 diabetic nephropathy (Sharma et al., 2003; Koya et al., 2000). Accordingly, we tested the hypothesis that the db/db mouse displays increased glucosuria and fluid excretion but reduced urine osmolality in association with decreased renal aquaporin 2 level. In addition, we examined potential impact of chromium picolinate (Cr(pic)3)) in the db/db mouse; Cr(pic)3 is a nutritional supplement purported to exert beneficial effects on glycemic control through amplification of insulin signaling (Lamson and Plaza, 2002; Cefalu and Hu, 2004; Mozaffari et al., 2009; 2011; Wang et al. 2006). Thus, we conjectured that improved glycemic control should reduce glucosuria and urinary fluid excretion associated with restoration of urine osmolality and renal AQP2 level towards that of the lean db/m control.
Methods and Materials
Five-week-old male db/m and db/db mice were obtained from the Jackson Laboratories (Boston, MA) and housed in the laboratory animal facilities at the Georgia Health Sciences University. The use of animals for these studies was approved by the Institutional Animal Care and Use Committee. At 6 weeks of age, the db/db mice were randomly assigned to either remain on the regular rodent diet (Harlan Teklad diet number 8604) or switched to the 8604-based diet that was supplemented with 100 mg/kg of chromium as chromium picolinate (i.e., db/db; 100 Cr; Harlan Teklad diet number 07604; n = 6–8 mice/group/condition). Lean, non-diabetic db/m controls were provided with the 8604 diet (without supplemental chromium). Based on measurement of food intake, the Cr(pic)3-enriched diet provided chromium at a dose of 9.7 ± 0.7 mg/kg/day. The dose of Cr(pic)3 was based on our recent studies which examined the effects of several doses of the nutritional supplement on the kidney and glycemic control (Mozaffari et al., 2011).
At about 6 months of age, two consecutive 48-hour urine samples were collected from each animal for determination of urinary excretion of glucose, urine osmolality and fractional excretion of water; the latter parameter was calculated using creatinine clearance which in turn was based on measurement of plasma and urinary creatinine as described previously (Mozaffari et al., 2009; 2011). For determination of plasma glucose and insulin concentrations, the animals were fasted overnight and blood samples were obtained from the retro-orbital plexus of the conscious animal. Plasma glucose concentration was measured using a Beckman Glucometer II and plasma insulin concentration determined using a radioimmunoassay Kit (MP Biomedicals, LLC; Solon, OH). As an index of insulin sensitivity, the quantitative insulin sensitivity check index (QUICKI) value was calculated as follows: 1/(log insulin (μU/ml)) + log glucose (mg/dL)). For assessment of chronic glycemic status, hemoglobin A1c was measured using a drop of blood from the tail (Bayer HealthCare - Diabetes Care, Sunnyvale, California).
After collection of metabolic data, the animals were anesthetized with sodium pentobarbital (40 mg/kg) and renal tissue procured for Western blot analysis (i.e., stored in liquid nitrogen until assayed) and histopathological studies (i.e., 10% formalin-fixed). Formalin-fixed and paraffin-embedded kidney blocks were cut in 4 μm sections for subsequent hematoxylin-eosin (H&E) staining which revealed increased proliferation of renal tubules. Thus, Ki67 immunostaining, using a rabbit polyclonal antibody (ABCAM, Cambridge, MA), was carried out to detect active cell proliferation. Determination of Ki67 positive cell number utilized the BIOQUANT computerized image analysis system (Nashville, TN). After obtaining images, in initial automated steps of the image analysis, digital color thresholds distinguished the immunoreactive objects followed by counting of immunoreactive cells in a specific area (1500 x 1125 μm; renal tissues from 3 db/m mice and 4 db/db mice/group were subjected to Ki67 immunostaining and image analysis. Also, immunohistochemical staining was carried out utilizing anti-aquaporin 2 primary antibody (SigmaAldrich, St. Louis, MO; Mozaffari et al., 2010).
For assessment of renal AQP2 level, renal tissue was pulverized and the tissue powder was added to the isolation buffer (10mM triethanolamine, 250mM Sucrose, PH 7.6, 1 μg/ml Leupeptin, PMSF (2 mg/ml)), sonicated and sodium dodecyl sulfate added to a final concentration of 1% prior to centrifugation; the supernatant was used for protein assay. Standard protocols were used for Western blot analysis as described previously (i.e., 10% gel, electrophoretic protein transfer to nitrocellulose membrane, primary antibody to aquaporin 2, secondary antibody and detection by enhanced chemiluminescence; GE Healthcare UK, Buckinghamshire UK; (Mozaffari et al., 2009; Mozaffari et al., 2010). The protein expression data were corrected for β-actin and expressed as percent of the control group.
Statistics
All data were analyzed by the analysis of variance. Newman-Keuls post hoc test was used for comparison of mean values (significance of criteria of p<0.05). Data are reported as means SEM.
Results
Body weight was significantly higher in the db/db mice than their lean counterparts (42.3 ± 0.8 vs. 29.4 ± 0.8 g); high-dose Cr(pic)3 treatment did not affect body weight (44.8 ± 1.5 g). As expected, plasma glucose concentration was significantly higher in the db/db mice than their lean control (37.3 ± 1.5 vs., 9.2 ± 0.4 mmol/L). Chronic treatment with high-dose Cr(pic)3 was associated with a mild, albeit, significant reduction in plasma glucose concentration (31.0 ± 1.9 mmol/L; p<0.05). Plasma insulin concentration was moderately elevated, albeit non-significant, in the untreated db/db (120.4 ± 11.0 pmol/L) and Cr(pic)3-treated (268 ± 77.6 pmol/L) db/db mice compared to the db/m control group (61.6 ± 4.8 pmol/L). As a result, the QUICKI insulin sensitivity index was significantly lower in the untreated db/db (0.247 ± 0.003) and Cr(pic)-treated db/db (0.243 ± 0.009) mice compared to the db/m group (0.320 ± 0.004). In addition, hemoglobin A1c was markedly higher in the db/db than db/m mice (12.1 ± 0.3 vs. 4.5 ± 0.8%). The Cr(pic)3-treated db/db showed a mild, albeit significant, reduction in Hemoglobin A1c value (10.1 ± 0.5%).
As shown in Figure 1A-C, water intake (4 fold), urine excretion (12 fold), fractional water excretion (50 fold) and the ratio of urine excretion to fluid intake (panel D) were markedly higher in the db/db mice than their lean counterparts. On the other hand, urine osmolality was markedly lower (about 3 fold) in the db/db than db/m control group (Figure 1E). While the db/m group excreted a negligible amount of glucose into the urine, the db/db mice displayed marked glucosuria which was nearly a thousand fold higher than the db/m controls (Figure 1F). Treatment of db/db mice with Cr(pic)3 did not significantly affect any of the renal functional parameters (Figure 1A-F). Urinary sodium excretion tended to be higher in the two diabetic groups (0.39 ± 0.10 and 0.63 ± 0.07μEq/ 48 hr in untreated and Cr(pic)3-treated db/db) than db/m mice (0.20 ± 0.04 μEq/ 48 hr). Also, the two db/db groups excreted more potassium compared to the db/m controls (2.44 ± 0.44 and 2.46 ± 0.22 μEq/ 48 hr, untreated and Cr(pic)3-treated db/db, respectively, vs. 1.39 ± 0.20 μEq/ 48 hr; p<0.05). Plasma sodium concentration (145.3 ± 2.4, 145.9 ± 2.3 and 146.7 ± 2.3 mEq/L) and potassium concentration (5.2 ± 0.3, 6.6 ± 0.7 and 5.5 ± 0.4 mEq/L) were similar in db/m, db/db and Cr(pic)3-treated db/db, respectively.
Figure 1.
Bar graphs show water intake (A), urine excretion (B), fractional water excretion (C), the ratio of urine excretion to water intake (D), urine osmolality (E) and glucose excretion (F) in lean db/m control, untreated diabetic obese db/db and chromium picolinate-treated db/db mice (db/db; 100 Cr). Data are means ± SEM of n=6–8 mice/ group.
* p<0.05 compared to other groups.
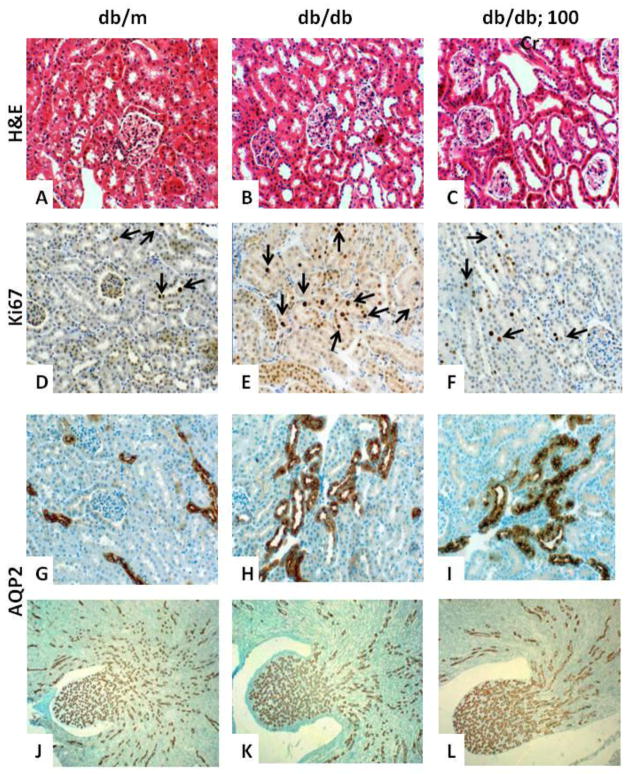
Histopathological examination of renal tissue sections revealed a number of abnormalities in the diabetic kidneys compared to that of the nondiabetic group. Of relevance to the focus of the present investigation was the apparent proliferation of the renal tubules in the db/db mouse compared to the kidney of db/m control (Figure 2, panel B vs. A). This was further verified by immunostaining for Ki67 which revealed increased number of Ki67 positive cells in the db/db than db/m group (36.4 ± 7.6 vs. 10.4 ± 3.2; Figure 2, panels D-E). To further establish that these proliferative tubules are of distal origin, kidney sections were immunostained for AQP2 which revealed intense reactions in cells of tightly packed proliferative tubules (Figure 2, panel H vs. G). However, the luminal and subapical localization of AQP2 as well as degree of AQP2 immunostaining intensity appeared generally similar among the diabetic and nondiabetic controls (Figure 2, panels G-H). The Cr(pic)3-treated db/db group showed generally similar renal distal tubule proliferation (Figure 2, panel C) although the number of Ki67 positive cells was lower than their untreated counterparts (16.3 ± 2.5 vs. 36.4 ± 7.6; p<0.05) and similar to the db/m control group (10.4 ± 3.2). However, immunostaining pattern and intensity for AQP2 (Figure 2, panel I) were similar to those of their untreated counterparts (Figure 2, panel H). Also of interest was the finding of seemingly enlarged renal pelvis of db/db groups (Figure 2K-L), compared to that of the db/m group (Figure 2J), displaying densely packed AQP2-positive collecting ducts.
Figure 2.
Representative panels show hematoxylin-eosin (H&E)-stained renal tissue from experimental groups, described in Figure 1. Renal tissues of db/db mice, irrespective of Cr(pic)3 treatment (panels B-C), show prominent renal tubule proliferation compared to their db/m control (panel A), a feature that was confirmed by Ki67 immunostaining (panels D-F, arrows). Also shown are immunostaining for renal AQP2 in the cortical (panels G-I) and pelvis (panels J-L) segments for each group. Panels are representative of n=6–8 mice/group for H&E and AQP2 immunostaining and n=3–4/group for Ki67 immunostaining. Panels A-I: 200x and panels J-L: 40x.
Western blot studies using a rabbit polyclonal antibody against renal AQP2 revealed the prominence of a 30–35 kDa protein band in nondiabetic and diabetic mice (Figure 3). However, the db/db mice, irrespective of Cr(pic)3, showed about 3–4 fold increase in AQP2 protein level (Figure 3).
Figure 3.
Bar graphs show percent expression of aquaporin (AQP)2 in renal tissue from experimental groups described in Figure 1. Protein expression is expressed as percent of the db/m control group. Also shown are representative blots for AQP2 and β-actin. Data are means ± SEM of n=6–8 mice/ group.
* p<0.05 compared to the other groups.
Discussion
The present study shows that the markedly hyperglycemic and glucosuric obese diabetic db/db mice display morphological evidence of proliferation of renal tubules which is consistent with increased Ki67 immunostaining compared to their db/m controls; however, these tubules show similar pattern and intensity of immunostaining for AQP2 compared to their lean nondiabetic controls. Further, renal tissue AQP2 protein level was markedly higher in the db/db compared to db/m mice, a feature consistent with increased proliferation of AQP2 positive tubules. These changes were associated with marked increases in fluid intake, urine excretion, fractional water excretion but prominent reduction in urine osmolality. Interestingly, Cr(pic)3 treatment was associated with a mild improvement in glycemic control of db/db mice. Nonetheless, Cr(pic)3-treated db/db mice remained markedly hyperglycemic and glucosuric and displayed similar AQP2 immunostaining pattern/ intensity and kidney AQP2 protein level compared to those of untreated db/db group. However, despite similar morphologic evidence for distal tubule proliferation, renal tissue of the Cr(pic)3-treated db/db mice showed reduced number of Ki67 positive cells compared to their untreated counterparts. To our knowledge, this is the first demonstration of proliferation of renal distal tubules, with preserved AQP2 expression, in an animal model of type 2 diabetes mellitus which likely reflects a compensatory mechanism to prevent excessive water loss and dehydration in the face of marked glucosuria.
The db/db mouse is one of the best characterized and most intensively studied animal models of human type 2 diabetic nephropathy; it lacks the leptin receptor thereby predisposing the animal to develop obesity and type 2 diabetes. Previous studies have primarily focused on abnormalities of glomerular structure and function of db/db mice (Sharma et al., 2003; Koya et al., 2000; Mozaffari et al., 2011). On the other hand, adaptive renal functional changes secondary to marked hyperglycemia and glucosuria in type 2 diabetes mellitus have received little attention.
The 6-month-old db/db mice of the present study displayed hallmark physical and metabolic features of type 2 diabetes mellitus. Further, the prominent renal functional abnormalities (e.g., increased fluid and glucose excretions but reduced urine osmolality) were associated with morphologic evidence of marked proliferation of renal cortical distal tubules which was further confirmed by increase in number of Ki67 positive cells. The Ki67 antigen is a cellular marker for proliferation which can be exclusively detected within the cell nucleus during the interphase; however, in mitosis, most of the protein is relocated to the surface of the chromosomes. The Ki67 protein is present during all active phases of the cell cycle but is absent from resting cells (Ye et al., 2011). Clearly, proliferation of renal distal tubules reflects functional adaptation to marked hyperglycemia and glucosuria in db/db mice.
A prominent function of renal distal tubules relates to regulation of urine osmolality with AQP2 playing a pivotal role in the process. We conjectured that the marked reduction in urine osmolality may reflect a downregulation of renal AQP2 expression despite prominent proliferation of renal distal tubules in db/db mice thereby facilitating urinary fluid excretion to cope with the need for excretion of excessive loads of glucose. Interestingly, however, kidney AQP2 protein level was markedly higher in the db/db mice compared to their db/m controls. Subsequent immunostaining for renal AQP2 revealed that, similar to kidneys of db/m mice, renal tissues of db/db mice displayed luminal and subapical localization of AQP2 of generally similar intensity. Clearly, the marked increase in total renal AQP2 protein level of db/db mice relates primarily to proliferative changes of renal distal tubules in the db/db mouse with preserved AQP2 expression.
Previous studies have used animal models of type 1 diabetes mellitus to explore the consequences of marked glucosuria on renal water channels. Earlier studies documented increased AQP2 abundance in rat kidneys after 2 weeks of streptozotocin-induced type 1 diabetes; the authors attributed upregulation of renal AQP2 to compensation for polyuria secondary to marked glucosuria (Nejsum et al., 2001; Bardoux et al., 2001). However, subsequent studies, utilizing streptozotocin-induced type 1 diabetes in rats and mice, have also reported decreased or no change in renal AQP2 level (Leung et al., 2005; Vidotti et al., 2008; Blount et al., 2008). Nonetheless, a more recent study explored the relation between renal AQPs and urine excretion in the setting of hyperglycemia in type 1 diabetic mice (Satake et al. 2010b). Accordingly, it was shown that inhibition of expression of renal AQPs, by lithium carbonate treatment, markedly increases urine excretion without affecting plasma glucose level. These observations led the authors to conclude that increased expression of AQP2 in the setting of hyperglycemia/glucosuria serves as a compensatory mechanism to alleviate dehydration. Another study using the KKAy mouse, a model of type 2 diabetes, also showed increased AQP2 protein level (Satake et al. 2010a). Importantly, however, these studies did not include histological examination of renal tissue to assess potential proliferative changes in renal distal tubules. On the other hand, using a highly relevant animal model of type 2 diabetes, we now show that the marked increase in AQP2 protein level is primarily related to proliferation of cortical collecting tubules. Clearly, unraveling of mechanisms by which type 2 diabetes impacts renal water channels will enhance our understanding of the fundamental processes that regulate renal function in the setting of marked glucosuria. This investigation would also be of relevance to increasing interest in developing agents that facilitate renal elimination of glucose (i.e., inhibitors of proximal tubule sodium-glucose cotransporters (SGLT)) as a novel approach to treatment of diabetes mellitus (Chao and Henry, 2010). This is an important consideration since compensatory mechanisms in various pathological conditions can become ineffective over time thereby resulting in further deterioration of the affected organ(s). Whether the use of SGLT inhibitors and the accompanying greater demand for renal glucose elimination would adversely impact proliferative changes of distal tubules and their function remains to be established. Although speculative, a failure of such renal adaptive mechanisms in the diabetic kidney could also potentially lead to therapeutic inefficacy of SGLT inhibitors.
The present study also examined potential effects of Cr(pic)3, a nutritional supplement purported to exert beneficial effects on glycemic control and body weight. Nonetheless, Cr(pic)3-treated mice displayed similar body weight to that of their untreated counterparts, a finding consistent with lack of an effect of the nutritional supplement on body weight of obese Zucker rats (Mozaffari et al., 2009). However, mild (~ 15–17%), albeit significant, reductions in fasting plasma glucose concentration and hemoglobin A1c were detected for Cr(pic)3-treated db/db mice. Nonetheless, the Cr(pic)3-treated db/db mice remained markedly hyperglycemic and were indistinguishable from their untreated counterparts with respect to water intake, urine excretion, glucosuria, urine excretion, AQP2 protein level and immunostaining pattern and intensity for AQP2. Interestingly, however, despite morphological evidence of similar renal distal tubule proliferation to that of untreated db/db mice, the Cr(pic)3-treated db/db mice displayed reduced number of Ki67 positive cells compared to their untreated counterparts. The reason(s) for this observation is not clear from this study. However, it is likely that the nutritional formulation affects the rate of proliferative changes, an aspect that requires establishing temporal relationship of functional and morphological changes associated with progression of type 2 diabetes.
In conclusion, the db/db mouse displays proliferative changes of renal distal tubules in association with increased total AQP2 protein level, polyuria and marked glucosuria but reduced urinary osmolality. These observations are suggestive of renal adaptive changes to cope with increased demand for glucose elimination but prevention of dehydration. Interestingly, however, dietary Cr(pic)3 treatment exerted a mild improvement in glycemic control without significant effects on renal AQP2 level, urine osmolality or morphological evidence of renal distal tubule proliferation despite reduced number of Ki67 positive cells. Collectively, our observations are of relevance to increasing interest for development of therapeutically useful agents that would facilitate renal elimination of glucose thereby achieving more effective glycemic control.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (1R21AT003012-01A2; MSM). The authors thank Nutrition 21 for the generous gift of Cr(pic)3.
Footnotes
Conflict of Interest: The authors do not have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardoux P, Ahloulay M, Le Maout S, Bankir L, Trinh-Trang-Tan MM. Aquaporin-2 and urea transporter-A1 are up-regulated in rats with type I diabetes mellitus. Diabetologia. 2001;44(5):637–645. doi: 10.1007/s001250051671. [DOI] [PubMed] [Google Scholar]
- Blount MA, Sands JM, Kent KJ, Smith TD, Price SR, Klein JD. Candesartan augments compensatory changes in medullary transport proteins in the diabetic rat kidney. Am J Physiol Renal Physiol. 2008;294(6):F1448–52. doi: 10.1152/ajprenal.00600.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27:2741–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- Chao EC, Henry RR. SGLT2 inhibition--a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9(7):551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Chu JYS, Chow BKC. Vasopressin-independent mechanisms in controlling water homeostasis. J Mol Endocrinol. 2009;43(3):81–92. doi: 10.1677/JME- 08-0123. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Knepper MA. Mouse models of the urinary concentrating mechanism in the new millennium. Physiol Rev. 2007;87(4):1083–1112. doi: 10.1152/physrev.00053.2006. [DOI] [PubMed] [Google Scholar]
- Frøkiaer J, Nielsen S. Do aquaporins have a role in nocturnal enuresis? Scand J Urol Nephrol Suppl. 1997;183:31–32. [PubMed] [Google Scholar]
- Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14(3):439–47. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- Lamson DW, Plaza SM. The safety and efficacy of high-dose chromium. Altern Med Rev. 2002;7(3):218–235. [PubMed] [Google Scholar]
- Leung JC, Chan LY, Tsang AW, Tang SC, Lai KN. Differential expression of aquaporins in the kidneys of streptozotocin-induced diabetic mice. Nephrology (Carlton) 2005;10(1):63–72. doi: 10.1111/j.1440-1797.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- McKenna K, Morris AD, Ryan M, Newton RW, Frier BM, Baylis PH, Saito T, Ishikawa S, Thompson CJ. Renal resistance to vasopressin in poorly controlled type 1 diabetes mellitus. Am J Physiol Endocrinol Metab. 2000;279(1):E155–60. doi: 10.1152/ajpendo.2000.279.1.E155. [DOI] [PubMed] [Google Scholar]
- Mozaffari MS, Abdelsayed R, Liu JY, Wimborne H, El-Remessy A, El-Marakby A. Effects of chromium picolinate on glycemic control and kidney of the obese Zucker rat. Nutr Metab. 2009;6:51. doi: 10.1186/1743-7075-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari MS, Abdelsayed R, Wimborne H, Patel C, Liu JY, Schaffer SW. Differential effects of taurine treatment and taurine deficiency on the outcome of renal ischemia reperfusion injury. J Biomed Sci. 2010;17(Suppl1):S32. doi: 10.1186/1423–0127-17-S1-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari MS, Baban B, Abdelsayed R, Liu JY, Wimborne H, Rodriguez N, Abebe W. Renal and Glycemic Effects of High-Dose Chromium Picolinate in db/db Mice: Assessment of DNA Damage. J Nutr Biochemistry. 2011 doi: 10.1016/j.jnutbio.2011.05.004. Accepted/In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejsum LN, Kwon TH, Marples D, Flyvbjerg A, Knepper MA, Frøkiaer J, Nielsen S. Compensatory increase in AQP2, p-AQP2, and AQP3 expression in rats with diabetes mellitus. Am J Physiol Renal Physiol. 2001;280(4):F715–26. doi: 10.1152/ajprenal.2001.280.4.F715. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Kwon TH, Frokiaer J, Agre P. Regulation and dysregulation of aquaporins in water balance disorders. J Intern Med. 2007;261(1):53–64. doi: 10.1111/j.1365-2796.2006.01760.x. [DOI] [PubMed] [Google Scholar]
- Satake M, Ikarashi N, Ichikawa Y, Maniwa A, Toda T, Ito K, Ochiai W, Sugiyama K. The role of renal aquaporin 2 in the alleviation of dehydration associated with diabetic polyuria in KKAy mice. Life Sci. 2010;87(15–16):475–80. doi: 10.1016/j.lfs.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Satake M, Ikarashi N, Kagami M, Ogiue N, Toda T, Kobayashi Y, Ochiai W, Sugiyama K. Increases in the expression levels of aquaporin-2 and aquaporin-3 in the renal collecting tubules alleviate dehydration associated with polyuria in diabetes mellitus. Biol Pharm Bull. 2010;33(12):1965–70. doi: 10.1248/bpb.33.1965. [DOI] [PubMed] [Google Scholar]
- Sharma K, McCue P, Dunn SR. Diabetic kidney disease in db/db mouse. Am J Physiol Renal Physiol. 2003;284(6):F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- Schrier RW. Aquaporin-related disorders of water homeostasis. Drug News Perspect. 2007;20(7):447–453. doi: 10.1358/dnp.2007.20.7.1138161. [DOI] [PubMed] [Google Scholar]
- Verbalis JG. Whole-body volume regulation and escape from antidiuresis. Am J Med. 2006;119(7 Suppl 1):S21–S29. doi: 10.1016/j.amjmed.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Vidotti DB, Arnoni CP, Maquigussa E, Boim MA. Effect of long-term type 1 diabetes on renal sodium and water transporters in rats. Am J Nephrol. 2008;28(1):107– 14. doi: 10.1159/000109967. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin- resistant JCR:LA-cp rats. J Nutr. 2006;136(2):415–420. doi: 10.1093/jn/136.2.415. [DOI] [PubMed] [Google Scholar]
- Ye Y, Wang B, Jiang X, Hu W, Feng J, Li H, Jin M, Ying Y, Wang W, Mao X, Jin K. Proliferative capacity of stem/progenitor-like cells in the kidney may associate with the outcome of patients with acute tubular necrosis. Hum Pathol. 2011 doi: 10.1016/j.humpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]