Abstract
Objective
Vascular impairment, a main feature of the pathogenesis of systemic sclerosis (SSc), involves both the macro- and the microvasculature. We compared and correlated simultaneously measured skin microvascular and brachial artery macrovascular post-occlusive hyperemia in 3 groups: patients with SSc, patients with primary Raynaud’s phenomenon (RP), and healthy volunteers.
Methods
Thirty-three healthy volunteers, 36 patients with primary RP, and 42 patients with SSc were enrolled. For each subject, brachial artery flow-mediated dilation (FMD) and cutaneous post-occlusive reactive hyperemia (PORH) were simultaneously recorded after 5-minute occlusion of the brachial artery. Local thermal hyperemia, nitroglycerin-mediated dilation (NMD), intima-media thickness (IMT), and pulse wave velocity (PWV) were also assessed.
Results
Digital cutaneous peak PORH was altered in patients with primary RP and SSc compared to healthy controls, whereas FMD was not significantly different among all groups. We observed a correlation between digital peak cutaneous vascular conductance and brachial FMD in healthy controls (r = 0.49; p = 0.004), but not in patients with primary RP or SSc. Thermal hyperemia was altered only in patients with SSc. Brachial NMD, IMT, and PWV were not different among all groups.
Conclusion
We observed a loss of the correlation between brachial FMD and digital cutaneous PORH in patients with SSc and primary RP. Microvascular function is impaired in SSc, whereas brachial artery endothelial function is normal. (J Rheumatol First Release June 15 2008)
Keywords: Systemic sclerosis, Raynaud's phenomenon, Microcirculation, Flow-mediated dilation, Post-occlusive hyperemia, Thermal Hyperemia
Keywords: Adult; Aged; Brachial Artery; physiology; Case-Control Studies; Female; Humans; Hyperemia; complications; diagnosis; physiopathology; Male; Microvessels; physiopathology; Middle Aged; Raynaud Disease; complications; physiopathology; Regional Blood Flow; physiology; Scleroderma, Systemic; complications; physiopathology; Skin; blood supply
Vascular impairment, a main feature of the pathogenesis of systemic sclerosis (SSc), involves both the macrovasculature and the microvasculature1. Microcirculatory abnormalities imply interdependent structural2 and functional changes, probably in part related to endothelial nitric oxide (NO)-dependent dysfunction3,4.
A number of different techniques have been used to assess microvascular function in human skin, including post-occlusive reactive hyperemia (PORH) and thermal hyperemia. The cutaneous PORH response is characterized by a precipitous increase in blood flow following a brief arterial occlusion. The local thermal hyperemic response is characterized by an initial peak within the first 5 minutes, a subsequent nadir, and a final sustained plateau. Both responses yield important information regarding microcirculatory function, and each can be assessed noninvasively using laser Doppler flowmetry5. Further, both tests differ with respect to the mechanisms involved in the endothelium-dependent part of the response. A recent study suggests that PORH is mostly dependent on endothelium-derived hyperpolarizing factors6, while the plateau phase of thermal hyperemia is NO-dependent7. Evidence for microvascular dysfunction in SSc comes from data demonstrating that both the amplitude and kinetics of the PORH and the thermal hyperemia are altered compared to healthy subjects8,9. In primary Raynaud’s phenomenon (RP), we previously observed a trend toward decreased PORH peak conductance but a normal thermal hyperemia9.
There is increasing evidence that the macrovasculature is involved in the SSc disease process, relating to structural abnormalities. For example, arterial stiffness is increased in patients with SSc compared to healthy controls10–13 or patients with primary RP14. In contrast, conflicting data exist regarding markers of macrovascular function, with observations suggesting both normal and abnormal brachial artery flow-mediated dilation (FMD)12,15–18. The mechanisms underlying both microvascular and macrovascular dysfunction in SSc are poorly understood. A common mechanism might affect both large arteries and microvessels, with the main hypothesis of impairment of the vessel’s ability to release endothelium-dependent relaxing factors, including NO. However, this assumption is contradictory to specific alterations of the microvasculature in SSc (e.g., neurovascular regulation). Whether micro- and macrovascular dysfunctions are linked, and as such occur at the same time in the disease process, is still unknown. Rajagopalan, et al showed an abnormal PORH in a heterogeneous group of patients with secondary RP compared to patients with primary RP, whereas FMD was preserved15. No data, however, are available about the correlation between macro- and microvascular dysfunction in primary RP and in SSc compared to healthy controls.
As a primary objective, we compared and correlated simultaneously tested microvascular (using PORH and laser Doppler flowmetry) and macrovascular function (using FMD and ultrasonography) between 2 patient groups (SSc and primary RP) and healthy controls. As a secondary objective we used alternative assessments of microvascular and macrovascular function to compare patients with SSc and RP and healthy controls.
MATERIALS AND METHODS
Study population
One hundred eleven subjects were enrolled in this study from March 2004 to July 2005, including 33 healthy volunteers, 36 patients with primary RP, and 42 patients with SSc. Healthy subjects and patients with primary RP were recruited through local newspaper advertisements. Patients with SSc were recruited from the Vascular Medicine Department, Grenoble University Hospital. Patients with primary RP were diagnosed according to the criteria of LeRoy and Medsger19. SSc was classified as the limited form of SSc (lSSc), limited cutaneous (lcSSc), or diffuse cutaneous SSc (dcSSc) with the criteria of LeRoy and Medsger20. All subjects were 18 years of age or older. Subjects taking calcium-channel blockers were instructed to stop medication 1 week before enrollment in the study.
For all subjects, exclusion criteria included cigarette smoking, diabetes mellitus, hypercholesterolemia, or any associated severe disease (cancer, cardiac and pulmonary failure, myocardial infarction, angina pectoris). Grenoble Institutional Review Board approval was obtained, and each subject gave written informed consent before participation.
Study design
This was an open-label physiology study performed in a temperature-controlled room (23°C ± 1°C). All subjects were in a fasting state. After clinical examination, blood pressure and heart rate were measured. Subsequently, the subject was placed in the supine position with each forearm resting at heart level.
After a 30-min resting period, blood flow was occluded for 5 min by inflating a cuff placed on the left arm to 50 mm Hg above the systolic blood pressure. The cuff was then released and brachial artery reactivity and cutaneous blood flow were recorded simultaneously (Figure 1) as described below. After a 30-min recovery period, sublingual administration of 0.4 mg nitroglycerin (an endothelium-independent vasodilator) was performed and the brachial response was again recorded. Thirty minutes later, digital thermal hyperemia was assessed, followed by common carotid intima-media thickness (IMT) and pulse wave velocity (PWV) measurements.
Figure 1.
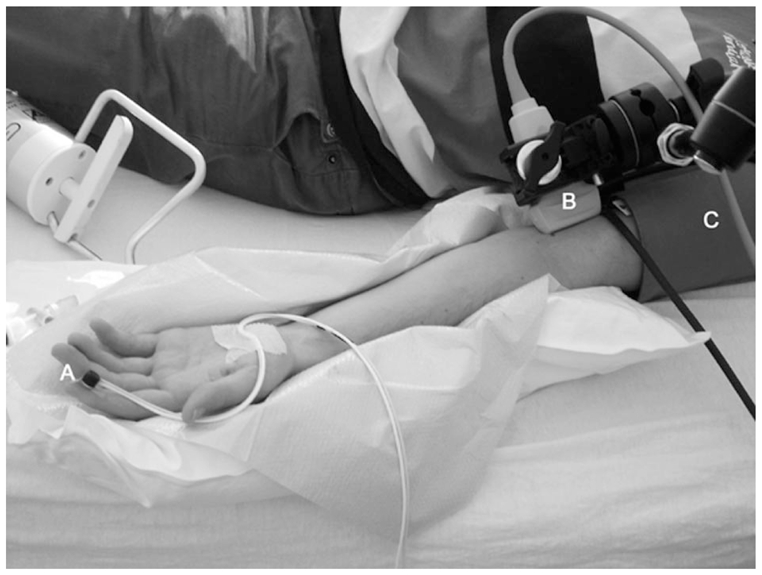
Simultaneous recording of digital skin microvascular and brachial artery macrovascular hyperemia after a 5-minute occlusion of the brachial artery. A: Laser Doppler probe; B: ultrasonography probe; C: pressure cuff.
Digital cutaneous blood flow measurements
Cutaneous blood flow was measured using laser Doppler flowmetry (Periflux System 5000; Perimed, Järfälla, Sweden). A laser probe with integrated local heater (Probe 457; Perimed) was attached to the distal pad of the middle finger of the left hand.
Blood flow was recorded continuously. The amplitude of the PORH was determined by the peak cutaneous vascular conductance as a main criterion (flux in mV divided by mean arterial pressure) following cuff release, and by the peak minus baseline cutaneous vascular conductance as a post hoc analysis. The kinetics of the response was determined by the time to peak hyperemia (time from cuff release to peak hyperemia, in seconds), as described9 (Figure 2).
Figure 2.
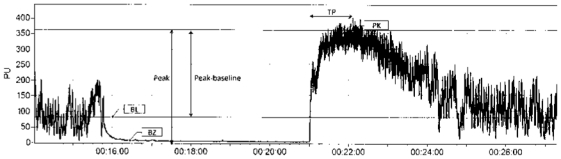
Typical trace of postocclusive reactive hyperemia on the finger pad. The biological zero corresponds to the laser Doppler signal recorded in the absence of blood flow. The amplitude of peak hyperemia is expressed as raw data (perfusion units or mV) or raw data minus baseline, and later corrected for mean arterial pressure to express data as conductances (mV/mm Hg) rather than flux. The kinetics of the response is determined by the time to peak hyperemia (time from cuff release to peak hyperemia). TP: time to peak; BZ: biological zero; BL: baseline; PK: peak.
Digital thermal hyperemia was performed by heating the probe from skin temperature to 42°C over 15 to 22 s and this temperature was maintained for 30 min. After 30 min, the skin sites were heated to 44°C for 5 min to achieve maximal skin blood flow. The amplitude of the thermal hyperemia was determined by baseline, thermal peak, and 42°C thermal plateau, and expressed as cutaneous vascular conductance (mV/mm Hg). Conductance values for the initial peak were averaged over a 1 min period. Conductance values for the 42°C plateau phase were averaged over a 3 min period. The day-to-day reproducibility of PORH and thermal hyperemia has been demonstrated9,21.
Data were digitized, stored on a computer, and analyzed off-line with signal processing software (PeriSoft 2.5.5; Perimed).
Brachial artery reactivity
The endothelial-dependent and -independent functions of the brachial artery were assessed as described22. Ultrasound scans were performed with a high-resolution ultrasonographic scanner (Titan®; SonoSite Inc., Bothell, WA, USA) equipped with a 5–10 MHz linear-array transducer. The brachial artery was scanned over a longitudinal section 3–5 cm above the antecubital fossa. When an adequate image was obtained, the ultrasound probe was secured in position using a clamp (Figure 1).
Postischemic artery diameter was recorded 1 minute after rapid deflation of the blood pressure cuff. The endothelium-dependent FMD was assessed as the percentage increase in arterial diameter from baseline to the post-occlusive period. For the endothelium-independent vasodilation, the maximal effect was assessed by measuring brachial artery diameter 4 minutes after nitroglycerin administration. The nitroglycerin-mediated dilation (NMD) was assessed as the percentage increase in arterial diameter from baseline to 4 minutes post-nitroglycerin administration. Ultrasound images were analyzed offline manually, averaging the arterial diameter along a 10-mm segment. Diameters from 3 consecutive end-diastolic frames (identified by the electrocardiographic R wave) were averaged to yield the brachial artery diameter during respective experimental stages. Two operators performed the FMD measurements. The intraobserver coefficient of variation was 0.8% and 17% for the baseline diameter and FMD, respectively. The interobserver coefficient of variation was 1.1% and 24% for the baseline diameter and FMD, respectively.
Intima-media thickness
B-mode ultrasonography was performed (HP Sonos 2500; Hewlett-Packard, Santa Clara, CA, USA) and IMT was defined as the distance separating the most internal parts of 2 hyperechogenic lines, as described23. For all patients, a zoom was used to define a zone of interest of 20 mm in length (stretching from 10 to 30 mm above the carotid bifurcation). The images were recorded in end-diastole and then stored on an optical disk for subsequent analysis by a specific validated program (TIMC Laboratory, Grenoble University Hospital). IMT measurements were carried out on areas free of atheroma and then averaged. The values of IMT for any subject were the mean values for the 2 common carotid arteries.
Carotid–femoral pulse wave velocity
To determine the carotid-femoral PWV, 2 pulse transducers were fixed on the skin over the right common carotid and femoral arteries as described23. The time delay of simultaneously recorded pulse waves was measured with a Complior device (Artech Medical, Pantin, France) and then averaged over 10 consecutive cycles. The carotid-femoral PWV was calculated as the distance between the arterial sites divided by the time delay.
Statistical analysis
Quantitative data are expressed as the median (interquartile). Qualitative data are expressed as the number and percentage. Normality and variance homogeneity analysis were tested prior to quantitative data analysis. As the amplitude of the hyperemic responses did not follow a normal distribution, nonparametric statistical methods were performed: Kruskal-Wallis test and Mann-Whitney test for between-group comparisons, and Spearman rank correlation test for the relationship between quantitative variables. Two-sided significance tests were used throughout. We considered p values < 0.05 as significant, corrected by Bonferroni’s method for multiple comparison. Sample size calculations for the main objective are based to detect a correlation of 0.4 between FMD and digital cutaneous PORH, with α = 0.05 and power (1 − β) = 0.8. Forty-one patients were required in the SSc group (nQuery Advisor® for Windows v 6.01; Statistical Solutions, Saugus, MA, USA).
RESULTS
Population characteristics
The demographic and clinical characteristics of the 111 subjects are listed in Table 1. Among the 42 patients with SSc, 16 were taking calcium-channel blockers, 3 angiotensin-converting enzyme inhibitors, 1 angiotensin II receptor blocker, 2 hydroxy-chloroquine, 2 cyclophosphamide, 1 corticosteroids, 2 aza-thioprine, and 1 methotrexate. No patient was taking prosta-cyclin analog at time of enrollment. Among the 36 patients with primary RP, 2 were taking calcium-channel blockers.
Table 1.
Demographic and clinical characteristics of healthy controls (HC), patients with primary Raynaud’s phenomenon (PRP) and systemic sclerosis (SSc). Quantitative data are expressed as mean (standard deviation). Qualitative data are expressed as number (percentage).
| Characteristic | HC, n = 33 | PRP, n = 36 | SSc, n = 42 |
|---|---|---|---|
| Age, yrs | 52 (14) | 51 (12.5) | 51 (13) |
| Female | 30 (91) | 30 (83) | 38 (90) |
| Body mass index | 21.8 (4.9) | 20.6 (3.3) | 23.1 (3.6) |
| RP | 0 (0) | 36 (100) | 42 (100) |
| Duration, yrs | NA | 22 (20.5) | 10 (14) |
| No. fingers involved | NA | 8 (3) | 10 (2) |
| Thumb involved | NA | 15 (42) | 32 (76) |
| Feet involved | NA | 16 (44) | 33 (79) |
| Disease duration, yrs | NA | NA | 5 (6) |
| Digital pitting scars | 0 (0) | 0 (0) | 23 (55) |
| Sclerodactyly | 0 (0) | 0 (0) | 35 (83) |
| Rodnan-modified skin score | 0 (0) | 0 (0) | 6 (6)* |
| lSSc/lcSSc/dcSSc | NA | NA 9/24/9 (21.5/57/21.5) | |
The Rodnan-modified skin score was 6 (2.5) for the limited cutaneous systemic sclerosis (lcSSc) and 23 (17) for the diffuse cutaneous systemic sclerosis (dcSSc). NA: not applicable. lSSc: limited systemic sclerosis.
Main objective. Comparison of simultaneous post-occlusive responses in the brachial artery and the digital microcirculation
Digital cutaneous vascular conductance after occlusion and brachial artery FMD responses are presented in Table 2. Digital cutaneous PORH amplitude was altered in patients with primary RP and SSc compared to healthy controls, whereas PORH delay was increased only in patients with SSc. When referring to the baseline, PORH amplitude was not significantly different between controls and patients with primary RP, but remained altered in patients with SSc. Simultaneously measured brachial vascular responses (FMD) were not significantly different among controls and primary RP and SSc patients.
Table 2.
Main objective. Brachial artery and digital cutaneous post-occlusive reactive hyperemia (PORH) in patients with primary Raynaud’s phenomenon (PRP) or systemic sclerosis (SSc) and healthy controls (HC). Data are expressed as median (interquartile).
| PORH | HC, n = 33 | PRP, n = 36 | SSc, n = 42 | p |
|---|---|---|---|---|
| Brachial PORH | ||||
| Baseline, mm | 3 (0.6) | 3.1 (0.8) | 3.1 (0.5) | NS |
| FMD, % | 11.4 (8.1) | 12.2 (8.3) | 13.6 (10.9) | NS |
| Digital cutaneous PORH | ||||
| Baseline, mV/mm Hg | 14.9 (27.5) | 7.5 (15.4) | 9.1 (14.4) | NS |
| Peak, mV/mm Hg | 42.1 (23.3) | 28.9* (20.9) | 28.4* (23.2) | 0.002 |
| Peak–baseline, mV/mm Hg | 18.3 (18.9) | 20.7 (11.8) | 10.6*† (10.9) | 0.01 |
| Time to peak, s | 47.6 (47.3) | 44.4 (33.9) | 80.2*† (120.7) | 0.003 |
p < 0.017 vs healthy volunteers;
p < 0.017 vs PRP. FMD: flow-mediated dilation. p values estimated with Kruskal-Wallis test and Mann-Whitney test for between-group comparisons, corrected by Bonferroni’s method for multiple comparison.
We observed a correlation (r = 0.49; p = 0.004) between digital peak cutaneous vascular conductance and brachial FMD in controls, whereas there was no correlation in patients with primary RP or SSc (r = –0.044 and r = –0.036, respectively; not significant; Figure 3). We observed no difference in FMD between patients with dcSSc, lSSc, or lcSSc.
Figure 3.
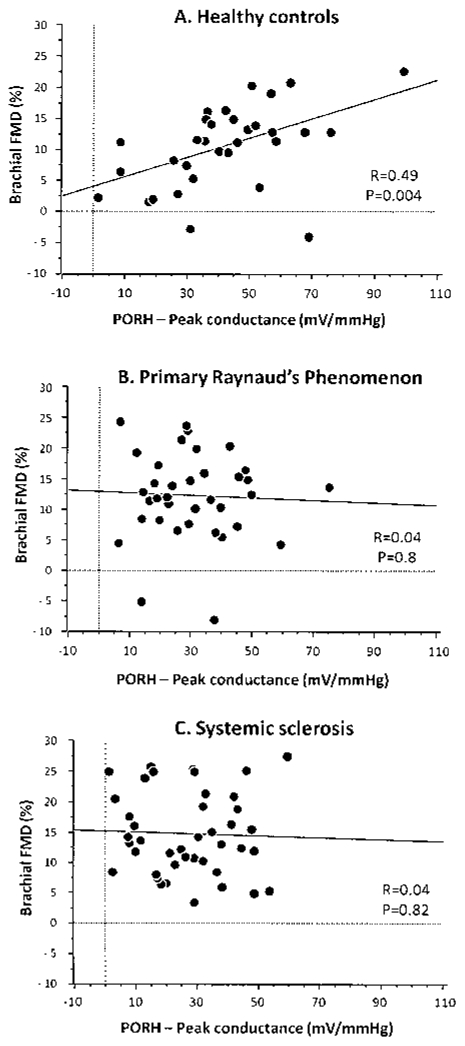
Correlation of digital peak cutaneous vascular conductance (mV/mm Hg) and brachial flow-mediated dilation (FMD) after 5-minute brachial occlusion in healthy controls (A) and patients with primary Raynaud’s phenomenon (B) and scleroderma (C) (Spearman correlation test). Both responses were recorded simultaneously for each subject.
Secondary objective. Assessment of other functional and structural markers of macro- and microvasculature in RP and SSc compared to healthy controls
Macro- and microvascular data corresponding to secondary outcomes are shown in Table 3. Thermal hyperemia was altered in patients with SSc compared to healthy controls, as both the axon reflex-mediated initial peak and the NO-dependent plateau were lower in patients with SSc. However, patients with primary RP displayed normal responses during thermal hyperemia.
Table 3.
Secondary objective. Markers of microvascular function and macrovascular structure in patients with primary Raynaud’s phenomenon (PRP) or systemic sclerosis (SSc) and healthy controls (HC). Data are expressed as median (interquartile).
| Marker | HC, n = 33 | PRP, n = 36 | SSc, n = 42 | p |
|---|---|---|---|---|
| Thermal hyperemia | ||||
| Baseline, mV/mm Hg | 11.8 (24) | 6.5 (10) | 5.7 (14.1) | NS |
| Peak, mV/mm Hg | 44.7 (24.5) | 47.8 (26.6) | 34.8*† (26.9) | < 0.001 |
| 42°C plateau | 36.4 (26.8) | 37.6 (22.4) | 28.7† (24.8) | 0.027 |
| Carotid IMT, mm | 0.51 (0.11) | 0.5 (0.1) | 0.53 (0.23) | NS |
| Cartoid-femoral PWV, m/s | 8.7 (1.7) | 8.5 (1.8) | 8.6 (2.3) | NS |
p values estimated with Kruskal-Wallis test and Mann-Whitney test for between group comparisons, corrected by Bonferroni’s method for multiple comparison.
p < 0.017 vs healthy volunteers;
p < 0.017 vs PRP. IMT: Intima-media thickness, PWV: pulse-wave velocity.
IMT and carotid-femoral PWV were not statistically different among all groups. In addition, median (interquartile) brachial NMD did not differ among groups [18.8% (10), 15.8% (8.4), and 20.7% (9.6), in controls, primary RP, and SSc patients, respectively; NS].
DISCUSSION
We observed an abnormal digital microvascular response to 5-minute brachial artery occlusion in patients with SSc. In contrast, FMD of the brachial artery, assessed simultaneously, was normal compared to healthy controls. In addition, we observed a loss of the correlation between the FMD and the digital PORH in such patients.
While there are numerous data that demonstrate impaired peripheral microvascular function in SSc, recent studies suggest that the macrovasculature may also be involved in this disease. However, most of these studies have demonstrated compliance abnormalities in large-conduit arteries (i.e., structural abnormalities). Moreover, the few studies addressing conduit artery function, with specific reference to endothelial function, have produced conflicting results. Our study was designed to investigate whether a correlation exists between macro- and microvascular function in SSc. We studied brachial artery FMD and cutaneous digital microvascular conductance after 5-minute occlusion of the brachial artery. Both tests are routinely performed as an index of macro- and microvascular functions, respectively. Brachial artery FMD is a standardized tool to investigate endothelial function in conduit arteries22. The amplitude of the response differs based on the cuff placement: it is higher when the cuff is placed on the upper arm, above the ultrasound probe, rather than on the forearm. In addition, the primary mechanism mediating the response also varies as a function of cuff placement: most of the response is NO-dependent when the cuff is located on the forearm, whereas it is only partially NO-dependent with the cuff placed on the upper arm24. We chose to place the pressure cuff on the upper arm because skin sclerosis on the forearm is more common than on the upper arm, and constriction of sclerosed skin was painful to some of our patients in pilot experiments. We chose a 5-minute occlusion time because this occlusion duration is generally accepted for use in both brachial artery FMD protocols and cutaneous PORH protocols, thus allowing simultaneous measurement of macrovascular and microvascular responses to a standardized stimulus5. It must be emphasized that while the brachial artery FMD is mostly NO-dependent22, cutaneous PORH is mostly endothelium-derived hyperpolarizing factor-dependent6,25. As recently reviewed, several measures can quantify the PORH flux response5. The measure most commonly used as the primary endpoint is peak hyperemia. However, no consensus exists concerning measure selection. It can be expressed as raw value of the peak minus baseline conductances and area under the curve26, percentage of baseline value27, or increase in postischemic flow using the area under the curve at baseline and postischemia28. The expression of data in terms of area under the curve provides more inter- and intra-subject variability than does the expression of data standardized to a maximal vasodilatation25. In addition, data expressed as absolute values have a lower variability than data expressed as a percentage of baseline29. Further, this intra-subject variability is related mostly to the site of measurement rather than to day-to-day variability30. On the finger pad, the high variability of the baseline conductance encouraged us to express our data in terms of absolute cutaneous vascular conductance for the main objective and peak conductance minus baseline conductance as a post hoc analysis (Figure 2) rather than a percentage of baseline values or area under the curve.
In patients with SSc, both the PORH response and the local thermal hyperemia were impaired. These results agree with previous reports8,15 and provide further evidence of microvascular dysfunction in SSc. Primary RP patients demonstrated impaired PORH peak response, while local thermal hyperemic responses were normal. However, when PORH data were expressed as peak minus baseline, the response was similar to controls. In other words, baseline digital skin blood flow is decreased in patients with primary RP (although not significantly), with a similar peak minus baseline response versus controls to the brachial artery occlusion, the absolute peak response remaining lower than controls. The impairment of the PORH peak response in this patient group agrees with previous work from our laboratory9,21. However, the discrepancy between the PORH peak response and local heating in these patients is enigmatic. It may be that the mechanism through which microvascular dysfunction is mediated in primary RP is not stressed (i.e., tested) during local thermal hyperemia. For example, the cutaneous vascular response to local thermal hyperemia is characterized by an initial peak, a subsequent nadir, and a final sustained plateau. The initial peak is axon reflex-dependent, while the sustained plateau is mediated by NO. Conversely, the cutaneous PORH response involves endothelial and metabolic vasodilators, a myogenic component, and contribution from sensory nerves5. Unlike thermal hyperemia, the contribution of NO to the PORH response in the human cutaneous vasculature is unlikely25,27,31,32. Further, a recent study suggests that large-conductance calcium-activated potassium channels play a major role in PORH, as endothelium-derived hyperpolarizing factors6. Therefore, one interpretation of the current data is that microvascular dysfunction exists in patients with primary RP, but the mechanisms responsible for this phenomenon are not stressed by the application of local thermal stress.
We were not the first to assess conduit-vessel function using brachial artery FMD in patients with SSc or primary RP. Andersen, et al observed no difference in brachial artery FMD between SSc patients and healthy controls using cuff placement on the forearm12. When Rajagopalan, et al used the same forearm cuff placement method, they observed similar FMD between patients with primary RP and secondary RP15. In contrast, using a cuff placed either on the fore-arm or the upper arm, other groups have shown lower brachial FMD in patients with SSc versus healthy controls16–18,33. Therefore, cuff placement alone cannot fully explain the disparate findings in this area. Importantly, the 3 studies showing normal FMD in SSc patients (including the current investigation) share common patient characteristics: most scleroderma patients had lSSc or lcSSc, with only a few dcSSc enrolled. In contrast, 2 of the 4 studies showing lower FMD enrolled dcSSc patients almost exclusively. No study has demonstrated an effect of scleroderma classification (i.e., limited, limited cutaneous, or diffuse cutaneous) on FMD. However, all of these studies (including our current investigation) were underpowered to detect such differences. Therefore, larger cohort studies are required to determine whether the disparate findings described above are linked to the differences in disease subtype or to disease duration. The NMD data from our study indicate that the capacity of the macrovasculature for vasodilation is not altered in SSc patients or primary RP patients, in agreement with previous findings12,16.
We are aware of only one other study enrolling patients with SSc in which comparisons were made between cutaneous PORH and brachial artery FMD15. In that study, FMD was similar in patients with secondary RP related to various connective tissue diseases including SSc, compared with primary RP, whereas PORH was lower in secondary RP15. In our study, we selected patients with secondary RP exclusively related to SSc, and added a sex- and age-matched group of healthy volunteers and patients with primary RP. We observed a correlation between simultaneously measured digital cutaneous PORH and brachial FMD. This observation agrees with the data of Hansell, et al, who demonstrated a correlation between acetylcholine ion-tophoresis (a test of skin microvasculature function) and brachial FMD34. In patients with SSc and primary RP, however, we observed no correlation between cutaneous and brachial vascular responses following occlusion.
In our investigation, neither IMT nor PWV were altered in patients with primary RP or SSc. Other investigators, using a similar methodology, have shown similar findings with respect to IMT in these patients groups13,16. In contrast, arterial stiffness, assessed with QKD60-10010,35,36 or calculated from changes in large-arteries diameter using ultra-sonography11,13,14, was increased in SSc. We evaluated PWV, and discrepancies may be related to the different methods used to assess arterial stiffness. An additional possibility is that impaired vascular distensibility appears at a later stage in disease progression. However, a 3-year followup of patients with SSc showed that progression to severe disease was predicted by arterial stiffness, suggesting that arterial involvement is an early event in SSc36. A final possibility is that differences may exist between elastic and muscular arteries. This would agree with the observations of Cheng, et al, who showed that patients with SSc lose carotid elasticity, whereas the elastic properties of the femoral wall are preserved13,14.
We observed a loss of the correlation between brachial FMD and digital cutaneous PORH in patients with SSc and primary RP. Microvascular function is impaired in SSc, whereas brachial artery endothelial function is normal.
Acknowledgments
We thank the patient association “Association des Sclérodermiques de France” for patient participation. We thank the Clinical Research Center of Grenoble University Hospital for reviewing the protocols corresponding to these studies. We also thank Dr. Muriel Salvat-Melis and Dr. Aude Boignard for assisting with the laser Doppler measurements and Dr. Olivier Ormezzano for reviewing the protocol.
Supported by the Association des Sclérodermiques de France; Groupe Français de Recherche sur la Sclérodermie; and Délégation Régionale à la Recherche Clinique de Grenoble.
References
- 1.Herrick AL. Vascular function in systemic sclerosis. Curr Opin Rheumatol. 2000;12:527–33. doi: 10.1097/00002281-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Carpentier PH, Maricq HR. Microvasculature in systemic sclerosis. Rheum Dis Clin North Am. 1990;16:75–91. [PubMed] [Google Scholar]
- 3.Matucci Cerinic M, Kahaleh MB. Beauty and the beast. The nitric oxide paradox in systemic sclerosis. Rheumatology Oxford. 2002;41:843–7. doi: 10.1093/rheumatology/41.8.843. [DOI] [PubMed] [Google Scholar]
- 4.Furst DE. The endothelium in the pathogenesis of systemic sclerosis: is it primary or secondary? J Mal Vasc. 1999;24:95–8. [PubMed] [Google Scholar]
- 5.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503–8. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo S, Minson C. Human cutaneous reactive hyperaemia: Role of BKCa channels and sensory nerves. J Physiol. 2007;585:295–303. doi: 10.1113/jphysiol.2007.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 8.Wigley FM, Wise RA, Mikdashi J, Schaefer S, Spence RJ. The post-occlusive hyperemic response in patients with systemic sclerosis. Arthritis Rheum. 1990;33:1620–5. doi: 10.1002/art.1780331103. [DOI] [PubMed] [Google Scholar]
- 9.Boignard A, Salvat-Melis M, Carpentier PH, et al. Local hyperemia to heating is impaired in secondary Raynaud’s phenomenon. Arthritis Res Ther. 2005;7:R1103–12. doi: 10.1186/ar1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constans J, Gosse P, Pellegrin JL, et al. Alteration of arterial distensibility in systemic sclerosis. J Intern Med. 1997;241:115–8. doi: 10.1046/j.1365-2796.1997.87110000.x. [DOI] [PubMed] [Google Scholar]
- 11.Moyssakis I, Gialafos E, Vassiliou V, et al. Aortic stiffness in systemic sclerosis is increased independently of the extent of skin involvement. Rheumatology Oxford. 2005;44:251–4. doi: 10.1093/rheumatology/keh478. [DOI] [PubMed] [Google Scholar]
- 12.Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum. 2002;46:1324–32. doi: 10.1002/art.10191. [DOI] [PubMed] [Google Scholar]
- 13.Cheng KS, Tiwari A, Boutin A, et al. Carotid and femoral arterial wall mechanics in scleroderma. Rheumatology Oxford. 2003;42:1299–305. doi: 10.1093/rheumatology/keg371. [DOI] [PubMed] [Google Scholar]
- 14.Cheng KS, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg. 2003;25:336–41. doi: 10.1053/ejvs.2002.1845. [DOI] [PubMed] [Google Scholar]
- 15.Rajagopalan S, Pfenninger D, Kehrer C, et al. Increased asymmetric dimethylarginine and endothelin 1 levels in secondary Raynaud’s phenomenon: implications for vascular dysfunction and progression of disease. Arthritis Rheum. 2003;48:1992–2000. doi: 10.1002/art.11060. [DOI] [PubMed] [Google Scholar]
- 16.Szucs G, Timar O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis — relevance for prevention of vascular complications. Rheumatology Oxford. 2007;46:759–62. doi: 10.1093/rheumatology/kel426. [DOI] [PubMed] [Google Scholar]
- 17.D’Andrea A, Stisi S, Caso P, et al. Associations between left ventricular myocardial involvement and endothelial dysfunction in systemic sclerosis: noninvasive assessment in asymptomatic patients. Echocardiography. 2007;24:587–97. doi: 10.1111/j.1540-8175.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 18.Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann NY Acad Sci. 2007;1108:283–90. doi: 10.1196/annals.1422.030. [DOI] [PubMed] [Google Scholar]
- 19.LeRoy EC, Medsger TA., Jr Raynaud’s phenomenon: a proposal for classification. Clin Exp Rheumatol. 1992;10:485–8. [PubMed] [Google Scholar]
- 20.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–6. [PubMed] [Google Scholar]
- 21.Salvat-Melis M, Carpentier PH, Minson CT, et al. Digital thermal hyperaemia impairment does not relate to skin fibrosis or macrovascular disease in systemic sclerosis. Rheumatology Oxford. 2006;45:1490–6. doi: 10.1093/rheumatology/kel116. [DOI] [PubMed] [Google Scholar]
- 22.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 23.Baguet JP, Hammer L, Levy P, et al. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest. 2005;128:3407–12. doi: 10.1378/chest.128.5.3407. [DOI] [PubMed] [Google Scholar]
- 24.Doshi S, Naka K, Payne N, et al. Flow-mediated dilation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci Lond. 2001;101:629–35. [PubMed] [Google Scholar]
- 25.Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol. 2003;95:504–10. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- 26.Stewart J, Kohen A, Brouder D, et al. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol. 2004;287:H2687–96. doi: 10.1152/ajpheart.00287.2004. [DOI] [PubMed] [Google Scholar]
- 27.Binggeli C, Spieker LE, Corti R, et al. Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients: a monitoring test of endothelial dysfunction for clinical practice? J Am Coll Cardiol. 2003;42:71–7. doi: 10.1016/s0735-1097(03)00505-9. [DOI] [PubMed] [Google Scholar]
- 28.Ruano J, Lopez-Miranda J, Fuentes F, et al. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J Am Coll Cardiol. 2005;46:1864–8. doi: 10.1016/j.jacc.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 29.Yvonne-Tee GB, Rasool AH, Halim AS, Rahman AR. Reproducibility of different laser Doppler fluximetry parameters of postocclusive reactive hyperemia in human forearm skin. J Pharmacol Toxicol Methods. 2005;52:286–92. doi: 10.1016/j.vascn.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Kubli S, Waeber B, Dalle-Ave A, Feihl F. Reproducibility of laser Doppler imaging of skin blood flow as a tool to assess endothelial function. J Cardiovasc Pharmacol. 2000;36:640–8. doi: 10.1097/00005344-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol. 1996;81:1807–14. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- 32.Medow MS, Taneja I, Stewart JM. Cyclooxygenase and nitric oxide synthase dependence of cutaneous reactive hyperemia in humans. Am J Physiol. 2007;293:H425–32. doi: 10.1152/ajpheart.01217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J. 1998;136:905–12. doi: 10.1016/s0002-8703(98)70137-1. [DOI] [PubMed] [Google Scholar]
- 34.Hansell J, Henareh L, Agewall S, Norman M. Non-invasive assessment of endothelial function — relation between vasodilatory responses in skin microcirculation and brachial artery. Clin Physiol Funct Imaging. 2004;24:317–22. doi: 10.1111/j.1475-097X.2004.00575.x. [DOI] [PubMed] [Google Scholar]
- 35.Gosse P, Taillard J, Constans J. Evolution of ambulatory measurement of blood pressure and parameters of arterial stiffness over a 1-year period in patients with systemic sclerosis: ERAMS study. J Hum Hypertens. 2002;16:627–30. doi: 10.1038/sj.jhh.1001466. [DOI] [PubMed] [Google Scholar]
- 36.Constans J, Germain C, Gosse P, et al. Arterial stiffness predicts severe progression in systemic sclerosis: the ERAMS study. J Hypertens. 2007;25:1900–6. doi: 10.1097/HJH.0b013e328244e1eb. [DOI] [PubMed] [Google Scholar]


