Abstract
Asthma has been hypothesized to be associated with lung cancer (LC) risk. We conducted a pooled analysis of 16 studies in the International Lung Cancer Consortium (ILCCO) to quantitatively assess this association and compared the results with 36 previously published studies. In total, information from 585 444 individuals was used. Study-specific measures were combined using random effects models. A meta-regression and subgroup meta-analyses were performed to identify sources of heterogeneity. The overall LC relative risk (RR) associated with asthma was 1.28 [95% confidence intervals (CIs) = 1.16–1.41] but with large heterogeneity (I2 = 73%, P < 0.001) between studies. Among ILCCO studies, an increased risk was found for squamous cell (RR = 1.69, 95%, CI = 1.26–2.26) and for small-cell carcinoma (RR = 1.71, 95% CI = 0.99–2.95) but was weaker for adenocarcinoma (RR = 1.09, 95% CI = 0.88–1.36). The increased LC risk was strongest in the 2 years after asthma diagnosis (RR = 2.13, 95% CI = 1.09–4.17) but subjects diagnosed with asthma over 10 years prior had no or little increased LC risk (RR = 1.10, 95% CI = 0.94–1.30). Because the increased incidence of LC was chiefly observed in small cell and squamous cell lung carcinomas, primarily within 2 years of asthma diagnosis and because the association was weak among never smokers, we conclude that the association may not reflect a causal effect of asthma on the risk of LC.
Introduction
The World Health Organization estimates that ∼300 million people worldwide suffer from asthma, one of the most frequent chronic diseases (1). The disease affects people of all ethnic groups, from infancy to old age (1,2). The prevalence of asthma, or more generally wheezing, differs remarkably between geographical regions and over time being more common in western developed countries (e.g. ∼4% in India and Algeria and 29% in Australia and Wales) (1,3). It poses substantial burden to individuals and families and is often a lifetime concern. Because asthma is a complex inflammatory disorder of the respiratory system, it has been hypothesized that this chronic condition may affect the risk of lung cancer (LC).
LC is the most common cause of cancer death worldwide with a 5 year survival probability of only 10% (4). Although tobacco smoking remains the predominant cause of LC, even in never-smokers, LC is an important public health issue. It is estimated that 10–29% of LC cases are attributable to factors other than smoking, representing between 16 000 and 24 000 LC deaths annually in the USA alone (5–7).
The association between asthma and LC risk has been investigated previously, the first report dating back to 1960 (8). Suggested hypotheses of the asthma–LC relationship are conflicting, i.e. that asthma is associated with either an increase or a decrease in LC. According to the enhanced immune surveillance theory, asthma may reduce the risk of LC by increased clearance of toxins and carcinogens from the bronchoalveolar epithelium (9–11) and by continual stimulation of cell regeneration to repair inflammatory lung damage (9,12). Conversely, asthma has been hypothesized to cause an increased risk of LC via chronic inflammation (the antigenic stimulation theory) (11,13). To shed light on these discrepancies, previous summaries of the relevant literature/evidence suggest that asthma is associated with an increased LC risk (9,11,14,15). However, heterogeneous results were found for case–control and cohort studies (16) and potential effect modifiers, such as latency period, were not or were rarely investigated. Stratification by histological subtypes was conducted by previous studies; however, the sample sizes tend in part to be too small to yield meaningful results (12,17–24). Since 2003, several additional studies of this association have become available.
We aimed to investigate the role of asthma as a potential risk factor for LC and, if present, to identify factors that might modify the strength of this association. To be able to conduct a detailed stratified analysis, with standardized adjustments for covariates, we conducted a pooled analysis based of individual-level data from 16 studies of the International Lung Cancer Consortium (ILCCO). To summarize the overall effect estimates, we also combined the results of the pooled analysis with all relevant studies published in the literatures up to October 2010.
Materials and methods
We performed meta-analyses based on study-specific estimates from ILCCO and published studies, separately and jointly. Sources of between-study heterogeneity were investigated by stratified analysis (25) and by a meta-regression (26).
Analysis of ILCCO studies
ILCCO was established in 2004 with the aim to pool comparable data and maximize resource sharing and statistical power of epidemiological studies of LC (http://ilcco.iarc.fr). Sixteen ILCCO studies are included in this pooled analysis. All studies were approved by ethical review boards. Written informed consent was obtained from all study participants. The data submitted were checked for inadmissible values, aberrant distributions, inconsistencies and missing values. Study participants with unknown sex, age, exposure (if distinguishable from ‘no asthma’), disease (LC) or smoking status (never-, former, current or ever-smoker) were excluded from the analysis. All studies considered primary and incident LC cases, histological confirmed. Asthma status was determined by questionnaire assessment (denoted as self-reported), by a verified physician’s diagnosis (denoted as physician’s diagnosis) or from entries in a national hospital registry (Danish Diet Cancer and Health Study, denoted as hospitalized).
The asthma–LC association was estimated in each study separately by fitting a logistic regression model for case–control studies and a Cox-regression model for the cohort study. The association estimates were adjusted for age at interview, sex and smoking (smoking status, pack years, time since quitting, age of start smoking and environmental tobacco smoke). Pack years were calculated based on smoking intensity in cigarettes equivalents and duration.
Analyses were repeated restricting the ILCCO study participants to never-smokers, defined as those who had smoked <100 cigarettes in their lifetime or had smoked <1 pack year to avoid potential bias by residual confounding owing to smoking and smoking-related effects.
Identification of published studies
We searched ‘PubMed’ and other databases via the ‘Deutsches Institut für Medizinische Dokumentation und Information’ for further publications concerning the epidemiology, etiology, classification or history of asthma or allergies or inflammation and LC (see Supplementary materials, available at Carcinogenesis Online) up to 27 October 2010 and tracked down references to identify relevant study reports. Studies needed to fulfill several criteria (see Supplementary materials, available at Carcinogenesis Online) to enter the meta-analysis. Usable data were extracted by two independent abstractors. No study from the ILCCO pooled analysis, for which individual-level data were available, was considered in this component.
Meta-analysis
To obtain a single estimate summarizing the asthma–LC association, for simplicity henceforth noted as a relative risk (RR), we fitted random effects models based on published or calculated odds ratios, RRs or hazard ratios. Methods of how the standard error was reconstructed if unreported are given in the Supplement (available at Carcinogenesis Online). To detect reporting bias, we visually inspected a funnel plot of precision versus effect estimates and performed Egger’s test for asymmetry (27). Consistency within studies is displayed by Galbraith radial plots (25).
We explored the between-study heterogeneity by performing an asymptotic test on Cochran’s Q. In addition, we calculated I2, the percentage of the variability in effect estimates that is due to heterogeneity and inspected Galbriath radial plots. The P-value for heterogeneity is noted as Phet. We conducted influence analysis where we performed a backward selection, excluding the study contributing most to the Q statistics at each step, until there was no evidence of heterogeneity (Phet < 0.05). The main purpose of this selection was to inspect changes in RR estimates in less heterogeneous subsets of studies and not to exclude of studies from the analysis. Therefore, summary statistics derived after exclusions are reported only when being meaningfully different from the overall dataset without exclusion.
To investigate sources of between-study heterogeneity, we conducted stratified analyses by smoking status, sex, age, history of other lung diseases, cancer histology and topography, age of asthma onset, extrinsic or intrinsic asthma and defined latency, whenever sufficient data were available or reported. ILCCO-studies with less than three observed cases or controls among asthmatics or non-asthmatics were excluded from subgroup analyses. In published case–control studies, a minimal latency time was set as an inclusion/exclusion criterion. Therefore, the effect of a minimal latency by design was investigated in case–control studies only, categorized into ‘within first year’, ‘1–2 years’, ‘3–10 years’ and ‘≥11 years’. If a reported stratification for latency does not perfectly fit to this classification used, we assigned the reports to the most overlapping strata.
In the meta-regression (26), we included all studies and considered 13 sources of heterogeneity as covariates (listed in the Supplement, available at Carcinogenesis Online). To avoid over-parameterization and to the limit ecologic bias due to the inclusion of aggregates of person characteristics (28), we performed a backward selection of covariates. The choice of the best fitting model was based on Akaike’s information criterion.
Results
Description of ILCCO studies
The characteristics of the 16 participating ILCCO studies are summarized in Table I (see also Supplementary Tables I—III, available at Carcinogenesis Online). Fifteen were case–control studies, with controls frequency matched to cases on at least age and sex. The 16th study was a register-based cohort study. Five studies were conducted in Europe, nine in North America and one each in Hawaii and China. In most studies, asthma was assessed using self-reported diagnosis. In total, 19 980 LC cases were compared with 79 723 controls. Ninety-three percent of the study population was European descendants and 33% were never-smokers. The most common histological subtypes were adenocarcinoma (33%) and squamous cell carcinoma (23%). More details are given in the Supplement, available at Carcinogenesis Online.
Table I.
Characteristics of ILCCO studies
| Study abbreviation Reference Principal investigator | Study name Organization | Country | Location | Perioda | Sex | Age (years) | Ethnicity | Source of study population |
Asthma |
Smoking |
Applicable sample size cases/controls | ||||||
| Cases | Controls | Ascertainment | Prior to LC | T | PY | A | TS | ETS | |||||||||
| Prospective (cohort) studies | |||||||||||||||||
| DDCHS (29) A.Tjønneland | Danish Diet Cancer and Health study | Demark | Copenhagen and Aarhus | 1993–2007 | Both | 50–65 | Caucasianb | IR | P | Hospitalized | — | • | • | • | • | 825/55 489 | |
| Retrospective (case–control) studies | |||||||||||||||||
| UCLA (30) Z.F.Zhang | University of California at Los Angeles | USA | Los Angeles | 1999–2004 | Both | 17–65 | Mixed | I | P | SR diagnosis | — | • | • | • | • | 608/1043 | |
| HMGU (31–34) E.Wichmann, H.Bickeböller | Helmholtz Lung Cancer Study | Germany | Nationwide | 2000–04 | Both | 22–54 | Caucasian | I | P | SR diagnosis | 2 | • | • | • | 661/7103 | ||
| CE (35) P.Boffetta | INCO Central Europe Health Study | Central/Eastern Europe | Several | 1998–2002 | Both | 25–86 | Caucasian | I | H | SR symptoms | — | • | • | • | • | 2633/2702 | |
| NCI-China (36,37) Q.Lan | National Cancer Institute | China | Xuan Wei | 1985–90 | Both | 22–80 | Asian | I | P | SR diagnosis | — | • | • | 120/124 | |||
| WSU/KCI-1 (38) A.G.Schwartz | Wayne State University and Karmanos Cancer Institute | USA | Detroit, Michigan | 1984–2005 | Both | 17–85 | Mixed | I | P | SR diagnosis | 9 | • | • | • | • | 1001/1183 | |
| Hawaii (39) L.Le Marchand | Study of Diet and Lunge Cancer III | USA | Hawaii | 1992–97 | Both | 31–84 | Mixed | I | P | SR diagnosis | — | • | • | • | • | 632/591 | |
| Toronto (40) J.McLaughlin | SLRI—Ontario Lung Cancer Study | Canada | Toronto, Ontario | 1997–2002 | Both | 20–85 | Mixed | I | Mixed | SR diagnosis | 1 | • | • | • | • | 451/940 | |
| LLP (41) J.Field | Liverpool Lung Project | UK | Liverpool | 1998–2006 | Both | 38–85 | Caucasian | I | P | SR diagnosis | — | • | • | • | • | 475/953 | |
| Mayo-H (42,43) P.Yang | Mayo Clinic | USA | Rochester, Minnesota | 1997–2006 | Both | 17–99 | Mixed | I | Mixed | Physician diagnosis | — | • | • | • | 5696/2271 | ||
| NELCS (44) E.Duell | New England Lung Cancer Study | USA | New Hampshire | 2005–08 | Both | 31–74 | Caucasian | I | P | SR diagnosis | — | • | • | • | • | 276/251 | |
| NYMS (45) J.Muscat | NY Multi-Center Study | USA | New York State | 1969–99 | Both | 24–83 | Mixed | I | H | SR diagnosis | — | • | • | • | 5133/4939 | ||
| CREST (46) M.Neri | Cancer of the Respiratory tract biorepository | Italy | Genoa | 2002–05 | Both | 19–94 | Caucasian | I | Mixed | SR diagnosis | — | • | • | • | • | • | 410/558 |
| UCSF (47) J.Wiencke | University of California at San Francisco | USA | San Francisco | 1998–2003 | Both | 26–95 | Mixed | I | Mixed | SR diagnosis | — | • | • | • | 424/903 | ||
| MSKCC (48) I.Orlow | Memorial Sloan Kettering Cancer Centre | USA | New York | 2003–05 | Both | 37–93 | Mixed | I | H | SR diagnosis | — | • | • | 101/102 | |||
| WSU/KCI-2 (49) A.G.Schwartz | Wayne State University and Karmanos Cancer Institute | USA | Detroit | 2001–05 | Women | 18–74 | Mixed | IR | P | SR diagnosis | — | • | • | • | • | 534/571 | |
| Total | 19 980/79 723 (Σ 99 703) | ||||||||||||||||
Type of cases: I, incidence cases; IR, incidence (register); MR, mortality (register); type of controls: H, hospital control; P, population control; N, neighborhood control; SI, comparison with incidence from standard population; asthma ascertainment: SR, self-report; smoking: T, type of smoker; PY, pack years; A, age start smoking; TS, time since quit smoking; ETS, exposed to environmental tobacco smoke.
Refers to the period within the used data were collected (some studies are still ongoing).
About half of the combined sample comes from the Danish Diet Cancer and Health study, a register based investigation. No ethnicity is provided for the single participants. Because the Danish population consists by far mostly of Caucasians, all participants are assigned to be white.
Description of published studies
Table II summarizes the characteristics of all 36 published studies identified through a literature search (see Supplementary materials, available at Carcinogenesis Online) and included in the meta-analysis, composed of 20 case–control studies, 1 nested case–control and 15 cohort studies. The studies were published between 1972 and 2010, providing information on 19 644 LC cases and 466 097 LC-free individuals. Seven studies investigated LC mortality and the remaining LC incidence. The majority of the studies included Caucasians, 17 exclusively. Asthma was self-reported in 23 studies and verified by a physician in 12 studies. Twenty-five studies controlled for smoking in the design or analysis (matching or adjusting), 6 studies restricted the exposure definition to asthma in the absence of other pulmonary disorders as exposure and 11 studies provided results for non-smokers.
Table II.
Characteristics of published studies
| Reference | Study | Period | Sex | Ethnicity | Source of study population |
Applicable sample size, cases/controls | Asthma |
Adjusted/match for smoking | Asthma only cases | Non-smokers | ||
| Cases | Controls | Ascertainment | Diagnosed prior to LC (years) | |||||||||
| Retrospective (case–control) studies | ||||||||||||
| (10) | Vena 1985 | 1957–65 | Both | Caucasian | I | H | 1186/4039 | SR diagnosis | 0–5 | Yes | Yes | SGA |
| (50) | Gabriel 1972 | 1969–69 | Male | Mixed | I | H | 150/150 | SR symptoms | 35a | No | No | No |
| (22) | Osann 1991 | 1969–77 | Female | Mixed | I | P | 217/217 | SR symptoms | Min. 2 | Yes | No | No |
| (51) | Markowe 1987 | 1970–76 | Both | Mixed | MR | P | 2547/2547 | Physician diagnosed | –– | No | No | No |
| (52) | Brown 2005 | 1976–80 | Both | Mixed | MR | P | 545/8542 | SR diagnosis | –– | Yes | No | SGA |
| (20) | Ramanakumar 2006 | 1979–86 | Male | Mixed | I | P | 755/512 | SR symptoms | 3–10 | Yes | No | No |
| 1995–2001 | Both | 1205/1541 | ||||||||||
| (53) | Samet 1986 | 1980–82 | Both | Caucasian | IR | HP | 518/769 | SR diagnosis | –– | No | No | SGA |
| (12) | Mayne 1999 | 1982–84 | Both | Mixed | IR | P | 437/437 | Physician diagnosed | Min. 5 | Yes | No | Yes |
| (54) | Wu 1988 | 1983–86 | Female | Mixed | I | N | 336/336 | SR diagnosis | Min. 3 | Yes | No | No |
| (17) | Wu 1995 | 1985–90 | Female | Mixed | I | HP | 412/1253 | Physician diagnosed | –– | No | No | No |
| (18) | Alavanja 1992 | 1986–91 | Female | Caucasian | I | P | 618/1402 | Physician diagnosed | Min. 2 | SGA | No | Yes |
| (21) | Osann 2000 | 1990–93 | Female | Caucasian | I | P | 98/204 | –– | Yes | No | No | |
| (55) | Brownson 2000 | 1993–94 | Female | Caucasian | I | P | 676/700 | Physician diagnosed | 1–3 | Yes | No | No |
| (56) | Brenner 2001 | 1994–98 | Both | Asia | I | P | 886/1765 | Physician diagnosed | 1–21 | Yes | SGA | SGA |
| (57) | Gorlova 2006 | 1995–2003 | Both | Mixed | I | H | 280/242 | SR diagnosis | 3–20 | No | No | No |
| (58) | Wang 2006 | 2000–03 | Both | Caucasian | I | HP | 196/4271 | SR diagnosis | –– | Yes | No | No |
| (24) | Liang 2009 | 2004–07 | Female | Asia | I | P | 226/253 | SR diagnosis | Min. 1 | Yes | No | Yes |
| (59) | El-Zein 2010 | 1979–86 | Male | Mixed | I | P | 755/512 | SR diagnosis | 0 | Yes | No | No |
| (60) | Koshiol 2009 | 2002–05 | Both | Caucasian | I | P | 1419/2104 | SR diagnosis | Min. 1 | Yes | No | SGA |
| Nested case–control studies | ||||||||||||
| (61) | González-Pérez 2006 | 1994–2001 | Both | Caucasian | IR | P | 866/18 792 | Physician diagnosed | Min. 2 | Yes | Yes | No |
| Prospective (cohort) studies | Follow-up period (years) | |||||||||||
| (62) | Alderson 1974 | 1936–79 | Both | Mixed | MR | SI | 16 of 1892/std. pop. | Hospitalized | Avg. 21 | No | No | No |
| (63) | Reynolds 1987 | 1965–65 | Both | Mixed | MR | P | 66 of 6815/std. pop. | SR symptoms | Max. 18 | Yes | No | No |
| (64) | Boffetta 2002 | 1965–95 | Both | Caucasian | I | SI | 713 of 92 986/std. pop. | Hospitalized | Avg. 8.5 | No | SGA | No |
| (65) | Ji 2009 | 1965–2004 | Both | Caucasian | IR | SI | 650 of140 425/std. pop. | Hospitalized | Max. 40 | No | No | No |
| (66) | Frostad 2008 | 1972–2002 | Both | Caucasian | IR | P | 352/17 318 | SR symptoms | Max. 30 | Yes | No | No |
| (67) | Vandentorren 2003 | 1974–98 | Both | Mixed | M | P | 178/13 149 | SR symptoms | Max. 25 | Yes | No | No |
| (68) | Eriksson 1995 | 1976–89 | Both | Caucasian | IR | SI | 1 of 2511/std. pop. | SR symptoms | Max. 14 | No | SGA | No |
| (69) | Huovinen 1997 | 1976–91 | Both | Caucasian | MR | twins | 115/30 134 | SR diagnosis | Max. 16 | SGA | No | No |
| (70) | Lange 1996 | 1976–95 | Both | Caucasian | IR | P | 380/13 160 | SR symptoms | Avg. 17 | Yes | No | No |
| (71) | Mills 1992 | 1977–82 | Both | Caucasian | IR | P | 62/34130 | Physician diagnosed | Max. 6 | Yes | No | Yes |
| (19) | Vesterinen 1993 | 1980–87 | Both | Caucasian | IR | SI | 783 of 77 952/std. pop. | Physician diagnosed | Max. 7 | No | No | No |
| (72) | Talbot-Smith 2003 | 1981–99 | Both | Caucasian | IR | P | 28/3280 | SR diagnosis | Max. 19 | Yes | No | No |
| (73) | Turner 2005 | 1982–2000 | Both | Mixed | MR | P | 892/18 987 | SR diagnosis | Max. 19 | Yes | SGA | SGA |
| (23) | Littman 2004 | 1985–96 | Both | Mixed | I | P | 1028/16 670 | SR diagnosis | Avg. 9.1 | Yes | No | Yes |
| (74) | Brown 2006 | 1995–2004 | Both | Mixed | I | P | 52/8845 | SR symptoms | Max. 9 | Yes | No | SGA |
| Total | 19 644/466 097(Σ485 741) | |||||||||||
Type of cases: I, incidence cases; IR, incidence (register); M, mortality (observed); MR, mortality (register); type of controls: H, hospital control; P, population control; N, neighborhood control; SI, comparison with incidence from standard population; asthma ascertainment: SR, self-report; asthma only: results available for asthmatics without any other lung disease; non-smokers, results available for never and/or former smokers; others: SGA, subgroup analysis; max., maximum; min., minimal; avg., average.
Asthma before the age of 15 years; participants in the age of 49–83 years.
ILCCO pooled analysis
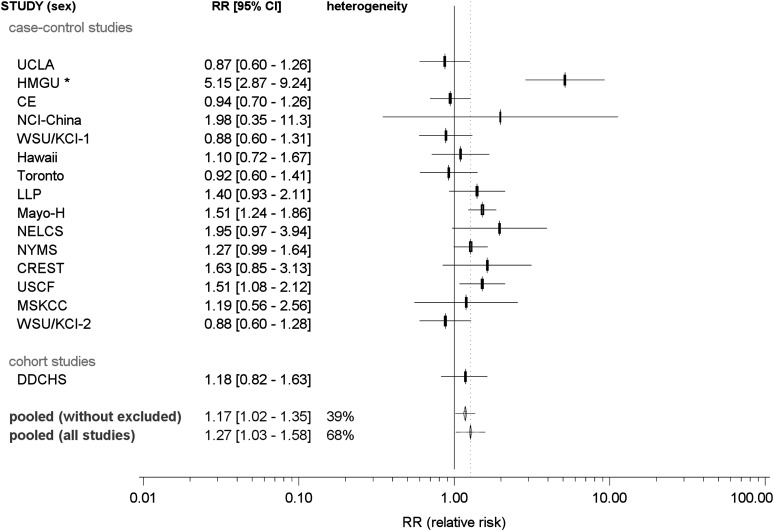
The estimated odds ratios of LC associated with asthma in case–control studies ranged from 0.88 (WSU/KCI-2 study) to 5.57 (Helmholtz lung cancer study) (Figure 1). The overall summary RR was 1.27 [95% confidence interval (CI) = 1.03–1.58]. However, appreciable significant heterogeneity was observed among these 16 studies (Phet < 0.001), with inter-study variability of 68%. The Helmholtz lung cancer study has an upper age limit of 50 years for cases, the largest amount of never-smokers in controls (71%) and the highest proportion of small-cell lung cancer (SCLC) cases (28%) and the largest proportion of cases with LC diagnosis within 2 years after asthma diagnosis (6% of cases). It is possible that the high proportion of SCLC may contribute to the large effect, although it cannot account for it completely. Excluding the HGMU study reduced the heterogeneity (Phet = 0.062, I2= 39%), and the strength of the association decreased considerably, although remained significant, with RR = 1.17 (95% CI = 1.02–1.35, P = 0.032).
Fig. 1.
Forest plot of the association between asthma and LC risk: ILCCO studies. Pooled, pooled RR according a random effects model; heterogeneity I2, percentage of inter-study heterogeneity; pooling all studies: the following eight studies were removed to reduce heterogeneity: HMGU.
Subgroup analysis
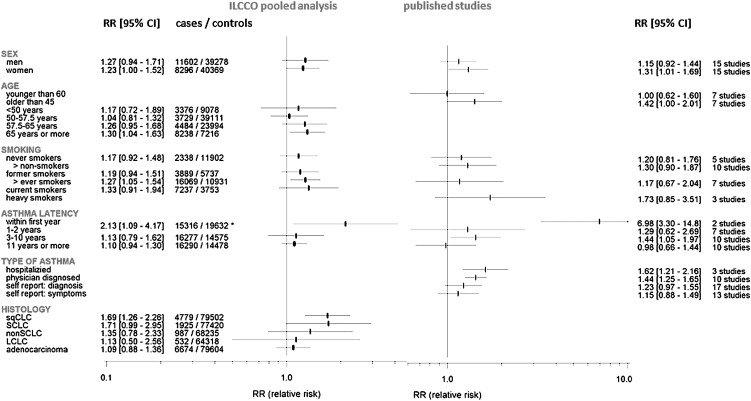
The results of the stratified analysis by smoking status, age, gender, asthma latency, asthma ascertainment method and histology are presented in Figure 2. When stratified by smoking status, we observed an association among ever-smokers (RR = 1.27, 95% CI = 1.05–1.54) and not in never-smokers (RR = 1.17, 95% CI = 0.72–1.48).
Fig. 2.
Subgroup meta-analysis. For some of the published studies, results from disjunctive subgroups (e.g. men and women) are reported. If so, subgroup estimates were used instead of overall results. For this reason, the number of subgroup results included need not to sum up to total number of selected studies. For published studies, asthma latency is defined as the minimal allowed latency by the design of a case–control study. Within the ILCCO pooled analysis, asthma latency was calculated for each study participant. Age, age at LC diagnosis (cases) or interview (controls); latency: asterisk indicates latency of 0–2 years; smoking: non-smokers, never- + former smokers + non-smokers (if so specified); ever smokers, former + current smokers + ever smokers (if so specified); heavy smokers, as defined in the original publication; histology: sqCLC, squamous cell carcinoma; non-SCLC, all types of LC apart from SCLC; LCLC, large cell lung carcinoma.
In terms of histology, we found a significantly elevated risk for squamous cell carcinoma (RR = 1.69, 95% CI = 1.26–2.26, P < 0.002) and borderline significantly for SCLC (RR = 1.71, 95% CI = 0.99–2.95, P = 0.052) but not for adenocarcinoma (RR = 1.09, 95% CI = 0.88–1.36), large cell lung cancer (RR = 1.13, 95% CI = 0.50–2.56) or for non-SCLC (RR = 1.35, 95% CI = 0.78–2.33), although the latter was based on small numbers.
When considering the latency period, the effect of asthma on LC risk appeared to be present and significant only for those who were diagnosed within 2 years of LC onset (RR = 2.13, 95% CI = 1.09–4.17). In contrast, for those having asthma for >11 years, no significant elevated risk for LC was observed: RR = 1.10 (95% CI = 0.94–1.30, P = 0.194).
When stratified by age of asthma onset, we observed a significant increased risk (RR = 1.30, 95% CI = 1.04–1.63) for those diagnosed at age ≥65 years as well as for those affected with asthma as adult (diagnosed after age 20 years: RR = 1.26, 95% CI = 1.05–1.51). We did not observe any effect modification by sex. Only three studies provided information to distinguish between ‘intrinsic’ (allergic) and ‘extrinsic’ asthma; therefore, we were not able to investigate this aspect. More details regarding subgroup analyses are given in the Supplement, available at Carcinogenesis Online.
Never-smokers
Three studies (NCI-China, Hawaii and LLP) needed to be excluded because of an insufficient number of observations. In general, we observed similar associations in non-smokers as those described above, though they were less precisely estimated due to the smaller sample size. RRs of LC were largest in short term after asthma diagnosis (RR = 4.26, 95% CI = 0.02–794) and close to null over 11 years after asthma diagnosis (RR = 1.02, 95% CI = 0.59–1.75). No increased risk for an adenocarcinoma (RR = 1.05, 95% CI = 0.78–1.40) was observed. More details are given in the Supplement, available at Carcinogenesis Online.
Meta-analysis of published studies
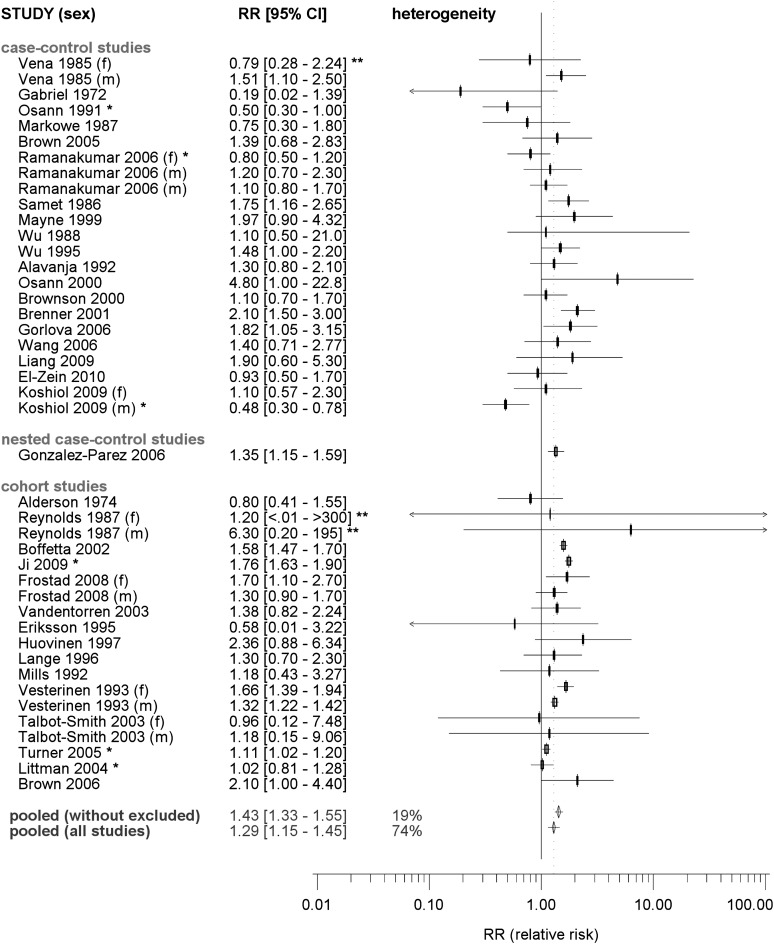
The reported RRs for LC associated with asthma ranged from 0.19 (50) to 6.3 (63) (Figure 3). As seven studies did not provide overall RRs, the reported sex-specific estimates were included in the analysis. The overall estimate of a summary RR was 1.29 (95% CI = 1.15–1.45). We observed appreciable heterogeneity (Phet < 0.001, I2 = 68%). Egger’s test of funnel plot asymmetry suggests likely reporting bias (P < 0.001). Smaller studies tended to have report lower RRs. After removing the six studies contributing to the largest heterogeneity [Ramanakumar (20) (f), Osann (22), Littman (23), Koshiol (60) (m), Ji (65), Turner (73)], the summary RR increased to 1.43 (95% CI = 1.33–1.55).
Fig. 3.
Forest plot of the association between asthma and LC risk: published studies. Gender-specific RRs were included if no overall results are available, indicated as (m), male, and (f), female. Pooled, pooled RR according a random effects model; heterogeneity I2, percentage of inter-study heterogeneity; pooling all studies: the following eight studies were removed to reduce heterogeneity: Vesterinen (19) (f), Osann (22), Brenner (56), Koshiol (60) (m), Boffetta (64), Ji (65), Turner (73), HMGU.
Subgroup analysis
The results of stratified analyses by smoking status, age, gender, asthma latency, asthma ascertainment method and histology are presented in Figure 3. More details are given in the Supplementary Table VIII, available at Carcinogenesis Online.
When considering ‘types of asthma assessment’, a clear difference between summary RRs was observed. The RRs of LC were 1.62 (95% CI = 1.21–2.16) and 1.44 (95% CI = 1.25–1.65) for asthma exposure considered as hospitalized asthmatics and physician-verified diagnoses, respectively. Associations were weaker for exposure defined as self-reported asthma (RR = 1.23, 95% CI = 0.97–1.55) or as having experienced asthma-like symptoms (RR = 1.15, 95% CI = 0.88–1.49).
Only two of the published studies provided information for asthmatics at a ‘minimal latency by design’ (here equally to ‘latency’) of <1 year ((63): RR = 6.3 and (65): 6.98), both highly significant. Similar to the observations in the ILCCO pooled analysis, this gives the largest summary RR of 6.98 (95% CI = 3.30–14.86). In contrast, the RR for asthmatics with a latency by design of 1–2 years was 1.29 (95% CI = 0.62–2.69) and was smallest for a latency by design of at least 10 years (RR = 0.98, 95% CI = 0.66–1.44).
Age (at LC diagnosis or interview), sex and the considered outcome parameter, type of controls and adjustment for smoking may additionally explain some of the observed heterogeneity. We found an increased risk associated with asthma over age 45 years (RR = 1.42, 95% CI = 1.00–2.01), but not if the study population was <60 years (RR = 1.00, 95% CI = 0.62–1.60). The pooled RR of women (RR = 1.31, 95% CI = 1.01–1.69) was larger than that for men (RR = 1.15, 95% CI = 0.92–1.44). We did not observe clear evidence of effect modification by smoking status in the published studies. However, the point estimate of pooled RR in never-smokers was lower (RR = 1.20, 95% CI = 0.81–1.76) than that in heavy smokers (RR = 1.73, 95% CI = 0.85–3.51).
The RR for asthmatics to die from LC (mortality) is estimated as RR = 1.12 (95% CI = 1.01–1.23) compared with RR = 1.31 (95% CI = 1.15–1.49) for LC (incidence). The summary RR estimates for cohort studies (RR = 1.39, 95% CI = 1.22–1.60) was larger than those for case–control studies (RR = 1.20, 95% CI = 0.99–1.45). Combined estimates of RRs that were controlled for smoking (base on models adjusted or matched for smoking: RR = 1.22, 95% CI = 1.06–1.41) were weaker than of unadjusted models (RR = 1.52, 95% CI = 1.34–1.73).
Meta-analysis of all studies
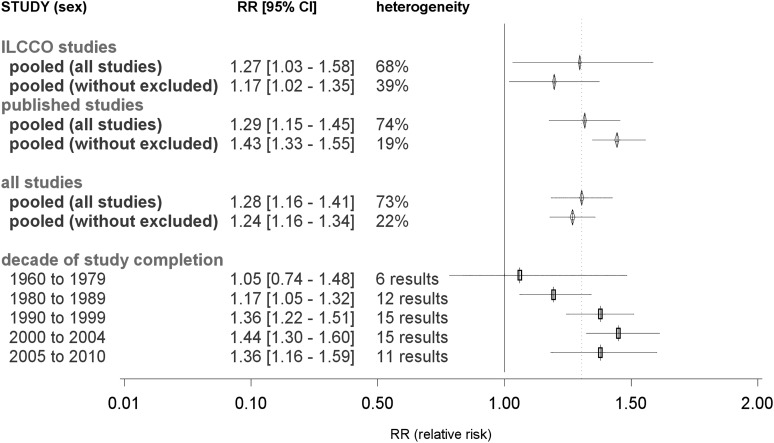
Based on all 52 studies with 59 reported RR estimates, the overall estimate of a summary RR of LC associated with asthma was 1.28 (95% CI = 1.16–1.41) (Figure 4). Significant heterogeneity between the RR estimates of all studies was observed (Phet < 0.001, I2 = 73%). After removing those eight studies contributing the most heterogeneity [Vesterinen (19) (f), Osann (22), Brenner (56), Koshiol (60) (m), Boffetta (64), Ji (65), Turner (73), Helmholtz lung cancer], the heterogeneity reduced substantially with I2 = 22% (Phet = 0.147). In this reduced subset, a positive association remained present (RR = 1.24, 95% CI = 1.16–1.34).
Fig. 4.
Forest plot of the association between asthma and LC risk: decade of study completion. Decade of study completion, RR estimate from the meta-regression for a study completed within the specified decade and of the ‘reference design’ defined as a case–control study with controls from a European Caucasian population of both sexes with mean age of 57 years, any type of smoking. Asthma should be assessed as self-reported diagnosis 3–10 years before the manifestation of LC. The analysis of such a ‘reference study’ was considered as adjusted for smoking but not for other lung diseases. Asterisk indicates excluded to reduce between-study heterogeneity; Double asterisk indicates 95% CI reconstructed; pooled, pooled RR according a random effects model; heterogeneity I2, percentage of inter-study heterogeneity; pooling all studies: the following eight studies were removed to reduce heterogeneity: Vesterinen (19) (f), Osann (22), Brenner (56), Koshiol (60) (m), Boffetta (64), Ji (65), Turner (73), HMGU.
Meta-regression
The ‘best fitting regression model’ based on the backward selection contained 13 covariates indicating that heterogeneity is not mainly caused by a single source. Significant covariates included in the model were other lung diseases (P < 0.001), a short latency period by design (0–2 years before LC, P < 0.001), type of controls (P < 0.001), type of controls within case–control studies (P <0.001–0.060), continent (Asia: P < 0.007), asthma ascertainment (P = 0.008) and age (P = 0.015). Additionally, men only (P = 0.301) were included, albeit not significant.
This model did not indicate a significant difference by study design (P = 0.811), but distinguished between published and ILCCO studies (P < 0.001). The adjusted pooled RR for ILCCO studies was RR = 1.29 (95% CI = 1.12–1.48), in contrast to those for published studies of RR = 1.82 (95% CI = 1.67–1.98). More details of the meta-regression analysis are given in the Supplementary Tables IV and V, available at Carcinogenesis Online. A linear increase in RR estimates over calendar time was observed (P < 0.001). Hereupon, reporting bias was evaluated by decade and does not appear to explain the increasing RR with decade of study completion. To avoid the linearity assumption, we fit the best fitting model again, having the years of study report/completion categorized into decades (Figure 4). For early studies, RR was not significant (RR1960–1979 = 1.05, 95% CI = 0.74–1.48) but thereafter, it steadily increased until the turn of the century (RR2000–2004 = 1.44, 95% CI = 1.30–1.60).
Discussion
In this investigation into the effect of asthma on the risk of LC, we considered study results from a pooled analysis of 16 studies and combined it with 36 published studies identified through a comprehensive literature review, which produced a summary RR of 1.28 (95% CI = 1.16–1.41). The association remained when excluding studies contributing to heterogeneity. However, the almost perfect concordance between the ILCCO pooled analysis (RR = 1.27, 95% CI = 1.03–1.58) and pooling published results (RR = 1.29, 95% CI = 1.15–1.45) should not be considered as an outright confirmation of findings because strong heterogeneity was indicated by inter-study variability of 73%. Confronted with such large heterogeneity, any estimate of a pooled RR for LC should be interpreted with caution. We have conducted a thorough investigation of the sources of heterogeneity and did not observe a single parameter that could explain the majority of the heterogeneity. However, we found that study period, histology and latency substantially contribute to heterogeneity as well as asthma ascertainment. Given that, we did not observe associations when considering latency of >10 years after asthma diagnosis and that the association was weakest among never-smokers, our findings provide evidence against a direct causal association between asthma and LC risk.
One of the most remarkable findings within the meta-regression is the difference between early and more recent studies, even adjusted for several design and population factors. The possible explanations include (i) studies investigating mortality were undertaken mainly before 1980. But we also observed a lower RR in studies performed during the 1980s (RR = 1.17, 95% CI = 1.05–1.32), all investigate LC incidence. Such case definition is unlikely to cause this observation. (ii) The rate of asthma misclassification is lower in more recent diagnoses of asthma. (iii) Decreases in asthma-related mortality, attributable to improvements in asthma health care (e.g. invention of the metered-dose inhaler), may have potentially increased the rate of long-term risks, such as LC. However, the regular use of long-acting beta-agonists of salmeterol has been shown to increase asthma-related deaths in comparison with placebo (75). (iv) Chance remains a possible explanation.
One major source of heterogeneity identified was ‘histology’. Considering the ILCCO pooled analysis, we found a positive association between asthma and squamous cell LC (RR = 1.69, 95% CI = 1.26–2.26) and SCLC LC (RR = 1.71, 95% CI = 0.99–2.95). In contrast to the previous meta-analysis (16), we neither found a significantly increased risk for adenocarcinoma within ILCCO studies (RR = 1.09, 95% CI = 0.88–1.36) nor within published studies (RR = 1.21, 95% CI = 0.89–1.67). However, the number of informative studies assessed was low (n = 9 of 34).
The actual exposure to active and passive smoking is difficult to quantify and residual confounding may affect these subtype-specific findings. Published studies unadjusted for smoking revealed a greater RR estimate (RR = 1.52, 95% CI = 1.34–1.73, n = 11) than studies adjusted for smoking (RR = 1.22, 95% CI = 1.06–1.41, n = 32). Tobacco smoke was shown to be more strongly associated with SCLC than with squamous cell LC and the least so with adenocarcinoma (76,77). In the case of inadequate adjustment for smoking, RRs for SCLC and squamous cell LC would be expected to have the greatest degree of bias and least for the observed weak association with adenocarcinoma. On the other hand, if asthma is an independent risk factor for LC, we would expect to see an association among never-smokers and with adenocarcinoma. However, in both cases, the observed association was weakest and statistically not significant. Our results demonstrate that the main association was observed among ever-smokers, which may be confounded by tobacco exposure despite the attempts to adjust for smoking exposure in the statistical analysis. Given that there has been a relative increase in adenocarcinoma and a concurrent relative decrease in squamous cell LC over the past several decades (78), one would expect a decreasing association between asthma and LC over time, in particular, in consideration of residual confounding. However, we observed the contrary. Consequently, inadequate adjustment for smoking may act as confounder in comparing histological subtypes, but it cannot be an explanation of the observed time trend.
The time between asthma diagnosis and LC diagnosis was identified as another factor that affects the summary RR. In both meta-analysis and pooled analysis, we found that the RR decreased with increasing time since asthma diagnosis, although in published studies, the latency period should be regarded as a study design parameter (the minimal allowed latency at the time of recruitment). The ILCCO pooled analysis revealed an RR of 2.13 (95% CI = 1.09–4.17) for a latency period of ≤2 years, but little is known about the risk within the first year. We know of only two published studies providing information for which we calculated a summary RR of 6.98 (95% CI = 3.30–14.78). However, LC does not appear to be a long-term consequence from asthma since the pooled RR in studies including asthma cases by a latency >10 years was between 1.10 (ILCCO pooled analysis) and 0.98 (subgroup meta-analysis of published studies). This stands in contrast to the previous meta-analysis, which revealed a borderline significant increased risk (RR = 1.8, 95% CI = 1.3–2.3), even for a latency of ≥20 years(16).
Asthma is a chronic inflammatory disorder and it is assumed that an insufficient anti-inflammatory response can lead to chronic inflammation and progress in tissue damage (13,79). If long-lasting chronic inflammation increases the risk of LC or acts as promoter in genesis of cancer, we would expect to see a positive association despite the long latency. On the contrary, our observation indicated that it is possible that the association between asthma and LC can be partially explained by the misdiagnosis of early LC symptoms as asthma. In addition, we cannot exclude the possibility of reverse causality, said to take place when an exposure is influenced by the early (subclinical) stages of the disease of interest (80). Hence, the observations might alternatively be explicable by an inflammatory immune response in a pre-diagnostic stage of the cancer manifesting as asthma.
This investigation is strengthened by the large sample size (in total 585 444 individuals) and large number of exposed individuals within the ILCCO pooled analysis, comprising 15 case–control studies and 1 cohort study. This also allowed us to perform some subgroup investigations among ever- and never-smokers. The respective comparison with published studies adds as a further merit by limiting the impact of reporting bias as we confronted the role of effect modifying factors on the individual level (e.g. sex or age) and on the study/population level (e.g. study design, asthma ascertainment or ethnicity). However, several limitations should be taken into considerations.
First the definition of asthma varied between studies, ranging from self-reported symptoms and verified physician-verified diagnosis to hospital registry data. Questionnaire assessment of self-reported asthma has been found to have satisfactory specificity (≥94%) but low sensitivity (∼68%) (81,82). Thus, combining these studies implies the combination of varying degrees of asthma severity (e.g. hospitalized versus verified physician diagnosed) and implies exposure misclassification (when self-reported) because mild or inactive forms of asthma are less probably to be atopic or exhibit bronchial hyperreactivity (83,84). Therefore, one can expect, that the estimated RR decreases by weaker asthma case definition, a gradient for severity/misclassification as we could observe by subgroup meta-analysis (hospitalized to self-reported symptoms: RR = 1.62 to RR = 1.15).
The definition of an asthma case may also have led to false classification of individuals suffering from other pulmonary diseases, e.g. chronic obstructive pulmonary diseases (COPD) (23,53,85). Because smoking is a strong risk factor for COPD (86,87), one would expect a higher proportion of non-smokers beyond COPD free individuals as we observed within non-asthmatic cases as in asthmatics with a long latency. But beyond asthmatics with a short latency, the proportion of never-smokers was almost equal between COPD and COPD free cases, as far as we were able to rate this reliably (data not shown). The complexity of this aspect is reflected in the so-called ‘Dutch hypothesis’; accordingly, all airway diseases should be considered as different expressions of a single malady (86–89). Analyzing the ILCCO studies, we found the estimated RRs adjusted for other lung diseases to be lower in general than unadjusted therefore, as reported previously (52,60). However, it remains unclear, if such an adjustment is necessary or leads to masking of effects, because all diseases should be considered as components of the same etiological path of LC.
In all studies, LC cases were defined by a histologically confirmed clinical diagnosis, but distribution of histological subtypes differed with respect to sex, age and smoking behavior (6,90). Eight studies focused solely on women, two on men and five on never-smokers. Two were restricted to a certain histological subtypes. The proportion of e.g. adenocarcinoma ranged from 11 (19) to 69% (17). The question of representativeness of the ‘total’ case sample of all studies is hence an obvious one. A pooled estimate for a RR of LC should therefore be considered as a rough approximation, depending on the weighting of more appropriate apposite estimates for specific subgroups of LC cases.
Further limitations arise due to several sources of bias and confounding, e.g. the discrepancy between newly diagnosed and newly developed cancer (Neyman’s bias) (80) or healthy worker effects owing to asthma-related job seeking (91,92). Finally, competing causes of asthma-related death, e.g. ischemic heart disease (93), or influenza and acute bronchitis (94,95), particularly before a clinical manifestation LC, may mask an increased risk (85,96).
The results can be considered from two perspectives. (i) Considering asthma as an epiphenomenon of LC, one can start to monitor those being newly diagnosed with asthma more intensively for LC; (ii) understanding the role of asthma in the etiology of LC. Some questions still cannot be answered from the data available today, e.g. differences between intrinsic and extrinsic asthma or the type of immune response causing an increased LC risk with respect to histological subtypes of LC.
To sum up, we did not gain clear evidence to support the hypothesis of an independent association between asthma and LC risk as the observed associations can at least in part be explained by residual confounding due to smoking and/or early symptoms/inverse causality.
For future studies, investigators may consider a better quantification of the severity of asthma and the use of asthma medication. A clear distinction between allergic and intrinsic asthma may clarify the role of atopy. Measuring biomarkers that indicate the different types of immune response may help to distinguish between subgroups of exposed and unexposed asthmatics.
Supplementary material
Supplementary material, supplement, Figures S1 and S2 and Tables I–VIII can be found at http://carcin.oxfordjournals.org/.
Funding
National Institute of Health (1U19CA148127-01); the German Research Foundation (GRK1034); the Canadian Cancer Society (CCSRI 020214); Cancer Care Ontario Research Chair Award. The individual studies were supported by the following sources:
Mayo Clinic (MAYO): Mayo Foundation Fund and National Institute of Health (HIN-CA77118, NIH-CA80127 to P.Y.]; Liverpool Lung Project (LLP): Roy Castle Lung Cancer Foundation UK; Study of the Memorial Sloan-Kettering Cancer Center (MSKCC): Steps for Breath, the Labrecque Foundation and the Society of MSKCC; Central Europe study (CE): World Cancer Research Fund and the European Commission’s INCO-COPERNICUS Program (contract number IC15-CT96-0313); The Warsaw part of CE: The Polish State Committee for Scientific Research (SPUB-M-COPERNICUS/P-05/DZ-30/99/2000); Studies of the Wayne State University, Karmanos Cancer Institute (WSU/KCI-1, WSU/KCI-2): National Institute of Health (R01CA060691, NIH R01CA87895, NIH N01-PC35145, NIH P30CA22453); Study of the University of California, San Francisco (UCSF): National Institutes of Environmental Health Sciences (ES06717); NCI (CA-113710 to S.S.O); Danish Diet Cancer and Health Study (DDCHS): Danish Cancer Society; Helmholtz lung cancer study: German Federal Ministry of Education, Science, Research and Technology, State of Bavaria, National Genome Research Network, German Research Foundation (BI 576/2-1, BI 576/2–2); Helmholtz Association and the Federal office for Radiation Protection (STSch4454).
Supplementary Material
Acknowledgments
We thank Mrs Verena Gullatz and Mrs Bernadette Stolz for their support in data extraction. We acknowledge the contribution of the CREST biorepository at the National Cancer Research Institute, Genoa. We thank all cases and controls for participating and providing personal and health-related information.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CI
95% confidence interval
- COPD
chronic obstructive pulmonary disease
- ILCCO
International Lung Cancer Consortium
- LC
lung cancer
- RR
relative risk
- SCLC
small-cell lung cancer
References
- 1.Masoli M, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Fanta CH. Asthma. N. Engl. J. Med. 2009;360:1002–1014. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 3.Beasley R, et al. Prevalence and etiology of asthma. J. Allergy Clin. Immunol. 2000;105:S466–S472. doi: 10.1016/s0091-6749(00)90044-7. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, et al. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Ezzati M, et al. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int. J. Cancer. 2005;116:963–971. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 6.Samet JM, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009;15:5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakelee HA, et al. Lung cancer incidence in never smokers. J. Clin. Oncol. 2007;25:472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisherman EW, et al. Does the allergic diathesis influence malignancy? J. Allergy. 1960;31:74–78. doi: 10.1016/0021-8707(60)90026-5. [DOI] [PubMed] [Google Scholar]
- 9.Turner MC, et al. An overview of the association between allergy and cancer. Int. J. Cancer. 2006;118:3124–3132. doi: 10.1002/ijc.21752. [DOI] [PubMed] [Google Scholar]
- 10.Vena JE, et al. Allergy-related diseases and cancer: an inverse association. Am. J. Epidemiol. 1985;122:66–74. doi: 10.1093/oxfordjournals.aje.a114087. [DOI] [PubMed] [Google Scholar]
- 11.Carrozzi L, et al. Allergy and cancer: a biological and epidemiological rebus. Allergy. 2005;60:1095–1097. doi: 10.1111/j.1398-9995.2005.00940.x. [DOI] [PubMed] [Google Scholar]
- 12.Mayne ST, et al. Previous lung disease and risk of lung cancer among men and women nonsmokers. Am. J. Epidemiol. 1999;149:13–20. doi: 10.1093/oxfordjournals.aje.a009722. [DOI] [PubMed] [Google Scholar]
- 13.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert. Rev. Anticancer Ther. 2008;8:605–615. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, et al. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy. 2005;60:1098–1111. doi: 10.1111/j.1398-9995.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 15.Sherman PW, et al. Allergies: their role in cancer prevention. Q. Rev. Biol. 2008;83:339–362. doi: 10.1086/592850. [DOI] [PubMed] [Google Scholar]
- 16.Santillan AA, et al. A meta-analysis of asthma and risk of lung cancer (United States) Cancer Causes Control. 2003;14:327–334. doi: 10.1023/a:1023982402137. [DOI] [PubMed] [Google Scholar]
- 17.Wu AH, et al. Previous lung disease and risk of lung cancer among lifetime nonsmoking women in the United States. Am. J. Epidemiol. 1995;141:1023–1032. doi: 10.1093/oxfordjournals.aje.a117366. [DOI] [PubMed] [Google Scholar]
- 18.Alavanja MC, et al. Preexisting lung disease and lung cancer among nonsmoking women. Am. J. Epidemiol. 1992;136:623–632. doi: 10.1093/oxfordjournals.aje.a116542. [DOI] [PubMed] [Google Scholar]
- 19.Vesterinen E, et al. Cancer incidence among 78,000 asthmatic patients. Int. J. Epidemiol. 1993;22:976–982. doi: 10.1093/ije/22.6.976. [DOI] [PubMed] [Google Scholar]
- 20.Ramanakumar AV, et al. Risk of lung cancer following nonmalignant respiratory conditions: evidence from two case-control studies in Montreal, Canada. Lung Cancer. 2006;53:5–12. doi: 10.1016/j.lungcan.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Osann KE, et al. Small cell lung cancer in women: risk associated with smoking, prior respiratory disease, and occupation. Lung Cancer. 2000;28:1–10. doi: 10.1016/s0169-5002(99)00106-3. [DOI] [PubMed] [Google Scholar]
- 22.Osann KE. Lung cancer in women: the importance of smoking, family history of cancer, and medical history of respiratory disease. Cancer Res. 1991;51:4893–4897. [PubMed] [Google Scholar]
- 23.Littman AJ, et al. Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes Control. 2004;15:819–827. doi: 10.1023/B:CACO.0000043432.71626.45. [DOI] [PubMed] [Google Scholar]
- 24.Liang H, et al. Risk of lung cancer following nonmalignant respiratory conditions among nonsmoking women living in Shenyang, Northeast China. J. Womens Health (Larchmt.) 2009;18:1989–1995. doi: 10.1089/jwh.2008.1355. [DOI] [PubMed] [Google Scholar]
- 25.Song F, et al. Methods for exploring heterogeneity in meta-analysis. Eval. Health Prof. 2001;24:126–151. doi: 10.1177/016327870102400203. [DOI] [PubMed] [Google Scholar]
- 26.van Houwelingen HC, et al. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat. Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 27. (2009) Cochrane Handbook for Systematic reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration.
- 28.Thompson SG, et al. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 29.Tjonneland A, et al. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand. J. Public Health. 2007;35:432–441. doi: 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- 30.Cui Y, et al. Dietary flavonoid intake and lung cancer–a population-based case-control study. Cancer. 2008;112:2241–2248. doi: 10.1002/cncr.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauter W, et al. Matrix metalloproteinase 1 (MMP1) is associated with early-onset lung cancer. Cancer Epidemiol. Biomarkers Prev. 2008;17:1127–1135. doi: 10.1158/1055-9965.EPI-07-2840. [DOI] [PubMed] [Google Scholar]
- 32.Kreienbrock L, et al. Case-control study on lung cancer and residential radon in western Germany. Am. J. Epidemiol. 2001;153:42–52. doi: 10.1093/aje/153.1.42. [DOI] [PubMed] [Google Scholar]
- 33.Kreuzer M, et al. Residential radon and risk of lung cancer in Eastern Germany. Epidemiology. 2003;14:559–568. doi: 10.1097/01.ede.0000071410.26053.c4. [DOI] [PubMed] [Google Scholar]
- 34.Holle R, et al. KORA–a research platform for population based health research. Gesundheitswesen, 67. Suppl. 2005;1:S19–S25. doi: 10.1055/s-2005-858235. [DOI] [PubMed] [Google Scholar]
- 35.Brennan P, et al. High cumulative risk of lung cancer death among smokers and nonsmokers in Central and Eastern Europe. Am. J. Epidemiol. 2006;164:1233–1241. doi: 10.1093/aje/kwj340. [DOI] [PubMed] [Google Scholar]
- 36.Lan Q, et al. Indoor coal combustion emissions, GSTM1 and GSTT1 genotypes, and lung cancer risk: a case-control study in Xuan Wei, China. Cancer Epidemiol. Biomarkers Prev. 2000;9:605–608. [PubMed] [Google Scholar]
- 37.The effect of tuberculosis control in China. Lancet. 2004;364:417–422. doi: 10.1016/S0140-6736(04)16764-0. [DOI] [PubMed] [Google Scholar]
- 38.Cote ML, et al. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA. 2005;293:3036–3042. doi: 10.1001/jama.293.24.3036. [DOI] [PubMed] [Google Scholar]
- 39.Le Marchand L, et al. Intake of flavonoids and lung cancer. J. Natl Cancer Inst. 2000;92:154–160. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 40.Brenner DR, et al. Lung cancer risk in never-smokers: a population-based case-control study of epidemiologic risk factors. BMC Cancer. 2010;10:285. doi: 10.1186/1471-2407-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Field JK, et al. The Liverpool lung Project research protocol. Int. J. Oncol. 2005;27:1633–1645. [PubMed] [Google Scholar]
- 42.Yang P, et al. Glutathione pathway genes and lung cancer risk in young and old populations. Carcinogenesis. 2004;25:1935–1944. doi: 10.1093/carcin/bgh203. [DOI] [PubMed] [Google Scholar]
- 43.Yang P, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 44.Heck JE, et al. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ. Health Perspect. 2009;117:1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muscat JE, et al. Insulation, asbestos, smoking habits, and lung cancer cell types. Am. J. Ind. Med. 1995;27:257–269. doi: 10.1002/ajim.4700270210. [DOI] [PubMed] [Google Scholar]
- 46.Ugolini D, et al. The CREST biorepository: a tool for molecular epidemiology and translational studies on malignant mesothelioma, lung cancer, and other respiratory tract diseases. Cancer Epidemiol. Biomarkers Prev. 2008;17:3013–3019. doi: 10.1158/1055-9965.EPI-08-0524. [DOI] [PubMed] [Google Scholar]
- 47.Wrensch MR, et al. CYP1A1 variants and smoking-related lung cancer in San Francisco Bay area Latinos and African Americans. Int. J. Cancer. 2005;113:141–147. doi: 10.1002/ijc.20537. [DOI] [PubMed] [Google Scholar]
- 48.Orlow I, et al. DNA damage and repair capacity in patients with lung cancer: prediction of multiple primary tumors. J. Clin. Oncol. 2008;26:3560–3566. doi: 10.1200/JCO.2007.13.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz AG, et al. Chronic obstructive lung diseases and risk of non-small cell lung cancer in women. J. Thorac. Oncol. 2009;4:291–299. doi: 10.1097/JTO.0b013e3181951cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabriel R, et al. Lung cancer and allergy. Br. J. Clin. Pract. 1972;26:202–204. [PubMed] [Google Scholar]
- 51.Markowe HL, et al. Prognosis in adult asthma: a national study. Br. Med. J. (Clin. Res. Ed) 1987;295:949–952. doi: 10.1136/bmj.295.6604.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown DW, et al. Asthma and risk of death from lung cancer: nHANES II Mortality Study. J. Asthma. 2005;42:597–600. doi: 10.1080/02770900500216234. [DOI] [PubMed] [Google Scholar]
- 53.Samet JM, et al. Personal and family history of respiratory disease and lung cancer risk. Am. Rev. Respir. Dis. 1986;134:466–470. doi: 10.1164/arrd.1986.134.3.466. [DOI] [PubMed] [Google Scholar]
- 54.Wu AH, et al. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer Res. 1988;48:7279–7284. [PubMed] [Google Scholar]
- 55.Brownson RC, et al. Previous lung disease and lung cancer risk among women (United States) Cancer Causes Control. 2000;11:853–858. doi: 10.1023/a:1008999202040. [DOI] [PubMed] [Google Scholar]
- 56.Brenner AV, et al. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int. J. Epidemiol. 2001;30:118–124. doi: 10.1093/ije/30.1.118. [DOI] [PubMed] [Google Scholar]
- 57.Gorlova OY, et al. Never smokers and lung cancer risk: a case-control study of epidemiological factors. Int. J. Cancer. 2006;118:1798–1804. doi: 10.1002/ijc.21561. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, et al. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int. J. Cancer. 2006;119:695–701. doi: 10.1002/ijc.21883. [DOI] [PubMed] [Google Scholar]
- 59.El Zein M, et al. History of asthma or eczema and cancer risk among men: a population-based case-control study in Montreal, Quebec, Canada. Ann. Allergy Asthma Immunol. 2010;104:378–384. doi: 10.1016/j.anai.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Koshiol J, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One. 2009;4:e7380. doi: 10.1371/journal.pone.0007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez-Perez A, et al. Cancer incidence in a general population of asthma patients. Pharmacoepidemiol. Drug Saf. 2006;15:131–138. doi: 10.1002/pds.1163. [DOI] [PubMed] [Google Scholar]
- 62.Alderson M. Mortality from malignant disease in patients with asthma. Lancet. 1974;2:1475–1477. doi: 10.1016/s0140-6736(74)90217-7. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds P, et al. Asthma and cancer. Am. J. Epidemiol. 1987;125:539–540. doi: 10.1093/oxfordjournals.aje.a114561. [DOI] [PubMed] [Google Scholar]
- 64.Boffetta P, et al. Lung cancer risk in a population-based cohort of patients hospitalized for asthma in Sweden. Eur. Respir. J. 2002;19:127–133. doi: 10.1183/09031936.02.00245802. [DOI] [PubMed] [Google Scholar]
- 65.Ji J, et al. Cancer risk in hospitalised asthma patients. Br. J. Cancer. 2009;100:829–833. doi: 10.1038/sj.bjc.6604890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frostad A, et al. Impact of respiratory symptoms on lung cancer: 30-year follow-up of an urban population. Lung Cancer. 2008;60:22–30. doi: 10.1016/j.lungcan.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Vandentorren S, et al. Long-term mortality among adults with or without asthma in the PAARC study. Eur. Respir. J. 2003;21:462–467. doi: 10.1183/09031936.03.00030303. [DOI] [PubMed] [Google Scholar]
- 68.Eriksson NE, et al. A prospective study of cancer incidence in a cohort examined for allergy. Allergy. 1995;50:718–722. doi: 10.1111/j.1398-9995.1995.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 69.Huovinen E, et al. Mortality of adults with asthma: a prospective cohort study. Thorax. 1997;52:49–54. doi: 10.1136/thx.52.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lange P, et al. Mortality in adults with self-reported asthma. Copenhagen City heart study group. Lancet. 1996;347:1285–1289. doi: 10.1016/s0140-6736(96)90937-x. [DOI] [PubMed] [Google Scholar]
- 71.Mills PK, et al. Allergy and cancer: organ site-specific results from the Adventist Health Study. Am. J. Epidemiol. 1992;136:287–295. doi: 10.1093/oxfordjournals.aje.a116494. [DOI] [PubMed] [Google Scholar]
- 72.Talbot-Smith A, et al. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. Am. J. Epidemiol. 2003;157:606–612. doi: 10.1093/aje/kwg020. [DOI] [PubMed] [Google Scholar]
- 73.Turner MC, et al. Cancer mortality among US men and women with asthma and hay fever. Am. J. Epidemiol. 2005;162:212–221. doi: 10.1093/aje/kwi193. [DOI] [PubMed] [Google Scholar]
- 74.Brown DW, et al. Re: asthma and the risk of lung cancer. findings from the Adverse Childhood Experiences (ACE) Cancer Causes Control. 2006;17:349–350. doi: 10.1007/s10552-005-0420-5. [DOI] [PubMed] [Google Scholar]
- 75.Salpeter SR, et al. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann. Intern. Med. 2006;144:904–912. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- 76.Kenfield SA, et al. Comparison of aspects of smoking among the four histological types of lung cancer. Tob. Control. 2008;17:198–204. doi: 10.1136/tc.2007.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sobue T, et al. Cigarette smoking and subsequent risk of lung cancer by histologic type in middle-aged Japanese men and women: the JPHC study. Int. J. Cancer. 2002;99:245–251. doi: 10.1002/ijc.10308. [DOI] [PubMed] [Google Scholar]
- 78.Burns DM, et al. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control. 2011;22:13–22. doi: 10.1007/s10552-010-9660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fitzpatrick FA. Inflammation, carcinogenesis and cancer. Int. Immunopharmacol. 2001;1:1651–1667. doi: 10.1016/s1567-5769(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 80.Delgado-Rodriguez M, et al. Bias. J. Epidemiol. Commun. Health. 2004;58:635–641. doi: 10.1136/jech.2003.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kilpelainen M, et al. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy. 2001;56:377–384. doi: 10.1034/j.1398-9995.2001.056005377.x. [DOI] [PubMed] [Google Scholar]
- 82.Toren K, et al. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest. 1993;104:600–608. doi: 10.1378/chest.104.2.600. [DOI] [PubMed] [Google Scholar]
- 83.Weissman DN. Epidemiology of asthma: severity matters. Chest. 2002;121:6–8. doi: 10.1378/chest.121.1.6. [DOI] [PubMed] [Google Scholar]
- 84.Ponsonby AL, et al. Which clinical subgroups within the spectrum of child asthma are attributable to atopy? Chest. 2002;121:135–142. doi: 10.1378/chest.121.1.135. [DOI] [PubMed] [Google Scholar]
- 85.Brenner DB, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International lung cancer ConsortiuPrevious lung diseases and lung cancer risk: a pooled analysis from the International lung cancer Consortium. In Preparation. 2010 doi: 10.1093/aje/kws151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barnes PJ. Against the Dutch hypothesis: asthma and chronic obstructive pulmonary disease are distinct diseases. Am. J. Respir. Crit. Care Med. 2006;174:240–243. doi: 10.1164/rccm.2604008. [DOI] [PubMed] [Google Scholar]
- 87.Hanania NA. The challenge of differentiating asthma from chronic obstructive pulmonary disease. Manag. Care. 2005;14:9–15. [PubMed] [Google Scholar]
- 88.Kabesch M. Asthma and… what is the link? Curr. Opin. Allergy Clin. Immunol. 2009;9:393–394. doi: 10.1097/aci.0b013e32833043fa. [DOI] [PubMed] [Google Scholar]
- 89.Chhabra SK. Asthma and chronic obstructive pulmonary disease: are they the same or are they distinct diseases? Am. J. Respir. Crit. Care Med. 2006;174:1056. doi: 10.1164/ajrccm.174.9.1056. [DOI] [PubMed] [Google Scholar]
- 90.Brownson RC, et al. Epidemiology and prevention of lung cancer in nonsmokers. Epidemiol. Rev. 1998;20:218–236. doi: 10.1093/oxfordjournals.epirev.a017982. [DOI] [PubMed] [Google Scholar]
- 91.Andersson E, et al. Mortality from asthma and cancer among sulfite mill workers. Scand. J. Work Environ. Health. 1998;24:12–17. doi: 10.5271/sjweh.273. [DOI] [PubMed] [Google Scholar]
- 92.Chenard L, et al. Lung function and farm size predict healthy worker effect in swine farmers. Chest. 2007;131:245–254. doi: 10.1378/chest.05-2238. [DOI] [PubMed] [Google Scholar]
- 93.Toren K, et al. Do patients with severe asthma run an increased risk from ischaemic heart disease? Int. J. Epidemiol. 1996;25:617–620. doi: 10.1093/ije/25.3.617. [DOI] [PubMed] [Google Scholar]
- 94.Fuhrman C, et al. Deaths with asthma in France, 2000-2005: a multiple-cause analysis. J. Asthma. 2009;46:402–406. doi: 10.1080/02770900902795553. [DOI] [PubMed] [Google Scholar]
- 95.McCoy L, et al. A multiple cause-of-death analysis of asthma mortality in the United States, 1990-2001. J. Asthma. 2005;42:757–763. doi: 10.1080/02770900500308189. [DOI] [PubMed] [Google Scholar]
- 96.Robinette CD, et al. Asthma and subsequent mortality in World War II veterans. J. Chronic. Dis. 1978;31:619–624. doi: 10.1016/0021-9681(78)90022-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.