Abstract
We earlier provided evidence that oral consumption of pomegranate fruit extract (PFE) inhibits prostate cancer (PCa) cell growth in nude mice. To ascertain convincing evidence of chemopreventive effects of PFE against PCa, its efficacy requires to be evaluated in animal models that closely emulate human disease. Here, we provide evidence of remarkable tumor growth inhibitory effects of PFE using the TRAMP model. Mice received 0.1 and 0.2% PFE, equivalent to 250 and 500 ml of pomegranate juice, in drinking water, starting at 6 weeks and examined at 12, 20 and 34 weeks of age. In water-fed group, 100% mice developed palpable tumors by 20 weeks compared with only 30 and 20% in the 0.1 and 0.2% PFE-supplemented groups, respectively. At 34 weeks, palpable tumors were observed in 70 of 0.1% and only 50 of 0.2% PFE-supplemented mice. Compared with median survival of 43 weeks in water-fed mice, 0.1 and 0.2% PFE-supplemented mice exhibited median life expectancy of 73 and 92 weeks, respectively. Compared with respective water-fed groups, none of the mice in PFE-supplemented groups exhibited metastases to any of the distant organs at 20 weeks and only 20% mice exhibited metastasis at 34 weeks of age. Many of the PFE-supplemented animals had multiple foci of well-differentiated carcinoma but no evidence of poorly differentiated carcinoma. PFE supplementation resulted in simultaneous and significant inhibition of IGF-I/Akt/mTOR pathways in the prostate tissues and tumors. We suggest that pomegranate juice be evaluated in clinical trials in patients at high risk for developing PCa.
Introduction
According to recent estimates, prostate cancer (PCa) continues to remain the most common cancer and the second leading cause of cancer-related deaths in American males (1) with similar trends in many other western countries. In the year 2011, a total of 240 890 men are estimated to be diagnosed with PCa and 33 720 PCa-related deaths are predicted in the USA alone (1). The success of the PCa prevention trials strongly suggests that the disease can be prevented by the use of non-toxic pharmacological agents (2,3). In fact, in recent years, there is great interest in developing chemopreventive strategies against PCa and more and more people are turning to the use of complementary and alternative approaches for prevention of this disease (4,5). Because of its age association and long latency, PCa represents an ideal candidate disease for chemoprevention and any modest delay achieved through pharmacological intervention could result in substantial reduction in the incidence of clinically detectable disease (6).
Pomegranate (Punica granatum), grown mainly in the Mediterranean region for its edible fruit, possesses many medicinal properties, such as being antioxidant and anti-inflammatory (6–9). The unique biochemical composition of the pomegranate fruit, being rich in antioxidant tannins and flavonoids, has drawn attention of many investigators to study its exceptional healing qualities (10). The antioxidant activity of flavonoids obtained from pomegranate juice was observed to be close to that of green tea and significantly greater than red wine (11,12). Recent research has shown that pomegranate extracts selectively inhibit the growth of breast, prostate, colon, leukemia and lung cancer cells in culture (13–25). In preclinical animal studies, oral consumption of pomegranate extract inhibited growth of lung, skin, colon and prostate tumors (26–36). An initial phase II clinical trial of pomegranate juice in patients with PCa reported significant prolongation of prostate-specific antigen doubling time (37). Pomegranate juice ingredients have been found to be bioavailable in human serum also found to localize in prostate tissues in mice (19). This observation could suggest that many of the effects of pomegranate juice could directly affect pathways within the prostate.
We have previously demonstrated that pomegranate fruit extract (PFE) possesses antiproliferative and proapoptotic properties against human PCa cells (35). We also demonstrated that oral administration of PFE to athymic nude mice implanted with androgen-sensitive CWR22Rν1 cells resulted in a significant inhibition in tumor growth with concomitant decrease in serum prostate-specific antigen levels (35). For a comprehensive evaluation of agents' cancer chemopreventive properties, it is essential that the compound be tested in more than one preclinical model before recommending its evaluation in humans. Therefore, in the present study, we tested the effects of PFE against PCa development using a classical chemoprevention protocol in the transgenic TRAMP mouse model. We observed significant inhibition of PCa growth and progression. Oral supplementation of PFE inhibited metastasis and increased overall survival possibly through inhibition of multiple pathways most notably the IGF-I/Akt/mTOR pathways.
Materials and methods
Animals
Male C57BL/6 and female heterozygous C57BL/TGN TRAMP mice, Line PB-Tag 8247NG, were purchased as breeding pairs from The Jackson Laboratory (Ann Arbor, MI). Transgenic males for these studies were routinely obtained as [TRAMP × C57BL/6] F1 or as [TRAMP × C57BL/6] F2 offspring. Identity of transgenic mice was established by the PCR-based DNA screening as described previously (38). The animals were bred and maintained at the AAALAC-accredited Animal Resource Facility of University of Wisconsin. Housing and care of the animals was approved by the University's Research Animal Resource Committee in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Study design and PFE supplementation
Six-week-old TRAMP mice were randomly divided into three groups. The first group of animals received normal drinking water and served as controls. The animals of groups 2 and 3 received the same drinking water supplemented with 0.1 and 0.2% PFE (wt/vol), respectively. As described previously (33), fresh pomegranates were peeled, and the edible portion was squeezed in 70% acetone/30% distilled water (1:20, wt/vol). The red extract was filtered through Whatman no. 1 filter paper, and the filtrate was condensed, freeze-dried and stored at 4°C. The extract as analyzed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry was found to contain anthocyanins, various ellagitannins and hydrolyzable tannins (33). Water bottles were changed every other day. The 0.1 and 0.2% doses of PFE selected were based on the assumption that a typical healthy individual (70 kg) may be persuaded to drink 250 or 500 ml of pomegranate juice extracted from one or two fruits, respectively (35). Body weight, diet and water consumption were recorded weekly throughout the study. PFE-supplemented water was provided to mice beginning at 6 weeks of age and was continued until the animals were 34 weeks old, at which time the experiment was terminated. Throughout the experiment, the animals had access to chow diet ad libitum. For each experiment, 30 male TRAMP mice of 6 weeks of age were divided into three equal groups of 10 mice. At the termination of the experiment, blood was collected from the retro-orbital plexus under anesthesia from both experimental and control groups. The genitourinary (GU) apparatus consisting of bladder, urethra, seminal vesicles, ampullary gland and the prostate was excised, removed and weighed. The prostate gland was then separately excised using a dissecting microscope. The wet weight of GU apparatus was recorded to the nearest 0.01 g. In a separate experiment, to investigate the effect of PFE supplementation on growth of palpable tumors and overall survival, 36 male TRAMP mice 6 weeks of age were equally divided into three groups. The control group of animals was provided with drinking water, whereas animals in the experimental group received PFE-supplemented water throughout the protocol. Animals in all groups were observed weekly for body weight, tumor progression by abdominal palpation and survival. For survival analyses, animals were allowed to remain in the protocol until they died or were moribund. Moribund animals were humanely killed and considered censored for statistical purposes.
Magnetic resonance imaging
Five animals each from control and experimental groups were randomly selected and monitored for tumor growth and volume by magnetic resonance imaging at 12, 20 and 34 weeks of age. Imaging in these animals was performed by using a whole-body Varain 4.5 Tesla imager (Varian, Inc. Magnetic Resonance Systems, Palo Alto, CA) horizontal bore imaging/spectroscopy system (7.0 cm ID) equipped with isoflurane gas anesthesia system. To identify prostate gland, urinary bladder was used as the reference point since the gland is located anatomically inferior and posterior to the bladder, encircling urethra (39). Tumor volumes were determined by image segmentation by manually outlining tumor boundaries in parallel slices separated by 50 μM in the two-dimensional images. The areas of the outlined contours were summed and multiplied by the interslice distance to compute tumor volume.
Ultrasound imaging
Ultrasound imaging was performed using the Vevo 770 microimaging system (Visualsonics, Ontario, Canada) with a 30 MHz transducer for three-dimensional image visualization and advanced analysis software. Mice were anesthetized by inhalation of isoflurane with 1–3% oxygen and ventral hair was removed using a mild depilatory cream. Mice were placed on a thermostatically controlled heating pad to help maintain mouse body temperature. Orientation of the prostate gland on ultrasound was accomplished by visualizing the bladder, which is less echogenic and appears as a dark area.
Metastasis examination
Metastasis to lymph nodes, lungs and the liver was observed under the microscope. The India ink method was used to examine cancer metastasis to lungs. Animals from control and treated groups were killed and the respiratory system was excised. India ink was injected through the trachea into the lungs until completely filled. The trachea was then tied with a thread. The ink fills normal respiratory structures and metastases stand out as unstained regions.
Histology and immunostaining
The dorsal and lateral prostates were excised, weighed and a small portion was fixed overnight in (10%) zinc-buffered formalin and then transferred to 70% ethanol. The sections were stained with hematoxylin and eosin. Histological sections were reviewed by light microscopy for the presence of PCa and classified as prostate intraepithelial neoplasia, PIN (epithelial stratification with occasional mitotic figures or cribriform pattern), well differentiated (multiple epithelial mitotic figures and apoptotic bodies, invasive glands with stromal hypercellularity), moderately differentiated (many acini completely filled with tumor yet still forming some glandular structures) or poorly differentiated (sheets of malignant cells with little or no gland formation) PCa or atrophic glands only (no identifiable tumor). For immunostaining, sections (4–6 μm) were cut from paraffin-embedded tissue and mounted on slides. Immunostaining was performed using specific antibodies with appropriate dilutions and was replaced with either normal host serum or block for negative controls, followed by staining with appropriate biotin-conjugated secondary antibodies followed by incubation with avidin–biotin conjugate. The slides were developed in diaminobenzidine and counter stained with a weak solution of hematoxylin stain. The stained slides were dehydrated and mounted in permount and visualized on a Zeiss-Axiophot DM HT microscope (Zeiss-Axiophot, Germany). Images were captured with an attached camera linked to a computer.
Immunoblot analysis
The dorsolateral prostate was removed from both treated and control groups, homogenized in lysis buffer to prepare cell lysates. The protein concentration was determined by BCA method and following the manufacturer's protocol (Pierce, Rockford, IL). Appropriate amount of protein (25–50 μg) was resolved over 8–14% Tris-glycine polyacrylamide gel and then transferred onto the nitrocellulose membrane. The blots were blocked using 5% non-fat dry milk and probed using appropriate primary antibodies: T-antigen (Santa Cruz Biotechnology, Santa Cruz, CA), Akt, pAkt (Ser473 and Thr308), phosphoinositide 3-kinase (PI3K) (p85), p-mTOR (Ser2448), p-p70S6K, p-AMPKα (Thr172), IGF-I, rictor and raptor obtained from Cell Signaling Technology (Danvers, MA). Anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were obtained from Cell Signaling Technology. Following incubation in the secondary antibody, blots were detected using chemiluminescence ECL kit (Amersham Life Sciences Inc., Arlington Heights, IL). Equal loading of protein was confirmed by stripping the membrane and reprobing it with β-actin antibody (Cell Signaling Technology). Protein expression was analyzed in five samples from each group. Because considerable variation in expression was observed, we selected two samples from each group for final immunoblotting that is presented in this study. The two samples selected from each group represent the variation observed.
IGF-I and IGFBP-3 assay
Serum was separated from the whole blood obtained from the retro-orbital venous plexus with heparinized capillary tubes, and IGF-I and IGFBP-3 levels were determined by commercially available ELISA kits (R&D Systems Inc., Minneapolis, MN) following the manufacturer's protocol.
Statistical analysis
Five tumors from each group were randomly selected for all histology and immunochemical analyses. For measurement of GU weight, Kruskal–Wallis test, a non-parametric test based on Wilcoxon scores was used to examine the difference between two groups. For other continuous measurements, the difference between two groups was examined by the Student's t-test after checking normality. Overall survival was estimated using Kaplan–Meier method and difference between groups was examined using log-rank test. Data are expressed as mean values with 95% confidence intervals for five mice. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
Results
PFE supplementation inhibits prostate tumorigenesis and metastasis
To investigate the effects of PFE supplementation on PCa growth and progression in TRAMP mice, 0.1 and 0.2% PFE was supplied to the animals in drinking water starting at the age of 6 weeks. Animals were examined at 12, 20 and 34 weeks of age for prostate tumor growth. PFE supplementation for 28 weeks to TRAMP mice did not result in any adverse side effects. No affect was observed on the body weight gain and water intake in mice infused with PFE when compared with the water-fed controls. Effect of PFE supplementation on prostate carcinogenesis and metastasis from two experiments are summarized in Table 1. As expected, 100% mice in the water-fed group developed palpable tumors by 20 weeks of age compared with only 30 and 20% in the 0.1 and 0.2% PFE-supplemented groups, respectively, which was assessed by abdominal pelvic palpation. Development of palpable tumors was further significantly delayed by oral supplementation of PFE. At 34 weeks of age, palpable tumors were observed in only 50 of 0.2% PFE-supplemented mice and in 70 of 0.1% PFE-supplemented TRAMP mice. Furthermore, we studied the effect of PFE supplementation on the metastases to the lungs, liver and the lymph nodes. While most of the metastatic lesions were observed in the lymph nodes and the lungs, there were some cases of liver metastasis in advanced stages. The cumulative data at 20 and 34 weeks of age from 10 animals, in the water-fed group showed 30 and 90% invasive tumors, which had metastasized to the lungs and the liver, respectively. In sharp contrast, in the 0.1 and 0.2% PFE-infused group, none of the 20 mice exhibited metastases to any of the distant organs studied at 20 weeks of age and only 20% mice exhibited distant site metastasis at 34 weeks of age (Table 1).
Table I.
Effect of oral supplementation of PFE on incidence of palpable tumors and metastasis
| Groups | Number of animals | Incidence of palpable tumors |
Metastasis to lungs, lymph, liver |
||||
| 12 weeks | 20 weeks | 34 weeks | 12 weeks | 20 weeks | 34 weeks | ||
| Experiment 1 | |||||||
| Water fed | 10 | 2 (20%) | 10 (100%) | 10 (100%) | 0 | 2 (20%) | 8 (80%) |
| PFE (0.1%) | 10 | 0 | 4 (40%) | 6 (60%) | 0 | 0 | 2 (20%) |
| PFE (0.2%) | 10 | 0 | 3 (30%) | 4 (40%) | 0 | 0 | 3 (30%) |
| Experiment 2 | |||||||
| Water fed | 10 | 2 (20%) | 10 (100%) | 10 (100%) | 0 | 4 (40%) | 10 (100%) |
| PFE (0.1%) | 10 | 0 | 2 (20%) | 8 (80%) | 0 | 0 | 2 (20%) |
| PFE (0.2%) | 10 | 0 | 1 (10%) | 6 (60%) | 0 | 0 | 1 (10) |
| Cumulative incidence of tumors and metastasis from the two experiments | |||||||
| Water fed | 20 | 4 (20%) | 20 (100%) | 20 (100%) | 0 | 6 (30%) | 18 (90%) |
| PFE (0.1%) | 20 | 0 | 6 (30%) | 14 (70%) | 0 | 0 | 4 (20%) |
| PFE (0.2%) | 20 | 0 | 4 (20%) | 10 (50%) | 0 | 0 | 4 (20) |
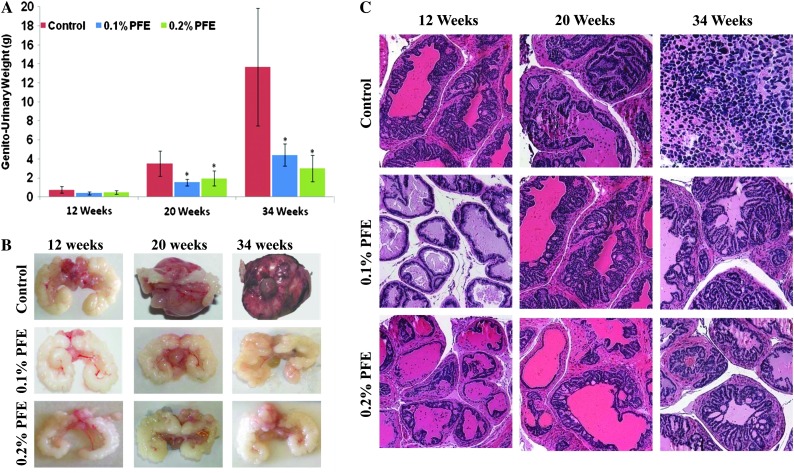
Furthermore, to determine the effect of PFE supplementation on PCa in TRAMP mice, gross biological indices (wet weights) were used to assess the tumorigenicity. An important observation in this experiment was that PFE supplementation resulted in a significant decrease in GU weight (>50% in both groups) compared with the water-fed TRAMP group at 20 weeks (Figure 1A). At 34 weeks, however, the GU wet weights were ∼69% lower in the 0.1% PFE-supplemented animals and ∼78% lower in the 0.2% PFE-supplemented animals compared with the water-fed animals, suggesting a dose-related effect (Figure 1A). As observed visually, PFE supplementation resulted in significant decrease in hyperplasia in the GU apparatus, especially in the seminal vesicles (Figure 1B).
Fig. 1.
Effect of oral supplementation of PFE on prostate carcinogenesis and pathology. Oral supplementation of PFE (0.1 and 0.2% in drinking water ad libitum) was initiated in animals at 6 weeks of age as described in the text. A control group that received water alone was run in parallel. (A) Histograms representing wet weight in grams (mean ± SE of five animals) of the GU apparatus recorded at 12, 20 and 34 weeks of age. *P < 0.01. (B) Representative photomicrographs of the GU apparatus taken from control and PFE-supplemented animals at 12, 20 and 34 weeks of age. (C) Representative photomicrographs of the prostate/tumor sections stained for hematoxylin and eosin from control water-fed and PFE-supplemented TRAMP mice. Control animals show varying degrees of abnormal pathology ranging from PIN lesions to poorly differentiated adenocarcinoma. In contrast, PFE-supplemented mice had mostly normal pathology and some evidence of well to moderate differentiation adenocarcinoma. None of the mice from PFE-supplemented groups exhibited any signs of poorly differentiated adenocarcinoma. Magnification, ×200.
PFE supplementation inhibits development of poorly differentiated adenocarcinoma
Histological findings in TRAMP mice of various ages have been well documented, and it is known that TRAMP mice older than 24 weeks typically have poorly differentiated prostate adenocarcinoma. We elected to evaluate the dorsolateral prostates of TRAMP mice in control and experimental groups of animals at 12, 20 and 34 weeks of age. Prostates of control TRAMP mice at 34 weeks of age, as expected, exhibited cancers of variable size, predominantly poorly differentiated carcinomas (>60%) composed of sheets of anaplastic cells with scant cytoplasm and marked nuclear pleomorphism (Figure 1C). The histological findings in prostates of PFE-supplemented TRAMP mice were significantly different. At 34 weeks of age, prostates of PFE-supplemented TRAMP mice contained well-differentiated adenocarcinoma that occupied ∼20% at surface area, admixed with lesser amounts of moderately differentiated adenocarcinoma (<3% of surface area), histology that was comparable with controls at 12 weeks of age. Many of the PFE-supplemented animals had multiple foci of well-differentiated carcinoma (7–15% of surface area) and some had moderately differentiated carcinoma (Figure 1C). Significantly, none of the mice in either group of PFE-supplemented animals exhibited any signs of poorly differentiated adenocarcinoma.
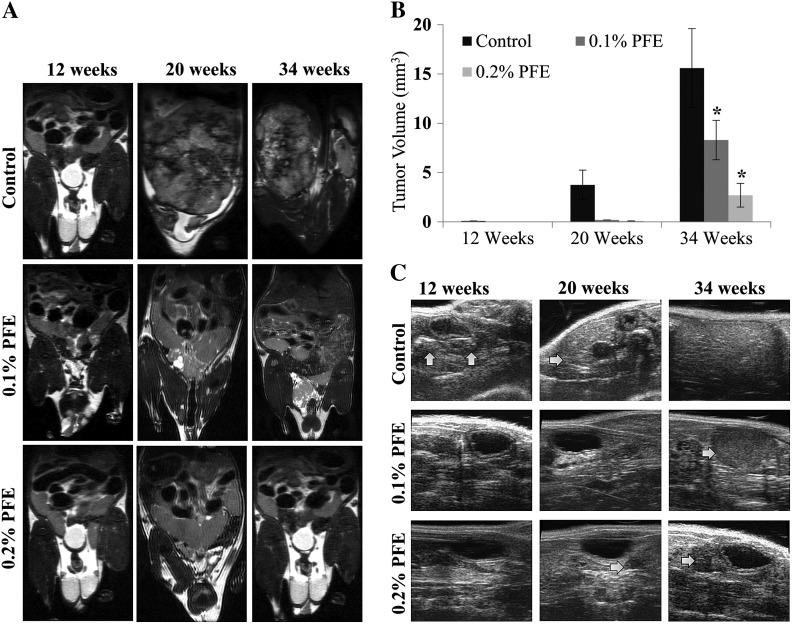
Progression of the disease was also monitored non-invasively by magnetic resonance and ultrasound based imaging. Prostate and tumor sizes assessed by magnetic resonance imaging at 12, 20 and 34 weeks of age in the control mice were consistent with the development and progression of PCa and significantly greater than those of PFE-supplemented mice. Prostate volumes in PFE-supplemented male TRAMP mice were substantially less than in the control water-fed animals (Figure 2A). PFE-supplemented TRAMP mice exhibited a significant reduction in the development of PCa measured at 20 and 34 weeks on test. Tumors were not quantifiable at 12 weeks and in PFE-supplemented animals at 20 weeks (Figure 2B). In water-fed animals, tumors occupied all of the pelvic space and masked other organs from visualization (Figure 2A). This inhibitory effect of PFE supplementation on prostate tumorigenesis was also evident by ultrasound imaging (Figure 2C). In the control water-fed animals, tumors were readily visible, especially at 20 and 34 weeks; however, in the PFE-supplemented animals, tumors were significantly smaller in size and difficult to locate (Figure 2C).
Fig. 2.
Effect of oral supplementation of PFE on PCa development in TRAMP mice. (A) Representative magnetic resonance imaging images of the pelvic region of TRAMP mice at 12, 20 and 34 weeks of age subjected to oral infusion of PFE starting at 6 (no cancer) weeks of age. Tumor growth was evaluated by longitudinal magnetic resonance imaging analysis. (B) Histograms represent mean tumor volumes ± SE in cubic centimeter as evaluated by two-dimensional magnetic resonance imaging-based imaging. *P < 0.01. (C) Representative ultrasound images of the pelvic region of TRAMP mice at 12, 20 and 34 weeks of age from control and PFE-supplemented animals starting at 6 (no cancer) weeks of age. Arrows point to tumor tissues.
PFE supplementation inhibits PI3K/Akt/mTOR signaling
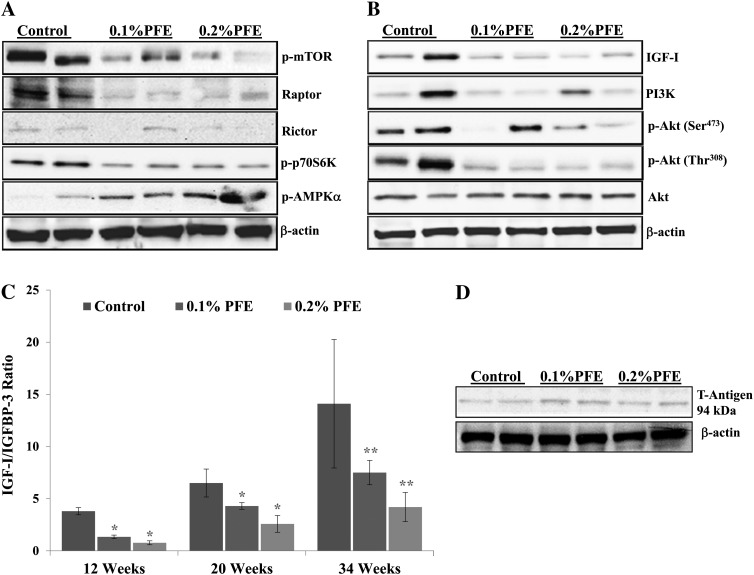
To ascertain the underlying molecular targets of PFE that could be responsible for its cancer preventive properties, we evaluated several molecules whose expression is deregulated during the progression of PCa in the TRAMP model. An important observation of our study was that treatment with PFE caused inhibition of the PI3K/Akt and mTOR signaling in the dorsolateral prostates of TRAMP mice. The mTOR has been identified as key kinase acting downstream of the activation of PI3K (40). Phosphorylation of mTOR at Ser2448 has been shown to be associated with the activity of mTOR and Ser2448 of mTOR is phosphorylated by Akt (41). We investigated the effect of PFE on the phosphorylation of mTOR and observed dose-dependent inhibition in the phosphorylation of mTOR at Ser2448 as detected by immunoblot analysis (Figure 3A). We next examined whether PFE affects mTOR complexes. Both raptor (forming mTOR complex 1, mTORC1) and rictor (forming mTOR complex 2, mTORC2) levels were found to be decreased by PFE supplementation. One of the best-characterized targets of mTOR is the ribosomal S6 kinases and PFE supplementation caused decrease in the phosphorylation of p70S6K protein, which is a downstream target of mTOR (Figure 3A). AMP-activated protein kinase (AMPK) is the central component of a protein kinase cascade that plays a major role in the regulation of energy control. It has been reported that there is a link between AMPK and the growth and survival of cancer cells (42). The phosphorylation of AMPK negatively regulates protein synthesis by directly phosphorylating and inhibiting mTOR (43). We found that there was a significant increase in the phosphorylation of AMPKα in the dorsolateral prostates of PFE-supplemented animals.
Fig. 3.
Effect of oral supplementation of PFE in TRAMP on PI3K/Akt/mTOR signaling and IGF-I/IGFBP-3 levels. (A, B) Oral PFE (0.1 and 0.2% in drinking water ad libitum) was initiated in animals at 6 weeks of age as described in the text. A control group that received water alone was run in parallel. At 12 weeks of age, the dorsolateral prostate was dissected, tissue lysates prepared and subjected to immunoblotting. Five samples from each group were analyzed by immunoblotting under identical conditions; however, only two bands representative of the variation in expression within each groups are shown. The blots were stripped and reprobed for analysis of β-actin to determine equal loading of the protein. (C) Histograms represent IGF-I/IGFBP-3 ratios (mean ± SE) in serum of control and PFE-supplemented animals. At 12, 20 and 34 weeks of age, blood was collected and serum separated for estimation of IGF-I and IGFBP-3 levels. The ratios were determined from the data obtained from analyses of IGF-I and IGFBP levels in serum. *P < 0.05, **P < 0.01. (D) Immunoblot representing protein expression of T-antigen in control and PFE-supplemented mice evaluated at 12 weeks of age. The blots were stripped and reprobed for analysis of β-actin to determine equal loading of the protein.
The PI3K family is involved in various cellular functions, including growth, proliferation, migration and survival. Supplementation of PFE in drinking water resulted in significant inhibition in the expression of regulatory (p85) subunit of PI3K (Figure 3B). PFE supplementation also resulted in the inhibition of phosphorylation of Akt at both Ser473 and Thr308 without affecting levels of total Akt (Figure 3B).
PFE supplementation inhibits IGF-I/IGFBP-3 ratio
Our previous studies have shown an increase in serum IGF-I levels with concomitant decrease in serum IGFBP-3 levels in TRAMP mice during PCa progression (44,45). Because IGFs are known to be produced locally by most tissues in which they act in an autocrine or a paracrine manner, we further determined IGF-I and IGFBP-3 expression in the serum of control and PFE-supplemented TRAMP mice. A progressive increase in the IGF-I protein expression was observed in TRAMP mice that was associated with concomitant decrease in the protein expression of IGFBP-3, the major binding protein for IGF-I and a shift in IGF-I to IGFBP-3 ratio favoring cancer progression (Figure 3C). PFE supplementation resulted in significant lowering of IGF-I/IGFBP-3 ratio. As seen in Figure 3B, continuous PFE supplementation to TRAMP mice resulted in significant inhibition in the protein expression of IGF-I as evaluated by immunoblotting in the dorsolateral prostate tissue of TRAMP mice.
PFE supplementation does not affect the expression of T-antigen
In the male TRAMP mice, prostate carcinogenesis is driven by the expression of a PB-Tag transgene consisting of the minimal regulatory element of the rat probasin promoter directing prostate-specific epithelial expression of the SV40 early genes. To rule out the possibility that the PFE effects could be related to direct or indirect effects on the transgene, we determined the protein expression of the T-antigen in the dorsolateral prostate tissues of control and PFE-supplemented mice at 12 weeks of age. No effect of PFE supplementation was observed on the expression of T-antigen suggesting that the inhibition of prostate carcinogenesis by PFE is not a consequence of inhibition of T-antigen levels (Figure 3D).
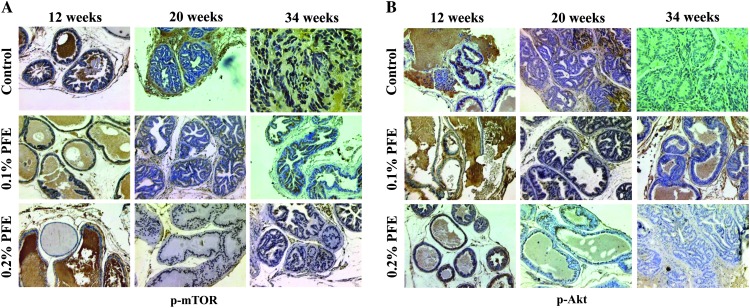
PFE supplementation decreases mTOR and pAkt expression in prostate tissues as evaluated by immunostaining
The effects on mTOR were also evaluated by immunostaining of the prostate tissues and tumors. Increased staining for mTOR was observed at all time points in water-fed animals and PFE-supplemented animals exhibited significant reduction in expression (Figure 4A). The effects on Akt were also evaluated by immunostaining of the prostate tissues and tumors. Increased staining for pAkt was observed at all time points in water-fed animals and PFE-supplemented animals exhibited significant reduction in expression (Figure 4B).
Fig. 4.
Effect of oral supplementation of PFE to TRAMP mice on mTOR and pAkt expression by immunostaining. Oral PFE (0.1 and 0.2% in drinking water ad libitum) was initiated in animals at ages representing different stages of the disease as described. At 12, 20 and 34 weeks of age, the dorsolateral prostate was dissected and processed for evaluation of p-mTOR (A) and pAkt (B) expression by immunostaining. Only representative photomicrographs of the prostate/tumor tissue from control water-fed and PFE-supplemented mice are shown. Control water-fed mice show high expression for phosphorylated mTOR and Akt compared with PFE-supplemented mice that show significantly low expression depending on the dose of PFE and age of mice. Magnification, ×200.
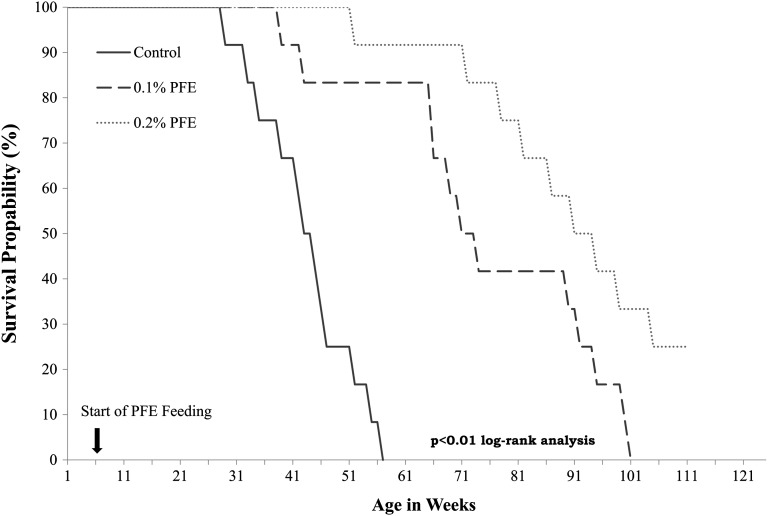
PFE supplementation increases overall survival
The outcome of any chemopreventive or chemotherapeutic agent is evaluated on the basis of its ability to increase overall survival and life span. We, therefore, in a separate experiment evaluated the effect of PFE supplementation on overall survival in 36 TRAMP mice divided into three groups of 12 each. Compared with the expected median survival of 43 weeks in water-fed mice, PFE-supplemented mice exhibited significant increase in life span with 0.1 and 0.2% PFE-supplemented mice exhibiting median life expectancy of 73 and 92 weeks, respectively (Figure 5). This significant increase in overall survival clearly suggests that PFE possesses high potential for PCa prevention.
Fig. 5.
Effect of oral supplementation of PFE on overall and tumor free survival. Oral PFE (0.1 and 0.2% in drinking water ad libitum) was initiated in animals at 6 weeks of age as described in the text. A control group that received water alone was run in parallel. Animals were allowed to remain in the protocol until death to ascertain overall survival. Animals were monitored biweekly for tumor development by abdominal pelvic palpation to analyze tumor free survival. Time in weeks is the start of oral supplementation of PFE. Survival probability by log-rank test.
Discussion
Cancer chemoprevention in gaining popularity among the scientific community because it is becoming increasingly evident that the best way to manage cancer is to delay or slowdown its progression (46). Because chemopreventive interventions are aimed at healthy populations at high risk for cancer development and usually would run over the course of many years, non-toxic dietary agents are preferred. There is considerable laboratory evidence demonstrating the usefulness of pomegranate juice in many disease conditions. Its beneficial properties against cancer have only recently been identified and there is optimism that it could be developed as a cancer chemopreventive agent (8–37). Studies from our and other laboratories indicate that pomegranate juice could be beneficial against the development of PCa (19,20,24,28,35–37). In this study, we report significant inhibition of PCa development in the TRAMP mice that were given free access to drinking water supplemented with PFE.
The most important finding of our study is that PFE supplementation resulted in significant inhibition in the development of advanced PCa and its metastasis and doubling the overall survival time. Tumor burden as analyzed by magnetic resonance imaging and ultrasound imaging was significantly lower in PFE-supplemented mice as was the histological evidence of absence of poorly differentiated adenocarcinoma. It is highly possible that inhibition of advanced disease resulted in high overall survival of mice.
To gain insight into the possible mechanisms by which pomegranate juice could exert its beneficial effects, we examined several molecules whose expression is deregulated during the process of carcinogenesis in the TRAMP mouse. We observed inhibition of PI3K/Akt and mTOR signaling in tumors and prostate tissues of PFE-supplemented TRAMP mice. It is speculated that PFE is rapidly taken up by cancer cells, whereas its uptake is slow and regulated in normal cells.
The mTOR has been identified as key kinase acting downstream of the activation of PI3K and acts as a master switch of cellular catabolism and anabolism, thereby determining whether cells, especially tumor cells grow and proliferate (40). The mTOR pathway has emerged as an important cancer therapeutic target and potent mTOR inhibitors such as rapamycin and its derivatives that specifically inhibit mTOR are now being actively evaluated in clinical trials (47). It is suggested that resistance to mTOR inhibitors is caused by a negative feedback loop in which mTOR inhibition leads to AKT activation and the relevance of this feedback is underscored by its existence in cancer patients (48). We observed that while PFE inhibits the mTOR pathway, it also simultaneously keeps the feedback loop in check by also inhibiting the PI3K/Akt pathway and inhibits cell survival and growth. The PI3K/Akt signaling represents a major cell survival pathway (49). mTOR functions downstream of the PI3K/Akt pathway and is phosphorylated in response to stimuli that activate the PI3K/Akt pathway. Since we observed a decrease in the phosphorylation of mTOR on treatment with PFE, we investigated the effect of PFE on PI3K/Akt pathway. PFE supplementation resulted in the inhibition of the expression of PI3K and inhibition of the phosphorylation of Akt, suggesting that PFE-induced decrease in mTOR phosphorylation is dependent on PI3K/Akt pathway as well. This dual inhibition of the mTOR and PI3K/Akt pathways, we believe is significant and responsible for the remarkable tumor inhibition by PFE observed in this study. PFE supplementation also resulted in upregulation of AMPK, which is a member of a metabolite-sensing protein kinase family and plays an essential role as an energy sensor mainly in ATP-deprived conditions (50). Therefore, AMPK is known to play a major protective role under metabolic stressed conditions. In the activated states, AMPK down-regulates several anabolic enzymes and thus shuts down the ATP-consuming metabolic pathways. Activation of AMPK inhibits mTOR signaling and is associated with inhibition of cancer cell growth. It is highly possible that inhibition of mTOR by PFE could be an indirect effect.
In a phase II clinical trial, Pantuck et al. (37) observed that PSA doubling time significantly increased with pomegranate juice treatment. No effect on androgen levels was observed suggesting the effect was not hormonal in natural. However, it was suggested based on other in vitro data that effects on PSA could be related to antioxidant and anti-inflammatory effects of pomegranate polyphenols. Several other studies have identified multiple targets to explain the bioactivity of pomegranate extracts (7,8,19). However, model system and extract source could partially explain the differences in the mechanisms observed in these studies. Our data imply that PFE modulates multiple signal transduction pathways to inhibit cancer development and progression.
PFE contains a mixture of phenols, flavonoids, anthocyanins and tannins that have wide effects on cellular biochemistry (10). It is therefore difficult to accurately assess the underlying mechanism that is responsible for its effects and relate it to a particular ingredient. Each of these ingredients could be targeting a different molecule or some of these ingredients could be competing for the same target, a possibility that cannot be ruled out. Most probably, different ingredients in PFE modulate different pathways resulting in inhibition of several pathways at the same time. Pomegranate juice ingredients are readily bioavailable in the human serum and have been found to localize in prostate tissues of mice (36).
In summary, we observed that continuous PFE supplementation starting at 6 weeks of age to TRAMP mice confers significant survival advantage and reduced tumor formation over water-fed TRAMP mice. Taken together, these findings clearly show that PFE, a natural fruit extract inhibits the development of PCa in the TRAMP mouse model. These observations need to be validated in additional preclinical studies and in appropriate human clinical trials before recommendations are made for the use of PFE in humans.
Funding
National Institutes of Health; National Cancer Institute (RO1CA120451).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AMPK
AMP-activated protein kinase
- GU
genitourinary
- PCa
prostate cancer
- PFE
pomegranate fruit extract
- PI3K
phosphoinositide 3-kinase
References
- 1.Siegel R, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Andriole GL, et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 4.Syed DN, et al. Dietary agents for chemoprevention of prostate cancer. Cancer Lett. 2008;265:167–176. doi: 10.1016/j.canlet.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan N, et al. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid. Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 6.Adhami VM, et al. Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. Mol. Biotechnol. 2007;37:52–57. doi: 10.1007/s12033-007-0047-8. [DOI] [PubMed] [Google Scholar]
- 7.Syed DN, et al. Pomegranate derived products for cancer chemoprevention. Semin. Cancer Biol. 2007;17:377–385. doi: 10.1016/j.semcancer.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Adhami VM, et al. Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutr. Cancer. 2009;61:811–815. doi: 10.1080/01635580903285064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longtin R. The pomegranate: nature's power fruit? J. Natl Cancer Inst. 2003;95:346–348. doi: 10.1093/jnci/95.5.346. [DOI] [PubMed] [Google Scholar]
- 10.El Kar C, et al. Pomegranate (Punica granatum) Juices: Chemical Composition, Micronutrient Cations, and Antioxidant Capacity. J Food Sci. 2011;76:C795–C800. doi: 10.1111/j.1750-3841.2011.02211.x. [DOI] [PubMed] [Google Scholar]
- 11.Gil MI, et al. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 12.Seeram NP, et al. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008;56:1415–1422. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- 13.Mehta R, et al. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur. J. Cancer Prev. 2004;13:345–348. doi: 10.1097/01.cej.0000136571.70998.5a. [DOI] [PubMed] [Google Scholar]
- 14.Adams LS, et al. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev. Res. 2010;3:108–113. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams LS, et al. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006;54:980–985. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- 16.Afaq F, et al. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009;18:553–561. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saruwatari A, et al. Pomegranate juice inhibits sulfoconjugation in Caco-2 human colon carcinoma cells. J. Med. Food. 2008;11:623–628. doi: 10.1089/jmf.2007.0050. [DOI] [PubMed] [Google Scholar]
- 18.Larrosa M, et al. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006;17:611–625. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Rettig MB, et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol. Cancer Ther. 2008;7:2662–2671. doi: 10.1158/1535-7163.MCT-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong MY, et al. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J. Nutr. Biochem. 2008;19:848–855. doi: 10.1016/j.jnutbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisburg JH, et al. Pomegranate extract, a prooxidant with antiproliferative and proapoptotic activities preferentially towards carcinoma cells. Anticancer Agents Med. Chem. 2010;10:634–644. doi: 10.2174/187152010794474000. [DOI] [PubMed] [Google Scholar]
- 22.Pacheco-Palencia LA, et al. Protective effects of standardized pomegranate (Punica granatum) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J. Agric. Food Chem. 2008;56:8434–8441. doi: 10.1021/jf8005307. [DOI] [PubMed] [Google Scholar]
- 23.Gasmi J, et al. Growth inhibitory, antiandrogenic, and pro-apoptotic effects of punicic acid in LNCaP human prostate cancer cells. J. Agric. Food Chem. 2010;58:12149–12156. doi: 10.1021/jf103306k. [DOI] [PubMed] [Google Scholar]
- 24.Koyama S, et al. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm. IGF Res. 2010;20:55–62. doi: 10.1016/j.ghir.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaii S, et al. Differentiation-promoting activity of pomegranate (Punica granatum) fruit extracts in HL-60 human promyelocytic leukemia cells. J. Med. Food. 2004;7:13–18. doi: 10.1089/109662004322984644. [DOI] [PubMed] [Google Scholar]
- 26.Khan N, et al. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67:3475–3482. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- 27.Khan N, et al. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28:163–173. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- 28.Albrecht M, et al. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J. Med. Food. 2004;7:274–283. doi: 10.1089/jmf.2004.7.274. [DOI] [PubMed] [Google Scholar]
- 29.Kim ND, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002;71:203–217. doi: 10.1023/a:1014405730585. [DOI] [PubMed] [Google Scholar]
- 30.Hora JJ, et al. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J. Med. Food. 2003;6:157–161. doi: 10.1089/10966200360716553. [DOI] [PubMed] [Google Scholar]
- 31.Toi M, et al. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis. 2003;6:121–128. doi: 10.1023/B:AGEN.0000011802.81320.e4. [DOI] [PubMed] [Google Scholar]
- 32.Kohno H, et al. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004;95:481–486. doi: 10.1111/j.1349-7006.2004.tb03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afaq F, et al. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 34.Afaq F, et al. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem. Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- 35.Malik A, et al. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl Acad. Sci. USA. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeram NP, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007;55:7732–7737. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 37.Pantuck AJ, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg NM, et al. Prostate cancer in a transgenic mouse. Proc. Natl Acad. Sci. USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rad AM, et al. Imaging mouse prostate gland by 3 Tesla clinical MRI system. Open Magn. Reson. Rev. 2008;1:60–63. doi: 10.2174/1874769800801010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Sekulic A, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 42.Xiang X, et al. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem. Biophys. Res. Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 43.Fryer LG, et al. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 44.Gupta S, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl Acad. Sci. USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adhami VM, et al. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 46.Brody H. Cancer prevention. Nature. 2011;471(suppl.):S1–S24. doi: 10.1038/471S1a. [DOI] [PubMed] [Google Scholar]
- 47.Faivre S, et al. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 48.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vivanco I, et al. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 50.Hardie DG, et al. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]