Abstract
Although platelet-activating factor (PAF) is a well-known acute inflammatory mediator, little is known regarding the role of PAF in chronic inflammation. Phorbol esters are known to stimulate PAF production. Moreover, the ability of repeated applications of phorbol esters to induce a sustained inflammatory response is crucial to their tumorigenic activity. We therefore examined whether PAF acts as a mediator of phorbol ester-induced inflammation and tumorigenesis. While PAF receptor knockout mice (PAFR (−/−)) showed an expected but modest reduction in the acute inflammatory response to phorbol 12-myristate 13-acetate (PMA), these mice exhibited a surprising increase in inflammation following chronic PMA application. This increased inflammation was documented by a number of findings that included: increased skin thickness, increased myeloperoxidase activity and expression and increased expression of known inflammatory mediators. Interestingly, vehicle-treated PAFR(−/−) mice also exhibited modest increases in levels of inflammatory markers. This suggests that the platelet activating factor receptor (PAFR) acts to suppress chronic inflammation in response to other stimuli, such as barrier disruption. The idea that chronic PAFR activation is anti-inflammatory was documented by repetitive topical PAFR agonist administration that resulted in reduced myeloperoxidase activity in skin. We next utilized a 7,12-dimethylbenz(a)anthracene/PMA carcinogenesis protocol to demonstrate that PAFR (−/−) mice exhibit significantly increased tumor formation and malignant progression compared with wild-type control mice. These studies provide evidence for two important, unexpected and possibly interrelated pathological roles for the PAFR: first, the PAFR acts to suppress PMA-induced chronic inflammation; secondly, the PAFR acts to suppress neoplastic development in response to chemical carcinogens.
Introduction
Platelet-activating factor (PAF) is a glycerophospholipid hormone that is synthesized by a wide variety of immune and non-immune cells in response to diverse stimuli (1). Numerous studies have detailed the important role that PAF plays as a positive mediator of acute inflammation, anaphylaxis and shock (1,2). PAF can be synthesized enzymatically through two distinct pathways: the first involves de novo synthesis of PAF from alkyl-acetylglycerol and the second occurs via transacetylation of lyso-PAF by the remodeling pathway (3,4). Once PAF is produced, its pro-inflammatory activities are mediated by binding to the platelet activating factor receptor (PAFR), a seven transmembrane G protein-coupled receptor expressed on a host of different cell types, including keratinocytes (1,5). The ability of PAF to promote inflammatory responses is due in part to its capacity to induce the expression of a variety of inflammatory mediators, such as interleukin (IL)-1, IL-6, IL-8 (CXCL8) and cyclooxygenase 2 (COX-2) (6–8) and to promote chemotaxis and activation of leukocytes (9,10). The importance of the PAFR in mediating acute inflammatory reactions, particularly type I hypersensitivity reactions, has been validated in transgenic mouse models (11,12).
Although there is wide-spread acceptance that PAF acts as a potent pro-inflammatory mediator, there have been several recent studies suggesting that PAF may play a more complex role in regulating the immune response. First, several groups have demonstrated that PAFR activation is necessary for ultraviolet B (UVB)-induced suppression of delayed type hypersensitivity responses (13,14). Second, in a study using an implanted sponge angiogenesis assay, PAFR knockout mice were shown to exhibit a paradoxical increase in the production of neutrophil and macrophage chemokines CXCL2 and CCL2 (15). Finally, a recent report indicates that systemic PAF treatment immediately after lipopolysaccharide challenge protected mice from endotoxic shock (16). This protection correlated with decreased production of proinflammatory cytokines. However, it should be noted that this study appears to conflict with other murine studies (11,12).
The ability of phorbol esters and other tumor promoters to induce chronic inflammation and increased oxidative stress are thought to be important in mediating their pro-neoplastic actions (17,18). Importantly, phorbol esters have been shown to activate both the de novo and the remodeling pathway of PAF synthesis (19,20). Moreover, phorbol esters can elicit a pronounced oxidative stress response in skin (21), and various oxidative stressors can induce non-enzymatic production of oxidized glycerophosphocholine (ox-GPCs) species with PAFR ligand activity (22). Thus, we hypothesized that phorbol ester tumor promoters would induce PAF production in murine skin and that this increased PAF production could then mediate many of the pro-inflammatory actions of phorbol ester application. This idea is supported by a previous study that demonstrated that a PAFR antagonist could inhibit the acute inflammatory response elicited by a single application of PMA (23). Thus, insofar as PAF is an important mediator of cutaneous inflammation and T-cell-mediated immune responses, we were interested in determining what role, if any, the PAFR plays in regulating PMA-induced tumor promotion.
In models of cutaneous carcinogenesis, a number of specific inflammatory changes have been described that provide a microenvironment favorable for epidermal neoplastic development. First, granulocyte and monocyte infiltration has been shown to be essential for cutaneous carcinogenesis (24,25). This could be due in part to the leukocyte oxidative burst leading to oxidative DNA damage in adjacent epithelial cells (26,27). In mice, this leukocyte-rich inflammatory infiltrate has been shown to be controlled by specific chemokines, in particular CXC chemokines containing a conserved glutamate–leucine–arginine (ELR+) motif, such as CXCL1-3 [or growth-related oncogenes (Gro-α, -β or –γ)] and CXCL5 [epithelial-derived neutrophil-activating peptide 78 (ENA-78)] (28,29). Of note, ELR+ chemokine (C-X-C motif) ligand (CXCL) chemokines have been shown to exert their chemotactic activity by binding primarily through the CXC chemokine receptor 2 (CXCR2) receptor (28).
Given that PMA is known to stimulate PAF production and that PMA induces a pronounced inflammatory effect, we sought to determine whether PAF mediates PMA-induced inflammatory responses. Our studies were surprising in that while PAFR knockout mice exhibit a reduction in PMA-induced acute inflammation, these mice were predisposed to develop an increase in chronic inflammation elicited by repetitive PMA applications. In additional studies, we provide compelling and surprising evidence that the PAFR may act to suppress tumor formation in response to chemical carcinogenesis.
Materials and methods
Reagents and Chemicals
PMA and caspase-(Glo) (R) 3/7 activity assay kit was purchased from Promega, Madison, WI. 7,12-dimethylbenz(a)anthracene (DMBA) was from Acros Organics, Fair Lawn, NJ. All murine PCR primers, first-strand synthesis kits and SYBR Green quantitative RT-PCR reagents were purchased from SuperArray Bioscience Corp (Frederick, MD). Carbamoyl-PAF (CPAF) was obtained from Sigma–Aldrich (St Louis, MO).
Animals
PAFR knockout (PAFR(−/−) in the C57Bl/6 background were originally obtained from Dr Satoshi Ishii (Department of Biochemistry and Molecular Biology, Faculty of Medicine, The University of Tokyo) as described previously (23). Age (8–12 week)-matched PAFR+/+ C57BL/6 [wild-type (WT)] were used as controls (The Jackson Laboratories, Bar Harbor, ME). SKH-1 hairless, albino mice were obtained from Charles Rivers (Wilmington, MA). Mice were housed under specific pathogen-free conditions at the Indiana University School of Medicine. All procedures were approved by the Animal Care and Use Committee of Indiana University School of Medicine.
Lipid extraction and intracellular calcium measurements
PAFR ligand production in mouse epidermis was assessed in mice treated topically with PMA or vehicle under anesthesia as described in the legend for Figure 1. After removing the epidermis by curettage, lipid extracts were prepared and assessed for PAFR ligand activity as previously detailed (30,31).
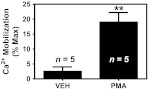
Fig. 1.
PMA treatment induces PAF-ligand activity in murine skin. The shaved dorsal skin of mice was treated with 40 nM of PMA in 0.2 ml in acetone/olive oil or vehicle (VEH). Two days later, PMA or VEH was reapplied. One hour after the second PMA or VEH application, the mice were killed and the treated skin excised. Whole skin lipid extracts were then prepared as described in the Materials and method section. Lipid extracts from 10 mg tissue were then tested for PAFR agonist activity using KB epidermoid carcinoma cells stably overexpressing the PAFR (KBP cells). Intracellular calcium mobilization was plotted as a percentage of the peak calcium response elicited by 1 μM of the stable PAFR ligand, CPAF. Results represent the mean + SD of calcium mobilization data obtained from lipid extracts of five different mice per treatment group. **, P < 0.01 compared with VEH, two-tailed t-test.
PMA-induced changes in ear thickness
The left ear of female WT C57BL/6 (WT) or PAFR (−/−) mice was treated with PMA and the right ear with vehicle 3 times a week (days 0, 2, 4, 7, 9, 11, 14 and 16). Ear thickness was measured in anesthetized mice using a constant pressure analog thickness gage (Peacock Model G, 0.4 N) on the days as indicated in Figure 2. For days in which the mice received PMA treatments, ear thickness was measured just prior to the treatments.
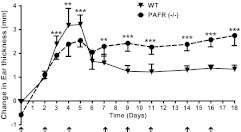
Fig. 2.
PAFR (−/−) mice exhibit enhanced chronic inflammation following repetitive PMA application. One ear of WT C57Bl/6 mice (WT; triangles) or PAF-R (−/−) mice (circles) were treated with 10 μg of PMA in 20 μl of acetone or vehicle (VEH) alone 3 times a week (arrows) for 16 days. Ear thickness was measured on the indicated days with a constant pressure analog thickness gage (Peacock Model G, 0.4 N). After subtracting the thickness of the VEH-treated ear, the PMA-induced increase in ear thickness was plotted. Results represent the mean and standard error of the mean of three independent experiments with a total of n = 8–11 mice per group. (**, P < 0.01; ***, P < 0.001: 2-tailed t-test).
PMA-induced inflammation in dorsal epidermis
The back skin of female WT or PAFR (−/−) mice was shaved and treated with 40 nM PMA in 0.2 ml acetone: olive oil in 1:1 ratio or vehicle twice a week for 3 weeks under anesthesia. On day 18, the mice were killed and the treated dorsal epidermis was excised for skin thickness measurements, myeloperoxidase (MPO) expression and MPO and N-acetyl-ß-d-glucosaminidase (NAG) activity assays as described in the figure legend (Figure 3) and below.
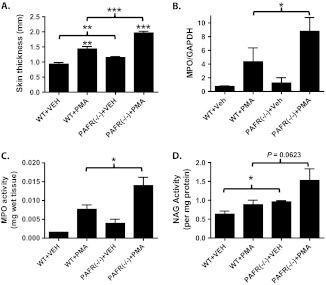
Fig. 3.
PAFR (−/−) mice treated for 3 weeks with PMA exhibit increased skin thickness as well as increased MPO and NAG expression and/or activity relative to WT mice. The dorsal epidermis of WT or PAFR (−/−) mice were treated with vehicle (VEH) or 40 nm of PMA twice weekly for 17 days. On day 18, the mice were killed and the treated dorsal epidermis excised. (A) Excised dorsal skin was bisected at the midline overlying the spinal column and frozen in liquid nitrogen. Midline skin thickness was measured using digital calipers at five different locations. (B) Quantitative RT-PCR for MPO expression normalized to glyceraldehyde 3-phosphate dehydrogenase expression. (C) MPO activity measured in dorsal skin extracts. (D) NAG activity measured in dorsal skin extracts. In A and B, data are shown as the mean ± SEM of n = 4 to 5 mice per group performed over two separate experiments. In C and D, data are shown as the mean ± SEM of three mice per group performed as a single experiment. (*, P < 0.05; **, P < 0.01; ***, P < 0.001, 2-tailed t-test).
MPO activity assay
Whole skin was collected in 0.5% hexadecyltrimethylammonium bromide (cetrimide) (Sigma, St Louis, MO) in 50 mM phosphate buffer, homogenized and centrifuged, and supernatants were collected. MPO activity was assessed in 10 μl of supernatant over a 5 min period at 450 nm as previously described (25). The data are expressed as mean units of MPO activity normalized by total protein content per experimental group.
NAG activity assay
For NAG assays, whole skin tissue was collected in Drabkin reagent (Sigma) and tissue homogenates were assessed for NAG activity in the presence of β-N-acetylglucosaminidase (Sigma) as previously described (32). The data expressed as mean units of NAG activity normalized by total protein content per experimental group.
RNA isolation and quantitative RT-PCR
A segment of mouse skin stored in RNA later was removed and RNA isolation was performed using an RNeasy kit (Qiagen) according to the manufacturer’s protocol. Following first-strand DNA synthesis, quantitative RT-PCR was performed using primers specific to murine MPO, CxCL2, CxCL3, CxCL5, CxCR2, COX-2, IL-12α, tumor necrosis factor α (TNFα) and glyceraldehyde 3-phosphate dehydrogenase with a StepOne™ real-time PCR instrument (Applied Biosystems, Foster City, CA). Results were normalized to glyceraldehyde 3-phosphate dehydrogenase using the ΔΔCt method (33).
Chemical carcinogenesis
For two-stage chemical carcinogenesis studies, the dorsal skin of C57Bl/6 WT mice and PAFR (−/−) mice were shaved and then treated with 100 μg of DMBA followed 1 week later by biweekly treatments with 40 nM of PMA [in 0.2 ml acetone: olive oil (1:1 ratio)] as previously described (34). These studies utilized relatively high doses of both DMBA and PMA since C57Bl/6 mice are relatively resistant to chemical tumorigenesis (34). For tumor counting, tumors > 1 mm in greatest diameter that persisted for >2 weeks were counted on a weekly basis. After 25 weeks of PMA treatment, mice were killed and tumor size (size at greatest dimension) was measured for each tumor. Tumors from WT and PAFR(−/−) mice were formalin fixed and paraffin-embedded. Formalin fixed and paraffin-embedded tumor sections were stained with H & E and the tumor type was assessed in blinded fashion by a board-certified dermatopathologist.
Statistical analysis
Statistical significance was assessed by Prism 5.0 software (Graph Pad Software, San Diego CA) and significance was set as P < 0.05.
Results
PMA induces PAFR ligand activity in mouse skin
We first verified that PMA could induce the production of PAFR ligands in mouse epidermis. In Figure 1, we show that a double application of PMA on mouse skin induces a marked increase in PAFR ligand activity. PAFR ligand activity was assessed by measuring the ability of mouse skin lipid extracts to induce a calcium mobilization response in KB epidermoid carcinoma cells that overexpress the PAFR (KBP cells) (30,31). Importantly, lipid extracts from PMA-treated mouse skin was unable to induce intracellular calcium mobilization in KBM cells that lack PAFR expression (data not shown).
PAFR (−/−) mice exhibit a reduction in acute inflammation but exhibit an increase in chronic PMA-induced inflammation following repetitive PMA applications
We next examined whether loss of the PAFR altered PMA-induced inflammatory responses. In particular, we wished to examine the role of the PAFR in regulating longer term inflammatory effects that are elicited by multiple PMA applications. For our initial studies, we utilized a mouse model of ear inflammation reported by Waskow et al. (9). Similar to the pattern of inflammation observed by Waskow’s group, we observed that PMA applications to our WT mice induced an acute inflammatory phase that peaked at 5 days (Figure 2). This acute inflammatory phase was followed by partial resolution and then a sustained chronic inflammatory phase. Given that the PAFR is known to be coupled to acute inflammatory effects, we expected that inflammation would be reduced in the PAFR (−/−) mice during the first 5 days of PMA treatment. Indeed, the PAFR (−/−) mice exhibited a statistically significant but modest reduction in the PMA-induced acute ear thickness changes. This is consistent with our previous study which demonstrated that a PAFR antagonist could inhibit the acute edema response elicited by a single application of PMA (23). However, the PAFR (−/−) mice unexpectedly failed to show any significant resolution of the initial acute inflammatory response and progressed directly into a stable chronic inflammatory phase that was increased roughly 2-fold relative to WT mice.
We next examined whether repetitive PMA application could induce an increase in chronic inflammation in the dorsal epidermis of PAFR knockout mice. We observed that loss of the PAFR was associated with a significant increase in skin thickness following repetitive PMA applications (Figure 3A). To determine whether this increase in skin thickness was due to increased inflammatory cell infiltrate rather than simply increased edema, we assessed leukocyte MPO mRNA expression and activity (26). In Figure 3B and C, we show that loss of the PAFR was associated with a statistically significant increase in MPO mRNA expression and MPO activity. We also measured the activity of another leukocyte marker that is more specific for macrophages, NAG (32). As with MPO, loss of the PAFR was associated with an augmented PMA-induced induction of NAG activity (Figure 3D).
PAFR knockout mice treated chronically with PMA exhibit increased expression of a number of chemokines associated with leukocyte infiltration
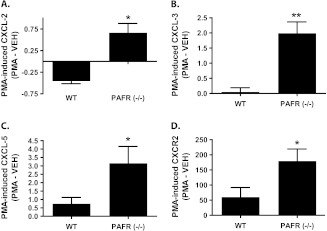
Given the increase in inflammation in PAFR (−/−) mice treated chronically with PMA, we sought to determine whether ELR+ CXC chemokines and the ELR+ CXC chemokine receptor (CXCR2) were upregulated in PAFR (−/−) mice. In Figure 4A–C a statistically significant increase in CXCL-2, CXCL-3 and CXCL-5 expression was seen in PAFR (−/−) mice relative to WT mice after 3 weeks of PMA treatment. This was associated with a similar PMA-induced increase in the expression of the ELR+ CXC chemokine receptor, CXCR2 (Figure 4D).
Fig. 4.
PAFR (−/−) mice treated for 3 weeks with PMA exhibit augmented expression of ELR+ CXC chemokines and the CXCR2 receptor. WT and PAFR (−/−) mice were treated with vehicle or PMA as in Figures 2 and 3. After total RNA was extracted from treated mouse skin, quantitative RT-PCR was performed for the following: (A) CXCL-2, (B) CXCL-3, (C) CXCL-5, and (D) CXCR-2. After normalizing for glyceraldehyde 3-phosphate dehydrogenase expression and subtracting the relevant vehicle-treated controls, the data are shown as the PMA-induced change in expression. The data represents the mean ± SEM of two (CXCL2) or three separate experiments (CXCL-3, 5 and CXCR2) of 3–5 mice per group. *, P < 0.05, **, P < 0.01, 2-tailed t-test.
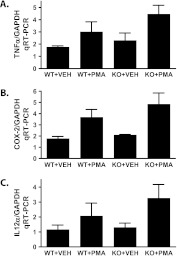
We next examined whether loss of the PAFR would alter the PMA-induced expression of known primary mediators of inflammation. TNFα, COX-2 and IL-12 are pro-inflammatory mediators that are known to be induced by acute PAF ligand exposure (6,7,35,36). Thus, based on previous reports, it would be expected that these inflammatory mediators would be reduced in PMA-treated PAFR (−/−) mice relative to WT controls. However, neither of these cytokines were reduced in PMA-treated PAFR (−/−) mice (Figure 5). In contrast, there was a trend toward increased expression of these pro-inflammatory mediators in PMA-treated PAFR (−/−) mice relative to WT mice.
Fig. 5.
PAFR (−/−) mice treated chronically with PMA fail to exhibit a decrease in primary cytokine production. PMA or vehicle (VEH) was topically applied for 17 days as described in Figures 2–4. On day 18, the mice were killed and treated dorsal epidermis excised and RNA was extracted. Quantitative RT-PCR was performed to assess the expression of inflammatory cytokines: (A) TNFα, (B) COX-2, and (C) Interleukin 12 (IL-12) by quantitative RT-PCR and normalized with glyceraldehyde 3-phosphate dehydrogenase as endogenous control using the ΔΔCT method. Results are expressed as the mean ± SEM of n = 3 to 6 mice per group.
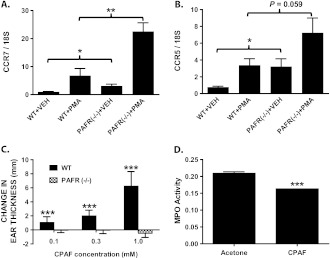
The above data indicates that mice lacking the PAFR exhibit increased inflammation in response to PMA. However, PAFR (−/−) mice treated with vehicle alone also exhibited a statistically significant increase in skin thickness (Figure 3A) and NAG activity (Figure 3D) relative to WT controls. In addition, while statistically insignificant, modest increases in MPO mRNA expression and activity (Figure 3B and C), CXCL-3 and 5 (data not shown) and CXCR2 (data not shown) were also noted in vehicle-treated knockout mice relative to control mice. In Figure 6A and B, we show that CC-chemokine receptors 5 and 7 are also significantly upregulated in vehicle-treated PAFR(−/−) mice relative to WT controls. PMA treatment also induced a further increase in the expression of these two inflammatory cell receptors (Figure 6A and B). C-C chemokine receptor type 7 is expressed on memory T cells and dendritic cells and metastatic tumor cells and is important in regulating lymph node trafficking of these cells (37,38). C-C chemokine receptor type 5 is a marker of CD4+ natural regulatory T cells (nTreg), Th1 T cells, monocytes, macrophages, NK cells and dendritic cells (39,40). Importantly, we did not note a significant change in skin thickness in WT relative to PAFR (−/−) mice in the absence of any topical treatment (data not shown). This suggests that the repetitive vehicle (acetone + olive oil) treatments alone induced inflammation that was augmented by loss of PAFR signaling. This is consistent with studies showing that organic solvents, such as acetone are pro-inflammatory when applied topically, presumably due to loss of epidermal barrier function (41,42). Collectively, these data raised the possibility that repetitive PAFR activation may act more broadly to suppress chronic inflammation. To further explore whether chronic and repetitive PAF-R activation can suppress chronic dermal inflammation, we treated WT and PAFR (−/−) mice with the stable PAF-R agonist CPAF (Figure 6C and D). We first showed that topical CPAF treatment (20 μl of 0.1, 0.3 or 1 mM CPAF in acetone) to mouse ears resulted in a dose-dependent and statistically significant increase in ear thickness at 2 h post-application for each dose (Figure 6C). This response was not observed in PAFR(−/−) mice. Using the middle dose to avoid receptor desensitization, we then show that chronic treatment of SKH-1 hairless mouse skin with CPAF resulted in a significant reduction in cutaneous MPO activity in these mice (Figure 6D).
Fig. 6.
Both vehicle and PMA treatment promote increased C-C chemokine receptor type 5 (CCR5)/C-C chemokine receptor type 7 (CCR7) expression in PAFR (−/−) mice while chronic CPAF treatment suppresses MPO activity in hairless mice. (A–C) PMA or VEH were topically applied to WT or PAFR (−/−) mice as described in Figure 5. The mRNA expression of (A) CCR7 or (B) CCR5 were then determined by quantitative RT-PCR and normalized to 18S rRNA expression. *, P < 0.05; **, P < 0.01: 2-sided t-test. (C) WT or PAFR (−/−) mice were treated with 20 μl of VEH (acetone) on the right ear and 20 μl of a 0.1, 0.3 or 1 mM solution of CPAF in acetone to the left ear. After 2 h, ear thickness was measured as described in Figure 2. CPAF-induced acute edema responses as the increase in ear thickness are shown. Results are the mean + SEM of n = 8 (0.1 and 0.3 mM) or three mice (1 mM) per treatment group. ***, P < 0.001: 2-sided t-test. (D) SKH-1 hairless mice were treated six times (days 0, 3, 7, 10, 14, 17) with 0.1 ml of 0.3 mM CPAF or VEH over their dorsal epidermis. On day 18, the mice were euthanized and MPO activity was measured in the treated skin as described in Figure 3. Results are mean + SD of MPO activity determined in three mice per group.
PAFR knockout mice exhibit increased cutaneous non-melanoma skin cancer formation following a two-stage chemical carcinogenesis protocol
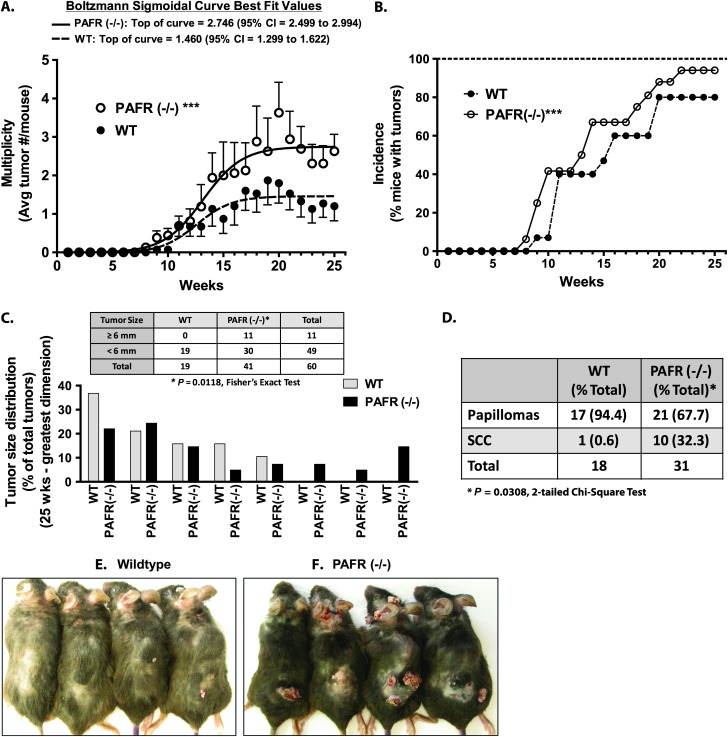
The above studies show that PAFR knockout mice exhibit a paradoxical increase in inflammation following chronic application of the tumor promoter PMA. Previous studies have demonstrated that persistent inflammation plays an important role in chemical and UVB-induced cutaneous carcinogenesis (25). We therefore examined whether loss of the PAFR would result in altered chemical carcinogenesis using the two-stage DMBA/PMA chemical carcinogenesis model. In Figure 7A, we show that PAFR (−/−) mice exhibit a roughly 2-fold increase in tumor multiplicity compared with WT mice. In Figure 7B, tumor incidence data is shown: PAFR (−/−) mice showed a trend toward earlier tumor appearance and a modest 14% increase in the maximal percentage of mice with tumors at the study conclusion (80 versus 94%; WT versus PAFR(−/−)). Interestingly, PAFR (−/−) mice also exhibited a statistically significant increase in larger tumors (tumors > 6 mm in greatest dimension represented 26.8% of the tumors in the PAFR (−/−) mice versus 0% in the WT mice) (Figure 7C). This increase in tumor size was associated with an increased frequency of malignant conversion in the PAFR (−/−) mice (Figure 7D): 32.3% of the tumors from PAFR (−/−) mice were classified as well-differentiated squamous cell carcinomas (SCCs). In contrast, only a single SCC was observed in the WT control mice (0.6% of WT tumors). This low rate of malignant conversion in the WT mice is consistent with previous studies, in which SCC’s occur at low frequency prior to 25 weeks of treatment in C57Bl/6 mice (43,44). Figure 7E and F shows representative images of tumors in WT and PAFR (−/−) mice.
Fig. 7.
PAF-R (−/−) mice show increased susceptibility to two-stage chemical carcinogenesis. WT or PAFR (−/−) mice were treated with DMBA followed by twice weekly applications of PMA for a total of 25 weeks. (A) Tumor multiplicity (mean ± SD per mouse) at each week are shown (***, P < 0.001; 2-way analysis of variance). The solid (PAFR (−/−)) and dotted (WT) lines represent Boltzmann sigmoidal curve fits for each genotype (Graph Pad Prism 5.0 Software). In addition, PAFR (−/−) mice exhibited significantly greater tumor multiplicity by 2-sided t-tests at weeks 20, 22–25 (P < 0.05 at each time point). (B) The percent of mice with at least one tumor as a percent of the total mice in each genotype at each week is shown (***, P < 0.001; 2-way analysis of variance). (C) Tumor size (size at greatest dimension) was measured for each tumor. Tumors were separated into bin sizes from ≥ 1 to ≥ 8 mm and plotted as the percent of the number of tumors in each bin size relative to the total tumor count. Insert: contingency table showing the number of tumors ≥ 6 mm or < 6 mm in greatest diameter for each genotype. The data in A–C represent the combined data of two independent experiments totaling n = 15 (WT) or n = 16 (PAFR (−/−)) mice. (D) Tumors in H & E stained sections were classified as either papillomas or SCCs by a dermatopathologist. PAFR (−/−) mice exhibit increased percentages of invasive SCCs relative to WT mice. (E and F) Representative photographs of WT and PAFR (−/−) mice following a two-stage chemical carcinogenesis protocol.
Given that our data show that PAFR (−/−) mice develop a greater number of tumors, we were interested in determining whether loss of the PAFR altered tumor proliferation, PMA-induced hyperplasia or the apoptotic response to DMBA-induced DNA damage. However, we did not detect any significant increase in tumor proliferation (Supplementary Figure 1A, available at Carcinogenesis Online) or PMA-induced hyperplasia (Supplementary Figure 1B, available at Carcinogenesis Online). Nor did we detect a significant change in apoptosis at 24 and 48 h following a single DMBA application (Supplementary Figure 2A and B is available at Carcinogenesis Online).
Discussion
Studies over many decades have provided a wealth of data demonstrating a pro-inflammatory role for PAF in acute inflammation. Moreover, studies in keratinocytes have shown that acute activation of the PAFR results in increased production of pro-inflammatory mediators TNFα, IL8 (CXCL-8), CXCL1-3 and COX-2 (6,7). Paradoxically, our data indicate that loss of the PAFR results in increased inflammation in the setting of chronic PMA application and possibly barrier disruption. To verify that chronic activation of the PAFR could suppress inflammation, we also show that repetitive CPAF application also suppresses cutaneous inflammation. Our study is the first to provide definitive evidence that PAF may act to suppress chronic inflammation mediated by leukocytes of the innate immune system. These findings are distinct from previous reports that PAF is necessary for UVB-induced suppression of T cell-mediated immune responses (13,14,45). Thus, our data provides strong evidence that PAF may serve a more complex role in modulating innate immune responses than previously believed.
The idea that PAF might suppress innate immune responses is supported by a report that intraperitoneal injection of PAF rescues mice from shock and death due to lethal doses of bacterial lipopolysaccharide (16). This activity was shown to be associated with decreased production of pro-inflammatory mediators, such as interferon gamma, IL-1 and TNFα but increased production of IL-10. Thus, while this study did not clearly implicate suppression of the innate immune response as the cause of the decreased mortality, it does provide additional support for the idea that PAF may exhibit anti-inflammatory effects on innate immune responses under some conditions. Finally, our data demonstrating a role for the PAFR in suppressing the expression of pro-inflammatory ELR+ CXC chemokines in a model of chronic inflammation is supported by a recent study using a model of sponge-induced angiogenesis (15). In this study, mice lacking the PAFR demonstrated increased angiogenesis, along with increased expression of CXCL-2 and chemokine (C-C motif) ligand 2 chemokines at 10–14 days post-sponge implantation. As in our study, they also demonstrated a slight but statistically insignificant increase in TNFα expression in PAFR (−/−) mice. However, they noted that loss of the PAFR resulted in decreased MPO and NAG activity over the 14 day study. Given the differences in the model system, this suggests that the ability of the PAFR to regulate chronic inflammatory cell infiltrates is context or tissue dependent. Interestingly, in addition to increased inflammation following chronic PMA exposure, we also note that vehicle-treated PAFR (−/−) mice also exhibit evidence of increased inflammation. Given that repetitive acetone treatment stimulates inflammation due to persistent epidermal permeability barrier disruption (41,42), this suggests that the ability of chronic PAFR activation to suppress chronic inflammation is not limited to phorbol ester-mediated effects alone. This idea is further supported by the decrease in MPO activity that we observed in a hairless mouse strain treated with repetitive PAFR agonist applications.
Given that loss of the PAFR was associated with an augmented inflammatory response to chronic PMA application, we next examined the role of the PAFR in a two-stage chemical carcinogenesis protocol. Thus, the second major finding of our studies is that loss of the PAFR is associated with increased chemical carcinogenesis, marked by increased tumor burden, tumor size and malignant progression. Although the mechanisms for the increase in chemical carcinogenesis in the PAFR (−/−) mice are unclear, the increase in chronic inflammation in the PAFR (−/−) mice following PMA application likely plays a key role. In this regard, it is interesting that a constitutive promoter phenotype is seen in transgenic mouse models with overexpression of other pro-inflammatory mediators, such as COX-2. In this case, transgenic mice exhibit increased tumorigenesis following DMBA treatment alone (46,47). Thus, future studies examining whether PAFR(−/−) mice exhibit increased tumorigenesis following DMBA application alone would be informative. However, it is important to note that in models such as the COX-2 transgenic mice, these mice exhibit baseline increases in inflammation and epidermal hyperplasia. This does not appear to occur in PAFR(−/−) mice in the absence of a pro-inflammatory physiological trigger (e.g. barrier disruption from acetone vehicle treatment) or chemical trigger (PMA application). Finally, it should be noted that there are numerous studies linking changes in PAF production or PAFR expression with the presence of neoplastic disease (48–50). In addition, other studies have also shown that exogenous PAF regulates the behavior of existing tumors or tumor cells: PAF has been shown to stimulate tumor growth (51), differentiation (51), angiogenesis (52), motility and metastatic behavior (53). However, none of these studies directly address the role of PAFR activation on the process of de novo tumor formation.
Inasmuch as the previous studies described above do not directly implicate PAF in the process of tumorigenesis, a recent study by Sreevidya et al. (54) is perhaps more relevant to our current findings. In this study, the PAFR antagonists, PCA-4248 and CV-3988, were reported to suppress UVB-induced tumor incidence and also to suppress continued tumor formation following removal of the UVB irradiations (54). In addition, they also noted that UVB-induced apoptosis was suppressed by PAFR antagonist treatment (54). Interestingly, we did not see a change in DMBA-induced apoptosis in mice lacking the PAFR. What could account for the discordance between these pharmacological studies and our current study? A trivial explanation for the differences with our data may be that these studies utilized different carcinogenesis protocols (UVB versus chemical carcinogenesis). In addition, Sreevidya et al. (54) counted only histologically confirmed SCCs but not papillomas. Thus, it is impossible to determine whether the total tumor burden (papilloma and SCC) were altered by PAFR antagonist treatment. Nonetheless, our data indicate that progression to SCC was significantly increased in our chemical carcinogenesis protocol. Another explanation for the discordant results would be that the PAFR antagonists exhibited off target effects. It should be noted that all the effects seen using PCA-4248 and CV-3988 were also seen using a serotonin receptor antagonist (54,55). In addition, PCA-4248 is a dual PAFR and serotonin receptor antagonist (56); thus, it is unclear whether the effects of PCA-4248 were due to its PAFR or serotonin receptor antagonist activity. Alternatively, we cannot exclude the possibility that germ-line deletion of the PAFR results in a compensatory adaptive change in these mice that predispose them to increased inflammatory reactions and increased tumor formation in response to phorbol esters. Additional ongoing studies using pharmacologic PAFR ligands in WT and PAFR (−/−) mice should provide crucial data to support the idea that the PAFR exhibits anti-inflammatory activity and suppresses tumorigenesis in response to chemical carcinogens. In addition, it will be of interest to determine whether genetic loss of the PAFR alters UVB-induced tumor formation.
In conclusion, we provide important data suggesting two novel and important functions for PAF in cutaneous pathophysiology. First, mice lacking the PAFR exhibited an increase in PMA-induced chronic inflammation. This observation runs counter to the well-known effects of PAF on acute inflammation. In addition, knockout mice exhibited increased tumorigenesis in response to a two-stage chemical carcinogenesis protocol.
Supplementary material
Supplementary Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (R01 HL062996, R21 ES017497, R03 AR053710 and U19A1070448); a Veterans Affairs Merit Award; a Prevent Cancer Foundation grant; a postdoctoral fellowship grant from the American Institute for Cancer Research.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Dan F.Spandau for his thoughtful discussion and editorial comments.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- COX-2
cyclooxygenase 2
- CPAF
carbamoyl-platelet activating factor
- CXCL
chemokine (C-X-C motif) ligand
- CXCR2
CXC chemokine receptor 2
- DMBA
7,12-dimethylbenz(a)anthracene
- IL
interleukin
- MPO
myeloperoxidase
- NAG
N-acetyl-ß-D-glucosaminidase
- PAF
platelet activating factor
- PAFR
platelet activating factor receptor
- PMA
phorbol 12-myristate 13-acetate
- SCC
squamous cell carcinoma
- TNFα
tumor necrosis factor alpha
- UVB
ultraviolet B
- WT
wild-type
References
- 1.Stafforini DM, et al. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit. Rev. Cl Lab. Sci. 2003;40:643–672. doi: 10.1080/714037693. [DOI] [PubMed] [Google Scholar]
- 2.Countryman NB, et al. Evidence for involvement of the epidermal platelet-activating factor receptor in ultraviolet-B-radiation-induced interleukin-8 production. J. Invest. Dermatol. 2000;115:267–272. doi: 10.1046/j.1523-1747.2000.00058.x. [DOI] [PubMed] [Google Scholar]
- 3.Snyder F. CDP-choline:alkylacetylglycerol cholinephosphotransferase catalyzes the final step in the de novo synthesis of platelet-activating factor. Biochimica et Biophysica Acta (BBA) 1997;1348:111–116. doi: 10.1016/s0005-2760(97)00109-4. [DOI] [PubMed] [Google Scholar]
- 4.Fragopoulou E, et al. Characterization of acetyl-CoA: lyso-PAF acetyltransferase of human mesangial cells. Mediators Inflamm. 2005;2004:263–272. doi: 10.1155/MI.2005.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travers JB, et al. Identification of functional platelet-activating factor receptors on human keratinocytes. J. Invest. Dermatol. 1995;105:816–823. doi: 10.1111/1523-1747.ep12326581. [DOI] [PubMed] [Google Scholar]
- 6.Pei Y, et al. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J. Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- 7.Travers JB, et al. Augmentation of UVB radiation-mediated early gene expression by the epidermal platelet-activating factor receptor. J. Invest. Dermatol. 2007;128:455–460. doi: 10.1038/sj.jid.5701083. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, et al. Activation of platelet-activating factor receptor in SZ95 sebocytes results in inflammatory cytokine and prostaglandin E2 production. Exp. Dermatol. 2006;15:769–774. doi: 10.1111/j.1600-0625.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 9.Waskow C, et al. Kit is essential for PMA-inflammation-induced mast-cell accumulation in the skin. Blood. 2007;109:5363–5370. doi: 10.1182/blood-2006-08-039131. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, et al. Polyunsaturated phospholipids promote the oxidation and fragmentation of Î3-hydroxyalkenals: formation and reactions of oxidatively truncated ether phospholipids. J. Lipid Res. 2008;49:832–846. doi: 10.1194/jlr.M700598-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii S, et al. Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J. 1997;16:133–142. doi: 10.1093/emboj/16.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii S, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J. Exp. Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, et al. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J. Invest. Dermatol. 2008;128:1780–1787. doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walterscheid JP, et al. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J. Exp. Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hikiji H, et al. Absence of platelet-activating factor receptor protects mice from osteoporosis following ovariectomy. J. Clin. Invest. 2004;114:85–93. doi: 10.1172/JCI20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong Y-I, et al. The novel role of platelet-activating factor in protecting mice against lipopolysaccharide-induced endotoxic shock. PLoS ONE. 2009;4:e6503. doi: 10.1371/journal.pone.0006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Bickers DR, et al. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 19.Elstad MR, et al. Protein kinase C regulates the synthesis of platelet-activating factor by human monocytes. Am. J. Respir. Cell Mol. Biol. 1991;4:148–155. doi: 10.1165/ajrcmb/4.2.148. [DOI] [PubMed] [Google Scholar]
- 20.Nieto ML, et al. Biosynthesis of platelet-activating factor in human polymorphonuclear leukocytes. Involvement of the cholinephosphotransferase pathway in response to the phorbol esters. J. Biol. Chem. 1988;263:2217–2222. [PubMed] [Google Scholar]
- 21.Nakamura Y, et al. Arachidonic acid cascade inhibitors modulate phorbol ester-induced oxidative stress in female ICR mouse skin: differential roles of 5-lipoxygenase and cyclooxygenase-2 in leukocyte infiltration and activation. Free Radic. Biol. Med. 2003;35:997–1007. doi: 10.1016/s0891-5849(03)00440-4. [DOI] [PubMed] [Google Scholar]
- 22.Murphy RC. Free radical-induced oxidation of glycerophosphocholine lipids and formation of biologically active products. Adv. Exp. Med. Biol. 1996;416:51–58. doi: 10.1007/978-1-4899-0179-8_10. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, et al. Staphylococcal lipoteichoic acid inhibits delayed-type hypersensitivity reactions via the platelet-activating factor receptor. J. Clin. Invest. 2005;115:2855–2861. doi: 10.1172/JCI25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albini A, et al. Tumor inflammatory angiogenesis and its chemoprevention. Cancer Res. 2005;65:10637–10641. doi: 10.1158/0008-5472.CAN-05-3473. [DOI] [PubMed] [Google Scholar]
- 25.Hatton JL, et al. Depletion of CD4+ cells exacerbates the cutaneous response to acute and chronic UVB exposure. J. Invest. Dermatol. 2007;127:1507–1515. doi: 10.1038/sj.jid.5700746. [DOI] [PubMed] [Google Scholar]
- 26.Knaapen AM, et al. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21:225–236. doi: 10.1093/mutage/gel032. [DOI] [PubMed] [Google Scholar]
- 27.Coussens LM, et al. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cataisson C, et al. CXCR2 ligands and G-CSF mediate PKCα-induced intraepidermal inflammation. J. Clin. Invest. 2006;116:2757–2766. doi: 10.1172/JCI27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cataisson C, et al. Inducible cutaneous inflammation reveals a protumorigenic role for keratinocyte CXCR2 in skin carcinogenesis. Cancer Res. 2009;69:319–328. doi: 10.1158/0008-5472.CAN-08-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marathe GK, et al. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J. Biol. Chem. 2005;280:35448–35457. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- 31.Yao Y, et al. Ultraviolet B radiation generated platelet-activating factor receptor agonist formation involves EGF-R-mediated reactive oxygen species. J. Immunol. 2009;182:2842–2848. doi: 10.4049/jimmunol.0802689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barcelos LS, et al. Impaired inflammatory angiogenesis, but not leukocyte influx, in mice lacking TNFR1. J. Leukoc. Biol. 2005;78:352–358. doi: 10.1189/jlb.1104682. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, et al. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods (Duluth) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Elmets CA, et al. Susceptibility to the biological effects of polyaromatic hydrocarbons is influenced by genes of the major histocompatibility complex. Proc. Natl Acad. Sci. USA. 1998;95:14915–14919. doi: 10.1073/pnas.95.25.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darst M, et al. Augmentation of chemotherapy-induced cytokine production by expression of the platelet-activating factor receptor in a human epithelial carcinoma cell line. J. Immunol. 2004;172:6330–6335. doi: 10.4049/jimmunol.172.10.6330. [DOI] [PubMed] [Google Scholar]
- 36.Al-Darmaki S, et al. Delineation of the role of platelet-activating factor in the immunoglobulin G2 antibody response. Clin. Diagn. Lab. Immunol. 2004;11:720–728. doi: 10.1128/CDLI.11.4.720-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angel CE, et al. Cutting edge: cD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J. Immunol. 2006;176:5730–5734. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- 38.Shields JD, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 39.de Groot M, et al. Expression of the chemokine receptor CCR5 in psoriasis and results of a randomized placebo controlled trial with a CCR5 inhibitor. Arch. Dermatol. Res. 2007;299:305–313. doi: 10.1007/s00403-007-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yurchenko E, et al. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J. Exp. Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ajani G, et al. Cellular responses to disruption of the permeability barrier in a three-dimensional organotypic epidermal model. Exp. Cell Res. 2007;313:3005–3015. doi: 10.1016/j.yexcr.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proksch E, et al. Integrity of the permeability barrier regulates epidermal Langerhans cell density. Br. J. Dermatol. 1996;134:630–638. [PubMed] [Google Scholar]
- 43.Allen SM, et al. Survivin expression in mouse skin prevents papilloma regression and promotes chemical-induced tumor progression. Cancer Res. 2003;63:567–572. [PubMed] [Google Scholar]
- 44.Hennings H, et al. Comparison of two-stage epidermal carcinogenesis initiated by 7,12-dimethylbenz(a)anthracene or N-methyl-Nâ€2-nitro-N-nitrosoguanidine in newborn and adult SENCAR and BALCB/c mice. Cancer Res. 1981;41:773–779. [PubMed] [Google Scholar]
- 45.Shreedhar V, et al. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J. Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]
- 46.Rundhaug JE, et al. The effect of cyclooxygenase-2 overexpression on skin carcinogenesis is context dependent. Mol. Carcinog. 2007;46:981–992. doi: 10.1002/mc.20340. [DOI] [PubMed] [Google Scholar]
- 47.Müller-Decker K, et al. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc. Natl Acad. Sci. USA. 2002;99:12483–12488. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denizot Y, et al. Is there a role of platelet-activating factor in human lung cancer? Lung Cancer. 2001;33:195–202. doi: 10.1016/s0169-5002(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 49.Denizot Y, et al. Tissue concentrations of platelet-activating factor in colorectal carcinoma: inverse relationships with Dukes’ stage of patients. Oncogene. 2003;22:7222–7224. doi: 10.1038/sj.onc.1207032. [DOI] [PubMed] [Google Scholar]
- 50.Kitagawa D, et al. Expression of platelet-activating factor receptor: a novel prognosticator in patients with hepatocellular carcinoma following hepatectomy. Oncology. 2007;72:381–387. doi: 10.1159/000113149. [DOI] [PubMed] [Google Scholar]
- 51.Cellai C, et al. Growth inhibition and differentiation of human breast cancer cells by the PAFR antagonist WEB-2086. Br. J. Cancer. 2006;94:1637–1642. doi: 10.1038/sj.bjc.6603156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bussolino F, et al. Platelet activating factor produced in vitro by Kaposi’s sarcoma cells induces and sustains in vivo angiogenesis. J. Clin. Invest. 1995;96:940–952. doi: 10.1172/JCI118142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Im S-Y, et al. Augmentation of tumor metastasis by platelet-activating factor. Cancer Res. 1996;56:2662–2665. [PubMed] [Google Scholar]
- 54.Sreevidya CS, et al. Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 2008;68:3978–3984. doi: 10.1158/0008-5472.CAN-07-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sreevidya CS, et al. Agents that reverse UV-Induced immune suppression and photocarcinogenesis affect DNA repair. J. Invest. Dermatol. 2010;130:1428–1437. doi: 10.1038/jid.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumura Y, et al. A role for inflammatory mediators in the induction of immunoregulatory B cells. J. Immunol. 2006;177:4810–4817. doi: 10.4049/jimmunol.177.7.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.