Abstract
The worsening of drug abuse by drug-associated social interaction is a well-studied phenomenon. In contrast, the molecular mechanisms of the beneficial effect of social interaction, if offered as a mutually exclusive choice to drugs of abuse, are under-investigated. In a rat place preference conditioning (CPP) paradigm, four 15 min episodes of social interaction with a gender- and weight-matched male early-adult conspecific inhibited cocaine-induced reinstatement of cocaine CPP, a model of relapse. These protective effects of social interaction were paralleled by a reduced activation, as assessed by Zif268 expression, in brain areas known to play pivotal roles in drug-seeking behavior. Here we show that social interaction during extinction of cocaine CPP also reduced cocaine-CPP-stimulated FosB expression in the nucleus accumbens shell and core. In addition, social interaction during cocaine CPP extinction increased pCREB (cAMP response element binding protein) expression in the nucleus accumbens shell and the cingulate cortex area 1 (Cg1). Our results show that FosB and pCREB may be implicated in the protective effect of social interaction against cocaine-induced reinstatement of CPP. Thus, social interaction, if offered in a context that is clearly distinct from the previously drug-associated one, may profoundly inhibit relapse to cocaine addiction.
Keywords: cocaine, conditioned place preference, social interaction, FosB/ΔFosB, pCREB, relapse, substance-use disorder
Introduction
Drug dependence is a multifactorial disorder resulting from an interaction between genetic, social, and environmental factors (Kreek et al., 2005; Enoch, 2006). There is compelling evidence that social experiences modify vulnerability to reinstatement, acting as prevention or risk factors in the development of drug addiction (Swadi, 1999). Using animal models, several studies have investigated how social interaction, if made available together with a drug of abuse, may worsen drug abuse and substance dependence (Gauvin et al., 1994; Thiel et al., 2008, 2009; Ribeiro Do Couto et al., 2009). However, there is little research on how social interaction, if offered as an alternative to drug consumption, affects neural circuits involved in drug reinforcement and substance dependence.
We have recently shown that only four social interaction episodes (15 min each) with a male early-adult conspecific as an alternative (i.e., non-drug-associated) stimulus completely reversed conditioned place preference (CPP) for cocaine (15mg/kg i.p.) and were even able to inhibit cocaine-induced reinstatement of cocaine CPP (Fritz et al., 2011a). The behavioral effects of social interaction were paralleled by effects on the brain circuitry known to be involved in drug reinforcement and reward. Social interaction during extinction of cocaine CPP reversed cocaine CPP-reinstatement-associated Zif268 expression in the nucleus accumbens shell, the central and basolateral amygdala, and the ventral tegmental area (Fritz et al., 2011a). We have also shown that the sigma1 receptor antagonist BD1047 enhances reversal of conditioned place preference from cocaine to social interaction (Fritz et al., 2011b). These findings suggest that social interaction, if offered in a context that is clearly distinct from the previously drug-associated one, may profoundly decrease the incentive salience of drug-associated contextual stimuli.
Drugs of abuse are known to cause several neuroadaptations in dopaminergic as well in other neurotransmitter systems. One of these adaptations is an altered expression of transcription factors that engender changes in gene expression and may possibly lead to alterations in sensitivity to drugs of abuse (Hyman and Malenka, 2001; Nestler et al., 2001). One of these transcription factors is ΔFosB. In contrast to the other Fos proteins, ΔFosB is induced to only a small degree in response to acute drug administration but because of its unique stability, after repeated drug administration, ΔFosB gradually accumulates in the striatum and stays elevated for weeks or months after discontinuation of drug exposure (Hope et al., 1994; Chen et al., 1995; Moratalla et al., 1996; Hiroi et al., 1997). Thus, it has been hypothesized that ΔFosB functions as a sustained molecular switch that mediates some of the more persistent adaptations of the brain that underlie addiction (McClung et al., 2004). The first study of fosB KO mice found that these mice develop a robust CPP to a lower dose of cocaine compared with wild type mice (Hiroi et al., 1997). However, it has been found that over-expression of ΔFosB leads to enhanced rewarding and reinforcing effects of drugs of abuse. Indeed, over-expression of ΔFosB in the striatum resulted in maximal CPP to a low dose of cocaine (Kelz et al., 1999) and facilitated the acquisition of cocaine self-administration at low doses (Colby et al., 2003). Another transcription factor implicated in the effects of drugs of abuse is CREB (cAMP response element binding protein). CREB is a constitutively expressed transcription factor the activity of which is tightly regulated by its phosphorylation at serine 133 (Lonze and Ginty, 2002). In the nucleus accumbens, it has been shown that increased CREB activity decreases the rewarding effects of cocaine and morphine in the CPP paradigm (Carlezon et al., 1998; Pliakas et al., 2001; Barrot et al., 2002; Dinieri et al., 2009). Furthermore, CREB activity in brain regions other than the nucleus accumbens seems to regulate the rewarding effects of drugs of abuse as well. Accordingly, it has been found that over-expression of CREB in the rostral ventral tegmental area enhanced the development of cocaine- and morphine-induced CPP (Olson et al., 2005).
Given that social interaction during extinction was able to prevent reinstatement of cocaine CPP and to reduce activation in reward-related brain areas as assessed by Zif268 protein expression (Fritz et al., 2011a), we aimed to expand our findings to explore the involvement of other transcription factors in social interaction preventive effect. Therefore, in the present study we investigated the expression of FosB/ΔFosB and pCREB proteins using immunohistochemistry in mesocorticolimbic areas in brains of (1) naïve rats, (2) rats that underwent cocaine CPP followed by saline extinction, and (3) rats that underwent cocaine CPP followed by social interaction during saline extinction (counterconditioning).
Materials and methods
Animals
Male Sprague–Dawley rats (150–250) g, corresponding to an age of (6–8 weeks) which can be considered at early adulthood (Spear, 2000), were obtained from the Research Institute of Laboratory Animal Breeding of the Medical University Vienna (Himberg, Austria) and were group-housed (six rats per cage) at 24°C. The animals received ad libitum access to tap water and pellet chow. A 12 h light/dark cycle, with lights on from 0800 h to 2000 h, was maintained. Single housing commenced at the start of the behavioral experiment and continued throughout the experiment which was conducted during the light period of the cycle. The animals used in this study were cared for in accordance with the guidelines of the National Institutes of Health Animal Care and Use Program and the NIDA-IRP Animal Program, and the present experiments were approved by the Austrian National Animal Experiment Ethics Committee.
Place conditioning apparatus
Conditioning was conducted in a homemade three chamber apparatus (63 cm wide × 33 cm deep × 30 cm high) made of plywood panels covered with plastic film. The middle (neutral) compartment (10 × 30 × 30 cm) had gray walls and a gray floor. The two conditioning chambers (25 × 30 × 30 cm) had either black walls with two vertical white stripes (5 × 30 cm) on each side and a stainless steel floor with 20 holes (diameter 0.5 cm) or black walls with five horizontal white stripes (3 × 25 cm) and a stainless steel floor with 15 slits (5 × 0.5 cm). Time spent in each compartment was taken with hand timers. If the added-up times for all three compartments were less than the total 900 s of the test session, the missing time was distributed equally among the three chambers to avoid any bias. After every single rat, the apparatus was cleaned with a 70% camphorated ethanol solution.
Place conditioning procedure
Training
Conditioned place preference was conducted as described previously (Fritz et al., 2011a). All experiments were performed by two individual experimenters using white light (15 Watt) and radio-generated white noise. During the pre-training test, rats were allowed to move freely between the three chambers for 15 min (900 s). Pre-test bias for the chambers was controlled for at the experimental group level by comparing means and SEMs of the times spent in any of chambers subsequently used for cocaine or saline pairing. If SEMs were overlapping, the group was advanced to CPP training. SEMs were chosen because they represent the numerically smallest measure of variance, thus yielding the most conservative group pre-test bias criterion. To further control for a pre-test bias at the individual level that may have escaped our group pre-test bias criterion, we chose the following arbitrary criterion: if any individual animal remained >349 s in one of the outer two compartments, cocaine was paired with the initially non-preferred side. This happened in about 10–15% of the animals. We have no indication that the application of these two criteria generated an “unbiased” vs. a “biased” population, which would have been evident as a bimodal distribution in pre-test times. Cocaine (hydrochloride salt, a gift from the National Institute on Drugs of Abuse to Gerald Zernig, corresponding to 15 mg/kg pure cocaine base in a volume of 1 ml/kg saline) or saline was injected intraperitoneally (i.p.) immediately before placing the rat into the closed dedicated chamber (cocaine- vs. saline allocation counterbalanced within-group). If a compartment was paired with social interaction during CPP training, each rat received an i.p. injection of saline and was placed in the compartment to allow for social interaction with a conspecific of the same weight and gender (male) during the whole 15 min conditioning session. Each rat was assigned a different partner, which stayed the same for the whole duration of the experiment. Gross observation indicated that only “agonistic” (i.e., “friendly”) social interaction, i.e., touching, crawling under, and grooming occurred, whereas “antagonistic” (i.e., threatening, boxing, fighting, biting, etc.) behavior was not observed during the training sessions. Both animals remained singly housed. Place preference was conditioned using an alternate-day design of four once-daily 15 min training sessions for each condition. The CPP test was performed 24 h after the last conditioning trial by placing the rat in the middle (neutral) compartment of the CPP apparatus and allowing it to move freely between the three compartments for 15 min. Only animals that had established cocaine CPP were used in the extinction and reinstatement experiments.
Extinction of cocaine CPP: effect of social interaction
After cocaine CPP had been established, subjects were divided into two groups. One group received i.p. saline injections immediately before being put into the former cocaine-paired chamber as well as into the previously saline-paired chamber for one extinction session each (during a total of two consecutive days). The second group received an i.p. saline injection immediately before being placed into the previously cocaine-paired chamber for 15 min on one day but, in contrast to the previous group, was also given the opportunity to have social interaction in the previously saline-paired chamber with a conspecific on the other day. On the third day, animals were tested for CPP (15 min; T1). The three-day cycle of training-training-test was repeated three more times (T2, T3, T4).
Cocaine-induced reinstatement of cocaine CPP: effect of social interaction
Twenty-four hours after the last extinction training, rats were administered a single i.p. cocaine injection in the previously cocaine-paired compartment. Another 24 h later, all rats were tested for CPP in a drug-free state.
Immunohistochemistry
FosB/ΔFosB and pCREB immunohistochemistry were per formed by an experimenter who was blind to treatment conditions. Two hours after the start of the cocaine reinstatement test i.e., 26 h after the last cocaine exposure, rats were deeply anesthetized using isoflurane and intracardially perfused with 0.1 M phosphate buffer followed by 4% paraformaldehyde (PFA) dissolved in 0.1 M phosphate buffered saline (PBS; pH 7.4). Brains were then removed and post-fixed in 4% PFA overnight, then stored in 30% sucrose at 4°C until the brain sank, and then at −80°C until sectioning. All serial brain sections (40 μ m) were cut using a Cryostat (Leica). Sections were stored in an assorter buffer (Tris buffer 0.25 M, NaN3 10%) at 4°C until processed for immunolabeling.
Free-floating sections from rats in different groups (naïve rats, cocaine CPP + saline extinction, and cocaine CPP + social interaction extinction) were processed simultaneously for FosB/ΔFosB or pCREB protein expression. Sections were washed in PBS 0.1 M and incubated for 30 min in 0.3% hydrogen peroxide/PBS 0.1 M. Then they were washed in PBS 0.1 M and incubated for 1 h in 0.3% Triton X-100 in PBS 0.1 M containing 3% normal goat serum. Subsequently sections were incubated for 24 h with N terminal anti-FosB rabbit primary antibody that recognizes both FosB and ΔFosB proteins at room temperature (1:5000, Santa Cruz Biotechnology) or pCREB rabbit primary antibody at 4°C (1:2000, Millipore) containing 0.3% Triton X-100, and 1% normal goat serum in PBS 0.1 M. Sections were washed in PBS and incubated for 1 h and 30 min in PBS containing biotinylated goat anti-rabbit antibody IgG (1:200, Vector Laboratories), 0.3% Triton X-100, and 1% normal goat serum. Afterward the tissue was given additional washes in PBS 0.1 M and incubated for 90 min in avidin-biotinylated horseradish peroxidase complex (ABC Elite kit, Vector Laboratories) diluted in PBS 0.1 M. Then, sections were washed in PBS 0.1 M followed by an incubation in 3,3-diaminobenzidine tetrahydrochloride (DAB tablets, Sigma). The reaction was terminated by rinsing the tissue in PBS 0.1 M. Finally, sections were then mounted onto gelatin-coated slides, dried, and dehydrated before coverslipping.
Brain sections were scanned using a Zeiss optical microscope set at 20× magnification equipped with a camera (Axioplan 2 Imaging) interfaced to a PC. Immunoreactive nuclei were counted by an observer who was blind to treatment conditions using Metamorph imaging software. Each rat contributed the averaged value of two sections per brain area to the group mean. Immunoreactivity was expressed as stained nuclei per mm2 of the respective brain regions (Figure 5) identified according to the atlas of Paxinos and Watson (Paxinos and Watson, 2007): the prelimbic (PrL) and infralimbic (IL) cortex, the anterior cingulate cortex areas Cg1 and Cg2, the dorsal striatum (caudate putamen, CPu), the nucleus accumbens core (AcbC) and shell (AcbSh) subregions, the central (Ce) and basolateral (BLA) amygdala, and the ventral tegmental area.
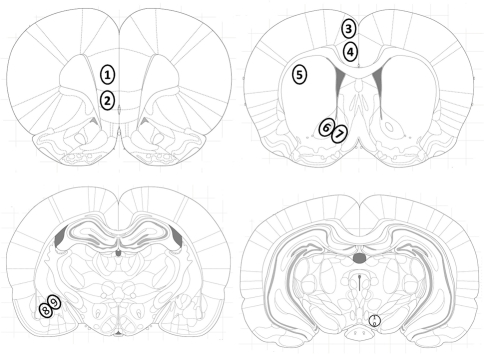
Figure 5.
Location of investigated brain areas. Brain areas were identified according to the atlas of Paxinos and Watson (Paxinos and Watson, 2007): the (1) prelimbic (PrL) and (2) infralimbic (IL) cortex, the cingulate cortex areas (3) Cg1 and (4) Cg2, (5) dorsolateral striatum (caudate putamen, CPu), the (6) nucleus accumbens core (AcbC) and (7) shell (AcbSh) subregions, (8) basolateral (BLA) and (9) central (Ce) amygdala, and (10) the ventral tegmental area (VTA).
Quantitative real time polymerase chain reaction (qRT-PCR)
All qRT-PCR experiments were performed by an experimenter who was blind to treatment conditions. Forty-five minutes after exposure to the cocaine-associated chamber, i.e., 30 min after the end of the reinstatement test, brains were rapidly removed and immediately frozen in −40°C isopentane on dry ice. Brain regions were removed by freehand using a sterile blade while viewing sections using magnifying goggles from thaw-mounted coronal 200 μ m sections at −15°C in a cryostat. The bregma coordinates according to the atlas of Paxinos and Watson (2007 ed.) was 2.52–0.84 mm for the Acb.
Total RNA was isolated from the dissected brain regions using Trizol (Invitrogen) according to the manufacturer's recommendations. To avoid contamination with genomic DNA, total RNA was treated with DNase (2 U/μ L) using the TURBO DNA-free Kit (Ambion). RNA was reverse transcribed in the presence of random hexamer primers and MultiScribe Reverse Transcriptase (50 U/μ L) in a total volume of 20 μ l employing the high-capacity cDNA reverse transcription kit with RNase Inhibitor (Applied Biosystems).
After dilution with 80 μ l of water, 3 μ l of the diluted cDNA was used as a template for amplification (duplicates) with AB Fast SYBR Green mastermix (Applied Biosystems). RT-PCR quantification was performed on a 7500 Fast Real Time PCR system (Applied Biosystems) using the following cycle settings: 20 s 95°C, 40 cycles of 95°C for 3 s, and 60°C for 30 s. All PCR primers were designed using PrimerSelect 5.05 software (DNASTAR). Ct and ΔCt values were calculated by using the 7500 software v2.0.1 and GAPDH as a reference gene.
Primer sequences used in the quantitative real time polymerase chain reaction:
Data analysis
All results are presented as group means ± SEM. Behavioral results were analyzed using One-Way or Two-Way repeated-measures analyses of variance (ANOVA). One-Way repeated-measures ANOVA models always included the within-subjects factor treatment (compartment), two-factor repeated-measures ANOVA models included the factors treatment and time. Statistical significance of both main effects and interactions was tested. Moreover, post-hoc comparisons between individual factor levels (e.g., cocaine vs. saline) were performed using Tukey's test. Differences in protein expression in individual brain regions and mRNA expression were performed using One-Way ANOVA. Post-hoc comparison of individual treatments was done in the same way as above. All statistical tests were performed at a 0.05 level of significance. All statistical analyses were conducted with stat view program.
Results
Extinction and reinstatement of cocaine CPP: effect of social interaction on behavior
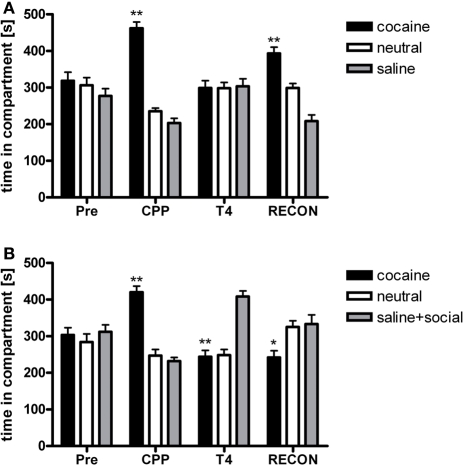
Animals acquired robust conditioned place preference to cocaine (P < 0.001; Figure 1). Cocaine CPP could be extinguished over four extinction cycles that were administered over a period of 12 days (Figure 1A; n = 17). Two-Way repeated-measures ANOVA with treatment and time (CPP to T4) as within-subject factors [of CPP tests T1–T4] performed during extinction revealed a significant treatment effect (previously cocaine-associated chamber vs. previously saline-paired chamber; P = 0.023), a significant time effect (P = 0.002) and a significant time-by-treatment interaction (P < 0.001). For T1 [Time in compartments (sec): drug-paired: 377 ± 34; neutral: 262 ± 14; saline-paired: 262 ± 29], a near-significant preference for the initially cocaine-associated compartment over the saline-associated compartment was found (P = 0.054), whereas tests T2 [Time in compartments (sec): drug-paired: 316 ± 22; neutral: 286 ± 13; saline-paired: 280 ± 23], T3 [Time in compartments (sec): drug-paired: 316 ± 26; neutral: 268 ± 16; saline-paired: 316 ± 22], and T4 (Figure 1A) did not yield any significant preference for the cocaine-associated compartment any more (P > 0.1).
Figure 1.
Social interaction during extinction reverses cocaine CPP and inhibits reinstatement of cocaine CPP. PRE, preconditioning test; CPP, conditioned place preference test; T4, conditioned place preference test during extinction in absence (panel A) or presence (panel B) of social interaction made available in the initially saline-associated compartment; RECON, reinstatement test. Shown is time spent in the cocaine, neutral, or saline-associated compartment (group means ± SEM). *P < 0.05 and **P < 0.01 compared to the time spent in the saline-associated chamber.
If social interaction was made available in the previously saline-paired chamber during extinction only once (i.e., during the first extinction cycle), conditioned place preference for cocaine was no longer observed during the subsequent CPP test (n = 18; T1). In the subsequent extinction (counterconditioning) cycles, pronounced preference for the social interaction-associated chamber developed (Figure 1B, T4), leading to a reversal of CPP from cocaine to social interaction. Two-Way repeated-measures ANOVA showed a non-significant treatment main effect (social interaction vs. saline, P > 0.1) and a non-significant time effect (P > 0.1), but a highly significant treatment × time interaction (P < 0.001). The non-significance of the main effects of the factors time and treatment is a consequence of the reversal of CPP described above. A significant preference of the saline-social interaction chamber over the initially cocaine-associated chamber was observed at CPP (P < 0.001), T3 [Time in compartments (sec): drug-paired: 242 ± 20; neutral: 243 ± 14; saline + social interaction-paired: 416 ± 21, P < 0.001] and T4 (Figure 1B, P < 0.001), whereas no significant difference between the times spent in the respective chambers was seen at T1 [Time in compartments (sec): drug-paired: 341 ± 37; neutral: 245 ± 20; saline + social interaction-paired: 315 ± 29] and T2 [Time in compartments (sec): drug-paired: 284 ± 26; neutral: 273 ± 19; saline + social interaction-paired: 342 ± 25].
If cocaine CPP extinction (saline extinction) was followed by one cocaine-chamber pairing session, fully extinguished cocaine CPP was reinstated (Figure 1A) [repeated-measures ANOVA, treatment effect (cocaine vs. neutral vs. saline), P < 0.001; cocaine vs. saline, P < 0.001]. However, if social interaction was available in the previously saline-paired chamber during cocaine CPP extinction, reinstatement of cocaine CPP was not only fully prevented but preference for the social interaction-paired compartment remained (Figure 1B) [repeated-measures ANOVA, treatment effect (social interaction vs. neutral vs. cocaine), P = 0.037; cocaine vs. social interaction, P = 0.045].
Reinstatement of cocaine CPP: effect of social interaction on the expression of the FosB/ΔFosB transcription factors
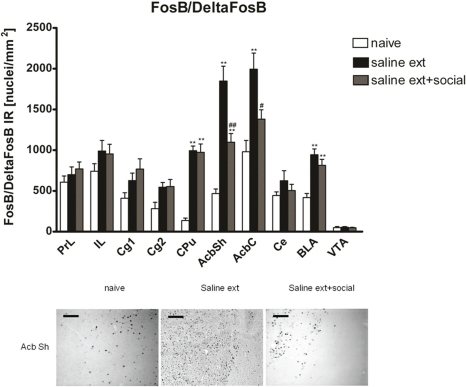
Two hours after the cocaine reinstatement test performed in the drug-free state, i.e., 26 h after the last cocaine exposure, we processed the rats' brain for immunohistochemistry to investigate changes in FosB/ΔFosB expression in brain areas implicated in drug reinforcement. FosB/ΔFosB expression was significantly different between treatments (naive n = 9, cocaine CPP followed by saline extinction n = 7, and cocaine CPP followed by social interaction n = 8) in the following brain areas (Figure 2): CPu [One-Way ANOVA, treatment effect: P < 0.0001], AcbSh [One-Way ANOVA, treatment effect: P < 0.0001], AcbC [One-Way ANOVA, treatment effect: P < 0.001], and BLA [One-Way ANOVA, treatment effect: P < 0.001]. Cocaine CPP in the absence of social interaction as an alternative (i.e., non-drug) stimulus during extinction significantly increased FosB/ΔFosB expression as compared to experiment-naïve animals (Figure 2) in the CPu (P < 0.01), AcbSh (P < 0.01), AcbC (P < 0.01), and the BLA (P < 0.01). While social interaction during extinction of cocaine CPP increased FosB/ΔFosB expression in the CPu (P < 0.01), AcbSh (P < 0.01), and the BLA (P < 0.01) in comparison to naive rats, it decreased cocaine CPP-reinstatement-associated FosB/ΔFosB expression (Figure 2) in the AcbSh (P < 0.01) and the AcbC (P < 0.05).
Figure 2.
Social interaction decreases cocaine CPP reinstatement-associated FosB/ΔFosB expression in nucleus accumbens shell and core. Shown are FosB/ΔFosB positive immuno-reactive nuclei per mm2 of the respective brain area. Shown are group means ± SEM. **P < 0.01 compared to naive group; #P < 0.05 and ##P < 0.01 compared to saline extinction group. In the dorsal striatum (CPu) and the basolateral amygdala (BLA), priming with cocaine after saline or saline + social extinction increased FosB/ΔFosB expression in comparison to naive rats (**P < 0.01). Brain area abbreviations follow the nomenclature of Paxinos and Watson (Paxinos and Watson, 2007). In the low panel, FosB-like immuno-reactive nuclei are given for the nucleus accumbens shell (AcbSh). Sections were taken from completely experiment-naïve rats (naïve), or from rats that underwent extinction before the cocaine reinstatement test in absence (sal ext) or presence of a weight-matched male conspecific (sal ext + social). Scale bar: 100 μ m.
As there is no antibody available to distinguish between FosB and the different isoforms of the truncated FosB protein ΔFosB and as it is not well known if exposure to reward-associated conditioned stimuli causes FosB expression (Harris et al., 2007), we proceeded as follows to investigate if the reduced expression of FosB/ΔFosB in the Acb (AcbSh and AcbC) of rats that express CPP and undergo social interaction is due to FosB or ΔFosB:
We assessed the expression of cdk5 and nfkb, targets of ΔFosB (Nestler, 2008) in the Acb of rats that underwent extinction of cocaine CPP in presence or absence of social interaction by qRT-PCR. Our results show that cocaine treatment used in this study [four injections of cocaine (15 mg/kg) during conditioning + one injection (15 mg/kg) to induce reinstatement] was not sufficient to induce the expression of ΔFosB targets cdk5 and nfkb. In addition, social interaction did not change expression of cdk5 [One-Way ANOVA, treatment effect: p > 0.05; naive (n = 4): 5.4 ± 0.4; saline extinction group (n = 5): 5.7 ± 0.4; social interaction group (n = 6): 5.3 ± 0.4] or nfkb [One-Way ANOVA, treatment effect: p > 0.05; naive (n = 4): 6.9 ± 0.1; saline extinction group (n = 5): 6.8 ± 0.2; social interaction group (n = 6): 6.7 ± 0.2].
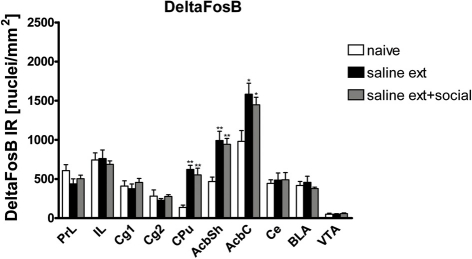
We trained another group of rats for cocaine CPP and extinguished cocaine CPP in presence or absence of social interaction. These rats were then administered an i.p. cocaine injection but were not exposed to the cocaine-associated CPP compartment (without the CPP reinstatement test done 24 h after the last cocaine injection) to eliminate the possibility of expression of FosB as an immediate early gene by the cues associated with cocaine CPP. Thus, the FosB/ΔFosB expression that we analyzed in this experiment 26 h after the last injection of cocaine should, in all likelihood, represent almost solely ΔFosB (Chen et al., 1997; Nestler, 2004; Ulery et al., 2006; Perrotti et al., 2008). The use of these animals enabled us not only to investigate the expression of ΔFosB protein alone but also consider any possible involvement of the full length FosB protein in social interaction protective effect by comparing ΔFosB expression to FosB/ΔFosB expression both analyzed 26 h after the last cocaine exposure. Using immunohistochemistry, we could investigate the shell and core subregions of the Acb in addition to other brain areas involved in drug rewarding effects. ΔFosB expression was significantly different between treatments (naive n = 9, cocaine CPP followed by saline extinction n = 5, and cocaine CPP followed by social interaction during saline extinction n = 6) in the following brain areas (Figure 3): CPu [One-Way ANOVA, treatment effect: P < 0.0001], AcbSh [One-Way ANOVA, treatment effect: P < 0.001], and AcbC [One-Way ANOVA, treatment effect: P < 0.05]. Cocaine CPP in the absence or presence of alternate social interaction during extinction increased similarly ΔFosB expression as compared to experiment-naïve animals (Figure 3) in the CPu (P < 0.01), AcbSh (P < 0.01), AcbC (P < 0.05).
Figure 3.
Cocaine CPP reinstatement-associated ΔFosB expression is increased similarly in the dorsal striatum (CPu), nucleus accumbens shell (AcbSh), and core (AcbC) of rats that underwent extinction before the cocaine priming in absence (sal ext) or presence of social interaction (sal ext + social). Shown are ΔFosB positive immuno-reactive nuclei per mm2 of the respective brain area. Shown are group means ± SEM. *P < 0.05 and **P < 0.01 compared to naive group. Brain area abbreviations follow the nomenclature of Paxinos and Watson (Paxinos and Watson, 2007).
The reduction of FosB/ΔFosB (Figure 2) and not ΔFosB (Figure 3) expression (both assessed 26 h after the last cocaine injection) in the AcbSh and AcbC after social interaction suggests that FosB is the transcription factor involved in the protective effect of social interaction.
Reinstatement of cocaine CPP: effect of social interaction on the expression of pCREB transcription factor
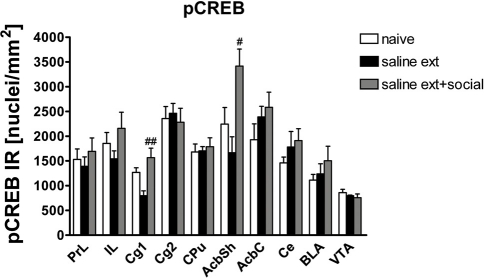
Given that CREB and ΔFosB usually produce opposite effects on either the expression of numerous genes or a number of behavioral phenotypes, we investigated pCREB expression in brain areas involved in drug rewarding effects of rats that did not undergo the reinstatement test (same animals used to assess ΔFosB expression) using immunohistochemistry. pCREB expression was significantly different between treatments (naive n = 7, cocaine CPP followed by saline extinction n = 5, and cocaine CPP followed by social interaction during saline extinction n = 6) in the following brain areas (Figure 4): Cg1 [One-Way ANOVA, treatment effect: P < 0.05] and AcbSh [One-Way ANOVA, treatment effect: P < 0.05]. Social interaction during extinction of cocaine CPP increased pCREB expression in the Cg1 (P < 0.01) and AcbSh (P < 0.05) as compared to rats that undergo cocaine CPP in the absence of alternate social interaction during extinction (Figure 4).
Figure 4.
Social interaction increases cocaine CPP reinstatement-associated pCREB expression in nucleus accumbens shell (AcbSh) and cingulated cortex (Cg1). Shown are pCREB positive immune-reactive nuclei per mm2 of the respective brain area. Shown are group means ± SEM. #P < 0.05 and ##P < 0.01 compared to saline extinction group. Brain area abbreviations follow the nomenclature of Paxinos and Watson (Paxinos and Watson, 2007).
Discussion
We have previously shown that social interaction during extinction of cocaine CPP reversed cocaine CPP-reinstatement-associated Zif268 expression in the nucleus accumbens shell, the central and basolateral amygdala, and the VTA (Fritz et al., 2011a). In the present study, we found that social interaction during extinction of cocaine CPP also decreased FosB expression in the nucleus AcbC and shell, and increased pCREB expression in the nucleus accumbens shell and Cg1 area of the cingulate cortex associated with cocaine-induced reinstatement of CPP.
Studies with gene modified animals and viral vectors have shown that an over-expression of ΔFosB in certain brain regions such as the nucleus accumbens creates a behavioral phenotype with increased sensitivity to drugs of abuse (Kelz et al., 1999; Colby et al., 2003; Peakman et al., 2003; Zachariou et al., 2006). Given that social interaction was able to prevent reinstatement of cocaine CPP, we expected that rats which had the opportunity to interact socially during cocaine CPP extinction would show decreased ΔFosB expression in the nucleus accumbens. Social interaction significantly decreased cocaine CPP-reinstatement-associated FosB/ΔFosB expression in the nucleus accumbens shell and core. However, as it is not well known if exposure to cues conditioned to rewards causes FosB expression (Harris et al., 2007), we cannot identify which transcription factor is implicated in the protective effect of social interaction. We first investigated ΔFosB targets cdk5 and nfkb mRNA expression in the nucleus accumbens (Nestler, 2008) of naïve and cocaine CPP-expressing rats followed by extinction in presence or absence of social interaction. Unexpectedly, we found no difference between these three groups of rats in the expression of either target of ΔFosB. These results suggest that (1) ΔFosB may not be the transcription factor implicated in the protective effect of social interaction or (2) As cdk5 mRNA expression depends highly on the dose of cocaine given, the protocol of injection (sensitization vs. desensitization), species (mice vs. rats), cocaine self-administration vs. injection (Wedzony et al., 2005); cocaine injections that the rat received during this study (four injections of 15 mg/kg each for CPP training every second day and one injection of 15 mg/kg to induce reinstatement) were not sufficient to produce changes in the targets of ΔFosB. While most of the studies investigating cdk5 mRNA expression are based on subsequent viral-mediated ΔFosB over-expression in mice (Bibb et al., 2001), the same group has also found a difference in cdk5 mRNA expression in the CPu and the nucleus accumbens of rats that received chronic injections of cocaine at the dose of 20 mg/kg i.p./day for eight days which represents a higher dose of cocaine and a different regimen of administration in comparison to the present study (Bibb et al., 2001). In order to explore separately ΔFoB expression in the nucleus accumbens subregions shell and core, we performed immunohistochemistry in rats that were treated exactly the same as before but without being exposed to the cues associated to cocaine CPP to eliminate possibilities of FosB induction. We found that cocaine CPP in absence or presence of social interaction as the non-drug (alternative) stimulus during cocaine CPP extinction increased ΔFosB expression to the same degree as compared to experiment-naïve animals in both subregions of the nucleus accumbens. Consequently, these results suggest that FosB could be induced by cues associated to drug's rewarding effects.
CPP depends on the ability to learn and remember associations between drug and environment (Bardo and Bevins, 2000). Based on the findings of the present study and the effects of social interaction on cocaine CPP-reinstatement-associated Zif268 expression shown previously (Fritz et al., 2011a), it appears that social interaction regulates the conditioned effects of drugs (immediate early genes) rather than the long-lasting effects of drugs of abuse (ΔFosB). Thus, social interaction could weaken the ability of cocaine CPP-associated cues to trigger relapse.
Contrary to ΔFosB, previous work has shown that elevated CREB in the nucleus accumbens shell reduces cocaine- and morphine-induced conditioned place preferences (Carlezon et al., 1998; Pliakas et al., 2001; Barrot et al., 2002). Increased CREB function in the striatum has recently been implicated in reduced motivation to self-administer cocaine (Hollander et al., 2010). Furthermore, elevated CREB function in the nucleus accumbens shell produced increases in intracranial self-stimulation thresholds (Muschamp et al., 2011). However, although stress is known to increase the reinforcing effects of drugs and reinstate extinguished drug-seeking behavior in rats (Lu et al., 2003), stress (footshock) has been shown to activate CREB within the nucleus accumbens shell (Muschamp et al., 2011). In addition,(Pliakas et al., 2001) showed that forced swim stress activates CREB via phosphorylation in the nucleus accumbens shell and that this neuroadaptive response has functional effects on behavior. Specifically, elevating CREB expression to mimic activation in the nucleus accumbens shell increased immobility behavior in the forced swim test whereas disruption of CREB in the nucleus accumbens shell produced opposite effects (Pliakas et al., 2001). Our results showed that social interaction during extinction of cocaine CPP increased pCREB expression in nucleus accumbens shell as compared to rats that undergo cocaine CPP in the absence of alternate social interaction during extinction. It is likely that CREB activation in the nucleus accumbens shell after social interaction in our study reflects a reduction of the rewarding effects of cocaine rather than stress-related effects. Indeed, while stress is known to induce reinstatement of extinguished drug-seeking behavior, we have shown that social interaction during extinction prevents reinstatement of cocaine CPP (Fritz et al., 2011a). Furthermore, it has been shown that social defeat or negative social interaction decreased functional activation as measured by zif268 mRNA expression in the medial prefrontal cortex (Covington et al., 2005) and that a temporary downregulation of zif268 expression in the medial prefrontal cortex of male rats produced social anxiety-like behaviors (Stack et al., 2010). In contrast, social interaction used in our study did not alter cocaine CPP-associated Zif268 expression in the medial prefrontal cortex (Fritz et al., 2011a). In addition, it has been shown that acute stress induced FosB/ΔFosB predominantly in the prefrontal cortex and the nucleus accumbens while chronic stress induced ΔFosB expression particularly in the prefrontal cortex, the nucleus accumbens and the basolateral amygdala (Perrotti et al., 2004). However, social interaction during extinction induced neither FosB/ΔFosB in any of the regions of the prefrontal cortex investigated nor ΔFosB expression in the prefrontal cortex or the basolateral amygdala. These evidences suggest that rats expressing cocaine CPP followed by social interaction during extinction did not exhibit signs of stress.
We expected to find a difference in pCREB expression after social interaction in the VTA. Unexpectedly, we found that social interaction during extinction increased pCREB in the cingulate cortex area 1 (Cg1). In support of this finding, it has been shown that morphine increased pCREB expression in the prefrontal cortex of alcohol-avoiding rats in comparison with alcohol non-avoiding rats (Kaste et al., 2009). To date, not much data has been generated on the role of pCREB in the cingulate cortex area. However, given the role of the cingulate cortex in decision-making (Schweimer and Hauber, 2006), our results suggest that the increased pCREB expression in Cg1 may be importantly involved in social interaction effects.
A few caveats for the interpretation of our study should be highlighted. First of all, in this study we focused on the correlation between the beneficial effects of social interaction and the changes in expression of transcription factors such as FosB and CREB. We did not investigate the functional effects of these changes on reinstatement of cocaine CPP. For example, Viral vector-mediated elevation or disruption of CREB expression within the nucleus accumbens shell could explore its eventual causal involvement in conditioned place preference to cocaine (Carlezon et al., 1998; Pliakas et al., 2001), forced swim stress (Pliakas et al., 2001), intracranial self-stimulation and fear conditioning (Muschamp et al., 2011). Thus, it would be important to study the causal relation between CREB and the protective effects of social interaction on reinstatement of cocaine CPP. In addition, the present study did not investigate the functional implication of discrete brain regions in the protective effects of social interaction on reinstatement of cocaine CPP. Interestingly, in a previous study, we showed that if rats were concurrently conditioned for place preference by pairing cocaine with one compartment and social interaction with the other (i.e., mutually exclusive stimulus presentation during training), pre-acquisition lesioning the AcbC or the basolateral amygdala shifted the animals' preference toward social interaction, whereas a bilateral shell lesion shifted the preference toward cocaine CPP (Fritz et al., 2011c). Given that both stimuli (15 min dyadic social interaction vs. 15 mg/kg i.p. cocaine) can produce equally strong CPP (Fritz et al., 2011a,c), these findings suggest a role of the nucleus accumbens shell in mediating alternative non-drug-(social interaction) associated conditioned contextual stimuli (Fritz et al., 2011c) that are in accordance with the present finding showing that social interaction during extinction increased the expression of pCREB in the shell of the nucleus accumbens. Also, in this study we focused on the naive group as the sole control group to interpret the reported neural correlates of the effects of social interaction on reinstatement of cocaine CPP. Therefore, other control groups that show different aspects of “conditioning” vs. “pharmacological” effects of cocaine before the reinstatement test should be added in future studies to better interpret the functional significance of the neural correlates of the “anti-relapse” effects of “friendly” social interaction.
Our results indicate that pCREB and FosB but not ΔFosB are implicated in the protective effect of social interaction against cocaine-induced reinstatement of CPP. Accordingly, it has been reported that gene expression after a short cocaine treatment, i.e., five injections (daily i.p. injections of cocaine 10 mg/kg for five days) is highly dependent on the actions of CREB and less dependent on ΔFosB (McClung and Nestler, 2003). However, long-term cocaine administration, i.e., 20 injections (cocaine 15 mg/kg for five days/week for four consecutive weeks) resulted in a gene expression profile that is highly dependent on ΔFosB and less dependent on CREB (McClung and Nestler, 2003). In conclusion, social interaction during extinction may (1) weaken the association between contextual cues and the rewarding effects of drugs of abuse and/or (2) reduce the drugs' rewarding effects. These findings suggest that social interaction, if offered in a context that is clearly distinct from the previously drug-associated one, may profoundly inhibit relapse to cocaine addiction.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was funded by the Austrian Science Fund (FWF): [M1169-B18] and [P23824-B18], and by the Verein für Experimentelle Psychiatrie, Psychotherapie, und Pharmakologie (VEPPP).
References
- Bardo M. T., Bevins R. A. (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl.) 153, 31–43 10.1007/s002130000569 [DOI] [PubMed] [Google Scholar]
- Barrot M., Olivier J. D., Perrotti L. I., DiLeone R. J., Berton O., Eisch A. J., Impey S., Storm D. R., Neve R. L., Yin J. C., Zachariou V., Nestler E. J. (2002). CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc. Natl. Acad. Sci. U.S.A. 99, 11435–11440 10.1073/pnas.172091899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb J. A., Chen J., Taylor J. R., Svenningsson P., Nishi A., Snyder G. L., Yan Z., Sagawa Z. K., Ouimet C. C., Nairn A. C., Nestler E. J., Greengard P. (2001). Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410, 376–380 10.1038/35066591 [DOI] [PubMed] [Google Scholar]
- Carlezon W. A. Jr., Thome J., Olson V. G., Lane-Ladd S. B., Brodkin E. S., Hiroi N., Duman R. S., Neve R. L., Nestler E. J. (1998). Regulation of cocaine reward by CREB. Science 282, 2272–2275 10.1126/science.282.5397.2272 [DOI] [PubMed] [Google Scholar]
- Chen J., Kelz M. B., Hope B. T., Nakabeppu Y., Nestler E. J. (1997). Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J. Neurosci. 17, 4933–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Nye H. E., Kelz M. B., Hiroi N., Nakabeppu Y., Hope B. T., Nestler E. J. (1995). Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol. Pharmacol. 48, 880–889 [PubMed] [Google Scholar]
- Colby C. R., Whisler K., Steffen C., Nestler E. J., Self D. W. (2003). Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J. Neurosci. 23, 2488–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington H. E., 3rd, Kikusui T., Goodhue J., Nikulina E. M., Hammer R. P. Jr., Miczek K. A. (2005). Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology 30, 310–321 10.1038/sj.npp.1300587 [DOI] [PubMed] [Google Scholar]
- Dinieri J. A., Nemeth C. L., Parsegian A., Carle T., Gurevich V. V., Gurevich E., Neve R. L., Nestler E. J., Carlezon W. A. Jr. (2009). Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. J. Neurosci. 29, 1855–1859 10.1523/JNEUROSCI.5104-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M. A. (2006). Genetic and environmental influences on the development of alcoholism: resilience vs risk. Ann. N.Y. Acad. Sci. 1094 193–201 10.1196/annals.1376.019 [DOI] [PubMed] [Google Scholar]
- Fritz M., El Rawas R., Salti A., Klement S., Bardo M. T., Kemmler G., Dechant G., Saria A., Zernig G. (2011a). Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict. Biol. 16, 273–284 10.1111/j.1369-1600.2010.00285.x [DOI] [PubMed] [Google Scholar]
- Fritz M., Klement S., El Rawas R., Saria A., Zernig G. (2011b). Sigma1 receptor antagonist BD1047 enhances reversal of conditioned place preference from cocaine to social interaction. Pharmacology 87, 45–48 10.1159/000322534 [DOI] [PubMed] [Google Scholar]
- Fritz M., El Rawas R., Klement S., Kummer K., Mayr M. J., Eggart V., Salti A., Bardo M. T., Saria A., Zernig G. (2011c). Differential effects of accumbens core vs. Shell lesions in a rat concurrent conditioned place preference paradigm for cocaine vs. social interaction. PLoS One 6:e26761 10.1371/journal.pone.0026761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin D. V., Briscoe R. J., Goulden K. L., Holloway F. A. (1994). Aversive attributes of ethanol can be attenuated by dyadic social interaction in the rat. Alcohol 11, 247–251 10.1016/0741-8329(94)90038-8 [DOI] [PubMed] [Google Scholar]
- Harris G. C., Hummel M., Wimmer M., Mague S. D., Aston-Jones G. (2007). Elevations of FosB in the nucleus accumbens during forced cocaine abstinence correlate with divergent changes in reward function. Neuroscience 147, 583–591 10.1016/j.neuroscience.2007.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N., Brown J. R., Haile C. N., Ye H., Greenberg M. E., Nestler E. J. (1997). FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc. Natl. Acad. Sci. U.S.A. 94, 10397–10402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander J. A., Im H. I., Amelio A. L., Kocerha J., Bali P., Lu Q., Willoughby D., Wahlestedt C., Conkright M. D., Kenny P. J. (2010). Striatal microRNA controls cocaine intake through CREB signalling. Nature 466, 197–202 10.1038/nature09202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B. T., Nye H. E., Kelz M. B., Self D. W., Iadarola M. J., Nakabeppu Y., Duman R. S., Nestler E. J. (1994). Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13, 1235–1244 10.1016/0896-6273(94)90061-2 [DOI] [PubMed] [Google Scholar]
- Hyman S. E., Malenka R. C. (2001). Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2, 695–703 10.1038/35094560 [DOI] [PubMed] [Google Scholar]
- Kaste K., Kivinummi T., Piepponen T. P., Kiianmaa K., Ahtee L. (2009). Differences in basal and morphine-induced FosB/DeltaFosB and pCREB immunoreactivities in dopaminergic brain regions of alcohol-preferring AA and alcohol-avoiding ANA rats. Pharmacol. Biochem. Behav. 92, 655–662 10.1016/j.pbb.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Kelz M. B., Chen J., Carlezon W. A. Jr., Whisler K., Gilden L., Beckmann A. M., Steffen C., Zhang Y. J., Marotti L., Self D. W., Tkatch T., Baranauskas G., Surmeier D. J., Neve R. L., Duman R. S., Picciotto M. R., Nestler E. J. (1999). Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature 401, 272–276 10.1038/45790 [DOI] [PubMed] [Google Scholar]
- Kreek M. J., Nielsen D. A., Butelman E. R., LaForge K. S. (2005). Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat. Neurosci. 8, 1450–1457 10.1038/nn1583 [DOI] [PubMed] [Google Scholar]
- Lonze B. E., Ginty D. D. (2002). Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 10.1016/S0896-6273(02)00828-0 [DOI] [PubMed] [Google Scholar]
- Lu L., Shepard J. D., Hall F. S., Shaham Y. (2003). Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci. Biobehav. Rev. 27, 457–491 10.1016/S0149-7634(03)00073-3 [DOI] [PubMed] [Google Scholar]
- McClung C. A., Nestler E. J. (2003). Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat. Neurosci. 6, 1208–1215 10.1038/nn1143 [DOI] [PubMed] [Google Scholar]
- McClung C. A., Ulery P. G., Perrotti L. I., Zachariou V., Berton O., Nestler E. J. (2004). DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res. Mol. Brain Res. 132, 146–154 10.1016/j.molbrainres.2004.05.014 [DOI] [PubMed] [Google Scholar]
- Moratalla R., Elibol B., Vallejo M., Graybiel A. M. (1996). Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron 17, 147–156 10.1016/S0896-6273(00)80288-3 [DOI] [PubMed] [Google Scholar]
- Muschamp J. W., Van't Veer A., Parsegian A., Gallo M. S., Chen M., Neve R. L., Meloni E. G., Carlezon W. A. Jr. (2011). Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J. Neurosci. 31, 3095–3103 10.1523/JNEUROSCI.5973-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J. (2004). Molecular mechanisms of drug addiction. Neuropharmacology 47(Suppl. 1), 24–32 10.1016/j.neuropharm.2004.06.031 [DOI] [PubMed] [Google Scholar]
- Nestler E. J. (2008). Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3245–3255 10.1098/rstb.2008.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Barrot M., Self D. W. (2001). DeltaFosB: a sustained molecular switch for addiction. Proc. Natl. Acad. Sci. U.S.A. 98, 11042–11046 10.1073/pnas.191352698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson V. G., Zabetian C. P., Bolanos C. A., Edwards S., Barrot M., Eisch A. J., Hughes T., Self D. W., Neve R. L., Nestler E. J. (2005). Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J. Neurosci. 25, 5553–5562 10.1523/JNEUROSCI.0345-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. (2007). The Rat Brain in Stereotaxic Coordinates. New York, NY: Academic Press; 6110810 [Google Scholar]
- Peakman M. C., Colby C., Perrotti L. I., Tekumalla P., Carle T., Ulery P., Chao J., Duman C., Steffen C., Monteggia L., Allen M. R., Stock J. L., Duman R. S., McNeish J. D., Barrot M., Self D. W., Nestler E. J., Schaeffer E. (2003). Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 970, 73–86 10.1016/S0006-8993(03)02230-3 [DOI] [PubMed] [Google Scholar]
- Perrotti L. I., Hadeishi Y., Ulery P. G., Barrot M., Monteggia L., Duman R. S., Nestler E. J. (2004). Induction of deltaFosB in reward-related brain structures after chronic stress. J. Neurosci. 24, 10594–10602 10.1523/JNEUROSCI.2542-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti L. I., Weaver R. R., Robison B., Renthal W., Maze I., Yazdani S., Elmore R. G., Knapp D. J., Selley D. E., Martin B. R., Sim-Selley L., Bachtell R. K., Self D. W., Nestler E. J. (2008). Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse 62, 358–369 10.1002/syn.20500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliakas A. M., Carlson R. R., Neve R. L., Konradi C., Nestler E. J., Carlezon W. A. Jr. (2001). Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J. Neurosci. 21, 7397–7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B., Aguilar M. A., Lluch J., Rodriguez-Arias M., Minarro J. (2009). Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology (Berl.) 207, 485–498 10.1007/s00213-009-1678-1 [DOI] [PubMed] [Google Scholar]
- Schweimer J., Hauber W. (2006). Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learn. Mem. 13, 777–782 10.1101/lm.409306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Stack A., Carrier N., Dietz D., Hollis F., Sorenson J., Kabbaj M. (2010). Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology 35, 570–580 10.1038/npp.2009.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadi H. (1999). Individual risk factors for adolescent substance use. Drug Alcohol Depend. 55, 209–224 10.1016/S0376-8716(99)00017-4 [DOI] [PubMed] [Google Scholar]
- Thiel K. J., Okun A. C., Neisewander J. L. (2008). Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 96, 202–212 10.1016/j.drugalcdep.2008.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel K. J., Sanabria F., Neisewander J. L. (2009). Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl.) 204, 391–402 10.1007/s00213-009-1470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery P. G., Rudenko G., Nestler E. J. (2006). Regulation of DeltaFosB stability by phosphorylation. J. Neurosci. 26, 5131–5142 10.1523/JNEUROSCI.4970-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K., Markowicz-Kula K., Chocyk A., Fijal K., Mackowiak M. (2005). The effect of ‘binge’ cocaine administration on the expression of cyclin-dependent kinase 5 and its activator p35 in various regions of rat brain. Brain Res. 1063, 195–200 10.1016/j.brainres.2005.09.034 [DOI] [PubMed] [Google Scholar]
- Zachariou V., Bolanos C. A., Selley D. E., Theobald D., Cassidy M. P., Kelz M. B., Shaw-Lutchman T., Berton O., Sim-Selley L. J., Dileone R. J., Kumar A., Nestler E. J. (2006). An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat. Neurosci. 9, 205–211 10.1038/nn1636 [DOI] [PubMed] [Google Scholar]