Abstract
Objective
To compare the short term financial costs of treating a patient in myasthenia gravis crisis (MGC) with intravenous immunoglobulin (IVIG) versus plasma exchange (PLEX).
Methods
An itemized comparative cost-minimization analysis of IVIG vs. plasma exchange for MGC was performed. Calculations were based on each therapy’s implementation cost, associated hospitalization times, and predicted cost to treat known complications. A cost superiority determination was proposed based on the total cost profile of each therapy.
Results
The difference in total cost favored IVIG over PLEX with an average savings of $22,326 per patient. Sensitivity analysis demonstrated that overall costs are highly dependent on IVIG dosing, hospital lengths of stay, and the number of plasma exchange days required.
Conclusions
The use of IVIG for MGC may be a short term cost minimizing therapy compared to PLEX. Additional prospective studies are required to evaluate the extended cost profile and efficacy of these therapies.
INTRODUCTION
Myasthenia gravis is a neuromuscular disease caused by autoantibodies that bind to postsynaptic nicotinic acetylcholine receptors. The primary clinical features of this disease include fatigability, weakness, double vision, problems speaking, and problems swallowing. Myasthenia gravis has an annual incidence of approximately 30 new cases per million.1 Approximately fifteen to twenty percent of these patients will experience respiratory failure, severe swallowing difficulty, or speech dysfunction; referred to as myasthenia gravis crisis (MGC).2 Approximately three to eight percent of all patients who go into myasthenia gravis crisis will die from this condition.2
Intravenous immunoglobulin (IVIG) is a biological agent obtained through the fractionation of blood from 2000–16,000 patients.3 Plasma exchange (PLEX) is a blood separation technique thought to remove autoantibodies.
Both IVIG and PLEX have been found to be effective disease stabilizing therapies for patients with MGC.4,5,6,7 To date, neither IVIG or PLEX has established clear clinical dominance over the other for the treatment of MGC.2,8,9,10
As the societal cost of health care increases, it is imperative to both optimize patient care and identify areas where costs can be reduced. Although cost should never be the primary reason for selecting a therapy, it is reasonable to consider this aspect in select cases where one beneficial therapy is not clearly clinically indicated over another. Here we identify the most critical factors which generate cost in MGC and perform a direct cost comparison of IVIG vs. PLEX for MGC.
MATERIALS AND METHODS
An itemized comparative cost-minimization analysis of IVIG vs. plasma exchange as a primary immunomodulating treatment for myasthenia gravis crisis was performed. The following items were used to determine the cost of each therapy: 1) The initial cost of each therapy; 2) The average length, location, and cost of hospitalization with each therapy; and, 3) The cost of secondary complications associated with each therapy. Local cost quotes, and a widespread literature review were utilized to determine each of the above items. MGC costs which accrue before the selection of treatment (e.g. ambulance costs, standard initial chest X-rays, standard initial laboratory testing costs) were not included in overall cost assessments. The cost of each possible outcome after treatment was modeled for both therapies. Possible outcomes arms included patients who have no complications with therapy and patients who experience known side effects from therapy. Prevalence and cost data were assigned to each outcome arm based on best available literature and utilized to calculate the total average short term societal cost of assigning a MGC patient to either IVIG of PLEX.
Average hospitalization times for each therapy were sought from studies representing a United States myasthenia population. The prevalence of known side effects for each therapy in the myasthenia gravis crisis population was evaluated. When treatment side effect profiles were not obtainable for myasthenia gravis crisis, a general myasthenia gravis population or comparable neuromuscular or neurological population was used. Recent American prospective studies with the largest myasthenia crisis study populations were prioritized. Sensitivity analysis was utilized in instances where there was a range of reported values for a cost variable. This analysis allowed for a graphical depiction of the overall cost of each therapy related to the reported ranges of these variables. TreeAge 4.0 was utilized to perform a sensitivity analysis, create a decision tree, and propose a cost superiority determination for IVIG and PLEX.
RESULTS
Average hospital and intensive care unit (ICU) length of stay data were based on two studies. The first study compared the use of IVIG (400mg/kg/day for 5 days) vs. PLEX during 54 episodes of myasthenia gravis crisis at four major United States tertiary centers.11 IVIG was associated with an average ICU stay of 14 days (hospital stay of 17.7) while plasma exchange was associated with an average ICU stay of 17.4 days (hospital stay of 25.7). The second study evaluated patients from a nationwide inpatient sample database with ICD-9-CM codes for myasthenia gravis or myasthenia gravis crisis.12 This cross-sectional study found a median MGC hospital stay of 10 days with PLEX and 5 days with IVIG; although intensive care time after each therapy were not reported.
The estimated prevalence of side effects during PLEX or IVIG use for MGC was obtained from the best available prospective myasthenia treatment studies. Plasma exchange side effect prevalence was obtained from a prospective study evaluating the use of this therapy in 41 MG patients.13 The prevalence of IVIG side effects was based on a prospective study of 88 myasthenia gravis patients who received 1 gram/kg of IVIG on 2 consecutive days for myasthenia gravis exacerbation.14 Two additional side effects not reported in these studies were also given consideration given their potential to substantially increase the costs associated with PLEX or IVIG treatment. Although uncommon, the estimated incidence of stroke with IVIG use (0.6%)15, and the potential incidence of death secondary to PLEX (0.02%)16 were included in the final cost assessments of these treatments.
Average costs for services and medicines (including daily professional fees, daily hospital rates, daily intensive care rates, costs for medicines, cost of IVIG, cost of albumin, cost of laboratory studies, cost of implementing PLEX, catheter costs, catheter placement costs, and catheter removal costs) were provided by the University of Rochester Billing Office and Lexi-comp. An average IVIG use of 400 mg/kg for five days, and an average exchange frequency of 5.5 was utilized.
All cost variables and their sources are provided in Table 1. Formulas utilized to calculate the total cost of each outcome arm are detailed in Supplemental Table 1.
TABLE 1.
Input Variables and Sources
| Base-Case Inputs | |||||
|---|---|---|---|---|---|
| Variable | Estimate ($) | Range | % Occurance | Source (Reference number) | |
| Plasma Exchange Costs | 1 Exchange | 2980 | Univerisity of Rochester Billing Office | ||
| 1 Dose Albumin | 1119 | Univerisity of Rochester Billing Office | |||
| Catheter | 520 | Univerisity of Rochester Billing Office | |||
| Catheter Placement | 859 | University of Rochester Billing Office | |||
| Fibrinogen Lab Test | 94 | Univerisity of Rochester Billing Office | |||
| Catheter Removal | 353 | Univerisity of Rochester Billing Office | |||
| Plasma Exchange | Death | 4700000 | 0-.02% | 0.02 | 16,18 |
| Side Effect % and Costs | Bleeding | 445 | 4.9 | 9, The University of Rochester Billing Office | |
| Catheter Occlusion | 1733 | 2.4 | 9, University of Rochester Billing Office | ||
| Nausea | 60 | 2.4 | 9, Lexi-comp | ||
| Hypotension | 14.9 | 4.9 | 9, Lexi-comp | ||
| DVT | 4100 | 2.4 | 9, Lexi-comp, The University of Rochester Billing Office | ||
| Infection | 1556 | 4.9 | 9, Lexi-comp, The University of Rochester Billing Office | ||
| Plasma Exchange Hospital Days | ICU | 17.4 | 10–17.4 | 8,11,12 | |
| Non-ICU | 8.3 | 8,11,12 | |||
| Number of Exchanges Required | 5.5 | 3 to 8 | 11 | ||
| Hospital Costs/Day | ICU cost | 2965 | Univerisity of Rochester Billing Office | ||
| ICU profession fee | 735 | Univerisity of Rochester Billing Office | |||
| Non-ICU hospital cost | 1015 | Univerisity of Rochester Billing Office | |||
| Non-ICU professional fee | 280 | Univerisity of Rochester Billing Office | |||
| IVIG Cost | IVIG Cost ($/gram) | 134.88 | Univerisity of Rochester Billing Office | ||
| Patient Mass (kg) | 80 | 40–120 | http://www.halls.md/chart/height-weight.htm | ||
| Cost of 2g/kg Treatment | 21581* | Univerisity of Rochester Billing Office | |||
| IVIG | Fever | 1556 | 14.8 | 14, Lexi-comp, University of Rochester Billing Office | |
| Side Effect % and Costs | Myalgia | 1 | 1 | 14, Lexi-comp | |
| Headache | 1 | 22.7 | 14, Lexi-comp | ||
| Nausea | 70 | 6.8 | 14, Lexi-comp | ||
| Increased Creatinine | 231 | 14.8 | 14, Univerisity of Rochester Billing Office | ||
| Increased LFTS | 224 | 8 | 14, Univerisity of Rochester Billing Office | ||
| Stroke | 59000 | 0.6 | 15,17 | ||
| IVIG Hosptial Days | ICU | 14 | 8 to 14 | 8,11,12 | |
| Non-ICU | 3.7 | 8,11,12 |
For a 80 kg person
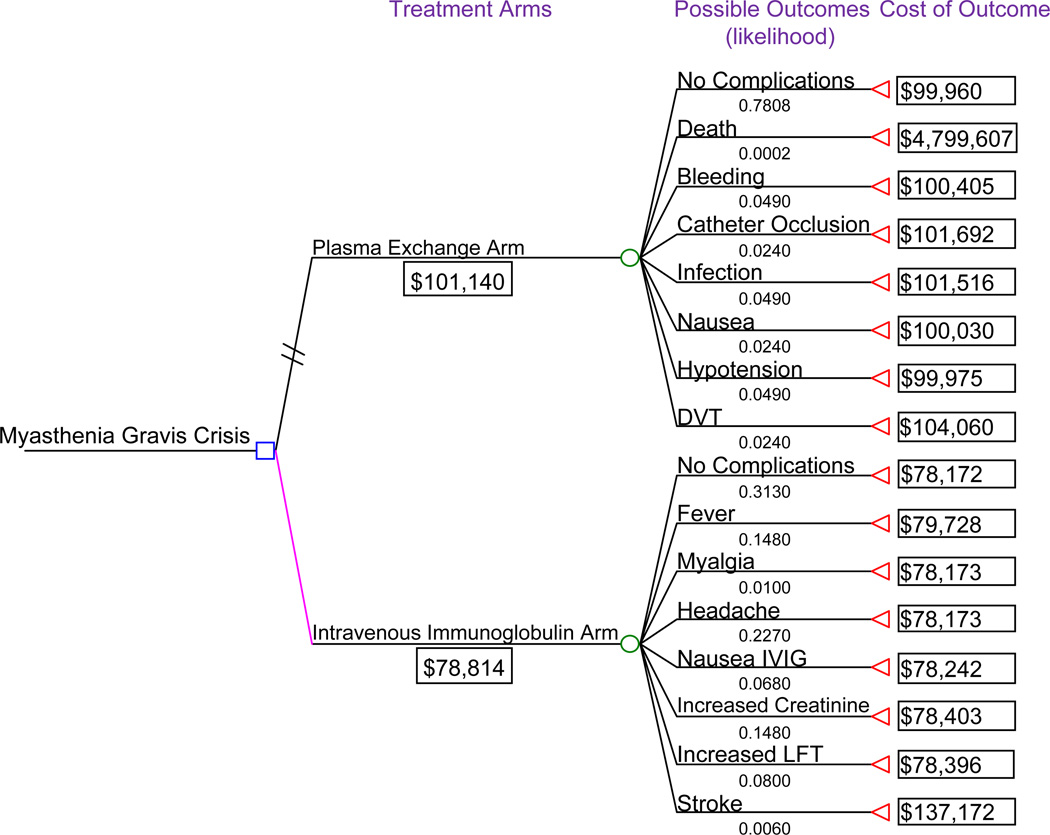
Using the above data, the average short term cost for utilizing plasma exchange for MGC was $101,140 per patient compared to IVIG which accrued an average cost per patient of $78,814. The TreeAge 4.0 cost analysis tree for these calculations is demonstrated in Figure 1. The total cost for both IVIG and PLEX utilized the cost of all possible outcomes for each treatment and the likelihood that each of these outcomes occur. The average total difference in cost favored the IVIG arm with an estimated savings of $22,326 per patient.
Figure 1.
Cost decision tree of IVIG vs. plasma exchange. Estimated total costs are provided for possible outcomes associated with each treatment arm. The average estimated cost for using each therapy ($101,140 for plasma exchange and $78,814 for IVIG) takes into account the cost and likelihood of occurrence of each specific outcome associated with the treatment.
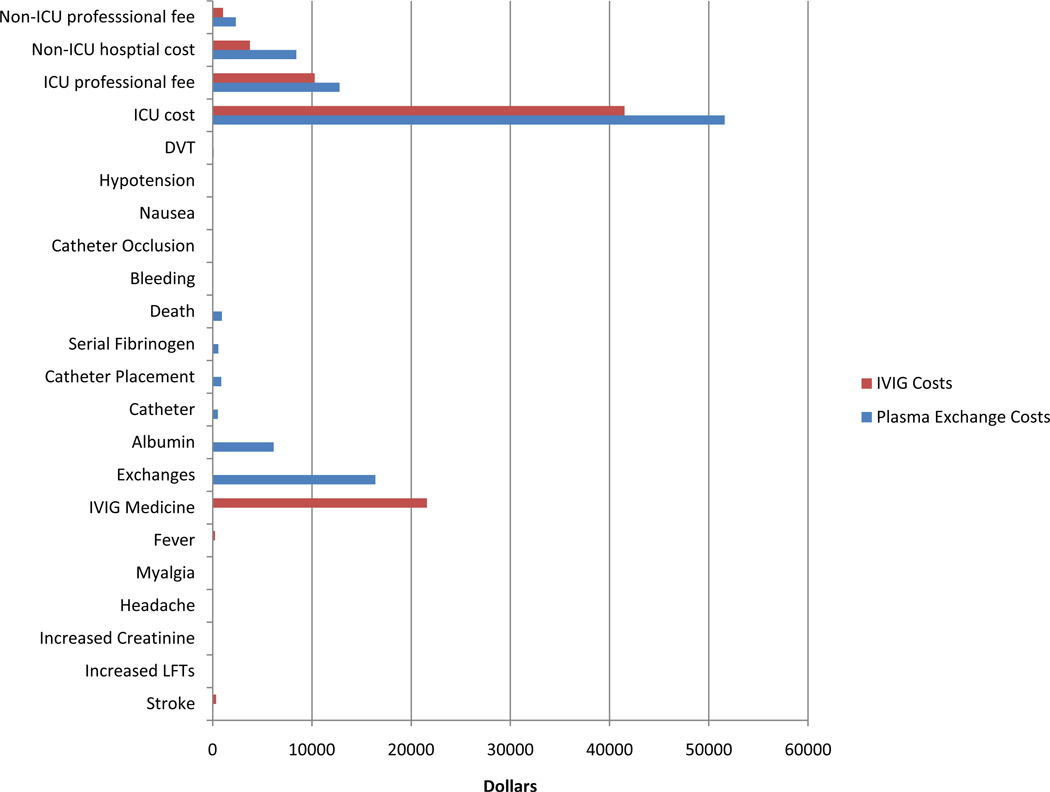
For the IVIG therapy arm the greatest cost was for room and care in the intensive care unit (Figure 2). At $21,581 the purchasing cost of IVIG comprised a significant portion of the total cost of implementing IVIG therapy. Although side effects with IVIG are reportedly common, the cost of treating these side effects was found to be low relative to other IVIG expenses.
Figure 2.
Average itemized cost of treating a myasthenia gravis crisis patient with IVIG ($78,814 per patient) vs. Plasma Exchange ($101,140 per patient). For IVIG, the two greatest costs are the fees associated with patient time spent in an intensive care unit and the purchase price of IVIG. For Plasma Exchange, the two greatest costs are the fees associated with patient time spent in an intensive care unit and the direct costs associated with implementing plasma exchange.
For plasma exchange, the greatest cost was for room and care in the intensive care unit (ICU) (Figure 2). Because patients receiving PLEX spent more time in the ICU, these costs were comparatively higher than those in the IVIG arm. Exchange costs, non-ICU hospital care and room fees, professional fees, and the cost for albumin also produced a significant cost burden. Similarly to IVIG, costs secondary to side effects were relatively insignificant for this treatment arm.
Sensitivity analysis demonstrated that variations in several key variables significantly change the cost comparison of IVIG and PLEX for MGC. Specifically, the number of plasma exchanges required, the time a patient is required to be in the ICU, and IVIG dosing were found to be three of the most critical MGC treatment cost variables.
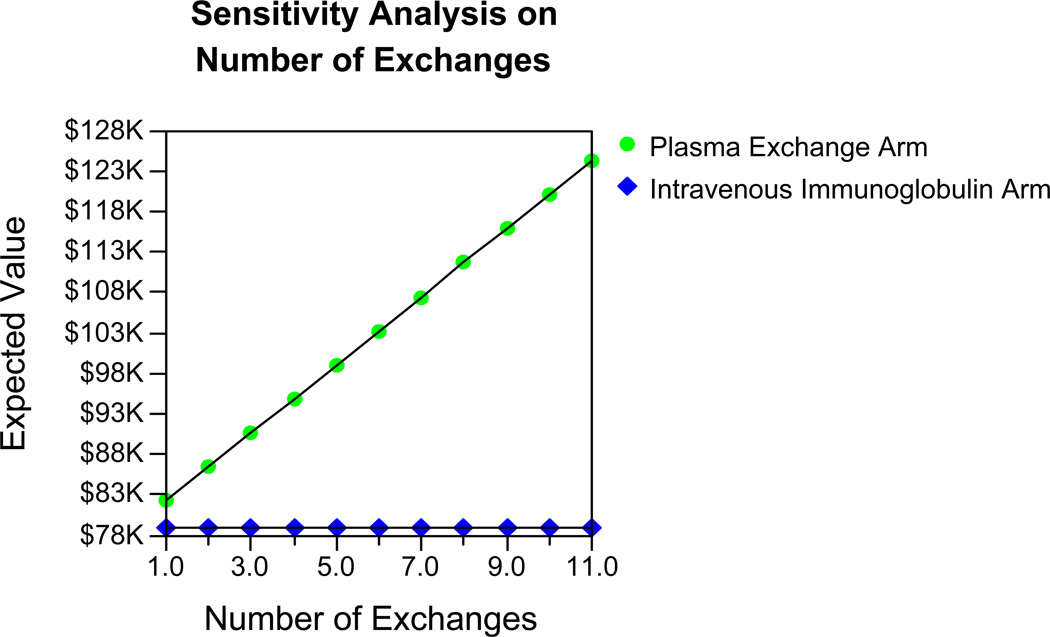
Historically, most MGC patients receive 4–6 exchanges; however, clinically this number can vary.11 A one-way sensitivity analysis of this specific variable demonstrated that the price of utilizing PLEX for MGC approaches (but does not equal) IVIG as the number of required exchanges becomes less (Figure 3).
Figure 3.
The comparative costs of IVIG vs. PLEX treatment of myasthenia gravis crisis based on the number of therapeutic plasma exchanges required.
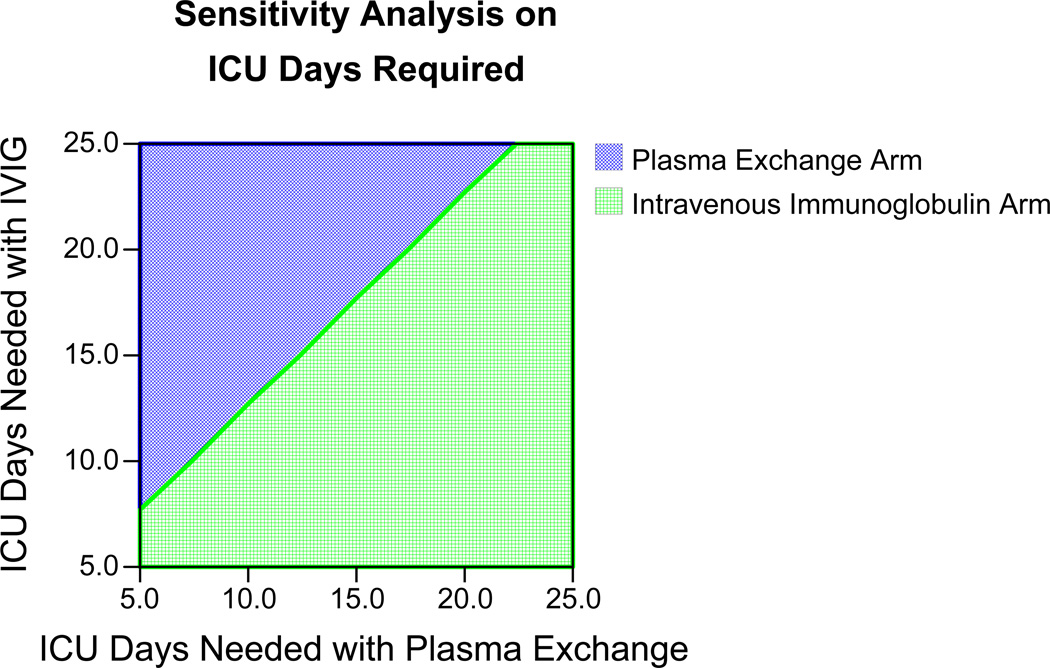
Time spent in the ICU was also a critical variable in comparing the cost of treating MGC patients. A two-way sensitivity analysis of required ICU time for both PLEX and IVIG arms demonstrated that the comparative cost of MGC therapy is highly dependent on this variable (Figure 4). In general, with equivalent or less ICU time, IVIG therapy was found to be the more cost saving therapy.
Figure 4.
Two-way sensitivity analysis of PLEX vs. IVIG for myasthenia gravis based on ICU days associated with each therapy. PLEX is more cost effective for all points in blue whereas IVIG is more cost effective for all points in green. The associated ICU times used for the cost calculations in this study were 17.4 days for PLEX and 14 days for IVIG therapy.11
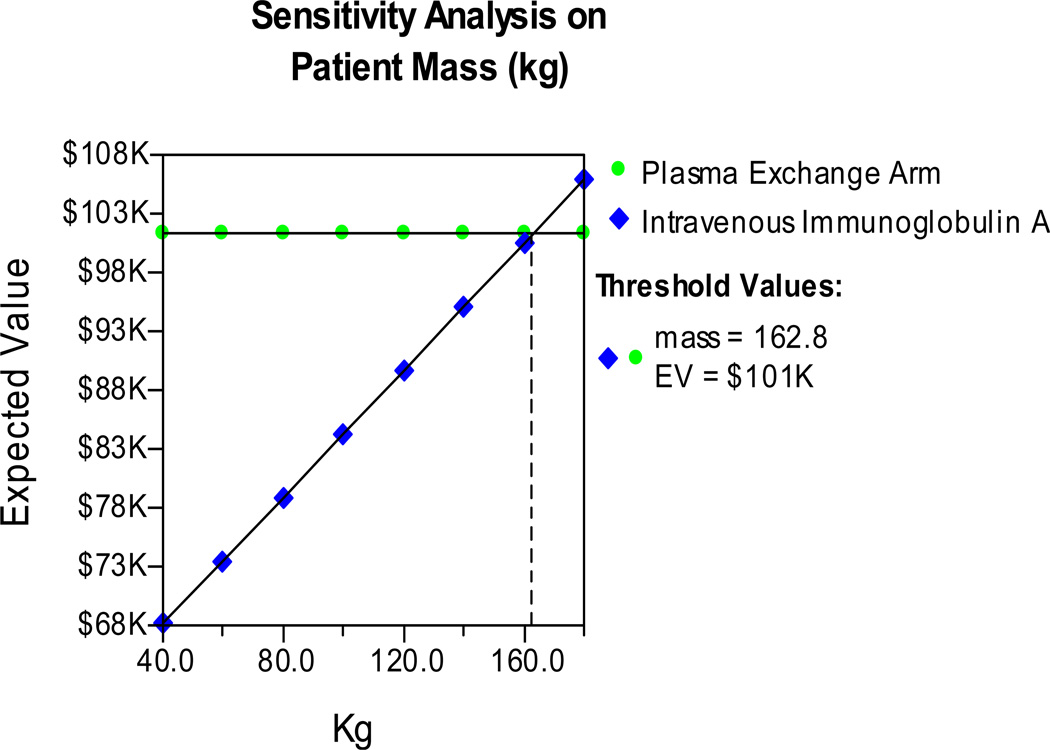
Lastly, a sensitivity analysis on the mass of the myasthenia gravis patient was performed (Figure 5). Dosing of IVIG is based on patient mass. This sensitivity analysis demonstrated that plasma exchange may become more economical than IVIG based on this variable alone, but only at patient masses over 162.8 kg. As most clinicians dose IVIG using ideal and not actual body weight, it is unlikely that IVIG dosing would frequently reach this level for MGC patients.
Figure 5.
The comparative costs of IVIG vs. PLEX treatment of myasthenia gravis crisis based on the mass of the patient and subsequent IVIG dosing (total dose 2.0 g/kg).
DISCUSSION
Here we estimate the short term financial costs of treating a patient in myasthenia gravis crisis (MGC) with intravenous immunoglobulin (IVIG) versus plasma exchange (PLEX). Through this research we identified several key variables that play a major role in determining the overall financial costs of these two effective MGC therapies. Given our model, we found that the use of IVIG for myasthenia gravis crisis is a more cost minimizing therapy compared to plasma exchange. Although other studies have retrospectively evaluated the cost of treating MGC, to the authors’ knowledge this is the first to systematically perform sensitivity analysis to determine which variables have the greatest impact on the relative cost of PLEX and IVIG for MGC. The identification of critical MGC cost variables has the potential to: 1) Reduce the cost of taking care of MGC patients; 2) Identify mechanisms for reducing the cost burden of IVIG and PLEX; and, 3) Assist clinicians who must select a MGC therapy in cases where one treatment is not clearly clinically indicated over the other.
The discrepancy in cost between IVIG and PLEX was largely due to the extended ICU and hospital stays associated with plasma exchange, and the high cost of performing plasma exchanges and purchasing albumin. In 2010 a retrospective study of MGC patients suggested that there may be a cost benefit for using IVIG for MGC; the results from our study support these findings. 12
Clinically, plasma exchange has been shown to provide a short term benefit in case series of myasthenia patients, especially those in crisis.4 In 2000, 79 of 94 myasthenia gravis patients received plasma exchange for poor clinical control, presurgical preparation, or crisis with all groups demonstrating improved muscle scores.5,6
Similarly, IVIG has been demonstrated to have a short term clinical benefit for progressive myasthenia gravis. Fifty-one patients with worsening myasthenia gravis were given either IVIG or IV dextrose. At 14 and 28 days the patients who received IVIG had a clinical improvement in their disability scores compared to dextrose.7
Although both IVIG and PLEX have been shown to be beneficial for MGC, comparison trials have failed to demonstrate a large clinical difference between these two therapies. In 1997 Gajdos et al. studied 87 patients with myasthenia gravis exacerbations. Patients were randomized to either plasma exchange or IVIG. There was no statistical difference in muscle scores after 14 days between these two groups.9 In 2001, a crossover trial of 12 patients showed no difference in quantified MG clinical score between plasma exchange and IVIG after one and 4 weeks; however, both therapies demonstrated improvements over baseline at the 4 week mark.10 Although the selection of a MGC therapy should never be determined solely on cost, its consideration in situations of clinical equipoise may be justified.
One of the major comparative components of cost was hospital and ICU stay length. To the authors’ knowledge there are only three studies comparing the hospital stays of IVIG and plasma exchange in this population.8,11,12 Only one of these studies adequately reported both ICU and hospital times for MGC patients receiving PLEX and IVIG and none of these studies were randomized. Data from this study documented 54 cases of MGC from four University Centers (Emory University Hospital, the Medical College of Virginia, Johns Hopkins Hospital, and the Medical College of Wisconsin) between the years 1990 and 1997.11 Although one may surmise that IVIG use may be associated with shorter hospital stays, this may not be the case and should be further investigated in future studies. It is possible that the dates of the studies, geographic location, and hospital policy (some centers require patients receiving PLEX to be monitored in an ICU setting) played a role on reported hospital stay times. Although IVIG patients statistically had a reduced ICU stays, we do not know the decision process in how patients were assigned IVIG rather than PLEX. It is possible that the “sicker” patients received one therapy over the other. Indeed, in the Qureshi study, 18/28 (64%) of PLEX patients were intubated before initiation of therapy compared to 15/26 (58%) of IVIG patients. However, after initiation of therapy, 5/10 (50%) of PLEX patients were intubated compared to only 4/11 (36%) of IVIG patients. 11 Therapy administration also likely played a role in the longer hospitalization stays associated with PLEX. In the Qureshi and Murthy studies PLEX was given for 5–6 cycles on an alternate day basis while IVIG was given daily for a total of 5 days. 8,11 Dosing schedules for both IVIG and PLEX can vary. These dosing schedules likely played a key role in longer reported hospital stays with PLEX and may be a cost modifying variable to be evaluated in future studies.
The limitations of this study are similar to any study evaluating a rare disease using historical data. Assigning costs to treating specific complications can lead to cost variability. The standard-of-care for treating side effects varies between clinicians and centers. The protocols and formulas utilized in this study represented the reviewed literature and the authors’ best judgment for proper clinical care. It should be noted that the side effect profiles for IVIG and PLEX were taken from prospective studies performed in different years.13,14 Although we would not expect side effects to markedly change over time, it is certainly possible that they could. Also, two known side effects of IVIG; namely deep vein thrombosis and aseptic meningitis were not reported in the IVIG side effect paper, and a two day dosing schedule was utilized in this study. IVIG regimens lasting from two to five days are commonly used. Similarly, pneumothorax during line placement for PLEX did not occur in the Gajdos paper; although this may periodically occur.
The exact specific costs for certain side effects (namely stroke) are not well known in the MGC population. Sullivan’s estimate was subsequently used to estimate the cost of IVIG induced stroke.17 Other costs, such as the treatment of rash, were excluded from the overall cost analysis as they frequently occur outside of the acute hospitalization and produce a comparably low cost burden. For other treatment costs (such as for headache and increased creatinine levels) our best estimation of treatment cost was used; however, it is fully recognized that in some instances the severity of a side effect may require varying management strategies that exceed the base costs we assigned. As for other costs, these represent local billing procedures, medicine costs, and brands used at a tertiary center in upstate New York. These costs may vary across centers and geographical locations.
Some basic assumptions were utilized for our model. It was assumed that patients would either experience one side effect or no side effects. Although the literature does not adequately quote the percentage of MGC patients who have more than one side effect, this could potentially occur in a crisis population. It is unlikely that this assumption affected the final result significantly as the itemized analysis demonstrated that the cost of side effects for each therapy was marginal compared to other stated costs. Ultimately, additional studies will be needed to know the exact incidence and prevalence of side effects associated with the treatment of MGC patients.
The variables identified through sensitivity analysis reflect some key points of our research. The major costs of crisis therapies are highly dependent on the number of exchanges, average ICU and hospital time, patient mass, as well as the cost of the exchanges, albumin, and IVIG. It is possible that as the cost of exchanges or albumin decreases, or as more peripheral veins are utilized for PLEX in place of central venous catheters, plasma exchange may become a less expensive option. Similarly, it is also possible that as methods used to extract IVIG become more efficient, the cost of this therapy could drop. The effects of the national and international market on these products and services could also ultimately affect treatment costs. Currently the short term cost assessment favors IVIG; however if in the future plasma exchange is found to dramatically reduce ICU and hospital stays over IVIG (a finding not clearly supported by prior studies) the cost comparison between these two therapies would change. Furthermore, the time frame of this research concentrated only on a patient’s immediate hospital visit. It is possible that there is a difference in re-admission rates for loss of myasthenia control based on the type of treatment received. An increased re-admission rate with IVIG would certainly change the overall cost analysis of this therapy. Additional prospective studies will be helpful to compare the extended societal cost, and more importantly the long term efficacy of IVIG vs. PLEX for MGC patients.
Supplementary Material
Acknowledgments
Dr. Heatwole receives grant support through the NIH (1K23AR055947), the New York State Empire Clinical Research Investigator Program (ECRIP), and the Muscular Dystrophy Association. Dr. Heatwole was also the recipient of a previous Clinical and Translational Science Institute Award and NIH Experimental Therapeutics for Neuromuscular Diseases Grant through the University of Rochester.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McGrogan A, Sneddon S, Vries C. The Incidence of Myasthenia Gravis: A Systematic Literature Review. Neuroepidemiology. 2010;34:171–183. doi: 10.1159/000279334. [DOI] [PubMed] [Google Scholar]
- 2.Alshekhlee A, Miles JD, Katirji B, Preston DC, Kaminski HJ. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology. 2009;72(18):1548–1554. doi: 10.1212/WNL.0b013e3181a41211. [DOI] [PubMed] [Google Scholar]
- 3.Donofrio PD, Berger A, Brannagan TH, 3rd, et al. Consensus statement: The use of intravenous immunoglobulin in the treatment of neuromuscular conditions report of the AANEM ad hoc committee. Muscle Nerve. 2009;40(5):890–900. doi: 10.1002/mus.21433. [DOI] [PubMed] [Google Scholar]
- 4.Gajdos P, Chevret S, Toyka K. Plasma exchange for myasthenia gravis. Cochrane Database Syst Rev. 2002;4(4) doi: 10.1002/14651858.CD002275. CD002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu HC, Chen WH, Yeh JH. The six year experience of plasmapheresis in patients with myasthenia gravis. Ther Apher. 2000;4(4):291–295. doi: 10.1046/j.1526-0968.2000.004004291.x. [DOI] [PubMed] [Google Scholar]
- 6.The utility of therapeutic plasmapheresis for neurological disorders. Natl Inst Health Consens Dev Conf Consens Statement. 1986;6(4):1–7. [PubMed] [Google Scholar]
- 7.Zinman L, Ng E, Bril V. IV immunoglobulin in patients with myasthenia gravis: A randomized controlled trial. Neurology. 2007;68(11):837–841. doi: 10.1212/01.wnl.0000256698.69121.45. [DOI] [PubMed] [Google Scholar]
- 8.Murthy JM, Meena AK, Chowdary GV, Naryanan JT. Myasthenic crisis: Clinical features, complications and mortality. Neurol India. 2005;53(1):37–40. doi: 10.4103/0028-3886.15050. discussion 40. [DOI] [PubMed] [Google Scholar]
- 9.Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. myasthenia gravis clinical study group. Ann Neurol. 1997;41(6):789–796. doi: 10.1002/ana.410410615. [DOI] [PubMed] [Google Scholar]
- 10.Ronager J, Ravnborg M, Hermansen I, Vorstrup S. Immunoglobulin treatment versus plasma exchange in patients with chronic moderate to severe myasthenia gravis. Artif Organs. 2001;25(12):967–973. doi: 10.1046/j.1525-1594.2001.06717.x. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi AI, Choudhry MA, Akbar MS, et al. Plasma exchange versus intravenous immunoglobulin treatment in myasthenic crisis. Neurology. 1999;52(3):629–632. doi: 10.1212/wnl.52.3.629. [DOI] [PubMed] [Google Scholar]
- 12.Mandawat A, Kaminski H, Cutter G, et al. Comparative Analysis of Therapeutic Options Used for Myasthenia Gravis. Ann Neurol. 2010;68:797–805. doi: 10.1002/ana.22139. [DOI] [PubMed] [Google Scholar]
- 13.Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. myasthenia gravis clinical study group. Ann Neurol. 1997;41(6):789–796. doi: 10.1002/ana.410410615. [DOI] [PubMed] [Google Scholar]
- 14.Gajdos P, Tranchant C, Clair B, et al. Treatment of myasthenia gravis exacerbation with intravenous immunoglobulin: A randomized double-blind clinical trial. Arch Neurol. 2005;62(11):1689–1693. doi: 10.1001/archneur.62.11.1689. [DOI] [PubMed] [Google Scholar]
- 15.Caress JB, Cartwright MS, Donofrio PD, Peacock JE., Jr The clinical features of 16 cases of stroke associated with administration of IVIg. Neurology. 2003;60(11):1822–1824. doi: 10.1212/01.wnl.0000068335.01620.9d. [DOI] [PubMed] [Google Scholar]
- 16.Korach JM, Petitpas D, Paris B, et al. Plasma exchange in france: Epidemiology 2001. Transfus Apher Sci. 2003;29(2):153–157. doi: 10.1016/S1473-0502(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan PW, Arant TW, Ellis SL, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics. 2006;24(10):1021–1033. doi: 10.2165/00019053-200624100-00009. [DOI] [PubMed] [Google Scholar]
- 18.Viscusi. The value of life: Estimates with risks by occupation and industry. Economic Inquiry. 2004;42(1):29–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.