Abstract
Objective This study examines the role of community-based health insurance (CBHI) in influencing health-seeking behaviour in Burkina Faso, West Africa. Community-based health insurance was introduced in Nouna district, Burkina Faso, in 2004 with the goal to improve access to contracted providers based at primary- and secondary-level facilities. The paper specifically examines the effect of CBHI enrolment on reducing the prevalence of seeking modern and traditional methods of self-treatment as the first choice in care among the insured population.
Methods Three stages of analysis were adopted to measure this effect. First, propensity score matching was used to minimize the observed baseline differences between the insured and uninsured populations. Second, through matching the average treatment effect on the treated, the effect of insurance enrolment on health-seeking behaviour was estimated. Finally, multinomial logistic regression was applied to model demand for available health care options, including no treatment, traditional self-treatment, modern self-treatment, traditional healers and facility-based care.
Results For the first choice in care sought, there was no significant difference in the prevalence of self-treatment among the insured and uninsured populations, reaching over 55% for each group. When comparing the alternative option of no treatment, CBHI played no significant role in reducing the demand for self-care (either traditional or modern) or utilization of traditional healers, while it did significantly increase consumption of facility-based care. The average treatment effect on the treated was insignificant for traditional self-care, modern self-care and traditional healer, but was significant with a positive effect for use of facility care.
Discussion While CBHI does have a positive impact on facility care utilization, its effect on reducing the prevalence of self-care is limited. The policy recommendations for improving the CBHI scheme’s responsiveness to population health care demand should incorporate community-based initiatives that offer attractive and appropriate alternatives to self-care.
Keywords: Treatment seeking, community-based health insurance, econometrics, health care utilization, informal sector
KEY MESSAGES.
Community-based health insurance plays a substantial role in increasing access to facility-based care, but an insignificant role in reducing unsupervised self-treatment of acute illnesses.
Community financing schemes may be ineffective in limiting consumption of services and medication accessed from the informal health sector.
Introduction
In recent years, the role of community-based health insurance (CBHI) in improving access to the formal health sector in low-income countries has been widely discussed (Russell et al. 1995; Mathiyazaghan 1998; Preker et al. 2002; Ekman 2004; Jütting 2004; Dror et al. 2007; Gnawali et al. 2008). The introduction of prepayment schemes in such settings aims to meet several goals, often with the intention of crossing several hurdles with one leap. Two explicit objectives of community financing schemes include the reduction of financial barriers to appropriate care and an increase in accessibility to contracted facility-based care (Preker et al. 2002; Ekman 2004; Jütting 2004; Smith and Sulzbach 2008). Yet a third implicit objective is rarely discussed. By improving access to and increasing utilization rates for the formal health sector, such interventions may have the ability to reduce consumption of informal, often ill-advised care through self-medication and treatment within the household.
Rationale for community-based health insurance
In many resource-poor settings, access to affordable and appropriate health care remains a major concern, with out-of-pocket expenditure for health care a major cause of impoverishment (Meessen et al. 2003; Frenk et al. 2006; McIntyre et al. 2006). One way to facilitate access to care and reduce unpredictable expenditure patterns is through an insurance mechanism, whereby risks are shared and financial inputs pooled (Abel-Smith and Dua 1988; Bennett and Gilson 2001; Carrin et al. 2005). Yet in low-income countries, the majority of the population works in the informal sector, with an insufficient tax base to develop formal health insurance schemes (Carrin et al. 2005). Community-based health insurance (CBHI) has been seen as an attractive solution to this problem and an alternative to out-of-pocket payments which have major consequences for the poor (Ekman 2004; Jütting 2004). Through enrolment in community financing schemes, payment is disassociated from the use of the health services, creating a financial buffer between service fees and seasonal fluctuations in income (Diop et al. 2000; McCord 2000; Preker et al. 2002). This is important principally in sub-Saharan Africa, where a large proportion of the population is active in rain-fed agriculture.
Hazardous medical care within the informal sector is of particular concern in sub-Saharan Africa, where infectious diseases such as malaria, diarrhoeal diseases and lower-respiratory infections still constitute the primary burden of illness (WHO 2008) and are commonly treated through unsupervised self-medication. Such treatment often leads to delays in accessing rational, appropriate care within the formal health sector, to over-prescribing and at times can even be life-threatening for childhood illnesses (Beiersmann et al. 2007). Until now, there has been limited discussion on how enrolment in community financing schemes affects the consumer’s decision to seek other methods of care, such as self-treatment and traditional healers.
The prevalence of self-treatment in low-income settings
Evidence suggests that self-treatment, or the seeking and consuming of unregulated care outside the formal health sector, can play a primary role in delaying access to professional medical attention (Kaseje et al. 1987; Ejov et al. 1999). Self-treatment has been previously defined as any treatment that does not involve consulting a health care provider or traditional healer (McCombie 2002). Modern methods of self-treatment include purchasing western medicine at local markets, private pharmacies or public pharmacies without the consultation of a health care provider. Methods also include informal consultations by pharmacists and local medicine sellers, as well as the use of leftover medication from previous consultations at health facilities. Traditional methods of self-care involve utilization of pharmacopoeia products, such as local plant and herbal remedies that are either prepared by members of the household or purchased from local suppliers. In Burkina Faso, traditional treatments usually comprise oral and/or skin applications of extracts from eucalyptus plants, acacia, citronella, papaya, guava and the neem tree (Okrah et al. 2002).
Often, including in the case of malaria, self-treatment with western medicine can be inappropriate and sub-optimal (McCombie 1996; Théra et al. 2000; Deressa et al. 2003; Muller et al. 2004), leading to the development of drug-resistant parasite strains and adverse health effects due to inappropriate dosages (WHO 2000; Malaria Knowledge Programme 2005). Equally hazardous, use of traditional medicine has been linked to delays in seeking formal care and at times can be life threatening (Beiersmann et al. 2007). Studies from countries in sub-Saharan Africa present self-treatment of malaria rates ranging from 4% to 87% (McCombie 1996). Socio-economic and demographic factors are often related to self-treatment, but vary greatly from country to country. Self-treatment with western medicine has been linked to high socio-economic status in Bombay, India (Kamat and Nichter 1998), low socio-economic status in Kerala, India (Saradamma et al. 2000), and to males and single people in Nigeria (Brieger et al. 1986). High prevalence of self-treatment with western medicine has also been linked to urban areas in Kenya (Brinkmann and Brinkmann 1991) and Ghana (Agyepong and Manderson 1994). In Burkina Faso, the most common reason for self-treatment was confidence in treating the disease (Mugisha et al. 2002).
Objective
This study examines the role of CBHI in influencing health-seeking behaviour in Burkina Faso, West Africa. Given the fact that CBHI schemes aim to improve access to formal, facility-based care, yet often operate within an environment where self-treatment remains highly prevalent, a central question is: ‘does CBHI play a central role in reducing self-medication within the informal health sector, particularly as a first choice in care for acute cases attributed to common infectious disease?’ For the first analysis, we investigate factors that influence the household decision to enrol in the insurance scheme. Second, controlling for observable differences in the two populations, we assess the role of enrolment in CBHI in the decision to seek various options of care. These options include no care at all, self-care (either traditional or modern methods), use of traditional healers and use of formal, public sector health facilities. Of particular interest to our study, we test the hypothesis that by improving access and increasing utilization rates for formal facility care, the CBHI scheme in Nouna district significantly reduces the prevalence of self-treatment among the insured population.
Methods
Study site
Nouna health district is located in northwest Burkina Faso in an area of dry orchard savannah, populated almost exclusively by subsistence farmers from five primary ethnic groups. The district has a population of approximately 304 000 living in 290 villages, who are served by 34 primary care health facilities and one secondary-level district hospital. The average distance to the closest primary care facility is 9.56 km (round-trip), slightly higher than the regional average of 8.25 km and the national average of 7.69 km (Ministère de la Santé 2007). The town of Nouna is the economic and political centre of Kossi province, lying roughly 300 km from the capital Ouagadougou. This study was carried out in the sub-portion of Nouna health district that is currently under demographic surveillance. The area covered includes over 85 000 inhabitants living in 58 villages and in Nouna town, including the catchment zone of Nouna hospital and 14 primary care clinics (Centre de Santé et de Promotion Sociale, CSPS).
Community-based health insurance in Nouna district
In early 2004, a CBHI scheme, Assurance Maladie à Base Communautaire (AMBC), was introduced in Nouna district. The household is the unit of enrolment for the scheme (candidates interested in enrolling can only join if they include the entire household), while premiums are paid annually and are set at the individual level. Children (≤14 years of age) pay 500 francs CFA, while adults (>14 years of age) pay 1500 francs CFA (US$ 1 = 500 francs CFA). Both outpatient services offered at primary care facilities (CSPS) and up to 15 days of inpatient care at the district hospital are covered, as well as all essential medicines offered at public facilities. There is no co-payment, ceiling or limit on number of services rendered, and members are assigned to one facility based on geographical location. At present, private clinics and pharmacies have not been included as contracted providers for the scheme. In order to avoid overprovision and overuse of services, health care providers are paid on a capitation basis, receiving a set amount of money for each member enrolled in CBHI, irrespective of whether he/she accesses services. This payment mechanism is applied to all 14 contracted CSPS, as well as the district hospital.
Since the introduction of CBHI in Nouna district, utilization rates for public facilities have increased substantially for the enrolled population (Gnawali et al. 2009). However, the district enrolment rate remains relatively low in comparison with pre-intervention estimates of 50% (Dong et al. 2003), while drop-out rates continue to be high. In 2009, the enrolment rate reached 8.6% of the target population, an increase from 7.2% in 2008 and 5.2% in 2006, the first year that CBHI was offered to the entire target population. In 2006, the drop-out rate rose to 45.7%, before reducing to 16.7% in 2007. According to the 2007 household survey, 28.4% of households that dropped out did so due to affordability reasons, while 32.7% dropped out due to quality of care concerns. These reasons include staff behaviour (19%), satisfaction with services (7.4%) and quality of drugs (6.3%).
Indirect costs incurred in order to benefit from CBHI services may also play a role in the low enrolment and high drop-out rates. Physical inaccessibility, caused either by a lack of transportation or by geographic barriers in the rainy season for remote, isolated villages, has resulted in poorer enrolment rates for lower socio-economic level and rural households. Long waiting time, limited hours of operation and insufficient numbers of providers also play a role in the limited accessibility of the CBHI benefit package, particularly for low-income clients who cannot afford to lose time waiting for services.
Data sources
The primary source of data was the 2007 Nouna District Household Survey (collected in April/May 2007), administered to a statistically representative sample of all households residing in the DSS area. The survey gathered relevant socio-demographic characteristics, health status and health-seeking behaviour, insurance membership, perceptions of quality of care and social capital/community networking characteristics. For health status and health-seeking behaviour data, a 1-month recall period was used to collect respondents’ information. The sample size was estimated in advance to have a 90% power of detecting an increase in health service utilization of one visit per year between insured and uninsured households, assuming a 2-sided Type 1 error probability of 0.05 and, given the results of the prior willingness-to-pay study, an enrolment of at least 50% (Dong et al. 2003). It was estimated that a sample size of 378 households would be sufficient (189 per intervention arm) to detect differences between insured and uninsured. Given cluster randomization, however, a design factor of 2.16 was applied to adjust for intra-cluster correlation. The minimum sample size agreed was 990 households across all 33 clusters, with 606 households selected in the rural area and 384 in the town of Nouna (De Allegri et al. 2008).
Statistical methods
For the first stage of analysis, we applied propensity score matching for the explanatory variable enrolment status, allowing us to control for observable differences between the insured and uninsured population. This technique allows us to make use of the information in two unlinked data sets. Secondly, we estimated the direct effect of insurance enrolment on the decision to seek treatment from available care options, which was done through matching on observable characteristics and estimating the average treatment effect on the treated (ATT). Finally, we described the various factors that influence different health-care user groups (no care, traditional and modern self-care, traditional healer, modern facility care) for the ill population (n = 1247). This was accomplished by estimating a health care demand model through multinomial logistic regression. STATA 10 was used for all statistical analyses.
Since we were primarily concerned with selection of CBHI enrolment based on observable characteristics, we adopted the propensity score matching technique to control for differences between the two populations. We hypothesized that there are inherent differences in the enrolled and non-enrolled populations, and these differences should be accounted for in the analysis of health-seeking behaviour. If X is the vector of observed covariates for a particular household, and binary variable Y is whether the household was insured (y = 1) or uninsured (y = 0) in the year of interest, then the propensity score can be written as:
This formula estimates the probability of enrolment conditional on the covariates X. Insured households and uninsured households that have the same e(X) value will have the same distribution of X; formally Y and X are conditionally independent given e(X) (Rosenbaum and Rubin 1985). The propensity scores were estimated by means of logit, using the Kernel matching method to estimate the ATT (Caliendo 2005).
Adjusting for the scalar propensity score fully removes all bias due to observables. As a result, most of the differences in the outcome of interest (type of treatment sought) can then be attributed to the intervention (CBHI enrolment) itself. Known as the ATT, this difference in the outcome of interest among the treatment group (in this case the insured population) can be estimated to see if it is statistically different from zero (Caliendo 2005). This method has been used to evaluate the impact of health insurance in Vietnam, China (Wagstaff 2009; Wagstaff et al. 2009) and Colombia (Trujillo et al. 2005). Finally, by using estimated propensity scores as an explanatory variable in the multinomial logistic (MNL) model, observable characteristics at the individual level can be simplified into one single score.
As the household is the unit of enrolment in Nouna’s CBHI scheme and previous studies have shown the household head as a prominent voice in the decision to enrol (de Allegri et al. 2006b), the logit model for the propensity of enrolling in CBHI used explanatory variables at the household level wherever possible. Household characteristics included household size, expenditure quintile, urban/rural residency, share of household under 5 years of age (as children are more prone to illness and consumption of various types of care) and distance to the assigned health facility (round-trip distance in kilometres). Household head characteristics included whether the head was literate, male or married (categorical 1–5 Likert scale, 1 = very bad, 5 = very good). Individual characteristics included religion, ethnicity and completion of primary school education.
Once propensity scores and the ATT were estimated, the MNL model was then used to estimate the relationship between utilization of available care options and various individual and household characteristics. The MNL model can be expressed as follows:
and
where for the ith individual, yi is the observed outcome and 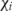 is the vector of explanatory variables (Kennedy 2008). All relative risk ratios for care options are in relation to no care, as such a model best fits the reality of decision-making patterns for choosing the first type of care sought. While it may be of particular interest to investigate statistical differences between different care-seeking sub-groups (such as those who choose modern self-medication vs those who choose facility-based care), such an investigation is not within the scope of this analysis.
is the vector of explanatory variables (Kennedy 2008). All relative risk ratios for care options are in relation to no care, as such a model best fits the reality of decision-making patterns for choosing the first type of care sought. While it may be of particular interest to investigate statistical differences between different care-seeking sub-groups (such as those who choose modern self-medication vs those who choose facility-based care), such an investigation is not within the scope of this analysis.
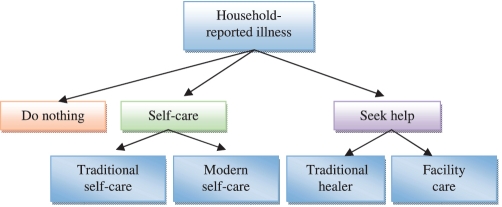
At the individual (i.e. the care-seeking person) level, insurance status, distance, employment status, literacy, expenditure quintile, age quartile, marital status, perceived severity of illness, urban residence, perceived quality of facility-based care, whether the illness was acute or not, and the propensity score were include as explanatory variables. Certain household head characteristics were also included, such as literacy level, sex and marital status. These attributes were included in the model because in Burkina Faso the household head is usually a prominent voice in making health care-seeking decisions within the household. Illness episodes are classified as being either acute or chronic according to the list of illnesses defined by the household survey. Examples of chronic illnesses include diabetes, haemorrhoids, hernia and general long-term body pains, while acute illnesses include malaria, diarrhoeal diseases, respiratory infections and fever. The health-seeking behaviour modelled in the MNL model is described in Figure 1.
Figure 1.
The decision-making process for seeking care Note: This figure presents a simplified decision-making tree highlighting options for care within Nouna health district. It should be noted that for any illness case, individuals may choose more than one care option during the search for treatment. Care-seeking pathways are not mutually exclusive
Limitations and alternative methods
There are several limitations to the methodology applied in this study. First, it should be noted that although propensity scores can balance observed baseline covariates between exposure groups, they do nothing to balance unmeasured characteristics and confounders. Thus one limitation is that remaining unmeasured confounding may be present. Secondly, the use of propensity scores does not overcome initial selection bias (Wolfgang and Winkelmayer 2004).
Two methods were used to control for unobservable differences in the two populations. First, the Heckman two-stage estimator for selection bias was applied (Heckman 1979). This correction method addresses the potential problem of non-random selection into the sample for the enrolled population. We used two variables to control for selection bias: ‘insurance rollout cluster phase’ and ‘chief enrolment’ into the scheme during the first 3 years (2004–06). Results from the Heckman model provided evidence that there was no significant selection bias present.
Secondly, instrumental variable (IV) estimation was applied to correct for potential endogeneity of insurance enrolment in the health care decision. If an individual or household exhibits a certain preference to utilize facility-based health care, this may have a direct effect of increasing their propensity to enrol in the insurance scheme. Simultaneously, enrolment in the insurance scheme will most likely lead to a higher probability of utilizing facility-based care. This problem of omitted variable bias (OVB), where certain individuals may have a personal preference for modern, facility-based care, could potentially lead to an overestimation and upward bias of the direct effect of insurance enrolment on use of facility care. We used ‘cluster phase’ and ‘chief enrolment’ as instruments to correct for this potential endogeneity. The instruments had only limited power in the first stage, which makes the interpretation of the IV estimates difficult, and were therefore dropped from the final analysis. Results of the two alternative methods are not presented in this paper.
Results
Table 1 presents the illness distribution by insurance group. Roughly 9% of 13 932 individuals with full information reported an illness episode during the 1-month recall period. The average number of illness episodes among respondents who reported an illness was 1.05 episodes for chronic illnesses and 1.01 for acute illnesses. The incidence of chronic illnesses was higher among uninsured adults (4.81% for insured adults and 4.92% for uninsured adults), with no significant difference between the two groups when the entire sample is considered.
Table 1.
Distribution of illness by enrolment status
| Illness | Children (<15 years) |
Adults (≥15 years) |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Insured (n = 727) | Uninsured (n = 3779) | Pearson chi-square | Insured (n = 1550) | Uninsured (n = 7856) | Pearson chi-square | Insured (n = 2277) | Uninsured (n = 11 655) | Pearson chi-square | |
| Acute (%) | 6.22 | 4.43 | 4.46* | 7.10 | 5.51 | 6.18* | 6.85 | 5.17 | 10.52** |
| Chronic (%) | 1.22 | 0.88 | 0.76 | 4.81 | 4.92 | 7.19* | 3.65 | 3.58 | 0.02 |
Table 2 presents care-seeking behaviour for the type of care sought during the first illness episode, given enrolment status. Second and third choices of care sought were not considered for this analysis. About 32% of insured individuals who reported an illness episode during the recall period visited a health facility, compared with only 14% of uninsured individuals (P < 0.001). Only 6.9% of the enrolled who were ill visited a traditional healer, while nearly 18% of the ill in the non-enrolled population did (P < 0.001). There was no significant difference between the insured and uninsured when it came to use of either modern (P = 0.66) or traditional (P = 0.90) self-treatment.
Table 2.
First type of treatment sought given illness and insurance status
| Type of treatment sought | Insured group (%) | Uninsured group (%) | Pearson chi-square | Significance |
|---|---|---|---|---|
| No care | 8.86 | 16.65 | 9.0426 | ** |
| Traditional self-care | 21.43 | 21.80 | 0.0159 | |
| Modern self-care | 31.09 | 29.63 | 0.1956 | |
| Traditional healer | 6.90 | 17.76 | 18.9262 | *** |
| Facility care | 31.72 | 14.16 | 35.3289 | *** |
| Observations (N = 1240) | 19.11 | 80.89 |
Notes: *P < 0.05; **P < 0.01; ***P < 0.001.
Propensity to enrol and average treatment effect on the treated
Table 3 reports the results from the logistic regression used to estimate the propensity scores for CBHI enrolment. Distance to facility, household size, household expenditure quintile, household share under 5 years, urban residency and Peul and Samo ethnic groups were all statistically significant and positively associated with insurance enrolment. The Dafine ethnic group was also significant but negatively associated with enrolment. One unexpected result is that a greater distance to health facilities has a positive effect on the probability of enrolment. This may be due to the fact that many of the households enrolling in the scheme do not see distance as a barrier to accessing facility care, where the benefit added through a reduction in financial constraints outweighs the residual problem of distance to care.
Table 3.
Logistic model results: propensity scores for CBHI enrolment at household level
| Variables | Insured (2007) |
|
|---|---|---|
| OR | SE | |
| Distance to facility (non-urban) | 1.03*** | (0.00) |
| Household size | 1.00 | (0.00) |
| Household expenditure quintile | 1.35*** | (0.03) |
| Household share under-5 | 2.03* | (0.73) |
| Urban | 2.48*** | (0.18) |
| Bwaba | 1.01 | (0.09) |
| Peul | 1.90*** | (0.16) |
| Mossi | 0.91 | (0.07) |
| Dafine | 0.74* | (0.11) |
| Samo | 1.26** | (0.10) |
| Muslim | 0.95 | (0.12) |
| Catholic | 0.90 | (0.11) |
| Animist | 1.37 | (0.22) |
| Household head literate | 2.34*** | (0.12) |
| Household head male | 0.64*** | (0.05) |
| Household male married | 0.84 | (0.08) |
| Observations | 13 817 | |
| Pseudo R-squared | 0.124 | |
| N | 13 817 | |
| LLR | −5414 | |
Notes: z-statistics in parentheses.
*P < 0.05; **P < 0.01; ***P < 0.001.
Table 4 reports the estimated average treatment effect for the treatment group, in this case the insured population. The ATT estimates provide a quantitative measure of the average effect of CBHI enrolment status across the enrolled population on the change in probability of choosing one of the four health care options. When matching observations on observable characteristics, the resulting treatment effect of insurance enrolment on choosing self-treatment (either modern or traditional) or traditional healer was negative (i.e. the probability of choosing such care options is reduced for the insured population), but not significantly different from zero. This suggests the impact of CBHI enrolment has no significant effect on reducing the prevalence of self-care among the insured population. On the other hand, enrolment in CBHI maintained a significant, positive effect on the utilization of facility-based care. These results suggest that while enrolment may lead to improved access to facility care within the formal health system, the insured population continues to actively seek drugs from the informal sector, resulting in a continued high prevalence of self-medication within the household.
Table 4.
Average treatment effect on the treated (ATT) for type of care (N = 1235)
| Dependent variable | ATT | SE | P-value |
|---|---|---|---|
| Traditional self-care | −0.039 | (0.05) | 0.442 |
| Modern self-care | −0.058 | (0.05) | 0.268 |
| Facility care | 0.202 | (0.05) | <0.001 |
| Traditional healer | −0.047 | (0.03) | 0.157 |
Note: Observations were matched using the same variables as in Table 3.
Factors that influence the decision to seek various types of care
The multinomial logistic regression model was valid. It passed the chi-squared test and has a Pseudo R2 of 0.133. Table 5 presents the results from the MNL model. In the model of no care vs traditional self-care, only perceived severity and distance to facility were significant predictors of traditional self-treatment. Both variables had a positive relationship with self-treatment through traditional means. Insurance enrolment had no significant relationship with self-treatment through traditional means, nor with expenditure quintile, urban residence and acute illness. In the model of no care vs modern self-care, distance to health facility, perceived severity of illness, urban residence and acute illness were all significant predictors of use of self-treatment with western drugs, each with a positive relationship with use of western self-care. Again, insurance enrolment had no significant relationship with self-care, even for modern methods of treatment. These results are in accordance with the estimated ATT of insurance enrolment, whereby upon controlling for the chosen vector of covariates, insurance enrolment plays no considerable role in reducing the incidence of self-treatment among the population.
Table 5.
Multinomial logistic (MNL) model describing factors that influence the decision to seek care
| Variables | Traditional self-care |
Modern self-care |
Traditional healer |
Public facility care |
||||
|---|---|---|---|---|---|---|---|---|
| Relative risk ratio | SE | Relative risk ratio | SE | Relative risk ratio | SE | Relative risk ratio | SE | |
| Insured (2007) | 1.42 | (0.66) | 1.09 | (0.50) | 0.91 | (0.51) | 2.73* | (1.26) |
| Distance to facility | 1.04** | (0.01) | 1.05*** | (0.01) | 1.02* | (0.01) | 1.02 | (0.01) |
| Employed | 1.40 | (0.93) | 0.57 | (0.33) | 0.62 | (0.40) | 0.52 | (0.29) |
| Sex (male = 1) | 0.93 | (0.35) | 0.77 | (0.30) | 0.65 | (0.26) | 0.80 | (0.32) |
| Literate | 1.46 | (0.58) | 2.00 | (0.73) | 0.29** | (0.14) | 1.85 | (0.73) |
| Expenditure quintile | 1.13 | (0.18) | 1.20 | (0.20) | 1.35 | (0.24) | 1.49* | (0.24) |
| Age quartile | 1.25 | (0.25) | 0.90 | (0.17) | 1.03 | (0.25) | 1.06 | (0.21) |
| Married | 0.54 | (0.22) | 0.90 | (0.37) | 1.40 | (0.68) | 0.75 | (0.34) |
| Perceived severity | 1.92* | (0.56) | 2.28** | (0.65) | 1.77* | (0.51) | 4.38*** | (1.27) |
| Urban | 1.64 | (0.65) | 5.09*** | (1.92) | 0.59 | (0.26) | 1.20 | (0.46) |
| Perceived facility quality | 0.85 | (0.18) | 1.00 | (0.21) | 0.89 | (0.19) | 1.04 | (0.24) |
| Acute illness | 1.51 | (0.47) | 2.94*** | (0.89) | 0.97 | (0.30) | 2.80*** | (0.86) |
| Household head literate | 0.87 | (0.43) | 0.25* | (0.15) | 0.72 | (0.37) | 0.35* | (0.19) |
| Household head male | 0.95 | (0.68) | 1.59 | (1.30) | 0.50 | (0.43) | 0.88 | (0.71) |
| Household head married | 0.79 | (0.51) | 0.72 | (0.48) | 1.57 | (1.29) | 1.52 | (1.08) |
| Propensity score | 1.00 | (0.01) | 1.00 | (0.00) | 0.99 | (0.01) | 1.00 | (0.01) |
| Observations | 645 | |||||||
| Pseudo R-squared | 0.133 | |||||||
Notes: Robust z-statistics in parentheses.
Treatment option 1 (no care) was used as the base outcome.
*P < 0.05; **P < 0.01; ***P < 0.001.
In the model of no care vs traditional healer, significant predictors of traditional healer utilization were distance to closest health facility, perceived severity of illness, if the individual was literate and the household head’s perception of the quality of facility care. Distance to facility and perceived severity were positively associated with use of traditional healers, while being literate and the household head’s perception of quality of care had a negative relationship. While we have already established that insurance enrolment does not lead to a reduction in self-care, the result that insurance does not reduce the demand for traditional healers is another important finding. Formal facility care and use of a traditional healer are both care options that occur once a household has decided to seek outside assistance.
In the model of no care vs modern facility care, insurance status, higher expenditure quintile, perceived severity of illness and if the illness was acute were all significant predictors of modern facility care use. All significant explanatory variables had a positive relationship on the relative risk of modern facility care in comparison with no care. Individuals who were insured had 2.78 times the relative risk of using modern care facilities than people who were not insured, while a one unit increase in severity increased the relative risk of facility use by 4.33.
Discussion
The following four key results of this analysis bring forth three important discussion points about the role of CBHI in care-seeking behavior in Burkina Faso. First, the incidence of chronic illnesses was higher among uninsured adults than insured adults, but with no significant difference between enrolment groups when the entire sample is considered. This finding suggests that there is no adverse selection for CBHI enrolment in Nouna district, as potential enrollees with chronic illnesses would be able to foretell the propensity to seek care when chronically ill and thus enrol to assure coverage of their expected medical costs. Secondly, enrolment in CBHI plays no significant role in reducing self-treatment of illnesses within the household. As noted in Table 2, both the insured and uninsured populations maintain self-treatment rates of over 55%. Even when controlling for observable characteristics, the ATT of insurance on the consumption of self-treatment remains statistically insignificant. Thirdly, the relative risk ratio of modern self-treatment to no treatment for acute illnesses is 2.92, while it is only 2.80 for modern facilities. That is to say, controlling for all other variables, acute illnesses have a greater association with modern self-treatment than they do with facility use. Finally, self-treatment within the household may have implications for cost-containment mechanisms inherent in the insurance scheme (such as the capitation payment method). Delays in accessing facility care brought forth by self-care within the household may lead to higher treatment costs for the insurance scheme, as the incidence of complex cases arriving at facilities may be higher than if enrollees bypass self-care and access formal treatment at an earlier stage of the illness.
Issue 1: Societal preference for self-treatment
One would expect that consumers of health care would have a certain preference for one type of care over another. This is especially true for enrollees who have already prepaid for their services, and who most likely have a personal partiality for facility-based care (Dong et al. 2003; Pokhrel et al. 2005; de Allegri et al. 2006b; Pokhrel 2006). Interestingly enough, our model suggests that many of the insured still continue to seek treatment through informal methods, such as care from traditional healers or purchasing drugs from local pharmacies (often with informal diagnosis/prescription from pharmacy merchants). On one hand, this may be due to a general social preference for traditional medicine for certain illnesses (McCombie 1996; McCombie 2002). Yet, of more importance for the insurance scheme, a continued high prevalence of self-care among the enrolled population may be influenced by low levels of expected benefits gained from enrolling in the programme (Baltussen et al. 2002; de Allegri et al. 2006a). This may be a primary cause for enrolment playing no significant role in reducing the preference for self-treatment in the first stage of the health-seeking decision-making process, as well as the low enrolment and high drop-out rates.
Issue 2: Acute illness, distance and self-medication
Acute illness has a stronger relationship with modern self-treatment than it does with facility-based treatment. The magnitude of the relationship increases as distance to the nearest health facility increases. This implies that for acute illnesses (such as malaria and upper respiratory infections for children), households turn to self-care for treatment as much as they do to facility care, especially when public facilities are far. This may be due to barriers in physical accessibility (Kroeger 1983; Kloos 1990; Alberts et al. 1998; Anson and Haanapel 1999; Akin et al. 2005), perceived low quality of facility-based care (Baltussen et al. 2002; De Allegri et al. 2006a), easy access to private pharmacies in urban areas (Goodman et al. 2007) or perceived costs of time loss for facility care use (Asenso-Okyere and Dzator 1997), all resulting in a preference to self-treat with western drugs. Households that partake in self-treatment may not be informed on how to treat complex illnesses in a rational, safe manner (Ndiaye et al. 2006; Ahmed et al. 2009). Health sector policy-makers should take this into consideration when searching for new ways to bring quality, affordable care closer to the population, and should consider incorporating strategies to educate the population on the dangers of uninformed self-medication.
Issue 3: Self-treatment and cost-containment for the CBHI scheme
Finally, as inappropriate self-care often leads to a delay in reaching effective, provider-based treatment, illnesses that can be efficiently treated during the preliminary stages often become severe before arriving at the health facility (Beiersmann et al. 2007). While this outcome is expensive for households that pay out-of-pocket (Mugisha et al. 2002), it is also expensive for the insurance scheme. As the insurance scheme is still in its infant state and remains financially vulnerable, it should consider pursuing alternative care options that reduce the incidence of delayed treatment. This will not only reduce the out-of-pocket costs for specialty drugs that are not included in the benefit package, but also reduce the costs incurred by the scheme.
Policy implications
If the CBHI scheme in Burkina Faso is to provide treatment options that truly respond to the care-seeking demand of its target population, it is in its best interest to acknowledge the high prevalence of self-treatment among the entire population, including the insured. Thus a critical discussion is needed regarding whether this result itself is a desirable outcome in the context of low-income countries, where pharmacies can play an important role within the formal and informal health care sectors. If the current benefit package of the CBHI scheme has no significant influence on reducing self-treatment, one option would be to incorporate strategies that improve the quality of diagnosis, prescription and treatment within households and throughout the informal sector. In the past decade, there has been a wealth of community-oriented initiatives in low-income countries that aim at reducing the prevalence of irrational, often hazardous self-treatment. These interventions include initiatives for the home management of malaria (Kidane and Morrow 2000; Sirima et al. 2003; Ajayi et al. 2008a; Ajayi et al. 2008b; Kouyate et al. 2008; Tiono et al. 2008; Pagnoni 2009), use of community health officers (Phillips et al. 2006), training of community pharmacists (Tasneem 2006; Ahmed 2008) and collaborating with local medicine sellers (Goodman et al. 2007).
Recent policy initiatives of Burkina Faso’s Ministry of Health have been aimed at reducing the average physical distance between patients and formal health facilities. A more affordable option may be to support the training and supervision of leaders, health workers and pharmacists from the community to facilitate health information campaigns that promote the rational use of drugs. One avenue through which this may be accomplished is a newly introduced government initiative to place clinically-trained nurses within the community setting, similar to the ‘community health officer’ intervention that was adopted in Navrongo district, Ghana, from 1996–2002 (Phillips et al. 2006).
Many of the above studies provide evidence that interventions which introduce community-based care for common illnesses like malaria can lead to significant declines in unregulated self-care, as well as a reduction in the burden of patient care at understaffed primary care facilities. While considering such alternatives, it should be noted that evidence supporting improvements in population health status due to community-based interventions remains limited, and that concerns remain about the depth and quality of supervised care produced through such initiatives.
Conclusion
As early as 1995, Foster argued that self-treatment would remain the main source of treatment for malaria for the foreseeable future, and should be understood and improved (Foster 1995). While CBHI has been seen as an attractive mechanism to increase access to the formal health sector, its effect on controlling self-treatment remains limited. In sub-Saharan Africa, where rates of self-medication are high, alternative strategies to combat unregulated care should be adopted in conjunction with community financing schemes. The findings and policy recommendations from this study can substantially contribute to improving the responsiveness of Nouna’s CBHI scheme to the population it serves. At present, CBHI in Burkina Faso may not be responding to the true health care demand structure of the population. Through further analysis, it will be possible to better evaluate whether the scheme is responsive to inherent preferences for certain types of care within the population of Nouna district.
Ethical clearance
The University of Heidelberg received approval for the research from their respective human subjects committee in Germany (130/2002) which was extended in 2005 and 2008, as well as the Nouna Health Research Center ethical committee (2005-005/CLE/CRSN).
Funding
This work was supported by the German Research Foundation (DFG) collaborative research grant SFB 544 ‘Control of tropical infectious diseases’, Project D2. The study sponsor had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Competing interests
The authors declare no competing interest.
Acknowledgements
The authors would like to thank Mr Cheik Bagagnan and Mr Alphonse Zakane for their support for data management as well as the team of Nouna Health Research Center. The authors would also like to thank Dr Günther Fink, Assistant Professor of International Health Economics, Harvard School of Public Health, Boston, Massachusetts, for his invaluable technical assistance throughout the process.
References
- Abel-Smith B, Dua A. Community financing in developing countries: the potential for the health sector. Health Policy and Planning. 1988;3:95–108. [Google Scholar]
- Agyepong IA, Manderson L. The diagnosis and management of fever at household level in the Greater Accra Region, Ghana. Acta Tropica. 1994;58:317–30. doi: 10.1016/0001-706x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Ahmed SM. Taking health care where the community is: the story of the Shasthya Sebikas of BRAC in Bangladesh. BRAC University Journal. 2008;5:39–45. [Google Scholar]
- Ahmed SM, Haque R, Haque U, Hossain A. Knowledge on the transmission, prevention and treatment of malaria among two endemic population of Bangladesh and their health-seeking behavior. Malaria Journal. 2009;8:173. doi: 10.1186/1475-2875-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi I, Browne E, Bateganya F, et al. Effectiveness of artemisinin-based combination therapy used in the context of home management of malaria: a report from three study sites in sub-Saharan Africa. Malaria Journal. 2008a;7:190. doi: 10.1186/1475-2875-7-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi I, Falade C, Olley B, et al. A qualitative study of the feasibility and community perception on the effectiveness of artemether-lumefantrine use in the context of home management of malaria in south-west Nigeria. BMC Health Services Research. 2008b;8:119. doi: 10.1186/1472-6963-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin J, Dow WH, Lance PM, Loh C. Changes in access to health care in China, 1989–1997. Health Policy and Planning. 2005;20:80–9. doi: 10.1093/heapol/czi010. [DOI] [PubMed] [Google Scholar]
- Alberts JF, Sanderman R, Gerstenbluth I, van den Heuvel WJA. Socio-cultural variations in help-seeking behaviour for everyday symptoms and chronic disorders. Health Policy. 1998;44:57–72. doi: 10.1016/s0168-8510(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Anson O, Haanappel FW. “Remnants of feudalism”? Women’s health and their utilization of health services in rural China. Women Health. 1999;30:105–23. doi: 10.1300/j013v30n01_07. [DOI] [PubMed] [Google Scholar]
- Asenso-Okyere WK, Dzator JA. Household cost of seeking malaria care: a retrospective study of two districts in Ghana. Social Science & Medicine. 1997;45:659–67. doi: 10.1016/s0277-9536(96)00383-8. [DOI] [PubMed] [Google Scholar]
- Baltussen Ye’ RM, Haddad S, Sauerborn R. Perceived quality of care of primary health care services in Burkina Faso. Health Policy and Planning. 2002;17:42–48. doi: 10.1093/heapol/17.1.42. [DOI] [PubMed] [Google Scholar]
- Beiersmann C, Sanou A, Wladarsch E, et al. Malaria in rural Burkina Faso: local illness concepts, patterns of traditional treatment and influence on health-seeking behaviour. Malaria Journal. 2007;6:106. doi: 10.1186/1475-2875-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S, Gilson L. Health financing: designing and implementing pro-poor policies. Issues Paper. London: DFID Health Systems Resource Centre; 2001. [Google Scholar]
- Brieger WR, Ramakrishna J, Adeniyi JD. Self-treatment in rural Nigeria: a community health education diagnosis. Hygiene. 1986;5:41–6. [PubMed] [Google Scholar]
- Brinkmann U, Brinkmann A. Malaria and health in Africa: the present situation and epidemiological trends. Tropical Medicine & Parasitology. 1991;42:204–13. [PubMed] [Google Scholar]
- Caliendo M, Kopeinig S. Some practical guidance for the implementation of propensity score matching. IZA Discussion Paper. Berlin: Department of Public Economics, DIW; 2005. [Google Scholar]
- Carrin G, Waelken MP, Criel B. Community-based health insurance in developing countries: a study of its contribution to the performance of health financing systems. Tropical Medicine & International Health. 2005;10:799–811. doi: 10.1111/j.1365-3156.2005.01455.x. [DOI] [PubMed] [Google Scholar]
- De Allegri M, Sanon M, Sauerborn R. “To enrol or not to enrol?”: a qualitative investigation of demand for health insurance in rural West Africa. Social Science & Medicine. 2006a;62:1520–7. doi: 10.1016/j.socscimed.2005.07.036. [DOI] [PubMed] [Google Scholar]
- De Allegri M, Kouyaté B, Becher H, et al. Understanding enrollment in community health insurance in sub-Saharan Africa: a population-based case-control study in rural Burkina Faso. Bulletin of the World Health Organization. 2006b;84:852–8. doi: 10.2471/blt.06.031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Allegri M, Pokhrel S, Becher H, et al. Step-wedge cluster-randomised community-based trials: an application to the study of the impact of community health insurance. Health Research Policy and Systems. 2008;6:10. doi: 10.1186/1478-4505-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deressa W, Ali A, Enqusellassie F. Self-treatment of malaria in rural communities, Butajira, southern Ethiopia. Bulletin of the World Health Organization. 2003;81:261–8. [PMC free article] [PubMed] [Google Scholar]
- Diop F, Schneider P, Butera D. Summary of Results: Prepayment Schemes in the Rwandan Districts of Byumba, Kabgayi, and Kabutare. Technical Report No. 59. Bethesda, MD: Abt Associates Inc; 2000. [Google Scholar]
- Dong H, Kouyate B, Cairns J, et al. Willingness-to-pay for community-based insurance in Burkina Faso. Health Economics. 2003;12:849–62. doi: 10.1002/hec.771. [DOI] [PubMed] [Google Scholar]
- Dror DM, Radermacher R, Koren R. Willingness to pay for health insurance among rural and poor persons: field evidence from seven micro health insurance units in India. Health Policy. 2007;82:12–27. doi: 10.1016/j.healthpol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Ejov MN, Aung S, Lwin S, Sein K. Hospital-based study of severe malaria and associated deaths in Myanmar. Bulletin of the World Health Organization. 1999;77:310–4. [PMC free article] [PubMed] [Google Scholar]
- Ekman B. Community-based health insurance in low-income countries: a systematic review of the evidence. Health Policy and Planning. 2004;19:249–70. doi: 10.1093/heapol/czh031. [DOI] [PubMed] [Google Scholar]
- Foster S. Treatment of malaria outside the formal health services. Journal of Tropical Medicine & Hygiene. 1995;98:29–34. [PubMed] [Google Scholar]
- Frenk J, Gonzalez-Pier E, Knaul FM, et al. Comprehensive reform to improve health system performance in Mexico. The Lancet. 2006;368:1524–34. doi: 10.1016/S0140-6736(06)69564-0. [DOI] [PubMed] [Google Scholar]
- Gnawali D, Pokhrel S, Sauerborn R, et al. The effect of community-based health insurance on the utilization of modern health care services: evidence from Burkina Faso. Health Policy. 2009;90:214–22. doi: 10.1016/j.healthpol.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Goodman C, Brieger W, Unwin A, et al. Medicine sellers and malaria treatment in sub-Saharan Africa: what do they do and how can their practice be improved? American Journal of Tropical Medicine & Hygiene. 2007;77(6 Suppl.):203–18. [PMC free article] [PubMed] [Google Scholar]
- Heckman J. Sample selection bias as a specification error. Econometrica. 1979;47:153–61. [Google Scholar]
- Hsiao W, Liu Y. Health care financing: assessing its relationship to health equity. In: Evans T, Whitehead M, Diderichsen F, Bhuiya A, Wirth M, editors. Challenging Inequities in Health: From Ethics to Action. New York: Oxford University Press; 2001. pp. 261–75. [Google Scholar]
- Jütting J. Do community based health insurance schemes improve poor people’s access to health care? Evidence from rural Senegal. World Development. 2004;32:273–88. [Google Scholar]
- Kamat V, Nichter M. Pharmacies, self-medication and pharmaceutical marketing in Bombay, India. Social Science and Medicine. 1998;47:779–94. doi: 10.1016/s0277-9536(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Kaseje D, Spencer HC, Sempebwa EK. Usage of community-based chloroquine treatment for malaria in Saradidi, Kenya. Annals of Tropical Medicine & Parisitology. 1987;81(Suppl. 1):111–5. doi: 10.1080/00034983.1987.11812196. [DOI] [PubMed] [Google Scholar]
- Kennedy P. A Guide to Econometrics. 6th. Malden: Blackwell Publishing; 2008. pp. 254–61. [Google Scholar]
- Kidane G, Morrow RH. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomised trial. The Lancet. 2000;356:550–55. doi: 10.1016/S0140-6736(00)02580-0. [DOI] [PubMed] [Google Scholar]
- Kloos H. Utilisation of selected hospitals, health centres and health stations in central, southern and western Ethiopia. Social Science & Medicine. 1990;31:1003–19. doi: 10.1016/0277-9536(90)90052-t. [DOI] [PubMed] [Google Scholar]
- Kouyate B, Some F, Jahn A, et al. Process and effects of a community intervention on malaria in rural Burkina Faso: randomized controlled trial. Malaria Journal. 2008;7:50. doi: 10.1186/1475-2875-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger A. Anthropological and socio-medical health care research in developing countries. Social Science & Medicine. 1983;17:147–61. doi: 10.1016/0277-9536(83)90248-4. [DOI] [PubMed] [Google Scholar]
- Malaria Knowledge Programme. Improving the quality of malaria diagnosis and laboratory services in resource-poor countries. 2005. MKP Policy Brief. Malaria Knowledge Programme, Liverpool School of Tropical Medicine. Online at: http://www.liv.ac.uk/lstm/majorprogs/malaria/outputs.htm, accessed 27 February 2009. [Google Scholar]
- Mathiyazaghan K. Willingness to pay for rural health insurance through community participation in India. International Journal of Health Planning and Management. 1998;13:47–67. doi: 10.1002/(SICI)1099-1751(199801/03)13:1<47::AID-HPM495>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- McCombie S. Treatment seeking for malaria: a review of recent research. Social Science & Medicine. 1996;43:933–45. doi: 10.1016/0277-9536(95)00446-7. [DOI] [PubMed] [Google Scholar]
- McCombie S. Self-treatment for malaria: the evidence and methodological issues. Health Policy & Planning. 2002;17:333–44. doi: 10.1093/heapol/17.4.333. [DOI] [PubMed] [Google Scholar]
- McCord MJ. Nairobi: Micro Save-Africa; 2000. Microinsurance: a case study of an example of the partner-agent model of micro insurance provision: NHHP/FINCA Uganda. [Google Scholar]
- McIntyre D, Thiede M, Dahlgren G, Whitehead M. What are the economic consequences for households of illness and of paying for health care in low- and middle-income country contexts? Social Science & Medicine. 2006;62:858–65. doi: 10.1016/j.socscimed.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Meessen B, Zhenzhong Z, Van Damme W, et al. Editorial: Iatrogenic poverty. Tropical Medicine & International Health. 2003;8:581–4. doi: 10.1046/j.1365-3156.2003.01081.x. [DOI] [PubMed] [Google Scholar]
- Ministère de la Santé, Burkina Faso. Annuaire Statistique. Ouagadougou: Ministère de la Santé; 2007. [Google Scholar]
- Mugisha F, Dong H, Sauerborn R. Costing health care interventions at primary health facilities in Nouna, Burkina Faso. African Journal of Health Sciences. 2002;9:69–79. doi: 10.4314/ajhs.v9i1.30757. [DOI] [PubMed] [Google Scholar]
- Muller O, Razum O, Traore C, Kouyate B. Community effectiveness of chloroquine and traditional remedies in the treatment of young children with falciparum malaria in rural Burkina Faso. Malaria Journal. 2004;3:36. doi: 10.1186/1475-2875-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye P, Tal-Dia A, Diedhiou A, Juergens-Behr A, Lemort JP. Self-treatment of fever in the northern district of Dakar, Senegal. Médicine Tropicale (Marseille) 2006;66:74–8. [PubMed] [Google Scholar]
- Okrah J, Traoré C, Palé A, Sommerfeld J, Müller O. Community factors associated with malaria prevention by mosquito nets: an exploratory study in rural Burkina Faso. Tropical Medicine & International Health. 2002;7:240–8. doi: 10.1046/j.1365-3156.2002.00856.x. [DOI] [PubMed] [Google Scholar]
- Pagnoni F. Malaria treatment: no place like home. Trends in Parasitology. 2009;25:115–9. doi: 10.1016/j.pt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Phillips J, Bawah A, Binka F. Accelerating reproductive and child health programme impact with community-based services: the Navrongo experiment in Ghana. Bulletin of the World Health Organization. 2006;84:949–55. doi: 10.2471/blt.06.030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhrel S. Scaling up health interventions in resource-poor countries: what role does research in stated-preference framework play? Health Research Policy and Systems. 2006;30:4. doi: 10.1186/1478-4505-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhrel S, Snow R, Dong H, Hidayat B, Flessa , et al. Gender role and child health care utilization in Nepal. Health Policy. 2005;74:100–9. doi: 10.1016/j.healthpol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Preker A, Carrin G, Dror D, et al. Effectiveness of community health financing in meeting the cost of illness. Bulletin of the World Health Organization. 2002;80:143. [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum P, Rubin D. The bias due to incomplete matching. Biometrics. 1985;41:103–16. [PubMed] [Google Scholar]
- Russell S, Fox-Rushby J, Arhin D. Willingness and ability to pay for health care: a selection of methods and issues. Health Policy and Planning. 1995;10:94–101. doi: 10.1093/heapol/10.1.94. [DOI] [PubMed] [Google Scholar]
- Saradamma RD, Higginbotham N, Nichter M. Social factors influencing the acquisition of antibiotics without prescription in Kerala State, south India. Social Science & Medicine. 2000;50:891–903. doi: 10.1016/s0277-9536(99)00380-9. [DOI] [PubMed] [Google Scholar]
- Sirima SB, Konate A, Tiono A, et al. Early treatment of childhood fevers with pre-packaged antimalarial drugs in the home reduces severe malaria morbidity in Burkina Faso. Tropical Medicine & International Health. 2003;8:133–9. doi: 10.1046/j.1365-3156.2003.00997.x. [DOI] [PubMed] [Google Scholar]
- Smith KV, Sulzbach S. Community-based health insurance and access to maternal health services: evidence from three West African countries. Social Science & Medicine. 2008;66:2460–73. doi: 10.1016/j.socscimed.2008.01.044. [DOI] [PubMed] [Google Scholar]
- Tasneem S. Community-based health care approach and ultra poor: exploring Shastha Sebikas’ performance over time. Dhaka: BRAC Research and Evaluation Division; 2006. [Google Scholar]
- Théra MA, D’Alessandro U, Thiéro M, et al. Child malaria treatment practices among mothers in the district of Yanfolila, Sikasso region, Mali. Tropical Medicine and International Health. 2000;5:876–81. doi: 10.1046/j.1365-3156.2000.00652.x. [DOI] [PubMed] [Google Scholar]
- Tiono A, Kaboré Y, Traoré A, et al. Implementation of home based management of malaria in children reduces the work load for peripheral health facilities in a rural district of Burkina Faso. Malaria Journal. 2008;7:201. doi: 10.1186/1475-2875-7-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo A, Portillo J, Vernon J. The impact of subsidized health insurance for the poor: evaluating the Colombian experience using propensity score matching. International Journal of Health Care Finance and Economics. 2005;5:211–39. doi: 10.1007/s10754-005-1792-5. [DOI] [PubMed] [Google Scholar]
- Wagstaff A. Estimating health insurance impacts under unobserved heterogeneity: the case of Vietnam’s health care fund for the poor. Health Economics. 2010;19:189–208. doi: 10.1002/hec.1466. [DOI] [PubMed] [Google Scholar]
- Wagstaff A, Lindelow M, Jun G, Ling X, Juncheng Q. Extending health insurance to the rural population: an impact evaluation of China’s new cooperative medical scheme. Journal of Health Economics. 2009;28:1–19. doi: 10.1016/j.jhealeco.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Wolfgang C, Winkelmayer K. Propensity scores: help or hype? Nephrology Dialysis Transplantation. 2004;19:1671–3. doi: 10.1093/ndt/gfh104. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) WHO Expert Committee on Malaria, Twentieth Report. WHO Technical Report Series 892. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- World Health Organization (WHO) The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; 2008. [Google Scholar]