Abstract
Tumor necrosis factor-α–converting enzyme (TACE; also known as ADAM17) is a proteolytic sheddase that is responsible for the cleavage of several membrane-bound molecules. We report that TACE cleaves neuregulin-1 (NRG1) type III in the epidermal growth factor domain, probably inactivating it (as assessed by deficient activation of the phosphatidylinositol-3-OH kinase pathway), and thereby negatively regulating peripheral nervous system (PNS) myelination. Lentivirus-mediated knockdown of TACE in vitro in dorsal root ganglia neurons accelerates the onset of myelination and results in hypermyelination. In agreement, motor neurons of conditional knockout mice lacking TACE specifically in these cells are significantly hypermyelinated, and small-caliber fibers are aberrantly myelinated. Further, reduced TACE activity rescues hypomyelination in NRG1 type III haploinsufficient mice in vivo. We also show that the inhibitory effect of TACE is neuron-autonomous, as Schwann cells lacking TACE elaborate myelin of normal thickness. Thus, TACE is a modulator of NRG1 type III activity and is a negative regulator of myelination in the PNS.
Myelin, the insulating membrane that is produced by Schwann cells in the PNS and by oligodendrocytes in the CNS, is an organelle whose assembly and function is crucial for proper transmission of the electric impulse. Myelination in the PNS is controlled at the molecular level by the amount of axonal NRG1 type III1,2. Thus, clarifying the mechanisms that control NRG1 type III expression is essential to establish the molecular events that regulate myelin formation. The β-secretase BACE1 (refs. 3–5) and the α-secretases belonging to the ADAM family of proteases6 control the classical regulated proteolysis of NRG1. Although the role of BACE1 in NRG1 type III cleavage has been investigated during myelination and remyelination3–5, the role of the α-secretases is unclear.
ADAM (a disintegrin and metalloprotease) proteins are a family of zinc-dependent, membrane-anchored metalloproteases that are implicated in ectodomain shedding of several growth factors. ADAMs control myogenesis, neurogenesis, fertilization, inflammation and myelination6. Several members of the ADAM secretases are expressed in the PNS, and some have been implicated in myelination. In particular, ADAM19 can process NRG1 (ref. 7), although recent studies suggest that ADAM19 targets NRG1 type I8, which is not implicated in myelination1,2. Accordingly, mice lacking ADAM19 myelinate normally, although they have delayed remyelination9. Another ADAM that is directly implicated in myelination is ADAM22, as transgenic mice lacking ADAM22 are hypomyelinated in the PNS10. However, it is unlikely that ADAM22 cleaves NRG1 type III or other growth factors, as this protein lacks a catalytic-site consensus sequence6. Rather, axonal ADAM22 functions as a receptor for secreted LGI4 (leucine-rich repeat LGI family, member 4)11. Recently, the role of ADAM10 in NRG1 cleavage was analyzed. However, mice with altered ADAM10 expression myelinate normally in the PNS12, suggesting that ADAM10 is dispensable for this process.
Another member of this family, TACE, has been implicated in NRG1 cleavage13. TACE mediates ectodomain shedding of several membrane-bound molecules, including tumor necrosis factor-α (TNF-α), transforming growth factor-α (TGF-α), p75 neurotrophin receptor (p75NTR; also known as NGFR), Notch and amyloid precursor protein (APP)6,14 in addition to NRG1. Transgenic mice lacking TACE die at birth, preventing the analysis of myelination in vivo15. We now report that TACE cleaves NRG1 type III and that ablation of TACE results in altered processing of axonal NRG1 type III. In addition, downregulation of TACE expression in vitro in myelinating cocultures and inactivation of TACE in vivo in motor neurons lead to precocious myelination, hypermyelination, ectopic myelination and enhanced activation of the phosphatidylinositol-3-OH kinase (PI3K) pathway. Furthermore, the reduction of TACE activity in vivo is sufficient to rescue myelination of NRG1 type III haploinsufficient mice. We also provide evidence that this phenotype is due to a cell-autonomous mechanism, as inactivation of TACE in Schwann cells in vitro and in vivo does not alter the timing of myelin production or the amount produced.
Thus, our study reveals a previously uncharacterized role for TACE in myelination. By cleaving NRG1 type III, TACE determines the amount of this critical regulator of myelination on the axonal surface and, unlike BACE1 (refs. 3,5), negatively regulates PNS myelination. To our knowledge, this is the first study reporting a negative mechanism for controlling NRG1 type III expression and myelination, further suggesting that secretases are important modulators of myelination.
RESULTS
TACE inactivation enhances myelination in vitro
To investigate the role of TACE in myelination, we first analyzed its mRNA and protein levels in vitro. TACE is highly expressed in Schwann cells and, at lower levels, in dorsal root ganglia (DRG) neurons, as shown by PCR with reverse transcription (RT-PCR) and western blot analyses of cDNAs or lysates prepared from Schwann cells and DRG neurons (Supplementary Fig. 1).
Tace–/– (Adam17–/–) mice die at birth15, precluding in vivo analysis of myelination. To determine the role of TACE in myelin formation, we first knocked down its expression using specific short hairpin RNA (shRNA)-encoding lentiviruses. For our studies, we used three different shRNA clones (TRCN0000031949, TRCN0000031952 and TRCN0000031953) that were obtained from the RNAi Consortium (Boston, Massachusetts, USA) together with a lentiviral vector expressing a scrambled artificial sequence as a negative control. Database searches confirmed that the sequence used in the scrambled shRNA did not recognize any mammalian DNA. To validate the specificity of Tace-targeting shRNAs, we tested their ability to modulate the cleavage of p75NTR and Notch1. Both molecules are important in myelination16,17, but only p75NTR is cleaved by TACE18, whereas Notch1 is extracellularly processed by ADAM10 (refs. 19,20). As expected, we observed impaired p75NTR cleavage in Schwann cell–DRG neuron cocultures infected with lentiviruses encoding Tace shRNAs, but the amount of Notch1 intracellular domain (NICD) was unaltered (Supplementary Fig. 2).
To corroborate the efficacy of knockdown, we infected primary rat Schwann cells for 2 d and then analyzed expression by western blotting on an Odyssey imaging system (Fig. 1a). All three tested shRNAs significantly decreased TACE expression by approximately 80% when compared with mock-infected or control uninfected Schwann cells (Fig. 1b). We then infected mouse DRG explant cultures, which contain a mix of Schwann cells and DRG neurons, with lentiviruses encoding Tace shRNA or a scrambled shRNA for 48 h. The cultures were grown without antimitotic agents to allow infection of both neurons and Schwann cells. Western blots confirmed effective TACE knockdown of infected cultures (data not shown). Myelination was induced by supplementing the cultures with 50 μg ml–1 ascorbic acid. Immunofluorescence analyses (Fig. 1) for myelin basic protein (MBP) and neurofilament showed that myelination was significantly greater in Tace shRNA–infected cultures and was already substantial 3 d after the addition of ascorbic acid. Thus, myelination in cocultures infected with Tace shRNA was greater and commenced earlier than myelination in scrambled shRNA–infected or control uninfected cultures, suggesting that TACE inhibits myelination. To further confirm this result, we counted the number of MBP-positive segments 3 d after ascorbic acid addition in Tace shRNA-infected versus uninfected or scrambled shRNA–infected cultures. Specific knockdown of Tace led to an approximately 15-fold increase in myelination (Fig. 1c). Western blotting for myelin proteins confirmed the hypermyelination. Fourteen days after the induction of myelination, myelin protein zero (MPZ) was appreciably upregulated in cocultures infected with lentiviruses encoding Tace shRNA (Fig. 1d). As Tace knockdown resulted in enhanced and precocious myelination, our data suggest that TACE controls the temporal activation of the myelinating program.
Figure 1.
TACE downregulation induces precocious myelination and hypermyelination in vitro. (a) Western blot of rat Schwann cells that were uninfected (WT) or infected with lentiviruses expressing one of three different shRNAs specific for Tace (sh1, sh2 and sh3) or a scrambled artificial sequence (shscr). Amounts of TACE and of actin, as a loading control, were determined 7 d after infection. TACE expression was significantly lower in Schwann cells infected with sh1, sh2 or sh3, but not in shscr-infected or uninfected samples. Full-length blots are shown in Supplementary Figure 10. (b) TACE expression in Schwann cells infected with sh1, sh2 or sh3 **P = 0.0019 (WT versus sh1), **P = 0.0025 (WT versus sh2), **P = 0.0018 (WT versus sh3). Data shown are the averages of three experiments; error bars, mean ± s.e.m. (c) Quantification of MBP-positive segments 3 d after the induction of myelination in control cultures and cultures infected with Tace shRNA or shscr. Quantification was performed on the entire culture (a total of three coverslips per experiment) **P = 0.0012 (WT versus sh1), **P = 0.0047 (WT versus sh2), ***P < 0.0001 (WT versus sh3). Data shown are averages of three experiments; error bars, mean ± s.e.m. (d) Western blot of organotypic rat Schwann cell neuronal cocultures that were uninfected or infected with lentiviruses expressing sh1, sh2 or sh3, or shscr. Lysates were tested for MPZ, and for actin as a loading control, 14 d after the induction of myelination. MPZ expression was significantly upregulated in cultures in which TACE was knocked down. (e) Organotypic rat Schwann cell neuronal cocultures infected with lentiviruses expressing TACE-specific shRNAs or shscr were maintained in myelinating conditions for 3 d, fixed and stained for MBP (rhodamine) and neurofilament (fluorescein). Numerous myelin segments were evident in Tace shRNA–infected cultures; none formed in shscr-infected cultures and only a few formed in uninfected cultures. Scale bar, 100 μm.
TACE activity in vitro is neuron-autonomous
These results indicate that TACE negatively controls myelination. As both Schwann cells and DRG neurons express TACE, we sought to determine whether these effects are specific to Schwann cells, neurons or both using a purified myelinating coculture system. We first infected mouse DRG neurons with lentiviruses encoding Tace shRNA or, as a control, with lentiviruses encoding scrambled shRNA. We then cultured these neurons with primary uninfected rat Schwann cells and induced myelination for 15 d by ascorbic acid addition; cultures were then stained for MBP and neurofilament. In comparison with control neurons, DRG neurons that were knocked down for Tace were substantially hypermyelinated (Fig. 2a and Supplementary Fig. 3a), and the number of myelinated segments in these neurons was approximately eightfold higher than the number in control neurons (Fig. 2b). We did not observe any effect on neurite outgrowth. We confirmed these results with western blots of MBP, myelin-associated glycoprotein (MAG) and neurofilament expression. As expected, the expression of myelin proteins was notably upregulated in the lysates of cocultures that were ablated for neuronal Tace (data not shown).
Figure 2.
In vitro hypermyelination is neuron-autonomous. (a) Cocultures of mouse neurons infected with Tace-specific shRNA (sh1) or a scrambled artificial sequence shRNA (shscr), rid of endogenous Schwann cells and repopulated with wild-type, uninfected rat Schwann cells, were maintained in myelinating conditions for 14 d and then stained for MBP (rhodamine) and neurofilament (fluorescein). Numerous myelin segments are evident in sh1-infected cultures and fewer in uninfected (WT) and shscr-infected cultures. Scale bar, 100 μm. (b) Quantification of MBP+ segments 14 d after the induction of myelination in control and infected (Tace shRNA or shscr) neuronal cocultures. Quantification was performed on the entire culture (three coverslips per experiment). ***P = 0.0002 (WT versus sh1), ***P = 0.0007 (WT versus sh2), ***P = 0.0009 (WT versus sh3). Data shown are averages of three experiments; error bars, mean ± s.e.m. (c) Cocultures of wild-type, uninfected mouse DRG neurons purified of endogenous Schwann cells and repopulated with rat Schwann cells that had previously been infected with sh1 or shscr. Cultures were maintained in myelinating conditions for 21 d, and then stained for MBP (rhodamine) and neurofilament (fluorescein). No difference in myelination was observed in infected versus control cultures. Scale bar, 100 μm. (d) Quantification of MBP+ segments 21 d after the induction of myelination in control and Schwann cells infected with Tace shRNA or shscr and cocultured with DRG neurons. Quantification was performed on the entire culture (three coverslips per experiment, for three experiments; P was not significant).
In a parallel set of experiments, we inactivated Tace in rat primary Schwann cells and cultured them with purified uninfected mouse DRG neurons. Cultures were maintained in myelinating conditions for 21 d. Immunofluorescence for MBP and neurofilament (Fig. 2c and Supplementary Fig. 3b) and western blots for MBP and MAG (data not shown) indicated that inactivation of Tace in Schwann cells did not enhance myelination, strongly suggesting that the inhibitory role of TACE in myelination is neuronal cell–autonomous. These results were further confirmed by assessing the numbers of MBP-positive segments in these cultures, which were comparable between Tace knockdown and control cultures (Fig. 2d). Thus, these studies indicate that TACE acts in a neuron-autonomous manner to inhibit myelination in an in vitro model of PNS myelination.
In vivo inactivation of axonal TACE causes hypermyelination
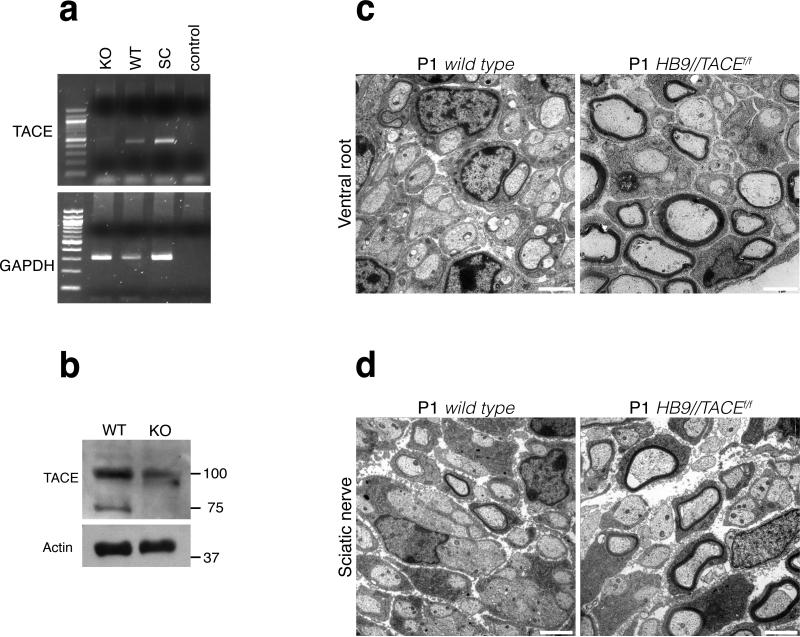
To evaluate the role of TACE in myelination during development and to determine whether neuronal TACE also inhibits myelination in vivo, we generated transgenic mice that lack TACE specifically in motor neurons. To this end, we crossed mice containing loxP sites flanking exon 2 of Tace (‘floxed’ Tace; Tacefl/fl)21 with the HB9-cre transgenic line, which has been used in previous studies to drive motor neuron-specific recombination using the promoter of the Mnx1 gene22. Ablation of Tace exon 2 leads to a functional null protein21. We screened the genotype by PCR analysis of genomic DNA that was prepared from the tail and spinal cord of HB9-cre; Tacefl/fl and wild-type controls (data not shown). As the HB9-cre transgene is expressed in motor neurons, we corroborated efficient recombination by determining Tace mRNA expression using RT-PCR on spinal cord of HB9-cre; Tacefl/fl and wild-type mice (Fig. 3a). We also assessed TACE protein amounts in lysates that were prepared from postnatal day (P) 30 wild-type and HB9-cre; Tacefl/fl femoral nerves (Fig. 3b). TACE expression was lower both at the mRNA and protein levels in HB9-cre; Tacefl/fl mice. Residual expression was probably due to un-recombined Tace that was present in other cells; in femoral nerves, these cells would be mainly Schwann cells and sensory nerve fibers.
Figure 3.
TACE inactivation in motor neurons leads to precocious myelination. (a) RT-PCR analysis of Tace mRNA expression in wild-type (WT) and HB9-cre; Tacefl/fl (KO) spinal cords. Schwann cell mRNA (SC) was used as a positive control. Gapdh expression was a control for amplification. (b) Lysates of wild-type and HB9-cre; Tacefl/fl P30 femoral nerves were fractionated by SDS-PAGE and blotted with antibodies to TACE and to actin as a loading control. TACE expression was lower in HB9-cre; Tacefl/fl femoral nerve extracts than in wild-type extracts. (c) Electron micrographs of P1 ventral roots from wild-type and HB9-cre; Tacefl/fl mice. In HB9-cre; Tacefl/fl mice, fibers are hypermyelinated as compared with controls. Scale bars, 2 μm. (d) Electron micrographs of P1 sciatic nerves from wild-type and HB9-cre; Tacefl/fl mice; most fibers in HB9-cre; Tacefl/fl mice were sorted, and many were already myelinated. Scale bars, 2 μm.
Ultrastructural analysis of the ventral roots and sciatic nerves from newborn (P1) HB9-cre; Tacefl/fl mice showed that the onset of myelination in the ventral roots of these mice was accelerated (Fig. 3c). As expected, we observed that some fibers in P1 sciatic nerves, which contain both sensory and motor fibers, were hypermyelinated (Fig. 3d). Quantification of the number of myelinated fibers demonstrated that there were significantly more in HB9-cre; Tacefl/fl mice (P = 0.0039); the total number of nerve fibers was similar in both genotypes (Supplementary Table 1).
To evaluate whether the hypermyelination of HB9-cre; Tacefl/fl mice is maintained during active phases of myelination, we analyzed peripheral nerve morphology in P7 and P15 sciatic nerves. At both time points, neurites were hypermyelinated, as evidenced in semithin sections (Fig. 4a,b, top panels) and in electron micrographs (Fig. 4a,b, lower panels). The hypermyelinating phenotype was also confirmed by g ratio (axon diameter/fiber diameter) measurements (Fig. 4c), which showed that the g ratio of P7 fibers of sciatic nerves was significantly lower in HB9-cre; Tacefl/fl mice than in wild-type mice (Supplementary Table 1). This change did not result in altered fiber diameters in HB9-cre; Tacefl/fl, which were similar to those of wild-type mice (Fig. 4d), strongly suggesting that the observed phenotype is due to changes in the myelin sheath. As suggested from our previous data, hypermyelination in HB9-cre; Tacefl/fl mice resulted in considerably more myelin lamellae surrounding fibers of similar caliber, with no alteration to myelin periodicity (Fig. 4e).
Figure 4.
HB9-cre; Tacefl/fl mice are hypermyelinated during development. (a) Semithin sections (top panels) and electron micrographs (bottom panels) of wild-type and HB9-cre; Tacefl/fl P7 nerves. Scale bars, 15 μm (top panels) and 2 μm (bottom panels). (b) Semithin sections (top panels) and electron micrographs (bottom panels) of wild-type and HB9-cre; Tacefl/fl P15 nerves. Scale bars, 20 μm (top panels) and 2 μm (bottom panels). (c) g ratios as a function of axon diameter were significantly different between wild-type (WT) P7 mice and HB9-cre; Tacefl/fl (KO) P7 mice (P = 0.0007). The graph represents the g ratios obtained from more than 500 myelinated axons per genotype (a total of three mice per genotype). (d) The distribution of myelinated fibers was similar in HB9-cre; Tacefl/fl and wild-type P7 sciatic nerves (P was not significant). (e) Myelinated axons of similar diameters demonstrate that myelin in wild-type versus HB9-cre; Tacefl/fl sciatic nerves had identical periodicity but differed considerably in the number of lamellae. Scale bar, 500 nm.
Morphological and ultrastructural analysis of P30 HB9-cre; Tacefl/fl ventral roots (Fig. 5a) and sciatic nerves (Fig. 5b) and g ratio measurements in sciatic nerves (Fig. 5 and Supplementary Table 1) confirmed hypermyelination in adult mice, strongly suggesting that this effect is maintained throughout development. Unexpectedly, we observed a significant number of myelinated fibers with axons of less than 1 μm diameter and markedly smaller axonal diameters for myelinated fibers in sciatic nerves of HB9-cre; Tacefl/fl, despite the total number of myelinated fibers being similar between genotypes (wild type, 1,717; HB9-cre; Tacefl/fl, 1,697) (Fig. 5g and Supplementary Table 1). Whether this difference is due to ectopic myelination or constriction of axonal caliber will require further investigation. Nonetheless, this alteration is unlikely to explain the hypermyelination, as both in vitro and in vivo studies indicate that axonal diameter increases with myelin formation23,24. Of note, we similarly observed myelin sheath surrounding several fibers whose diameter was less than 1 μm in ventral roots of HB9-cre; Tacefl/fl (Fig. 5a, asterisks). To determine whether the myelin protein content parallels the hypermyelinating phenotype, we checked MBP expression in P15 and P30 femoral nerves of wild-type and HB9-cre; Tacefl/fl by Western blotting. As expected, hypermyelination was accompanied by higher MBP expression (Fig. 5c). Of note, even amounts of phosphorylated AKT (p-AKT) (Fig. 5d)—a key effector of the PI3K pathway, which acts downstream of NRG1 type III1 and is important for PNS myelination25,26—were higher in P30 femoral nerves of HB9-cre; Tacefl/fl mice than in those of wild-type mice.
Figure 5.
HB9-cre; Tacefl/fl adult mice were hypermyelinated and Remak fibers were aberrantly ensheathed. (a) Semithin sections (top panels) and electron micrographs (bottom panels) of wild-type and HB9-cre; Tacefl/fl P30 ventral roots. Scale bars, 20 μm (top panels) and 2 μm (bottom panels). Asterisks indicate heavily myelinated fibers of less than 1 μm diameter. (b) Semithin sections (top panels) and electron micrographs (bottom panels) of wild-type and HB9-cre; Tacefl/fl P30 sciatic nerves. Scale bars, 20 μm (top panels) and 2 μm (bottom panels). (c) Lysates of wild-type (WT) and HB9-cre; Tacefl/fl (KO) P15 and P30 femoral nerves were blotted with antibodies to MBP and to actin as a loading control. MBP expression was upregulated in femoral nerve extracts from HB9-cre; Tacefl/fl mice. (d) Lysates of wild-type and HB9-cre; Tacefl/fl P30 femoral nerves were blotted with antibodies to p-AKT, total AKT (tot-AKT) and actin as a loading control. p-AKT expression was notably upregulated in HB9-cre; Tacefl/fl femoral nerve extracts. (e) Impaired sorting of Remak fibers of HB9-cre; Tacefl/fl sciatic nerves. In wild-type mice, unmyelinated axons are segregated into separate pockets of Remak bundles and fully wrapped by Schwann cells. In HB9-cre; Tacefl/fl mice, Remak bundles contained abnormally large-caliber axons (asterisks), and Schwann cells failed to ensheath the axons. Scale bar, 1 μm. (f) Significant difference in g ratios as a function of axon diameter between P30 sciatic nerve fibers from wild-type and HB9-cre; Tacefl/fl mice (P < 0.0001). The graph represents the g ratio obtained from more than 700 myelinated axons (three mice per genotype). (g) Myelinated axons in HB9-cre; Tacefl/fl P30 sciatic nerves were significantly smaller (***P < 0.0001, **P = 0.0051). Approximately 7% of myelinated fibers were <1 μm in diameter in HB9-cre; Tacefl/fl sciatic nerves. In each genotype, we counted >700 axons in a total of three mice. Error bars, mean ± s.e.m.
Our ultrastructural analysis also showed that ablation of neuronal Tace altered the organization of C fibers, which are engulfed by non-myelinated Schwann cells in Remak bundles. In fact, we observed unsorted and unmyelinated fibers that were larger than 1 μm in diameter in HB9-cre; Tacefl/fl Remak bundles (Fig. 5e, asterisks), suggesting an impaired sorting process. Furthermore, in many cases Remak bundles lacked intervening Schwann cell processes, suggesting that TACE also affects the ensheathment process of non-myelinated fibers.
To test whether TACE haploinsufficiency induces hypermyelination, we generated a complete Tace+/– transgenic line by crossing Tacefl/fl mice with mice with a CMV-cre transgene that drives recombination in all cells27. Morphological analysis, g ratio measurements and western blotting for myelin proteins showed that Tace+/– P30 sciatic nerves were myelinated normally, indicating that residual TACE activity is sufficient to regulate myelin formation (Supplementary Fig. 4 and Supplementary Table 1).
Only axonal TACE determines hypermyelination
To corroborate our previous results, which indicate that hypermyelination is due to a neuronal cell–autonomous mechanism, we deleted Tace in Schwann cells in vivo by crossing Tace-floxed transgenic mice with a Mpz-cre transgenic line (creating Mpz-cre; Tacefl/fl mice)28. We confirmed efficient recombination in genomic DNA of P15 sciatic nerves of Mpz-cre; Tacefl/fl mice by PCR analyses (Fig. 6a). Myelination in P7 and P30 Mpz-cre; Tacefl/fl sciatic nerves was similar to that of wild-type control mice, as assessed by semithin sections (top panels in Fig. 6b,c) and ultrastructural analysis (lower panels in Fig. 6b,c). This result was further confirmed by g ratio measurements (Fig. 6d), which were not significantly different in wild-type and Mpz-cre; Tacefl/fl mice (Supplementary Table 1). Furthermore, MBP and MAG protein levels were similar in lysates of wild-type and Mpz-cre; Tacefl/fl P15 sciatic nerves (Fig. 6e). Surprisingly, despite the similar numbers of myelinated fibers in wild-type and Mpz-cre; Tacefl/fl mice (wild type, 1,298; Mpz-cre; Tacefl/fl, 1,244), axonal diameters were slightly greater in Mpz-cre; Tacefl/fl sciatic nerves (Fig. 6f and Supplementary Table 1). Furthermore, the myelinated fibers of Mpz-cre; Tacefl/fl mice had more periaxonal space and an accumulation of organelles in the inner cytoplasmic collar (Supplementary Fig. 5), suggesting that glial TACE may process molecules that are implicated in myelin compaction and/or adhesion.
Figure 6.
Mpz-cre; Tacefl/fl mice were normally myelinated. (a) Genotyping PCR on genomic DNA prepared from sciatic nerves of P15 wild-type (+/+) and Mpz-cre; Tacefl/fl mice. Tace recombination was present in nerves of Tacefl/fl mice expressing the Cre recombinase also. Flx, amplification of the fl allele; Cre, amplification of cre; null, amplification of the recombined allele21. (b) Mpz-cre; Tacefl/fl mice had a comparable myelin thickness to wild-type mice. Semithin sections (top panels) and electron micrographs (bottom panels) of wild-type and Mpz-cre; Tacefl/fl P7 sciatic nerves. Scale bars, 20 μm (top panels) and 2 μm (bottom panels). (c) Semithin sections (top panels) and electron micrographs (bottom panels) of wild-type and Mpz-cre; Tacefl/fl P30 sciatic nerves. Scale bars, 20 μm (top panels) and 2 μm (bottom panels). (d) g ratios as a function of axon diameter were identical in wild-type (WT) and Mpz-cre; Tacefl/fl (KO) P30 sciatic nerve fibers (P was not significant). The graph represents the g ratios obtained from >300 myelinated axons (three mice per genotype). (e) Lysates of wild-type and Mpz-cre; Tacefl/fl P15 sciatic nerves were fractionated by SDS-PAGE and blotted with antibodies to myelin proteins (MAG and MBP) and to actin as a loading control. No alteration in myelin protein expression was observed among wild-type and Mpz-cre; Tacefl/fl mice. (f) Mpz-cre; Tacefl/fl mice have a slightly but significantly greater axonal diameters than wild-type mice (*P < 0.036, **P < 0.001, ***P < 0.0001). Myelinated axons of P30 sciatic nerves were binned based on their axonal diameters. >1,200 axons were counted from three different mice per genotype. Error bars, mean ± s.e.m.
Taken together, these in vivo analyses provide compelling evidence that neuronal TACE inhibits myelination in a cell-autonomous mechanism. Ablation of Tace in motor neurons accelerates the myelination process and results in hypermyelination, leading to a greater number of myelin lamellae than in wild-type fibers with similar caliber, and ectopic myelination of small-caliber fibers that would normally be unmyelinated. In addition, our findings indicate that glial TACE also participates in the myelinating process, although it does not regulate myelin thickness.
TACE cleaves NRG1 and controls axonal NRG1 type III levels
The phenotype observed in HB9-cre; Tacefl/fl mice, particularly the greater number of myelin lamellae in vivo, strongly resembles the hypermyelinating phenotype of mice overexpressing NRG1 type III2. In addition, ablation of Tace is sufficient to determine myelination of fibers that would normally be amyelinated, mirroring what happens when NRG1 type III is ectopically expressed1. Thus, by cleaving NRG1 (ref. 13), TACE might regulate the amount of axonal NRG1 type III and, hence, PNS myelination. To determine whether NRG1 shedding is altered in the absence of TACE, we analyzed NRG1 expression in lysates of wild-type, Tace+/– and Tace–/– DRG neurons prepared from embryonic day 14.5 embryos (Fig. 7a) and in lysates of P30 HB9-cre; Tacefl/fl femoral nerves (Fig. 7b). In the absence of TACE, NRG1 processing was impaired, as there was more of the unprocessed pro-protein form in HB9-cre; Tacefl/fl DRG neurons. Of note, unprocessed NRG1 proprotein was absent in HB9-cre; Tacefl/fl femoral nerves and barely present in wild-type control extracts. This difference is likely to be due to altered NRG1 expression in DRG neurons in culture and in sciatic nerves1. More importantly, the amount of cleaved NRG1 was similarly greater in the absence of TACE, probably owing to enhanced activity of other secretases (such as BACE1).
Figure 7.
TACE cleaves NRG1 type III. (a) Lysates of wild-type, Tace+/– and Tace–/– DRG neurons were blotted with antibodies to NRG1 and to actin as loading control. The 135-kDa band corresponding to the full-length NRG1 pro-protein (arrowhead) was increased in Tace+/– and, to a greater extent, in Tace–/– lysates. The active cleaved fragment of NRG1 (arrow) was upregulated in Tace+/– and Tace–/– samples. (b) Lysates of wild-type (WT) and HB9-cre; Tacefl/fl (KO) P30 femoral nerves were blotted with antibodies to NRG1 and to actin as a loading control. Uncleaved NRG1 was present in wild-type but not in Tace–/– nerves (arrowhead), contrary to the cleaved, active NRG1 fragment, which is upregulated in HB9-cre; Tacefl/fl samples (arrow). (c) In vitro cleavage of the EGF domain of human recombinant NRG1 β1. NRG1 β1 was incubated with human recombinant TACE and then separated on an SDS-PAGE gel. The corresponding cleaved band (arrow) was digested with trypsin and analyzed by MALDI-TOF mass spectrometry. (d) MALDI-TOF spectrum of the 6.5 kDa band digested with endoproteinase Glu-C. Peaks with arrows are from NRG1, and unlabeled peaks are from autolysis mediated by endoproteinase Glu-C. (e) NRG1 β1 sequence representing the EGF domain (red), the β1 exon (yellow box) and the transmembrane domain (blue). TACE (α) and BACE1 (β) cleavage sites are also indicated. (f) Cultures of wild-type and Tace–/– DRG neurons were incubated with ERBB2-ERBB3–Fc and the binding visualized with rhodamine-conjugated antibodies to human Fc (rhodamine); neurofilament staining from corresponding fields is shown (fluorescein). Scale bar, 50 μm. (g) Quantification of the ERBB2-ERBB3 binding signal. Quantification was performed on confocal images that were acquired with the same z-stack and laser intensity (a total of five coverslips per experiment, for three experiments). ***P < 0.0001. Error bars, mean ± s.e.m.
To further confirm these data, we mapped the TACE cleavage site of NRG1 by mass spectrometry. To this end, we incubated 2 μg of human recombinant NRG1 containing the EGF β-exon domain (NRG β1), the most abundant NRG1 isoform expressed in the nervous system, with 1 μg of human recombinant TACE. The proteins were then separated on a Tris-tricine gel (to favor the resolution of low-molecular-mass bands) together with native, uncleaved recombinant NRG1 β1 for comparison (Fig. 7c). The reaction generated a cleavage product of ~6 kDa, which was digested with endoproteinase Glu-C and analyzed by matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry. In the MALDI-TOF spectrum, two potential C-terminal peptides were detected that did not correspond to endoproteinase Glu-C cleavage (Supplementary Fig. 6) and therefore derived from TACE activity (Fig. 7d). These peptides, with masses of 1,858.812 Da and 2,294.057 Da, corresponded to the peptide sequences FTGDRCQNYVMASFY and FTGDRCQNYVMASFYKHLG, respectively, suggesting that TACE cleaves NRG1 β1 either upstream or in the β-exon of the EGF domain, 3–6 amino acids before the previously identified BACE1 site (Fig. 7e)4. In our analysis, we did not observe cleavage at the BACE1 site of NRG1.
To determine whether the activity of NRG1 type III is altered following TACE cleavage, we incubated wild-type, control and Tace–/– DRG neurons, obtained by crossing Tace+/– mice, with the receptor ERBB2-ERBB3 fused to the Fc portion of human immunoglobulin G (ERBB2-ERBB3–Fc); this chimeric protein binds to NRG1 type III molecules, the only NRG1 molecules that are expressed on the axonal surface1. Chimeric ERBB2-ERBB3–Fc bound more robustly to DRG neurons lacking TACE than to wild-type uninfected or control-shRNA-infected DRG neurons (Fig. 7f). Similar results were obtained in DRG neurons in which Tace expression had been ablated by shRNA infection (Supplementary Fig. 7). Inhibition of Tace expression resulted in an ~50% increase in the amount of NRG1 type III on the axonal surface (Fig. 7g). These results confirm that TACE cleaves NRG1 and suggest that TACE inhibits myelination by limiting the amount of functional NRG1 type III on the axonal surface.
TACE alters NRG1 type III signaling and myelination
Despite the key role of NRG1 type III in myelination, this is not the sole NRG1 isoform to be expressed by DRG neurons. In particular, DRG neurons also express NRG1 type I29, which is also processed by α-secretases1 and has an amino acid sequence that is identical to that of NRG1 type III in the region cleaved by TACE. Thus, TACE could affect myelination by inhibiting the processing of NRG1 type I. Similarly, TACE could process other molecules that regulate myelin formation.
To further investigate the role of TACE-specific processing of NRG1 type III, we downregulated TACE activity in neurons in which the NRG1 type III isoform was disrupted (Nrg1 type III–/– neurons) (Supplementary Fig. 8b) and investigated whether ablation of Tace could rescue myelination in these neurons1. We infected Nrg1 type III–/– DRG neurons with Tace-specific shRNA or with a scrambled shRNA control and rid the cultures of endogenous Schwann cells and fibroblasts (Fig. 8 and Supplementary Fig. 8a). We then repopulated the neurons with rat primary Schwann cells and, 21 d after the induction of myelination, we examined MBP and neurofilament expression by immunofluorescence (Fig. 8a) and western blot (Supplementary Fig. 8c) analyses. Ablation of Tace in Nrg1 type III–/– neurons did not rescue myelination (Fig. 8).
Figure 8.
TACE regulates NRG1 type III activity. (a) Cocultures of wild-type (WT) and Nrg1 type III–/– (KO) DRG neurons that were uninfected or infected with Tace-specific shRNA (sh1) or scrambled shRNA (shscr) were maintained in myelinating conditions with rat Schwann cells for 21 d and then stained for MBP (rhodamine) and neurofilament (fluorescein). Tace knockdown does not rescue myelination in Nrg1 type III–/– neurons. Scale bar, 50 μm. (b) Cocultures of wild-type or Nrg1 type III–/– DRG neurons were uninfected or infected with full-length NRG1 type III or NRG1 cleaved at the TACE cleavage site. These cocultures were maintained in myelinating conditions with wild-type rat Schwann cells for 14 d, fixed and stained for MBP (rhodamine) and neurofilament (fluorescein). NRG1 cleaved by TACE inefficiently rescued myelination in Nrg1 type III–/– neurons. The few MBP-positive segments observed (nine coverslips from three experiments) are shown. Scale bar, 50 μm. (c) Rat primary Schwann cells were starved for 16 h and not stimulated (control) or stimulated with 50 ng ml–1 NRG1 β1 (NRG1), 50 ng ml–1 NRG1 β1 cleaved by TACE (NRG1 + TACE) or 50 ng ml–1 TACE alone (TACE). Lysates of these cells were blotted with antibodies to p-AKT, total AKT (tot-AKT) and calnexin as a loading control. (d) Rat primary Schwann cells were starved for 16 h and not stimulated (control) or stimulated with 50 ng ml–1 NRG1 β1 (NRG1), 50 ng ml–1 NRG1 β1 cleaved by BACE1 (NRG1+BACE1) or 50 ng ml–1 BACE1 alone (BACE1). Lysates from these cells were blotted with antibodies to p-AKT, total AKT and calnexin as a loading control. (e) NRG1 cleaved by TACE was detected on the axonal surface of Nrg1 type III–/– neurons infected with lentiviruses expressing NRG1 processed at the TACE cleavage site by live staining for the NRG1 hemagglutinin (HA) epitope (rhodamine) and neurofilament (fluorescein). Scale bar, 50 μm. (f) Electron micrographs of NRG1 type III+/–, NRG1 type III+/–; Tace+/– and wild-type P30 sciatic nerves. NRG1 type III+/–; Tace+/– fibers are normally myelinated. Scale bar, 2 μm. (g) g ratios of P30 sciatic nerve fibers as a function of axon diameter were lower in NRG1 type III+/–; Tace+/– mice than in NRG1 type III+/– mice (P < 0.0001). The graph represents the g ratios obtained from >200 myelinated axons (three mice per genotype).
As PI3K is a key effector of NRG1 signaling and myelination, we tested whether NRG1 cleaved by TACE or BACE1 modulated this pathway. Thus, we incubated NRG1 β1 with recombinant human TACE or BACE1 and determined p-AKT activation in starved primary rat Schwann cells. Importantly, TACE-cleaved NRG1 did not activate p-AKT (Fig. 8c), unlike BACE1-cleaved NRG1 (Fig. 8d). This result suggests that TACE and BACE1 differentially activate NRG1, leading to either impairment (TACE) or activation (BACE1) of PI3K signaling and, hence, of myelination in Schwann cells. Furthermore, unlike a lentiviral construct expressing full-length NRG1 type III, a lentiviral construct expressing a cDNA encoding the portion of NRG1 type III from the N terminus to the histidine located between the identified TACE cleavage sites (Fig 7e), rescued myelination of Nrg1 type III–/– DRGs inefficiently (Fig. 8b), despite being expressed on the axonal surface (Fig. 8e).
To further confirm the inhibitory role of TACE on NRG1 type III, we tested whether a cross with Tace+/– mice, whose neurons are myelinated normally, would rescue hypomyelination of Nrg1 type III+/– mice. Morphological analysis of compound Nrg1 type III+/–; Tace+/– P30 sciatic nerves (Fig. 8f) and g ratio measurements (Fig. 8g and Supplementary Table 1) showed that a 50% reduction in TACE activity was sufficient to rescue myelination in Nrg1 type III+/– mice.
Taken together, these results provide strong evidence that TACE inhibits myelination by modulating the amount of functional NRG1 type III on axonal membranes. They also describe a newly discovered level of regulation of PNS myelination.
DISCUSSION
In this study, we describe a previously uncharacterized inhibitory mechanism that regulates the extent of myelination in the PNS. As myelin is required for the correct transmission of electrical impulses along axons and for the preservation of axonal integrity, understanding the processes that direct myelin formation and assembly is important for the development of effective treatments for demyelinating diseases. Using TACE knockdown strategies in vitro and conditional inactivation of TACE in vivo, we show that this inhibitory role is neuron-autonomous. We further demonstrate that TACE activity regulates NRG1 type III processing on axons and modulates the PI3K pathway in Schwann cells, thereby ensuring the correct timing and and myelination levels of PNS axons. These results underscore the existence of several components that control myelination and, to our knowledge, represent the first example of a negative control of myelination by secretases.
Several secretases have previously been implicated in regulating myelination. Among these, only BACE1 (refs. 3,5) and the zinc peptidase nardylisin (NRD1)30 have been directly linked to NRG1 type III cleavage; both positively regulate myelination. Biochemical studies have shown that BACE1 cleaves NRG1 type III4. As Bace1–/– mice are hypomyelinated in the CNS5 and PNS3, similarly to NRG1 type III+/– mice1,2, BACE1 cleavage should activate NRG1 type III. NRD1 was originally identified as an enhancer of the activity of ADAM proteases, including TACE30. However, Nrd1–/– mice are hypomyelinated in the CNS and PNS, rather than hypermyelinated, as would be expected for a TACE hypomorph based on our results. This suggests that NRD1 may principally regulate other secretases that mediate NRG1 cleavage, including BACE1 (ref. 30). Moreover, in this study TACE was shown to mediate the cleavage of NRG1 type I30, which we and others have shown is not involved in PNS myelination1,2.
A key finding is that these inhibitory effects of TACE directly oppose those of BACE1 (refs. 3–5), as indicated by differential activation of the PI3K pathway in Schwann cells. TACE inactivation leads to an acceleration of the myelinating program in vitro and in vivo. Together, these data indicate that secretases provide a post-transcriptional control of the amount of functional NRG1 type III and suggest that the balance between BACE1 and TACE activity is a key determinant of the timing and extent of PNS myelination (Supplementary Fig. 9).
An important question is how the balance of NRG1 cleavage by these distinct secretases is regulated, including whether cleavage by one secretase precludes processing by the others. The specificity that is achieved by sheddases, which normally process multiple substrates, has not been completely clarified. Several studies have reported that secretases do not recognize a specific amino acid sequence31. This complexity is further enhanced by the fact that the same sheddase can process a single protein at different sites, as in the case of APP32. Thus, even though this is a highly conserved process, the actual mechanism by which regulated shedding occurs is unknown. Nonetheless, comparisons of the amino acid sequences of different substrates that are cleaved by the same secretase, together with the use of peptide libraries to identify putative consensus cleavage sequences, have helped in the identification of preferential amino acids that are targeted by secretases. Recent studies have reported that TACE selects small aliphatic residues immediately downstream of the cleavage site (P1′ site). Of note, for one of the cleavage sites identified by mass spec-trometry, the isoleucine in the P1′ position is among the preferred amino acids for TACE cleavage31.
The TACE cleavage site is very close to the previously identified BACE1 cleavage site of NRG1 type III4, suggesting that the two processing sites are mutually exclusive. TACE and BACE1 frequently have common substrates, although normally the corresponding cleavage sites are not nearby33. TACE-mediated processing and BACE1-mediated processing of NRG1 type III most probably occur at different subcellular locations, further strengthening the possibility that that these two events are distinct. The mature form of TACE is mainly located in the perinuclear space34 and in the plasma membrane35. In addition, TACE and NRG1 colocalize in lipid rafts, where they could interact36,37. By contrast, BACE1 is internalized from the plasma membrane to the endosomal compartments and then recycled to the late Golgi33,38. As NRG1 molecules are glycosylated39, they are probably delivered to the plasma membrane through the trans-Golgi network, where they may be activated by BACE1. Whether BACE1-mediated cleavage of NRG1 type III also occurs in endosomes and determines NRG1 type III recycling or degradation, as in the case of cleavage of APP40, is unknown. Further studies are required to establish the identity of the cellular organelles in which NRG1 type III processing occurs, as the different localization could influence NRG1 type III-mediated effects on myelination.
Our study suggests that TACE inhibits myelination by modulating NRG1 type III activity rather than total surface levels. Thus, in the absence of TACE, the active form of NRG1 was upregulated (Fig. 7). Our results indicate that TACE cleaves NRG1 type III in the β exon of the EGF domain, generating a nonfunctional molecule that inefficiently rescues myelination of Nrg1 type III–/– DRG neurons (Fig. 8). We suggest that this cleavage affects the affinity of NRG1 type III for the ERBB2-ERBB3 heterodimer, as indicated by enhanced ERBB2-ERBB3–Fc binding in the absence of TACE (Fig. 7). In agreement with this model, there was more p-AKT in femoral nerves of Tace–/– mice (Fig. 5), and NRG1 cleaved by TACE did not activate the PI3K pathway in Schwann cells (Fig. 8). A corollary to these findings is that nearby cleavage of NRG1 by BACE1 enhanced the affinity of NRG1 for ERBB receptors, as suggested by higher levels of AKT phosphorylation (Fig. 8).
The effects of TACE cleavage are neuron-autonomous. We observed the hypermyelinating phenotype only when Tace was ablated in neurons, further strengthening the likelihood that TACE acts on axonal NRG1 type III. More importantly, this hypermyelination closely resembled that of mice overexpressing Nrg1 type III–/–2 and was due to the higher number of myelin lamellae. In addition, reduced TACE activity was sufficient to rescue myelination in NRG1 type III+/– mice. Further, ablation of neuronal Tace determined aberrant myelination of small-caliber axons that would normally be unmyelinated, probably by altering the threshold levels of expression of axonal NRG1 type III1. Finally, inhibition of TACE in Nrg1 type III–/– neurons was not enough to rescue the myelination defect of these neurons, further suggesting that TACE processes NRG1 type III. Nonetheless, we cannot exclude the possibility that TACE might act on a separate molecule that is not functional in Nrg1 type III–/– neurons because it is inactive or absent owing to the absence of NRG1 type III. A likely candidate is Notch1, an inhibitor of myelination17, although Notch1 processing is not altered in cocultures of Schwann cells and DRG neurons infected with Tace shRNAs (Supplementary Fig. 2). In agreement, it has been shown recently that ADAM10 is the essential protease that regulates Notch1 cleavage, and does so in a ligand-dependent manner, whereas TACE participates in substrate cleavage in a ligand-independent manner41.
Surprisingly, defects in the Remak bundles of HB9-cre; Tacefl/fl mice partially resemble those of NRG1 type III+/– mice1. Unlike these NRG1-haploinsufficient mice, however, HB9-cre; Tacefl/fl mice were also characterized by unsorted fibers of large diameter, suggesting that TACE cleaves a different substrate in Remak bundles. Of note, MAL-overexpressing mice42 have similar alterations to and accumulation of p75NTR, another target of TACE6, in nonmyelinating Schwann cells. Accordingly, TACE inhibition altered the processing of p75NTR. Although we cannot exclude the contribution of p75NTR, it has been suggested that glia and not axonal p75NTR promotes myelination in vitro16 and remyelination in vivo43. That TACE might cleave molecules other than NRG1 type III that are also implicated in myelination is also suggested by our analyses of Mpz-cre; Tacefl/fl mice and by the reductions in the caliber of myelinated fibers in HB9-cre; Tacefl/fl mice.
In summary, we describe a previously uncharacterized mechanism that controls the regulation of myelination during PNS development, by modulating a signaling pathway. Recent studies suggest that NRG1 type III is dispensable for myelin maintenance but important for remyelination44,45. The question of whether TACE plays a part during myelin maintenance or remyelination will require further investigations. The therapeutic potential of TACE modulation is underscored by the development of several compounds that specifically target TACE and have already been tested in clinical trials for rheumatoid arthritis46. Thus, TACE inhibition may be a useful strategy to promote myelination in dysmyelinating peripheral neuropathies.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
Supplementary Table 1. Morphometric analyses of wild type and TACE–/– mice
SUPPLEMENTARY FIGURES
Supplementary Figure S1. TACE is expressed in Schwann and DRG neurons
RT-PCR (a) and Western blotting analyses (b), showing high level of TACE mRNA and protein expression in Schwann cells and lower levels in DRG neurons. Actin expression is a control for equal loading. In B) CHO cells extracts are used as positive control.
Supplementary Figure S2. TACE acts in a neuronal cell autonomous manner.
a-f) Cocultures of wild type DRG neurons infected with TACE sh2 and sh3, purified of endogenous Schwann cells and grown with wild type not infected rat Schwann cells. Cultures were maintained in myelinating conditions for 14 days, fixed and stained for the myelin protein MBP (rhodamine; a-c) and neurofilament (fluorescein; d-f). Numerous myelin segments are evident in sh TACE infected; fewer segments are present in control not infected cultures. Bars: 100 μm.
g-l) Cocultures of purified wild type not infected mouse DRG neurons repopulated with wild type rat Schwann cells previously infected with TACE sh2 and sh3. Cultures were maintained in myelinating conditions for 21 days, fixed and stained for the myelin protein MBP (rhodamine; g-i) and neurofilament (fluorescein; j-l). No difference in myelination extent is present in infected versus not infected or scramble infected cultures. Bars: 100 μm.
Supplementary Figure S3. Abnormalities in P0Cre//TACEflx/flx mice.
Ultrastructural analyses of P7 (a-d) and P30 (e-h) P0Cre//TACEflx/flx sciatic nerves. Mitochondria and vacuoles accumulate at both ages in the inner cytoplasmic collar of null animals but not in control wild type mice. Of note in f) the presence of large unsegregated and unmyelinated axons in a Remak bundles (asterisk). Bars: 1 μm.
Supplementary Figure S4. MALDI-TOF spectra of TACE cleavage sites of NRG1 type III.
Spectra generated by the Endopeptidase Glu-C alone (upper panels) and specific peaks identified upon digestion of NRG1 by TACE by the Endopeptidase Glu-C (lower panels).
Supplementary figure S5. TACE ablation does not rescue myelination of type III NRG1 null neurons.
a-d) Cocultures type III NRG1 null neurons infected with TACE specific shRNA (sh2 or sh3) were maintained in myelinating conditions together with wild type rat Schwann cells for 21 days; fixed and stained for MBP (rhodamine; a-b) and neurofilament (fluorescein; e-f). TACE knock down is not sufficient to rescue myelination in type III NRG1 null neurons Bar: 100 μm. e) Extracts prepared from these cocultures were probed for MBP and actin as loading control. MBP is detected only in extracts of wild type cocultures, but not in type III NRG1 null neurons either infected or not.
AcknowledgmenTS
We thank S. Arber (University of Basel) for providing the HB9-cre transgenic line, M. Filbin (Hunter College) for antibodies, P. Podini for assistance with electron microscopy, G. Dina and A. Cattaneo for technical support and Y. Poitelon for artwork. This study was supported by the Federazione Italiana Sclerosi Multipla (FISM) (grant 2007/PC/01) and the Compagnia di San Paolo (C.T.); by the US National Institute of Health (grants R01-NS045630 (M.L.F.), R01-NS055256 (L.W.), R01-GM64750 (C.P.B.) and RO1-NS26001 (J.L.S.)); by Telethon Italia (grants GGP08021 (M.L.F.), GGP071100 (L.W.) and GPP10007 (C.T., L.W. and M.L.F.)). C.T. is a recipient of a FISM Transition Career Award.
ONLINE METHODS
Mice and genotyping
Generation of Tace-floxed mice has been previously reported21. Mice were genotyped by PCR using the following primers: 5′-TTA CTCTTCTTACTAACAGTCCCCTG-3′ and 5′-AACTATCTCAAACAATAAG CTGAAGTG-3′. Homozygous Tace-floxed mice were crossed with the HB9-cre transgenic line to specifically delete Tace in motor neurons, with the mP0TOT-cre (Mpz-cre) transgenic line to ablate Tace in Schwann cells and with the CMV-cre transgenic line to generate complete Tace+/– mice. Generation of the HB9-cre, Mpz-cre and CMV-cre transgenic lines has been previously described22,27,28. The genotyping was performed by PCR analysis on genomic DNA. PCR was carried out at 94 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, followed by a 5 min extension at 72 °C for 40 cycles. The expected 850-nucleotide (nt) product for the wild-type allele and 1,000-nt product for the floxed allele were separated on a 1% agarose gel. The presence of the null allele was also determined by PCR on genomic DNA using the following primers: 5′-TTACTCTTCTTAC TAACAGTCCCCTG-3′ and 5′-GGGAGAGCCACACCTTGACC-3′. PCR was carried out at 94 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, followed by a 5-min extension at 72 °C for 40 cycles. The amplified fragment of 400 nt, corresponding to the null allele, was analyzed on a 1% agarose gel. Generation and analysis of Nrg1 type III–/– and Nrg1 type III+/– mice have been previously described1. All experiments involving animals followed protocols approved by the Animal Care and Use Committee of San Raffaele Scientific Institute.
Cell cultures
Mouse DRG neurons were isolated from embryonic day 14.5 embryos and established on collagen-coated glass coverslips as described1. Explants were cycled with fluoroxidine (FUDR, Sigma-Aldrich) to eliminate all non-neuronal cells. Neuronal medium was supplemented with 50 ng ml–1 NGF (Harlan, Bioproducts for Science). Primary rat Schwann cells were prepared as described1 and maintained in DMEM (BioWhittaker), 10% FBS (Invitrogen) and 2 mM l-glutamine (Invitrogen) until used. Rat Schwann cells were added (200,000 cells per coverslip) to establish explant cultures of DRG neurons, and myelination was initiated by supplementing the medium with 50 μg ml–1 ascorbic acid (Sigma-Aldrich).
Fc fusion proteins and binding experiments
Established explants of Tace–/–, wild-type, and DRG neurons previously infected with shRNA against Tace were incubated with supernatant containing ERBB2-ERBB3–Fc and an antibody to human Fc (Jackson ImmunoResearch) as described1. Quantification was performed on confocal images from three different experiments (five coverslips per experiment). All images with the same magnification, z-stack and laser intensity were analyzed using Image J version 1.44 (US National Institutes of Health).
Lentivirus production and infection
Individual shRNAs clones (TRCN0000031949, TRCN0000031952 and TRCN0000031953) specifically targeting mouse Tace were obtained through the RNAi Consortium (Open Biosystems). Lentiviral vectors were transfected into 293FT cells (Invitrogen) together with packaging plasmids pLP1, pLP2 and pLP/VSVG (Invitrogen) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. As a control, we used a vector encoding an shRNA directed against an oligonucleotide (5′-TCGTACGCGCAATACTTCGA-3′) whose sequence had been verified to be nonspecific by a database search against the human and the mouse genomes. The scrambled shRNA–encoding lentiviral vector was cloned in pLL3.7 and produced as described47. Lentivirus encoding full length NRG1 type III was generated as described1. The cDNA encoding NRG1 type III from the N terminus until the histidine between the identified TACE cleavage sites was amplified by PCR with the following primers: 5′ CAGATCACTAGTATGGAGATTTATTCC CCAGAC 3′ and 5′CACTTTCTCGAGTTAATGTTTGTAGAAGCTGGC 3′; the template used was full length NRG1 type III that was hemagglutinin-tagged in the extracellular region, cloned in a pLenti6/V5 plasmid with standard molecular biology techniques and confirmed by sequencing.
Viral supernatants were collected 48 h after transfection, centrifuged at 1,500 g for 15 min, divided into aliquots for one-time use and frozen at –80 °C. Freshly plated Schwann cells (106 cells per 100-mm plate) were incubated for 2 d with viruses at a 2/3 dilution (vol/vol) in DMEM, 10% FBS and 2 mM l-glutamine supplemented with forskolin and recombinant human NRG1 (EGF domain, R&D Systems ). Cells were expanded for 1 week more and maintained for 3 d in Schwann cell medium before use. Protein knockdowns were confirmed by western blotting. Mouse DRG neuronal explants were infected the day after the dissection and left in the presence of the virus for 24 h, after which cultures were either purified of endogenous Schwann cells to obtain pure DRG neurons by cycling them with antimitotic reagents, or cultured with endogenous Schwann cells. In the latter case, both DRG neurons and mouse primary Schwann cells were infected.
Electron microscopy and morphological analyses
Semithin and ultrathin sections were obtained as previously described48. Tissues were removed and fixed with 2% (vol/vol) glutaraldehyde in 0.12 M phosphate buffer, postfixed with 1% (wt/vol) osmium tetroxide and embedded in Epon (Fluka). Semithin sections (0.5 to 1 μm thick) were stained with toluidine blue and examined by light microscopy (Olympus BX51). Ultrathin sections (100 to 120 nm thick) were stained with uranyl acetate and lead citrate and examined by electron microscopy (Leo 912 Omega). Digitized nonoverlapping semithin sections images from corresponding levels of the sciatic nerve were obtained with a digital camera (Leica DFC300F) using a ×100 objective. g ratio measurements were performed on digitized nonoverlapping electron micrographs images. g ratios were determined by dividing the mean diameter of an axon without myelin by the mean diameter of the same axon with myelin.
To determine the size distribution of myelinated fibers in sciatic nerves, diameters of all fibers in at least ten images of randomly chosen representative images were measured and binned based on their width. All measurements were acquired on ×100 semithin section images using the ImageJ software (US National Institutes of Health).
TACE and BACE1 cleavage reaction
Human recombinant TACE (1 μg; R&D Systems cat. no. 930-ADB) was incubated with 2 μg of human recombinant NRG1 β1 (R&D Systems cat. no. 396-HB, residues 176–246), which differs from the mouse sequence by one amino acid upstream of the EGF domain, for 16 h at 37 °C in the presence of ZnCl2 and then separated on a Tris-tricine gel together with native, uncleaved NRG1 β1 protein for comparison. The gel was stained with Coomassie blue and then processed for mass spectrometry analysis.
To test TACE and BACE1 cleavage effects of NRG1 signaling, 1 or 2 μg of human recombinant TACE or BACE1 (R&D Systems cat. no. 931-AS) were incubated with 0.5 μg of human recombinant NRG1 β1 for 16 h at 37 °C. Cleaved NRG1 β1 was used to stimulate serum-starved rat primary Schwann cells for 20 min at 37 °C. Cells were then lysed and analyzed by western blot for PI3K-intermediate pathways.
MALDI-TOF MS analyses
Bands of interest were excised from gels, subjected to reduction by 10 mM dithiothreitol and alkylation by 55 mM iodoacetamide, and finally digested overnight with endoproteinase Glu-C (Roche). Supernatant mixture from the digestion (10 μl) was acidified with formic acid up to a final concentration of 10% (vol/vol), desalted with Stage Tip μC18 (Stage Tip packed with uC18 reverse phase resin Proxeon Biosystem)49 and analyzed on a MALDI-TOF Voyager-DE STR (Applied Biosystems) mass spectrometer using the dried droplet technique and α-cyano-4-hydroxycinnamic acid as matrix. Spectra were acquired in the reflector positive-ion mode, accumulated over a mass range of 750–4,000 Da with a mean resolution of about 15,000, then internally calibrated using matrix signals and Glu-C autolysis peaks and processed using Data Explorer software version 4.0.0.0 (Applied Biosystems). Assignment of singly protonated species peaks was carried out manually using GPMAW software (version 8.10).
RNA isolation and measurements
Total RNA was isolated from purified rat Schwann cells, purified mouse DRG neurons and mouse spinal cord using Trizol (Roche), according to the manufacturer's instructions. Total RNA was reverse-transcribed to cDNA as previously described50. Aliquots of the reverse transcription products were tested in parallel using a primer pair for Tace (5′-TTGAAGAATACTTGTAAATT-3′ and 5′-GGGTTGTAATAAGCTTTT GG-3′; 94 °C for 30 s, 52 °C for 30 s and 72 °C for 60 s, followed by a 5 min extension at 72 °C for 30 cycles).
Preparation of detergent lysates and immunoblotting
Tissues and cell cultures were Dounce-homogenized in a lysis buffer containing 2% (wt/vol) SDS, 95 mM NaCl, 10 mM EDTA, phosphatase inhibitor (PhoSTOP, Roche) and pro-tease inhibitors (Complete-Mini EDTA free, Roche). Homogenates were boiled for 5 min and centrifuged for 10 min at 16,800 g at 16 °C. Supernatants were divided into aliquots and stored at –80 °C until used. Protein concentrations were determined by the bicinchoninic acid method (Pierce); samples (20–40 μg of protein) were fractionated by SDS-PAGE and blotted onto nitrocellulose (Protran Biosciences). Membranes were blocked in 5% BSA, 0.05% sodium azide in TBST (0.1% Triton X-100 in TBS). Appropriate regions were excised, incubated with specific primary and secondary antibodies, washed in TBST and developed with the SuperSignal chemiluminescent substrate (Pierce). For quantitative western blotting, filters were analyzed using the Odyssey Infrared Imaging System (LI-COR Biosciences) according to the manufacturer's instructions.
Antibodies and immunofluorescence
Mouse monoclonal antibodies included antibodies to MBP (SMI-94, SMI-99) and neurofilament (SMI-31 and SMI-32) (Sternberger Monoclonals) and to Notch-1 (NICD) (Chemicon). Rabbit polyclonal antibodies included antibodies to NRG1 (sc-348) (Santa Cruz); MAG1; MPZ (M. Filbin); actin (Sigma-Aldrich); peripherin, TACE and p75NTR (Chemicon); and Ser473 p-AKT and total AKT (Cell Signaling). Chicken antibodies were to neurofilament M (Covance). Secondary antibodies conjugated to rhodamine, fluorescein, coumarin, Cy5, or horseradish peroxidase were obtained from Jackson ImmunoResearch. Infrared secondary antibodies for quantitative western blotting (goat anti-rabbit and goat anti-mouse IRDye 680 or IRDye 800) were obtained from LI-COR Biosciences. Cocultures were fixed in 4% (wt/vol) paraformaldehyde, permeabilized in 100% methanol at –20 °C for 15 min, stained as described1 and examined by epifluorescence on a Nikon E800 microscope and by confocal microscopy on a Zeiss LSM 510 or on a Leica SP5.
Statistical analyses
Statistical analyses (Fisher's exact test, the chi-squared test and Student's t-test) were performed using the Prism Software package (GraphPad). P < 0.05 was taken as the limit of statistical significance.
Footnotes
AUTHOR CONTRIBUTIONS
R.L.M. conducted most of the experiments. F.C. and A.Q. performed morphological and ultrastructural analyses of sciatic nerves and ventral roots. K.H., C.P.B., M.L.F. and L.W. provided transgenic lines and helped with discussions. A.B. performed the mass spectrometry analyses. J.L.S. provided support and initially contributed to the experimental design. C.T. designed the experimental plan, supervised the project and wrote the paper. All authors commented on the paper.
Note: Supplementary information is available on the Nature Neuroscience website.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 3.Willem M, et al. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 4.Hu X, et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Baker KA, Hagg T. The ADAMs family: coordinators of nervous system development, plasticity and repair. Prog. Neurobiol. 2006;79:73–94. doi: 10.1016/j.pneurobio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Shirakabe K, Wakatsuki S, Kurisaki T, Fujisawa-Sehara A. Roles of Meltrin β/ADAM19 in the processing of neuregulin. J. Biol. Chem. 2001;276:9352–9358. doi: 10.1074/jbc.M007913200. [DOI] [PubMed] [Google Scholar]
- 8.Yokozeki T, et al. Meltrin beta (ADAM19) mediates ectodomain shedding of Neuregulin beta1 in the Golgi apparatus: fluorescence correlation spectroscopic observation of the dynamics of ectodomain shedding in living cells. Genes Cells. 2007;12:329–343. doi: 10.1111/j.1365-2443.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 9.Wakatsuki S, Yumoto N, Komatsu K, Araki T, Sehara-Fujisawa A. Roles of meltrin-β/ADAM19 in progression of Schwann cell differentiation and myelination during sciatic nerve regeneration. J. Biol. Chem. 2009;284:2957–2966. doi: 10.1074/jbc.M803191200. [DOI] [PubMed] [Google Scholar]
- 10.Sagane K, et al. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6:33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozkaynak E, et al. Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling. J. Neurosci. 2010;30:3857–3864. doi: 10.1523/JNEUROSCI.6287-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freese C, Garratt AN, Fahrenholz F, Endres K. The effects of alpha-secretase ADAM10 on the proteolysis of neuregulin-1. FEBS J. 2009;276:1568–1580. doi: 10.1111/j.1742-4658.2009.06889.x. [DOI] [PubMed] [Google Scholar]
- 13.Horiuchi K, Zhou HM, Kelly K, Manova K, Blobel CP. Evaluation of the contributions of ADAMs 9, 12, 15, 17, and 19 to heart development and ectodomain shedding of neuregulins β1 and β2. Dev. Biol. 2005;283:459–471. doi: 10.1016/j.ydbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 15.Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 16.Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298:1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- 17.Woodhoo A, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci. 2009;12:839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zampieri N, Xu CF, Neubert TA, Chao MV. Cleavage of p75 neurotrophin receptor by α-secretase and γ-secretase requires specific receptor domains. J. Biol. Chem. 2005;280:14563–14571. doi: 10.1074/jbc.M412957200. [DOI] [PubMed] [Google Scholar]
- 19.van Tetering G, et al. Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J. Biol. Chem. 2009;284:31018–31027. doi: 10.1074/jbc.M109.006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber S, et al. The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development. 2011;138:495–505. doi: 10.1242/dev.055210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horiuchi K, et al. Cutting edge: TNF-α-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J. Immunol. 2007;179:2686–2689. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 23.Donald D. A relation between axone diameter and myelination determined by measurement of myelinated spinal root fibers. J. Comp. Neurol. 1934;60:437–471. [Google Scholar]
- 24.Windebank AJ, Wood P, Bunge RP, Dyck PJ. Myelination determines the caliber of dorsal root ganglion neurons in culture. J. Neurosci. 1985;5:1563–1569. doi: 10.1523/JNEUROSCI.05-06-01563.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J. Neurosci. 2000;20:4635–4645. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogata T, et al. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feltri ML, et al. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer D, et al. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 30.Ohno M, et al. Nardilysin regulates axonal maturation and myelination in the central and peripheral nervous system. Nat. Neurosci. 2009;12:1506–1513. doi: 10.1038/nn.2438. [DOI] [PubMed] [Google Scholar]
- 31.Caescu CI, Jeschke GR, Turk BE. Active-site determinants of substrate recognition by the metalloproteinases TACE and ADAM10. Biochem. J. 2009;424:79–88. doi: 10.1042/BJ20090549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang HC, et al. Biochemical and kinetic characterization of BACE1: investigation into the putative species-specificity for β- and β′-cleavage sites by human and murine BACE1. J. Neurochem. 2004;91:1249–1259. doi: 10.1111/j.1471-4159.2004.02764.x. [DOI] [PubMed] [Google Scholar]
- 33.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlöndorff J, Becherer JD, Blobel CP. Intracellular maturation and localization of the tumour necrosis factor α convertase (TACE). Biochem. J. 2000;347:131–138. [PMC free article] [PubMed] [Google Scholar]
- 35.Doedens JR, Black RA. Stimulation-induced down-regulation of tumor necrosis factor-α converting enzyme. J. Biol. Chem. 2000;275:14598–14607. doi: 10.1074/jbc.275.19.14598. [DOI] [PubMed] [Google Scholar]
- 36.Tellier E, et al. The shedding activity of ADAM17 is sequestered in lipid rafts. Exp. Cell Res. 2006;312:3969–3980. doi: 10.1016/j.yexcr.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Frenzel KE, Falls DL. Neuregulin-1 proteins in rat brain and transfected cells are localized to lipid rafts. J. Neurochem. 2001;77:1–12. doi: 10.1046/j.1471-4159.2001.t01-1-00132.x. [DOI] [PubMed] [Google Scholar]
- 38.Koo EH, Squazzo SL. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J. Biol. Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 39.Burgess TL, Ross SL, Qian YX, Brankow D, Hu S. Biosynthetic processing of neu differentiation factor. Glycosylation trafficking, and regulated cleavage from the cell surface. J. Biol. Chem. 1995;270:19188–19196. doi: 10.1074/jbc.270.32.19188. [DOI] [PubMed] [Google Scholar]
- 40.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357:500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 41.Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol. Cell. Biol. 2009;29:5679–5695. doi: 10.1128/MCB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buser AM, et al. The myelin protein MAL affects peripheral nerve myelination: a new player influencing p75 neurotrophin receptor expression. Eur. J. Neurosci. 2009;29:2276–2290. doi: 10.1111/j.1460-9568.2009.06785.x. [DOI] [PubMed] [Google Scholar]
- 43.Tomita K, et al. The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia. 2007;55:1199–1208. doi: 10.1002/glia.20533. [DOI] [PubMed] [Google Scholar]
- 44.Fricker FR, et al. Sensory axon-derived neuregulin-1 is required for axoglial signaling and normal sensory function but not for long-term axon maintenance. J. Neurosci. 2009;29:7667–7678. doi: 10.1523/JNEUROSCI.6053-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fricker FR, et al. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J. Neurosci. 2011;31:3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moss ML, Sklair-Tavron L, Nudelman R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2008;4:300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 47.Maurel P, et al. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J. Cell Biol. 2007;178:861–874. doi: 10.1083/jcb.200705132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quattrini A, et al. β4 integrin and other Schwann cell markers in axonal neuropathy. Glia. 1996;17:294–306. doi: 10.1002/(SICI)1098-1136(199608)17:4<294::AID-GLIA4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 50.Wrabetz L, et al. A minimal human MBP promoter-lacZ transgene is appropriately regulated in developing brain and after optic enucleation, but not in shiverer mutant mice. J. Neurobiol. 1998;34:10–26. doi: 10.1002/(sici)1097-4695(199801)34:1<10::aid-neu2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Morphometric analyses of wild type and TACE–/– mice
SUPPLEMENTARY FIGURES
Supplementary Figure S1. TACE is expressed in Schwann and DRG neurons
RT-PCR (a) and Western blotting analyses (b), showing high level of TACE mRNA and protein expression in Schwann cells and lower levels in DRG neurons. Actin expression is a control for equal loading. In B) CHO cells extracts are used as positive control.
Supplementary Figure S2. TACE acts in a neuronal cell autonomous manner.
a-f) Cocultures of wild type DRG neurons infected with TACE sh2 and sh3, purified of endogenous Schwann cells and grown with wild type not infected rat Schwann cells. Cultures were maintained in myelinating conditions for 14 days, fixed and stained for the myelin protein MBP (rhodamine; a-c) and neurofilament (fluorescein; d-f). Numerous myelin segments are evident in sh TACE infected; fewer segments are present in control not infected cultures. Bars: 100 μm.
g-l) Cocultures of purified wild type not infected mouse DRG neurons repopulated with wild type rat Schwann cells previously infected with TACE sh2 and sh3. Cultures were maintained in myelinating conditions for 21 days, fixed and stained for the myelin protein MBP (rhodamine; g-i) and neurofilament (fluorescein; j-l). No difference in myelination extent is present in infected versus not infected or scramble infected cultures. Bars: 100 μm.
Supplementary Figure S3. Abnormalities in P0Cre//TACEflx/flx mice.
Ultrastructural analyses of P7 (a-d) and P30 (e-h) P0Cre//TACEflx/flx sciatic nerves. Mitochondria and vacuoles accumulate at both ages in the inner cytoplasmic collar of null animals but not in control wild type mice. Of note in f) the presence of large unsegregated and unmyelinated axons in a Remak bundles (asterisk). Bars: 1 μm.
Supplementary Figure S4. MALDI-TOF spectra of TACE cleavage sites of NRG1 type III.
Spectra generated by the Endopeptidase Glu-C alone (upper panels) and specific peaks identified upon digestion of NRG1 by TACE by the Endopeptidase Glu-C (lower panels).
Supplementary figure S5. TACE ablation does not rescue myelination of type III NRG1 null neurons.
a-d) Cocultures type III NRG1 null neurons infected with TACE specific shRNA (sh2 or sh3) were maintained in myelinating conditions together with wild type rat Schwann cells for 21 days; fixed and stained for MBP (rhodamine; a-b) and neurofilament (fluorescein; e-f). TACE knock down is not sufficient to rescue myelination in type III NRG1 null neurons Bar: 100 μm. e) Extracts prepared from these cocultures were probed for MBP and actin as loading control. MBP is detected only in extracts of wild type cocultures, but not in type III NRG1 null neurons either infected or not.