Abstract
Objective
To investigate the effects of peroxisome proliferator-activated receptor delta (PPARδ) in the cerebral vasculature following stroke-induced brain injury.
Methods and Results
Here we report a novel finding that selective PPARδ genetic deletion in vascular smooth muscle cells (VSMCs) resulted in increased cerebrovascular permeability and brain infarction in mice after middle cerebral artery occlusion (MCAO). Mechanistically, we revealed for the first time that PPARδ expression is reduced, but MMP-9 activity is increased in cultured VSMCs after oxygen-glucose deprivation (OGD) and also in the cerebral cortex of mice following MCAO. Moreover, gain- and loss-of-PPARδ function in VSMCs significantly reduces and increases OGD-induced MMP-9 activity, respectively. We have further identified that MMP-9 is a direct target of PPARδ-mediated transrepression by chromatin immunoprecipitation and PPARδ transcriptional activity assays. Furthermore, inhibition of MMP-9 activity by lentiviral MMP-9 shRNA effectively improves cerebrovascular permeability and reduces brain infarction in VSMC-selective PPARδ conditional knockout mice after MCAO.
Conclusion
Our data demonstrate that PPARδ in VSMCs can prevent ischemic brain injury by inhibition of MMP-9 activation and attenuation of post-ischemic inflammation. The pharmacological activation of PPARδ may provide a new therapeutic strategy to treat stroke-induced vascular and neuronal damage.
Keywords: PPARδ, MMP-9, inflammation, cerebral vascular smooth muscle cell, cerebral ischemia
Introduction
The cerebral vasculature is a major target of ischemic insult in the brain. Activation of vascular cells, along with attendant extracellular matrix (ECM) degradation and subsequent inflammatory responses, contribute to vascular injury, resulting in blood brain barrier (BBB) disruption, hemorrhagic transformation, and eventual neuronal loss after cerebral ischemia 1, 2. As one of the major cell types in the brain vasculature, vascular smooth muscle cells (VSMCs) play a dominant role in the regulation of cerebral vascular tone, vascular integrity and cerebral homeostasis in physiological conditions. However, the pathological role and molecular mechanisms of VSMCs in cerebral ischemia are largely unknown.
The peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors in which three distinct isoforms (PPARα, γ and δ) have been identified in tissue. Beyond the initially-identified metabolic effects, PPAR activation also induces anti-inflammatory effects in the brain, and thus may serve as a new pharmacological target for the treatment of neurological diseases 3. Recently, accumulating evidence suggests that PPARγ activation by thiazolidinediones (TZDs), a class of synthetic ligands used to treat type 2 diabetes 4, reduces infarct size and improves functional recovery from stroke in rodent models 5, 6. While most studies have shown that PPARγ and PPARα agonists exert neuroprotective effects in rodent models of cerebral ischemia, the role of PPARδ, the highest expressed PPAR subtype in brain parenchyma and the cerebral vasculature, is poorly understood in the context of ischemic stroke 7.
The matrix metalloproteinase (MMP) family is a class of zinc-dependent endoproteases responsible for degradation of structural proteins in the extracellular matrix 8. Activation of MMPs plays an important role in the pathogenesis of various neurological diseases, including stroke. For example, mice with a genetic deletion of either the MMP-2 or MMP-9 gene are resistant to stroke-triggered vascular and brain parenchymal damage 9, 10. Of significance, because of their destructive potential, MMPs are tightly controlled at multiple levels in physiological conditions 11. Several recent studies have documented that MMP activity in vascular cells is negatively regulated by PPARγ 12, 13. However, whether PPARδ is able to regulate MMP expression is still largely unexplored.
In the present study, we utilized mice with VSMC-selective PPARδ deletion to explore the effects and molecular mechanisms of vascular PPARδ on ischemia-induced brain injury. We have identified for the first time that MMP-9 is a novel target of PPARδ trans-repression, and this inhibition contributes to PPARδ-mediated vascular and neuronal protection against ischemic insults.
Methods
Mouse model of transient focal cerebral ischemia
VSMC-selective PPARδ knockout (SMPδ cKO) and littermate control mice (LC) were subjected to middle cerebral artery occlusion (MCAO) for 30 minutes and followed by 24 h reperfusion 14.
Cell cultures
Primary VSMCs were obtained from wild-type mouse aortas 15.
Oxygen-glucose deprivation (OGD)
To mimic ischemia-like conditions in vitro, cell cultures were exposed to OGD 14.
Statistical analysis
Quantitative data were expressed as mean ± SD or SEM and analyzed using Prism Software. Statistical comparisons between two groups were performed by Student’s t test, and comparisons among three or more groups were analyzed by one-way ANOVA. Groups were considered significantly different if P values were <0.05.
Results
VSMC-specific PPARδ deletion potentiates brain injury in mice after focal cerebral ischemia
To investigate the role of PPARδ in VSMCs, we generated VSMC-selective PPARδ knockout (SMPδ cKO) mice by crossing PPARδflox/flox mice 16 with SM22α-Cre knock-in mice that we previously developed 17. The western blot, as shown in the Supplemental Figure I, confirms that PPARδ protein expression was completely absent in aortic smooth muscle cells of SMPδ cKO mice, while PPARδ expression was significantly decreased in the cerebral vessels.
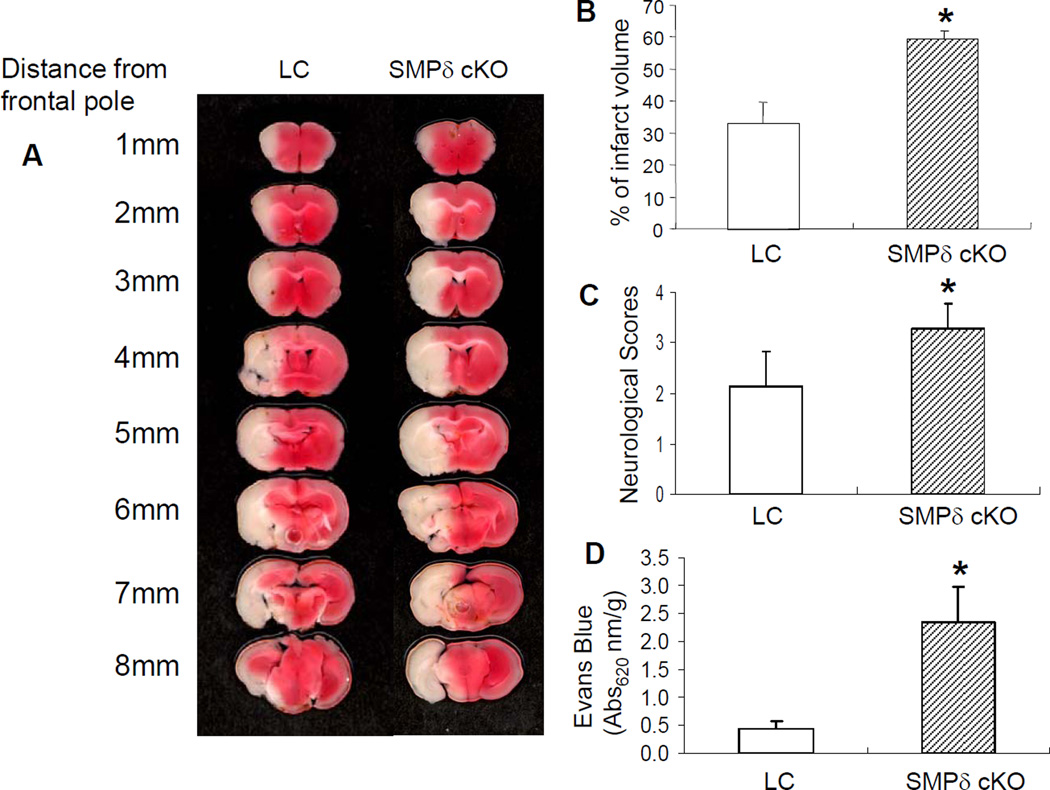
To determine the role of VSMC-specific PPARδ function in ischemic brain injury, SMPδ cKO and littermate control (LC) mice were subjected to transient MCAO for 30 min followed by 24 h reperfusion (n= 8–10). Cerebral infarction, neurological outcomes, and cerebral vascular permeability were determined by 2% 2,3,5-triphenyltetrazolium chloride (TTC) staining and Evans Blue extravasation, respectively. In comparison with the LC mice, SMPδ cKO mice showed a larger cerebral infarct volume (Figure 1A–B) and a significantly severer neurological deficit (Figure 1C) in response to ischemic insults. Of significance, VSMC-selective PPARδ deletion also aggravated ischemia-induced BBB disruption by increasing cerebrovascular permeability (Figure 1D). Taken together, these results suggest that PPARδ in VSMCs plays a critical role in the maintenance of vascular structure and function. Loss-of-PPARδ function in the cerebral vasculature appears to exacerbate ischemic brain damage.
Figure 1.
The effect of VSMC PPARδ on ischemia-induced brain infarction and cerebrovascular permeability. VSMC-selective PPARδ knockout (SMPδ cKO) and littermate control (LC) mice were subjected to 30 min MCAO and 24 h reperfusion. 2% TTC-stained coronal sections are shown at different brain levels posterior to the frontal pole (A). Quantitative analysis was performed on infarct volume (B) and neurological deficits (C) in mice after stroke. Quantitative analysis of cerebrovascular permeability was determined 1 h after Evans Blue injection (D). Compared to LC mice, SMPδ cKO mice show potentiated ischemia-induced brain infarction (n=7), neurological outcomes (n=7) and cerebrovascular permeability (n=6). Data are expressed as mean ± SD. *p < 0.05 vs the LC group.
PPARδ deletion in VSMCs increases post-ischemic inflammatory responses in mouse brain
It is well-established that inflammatory responses contribute to the development of ischemic brain damage 18. To determine whether genetic deficiency of PPARδ in VSMCs affects post-ischemic inflammatory mechanisms, we examined the expression of several pro-inflammatory cytokines in the mouse brain after 24 h MCAO. As indicated in Supplemental Figure II, loss-of-PPARδ function in VSMCs had no significant effect on the endogenous expression of most cytokines. However, MCAO caused a significant increase in the mRNA levels of cytokines, including IL-1β, IL-6, ICAM-1, and MCP-1 in LC mouse brains. Intriguingly, VSMC deletion of PPARδ significantly exacerbated ischemia-induced elevations of IL-1β, IL-6, ICAM-1 and MCP-1 mRNA levels, whereas no changes were found in the expression of TNF-α and VCAM-1 in SMPδ cKO mouse brains.
VSMC-selective PPARδ deletion exacerbates ischemia-induced activation of MMP-9
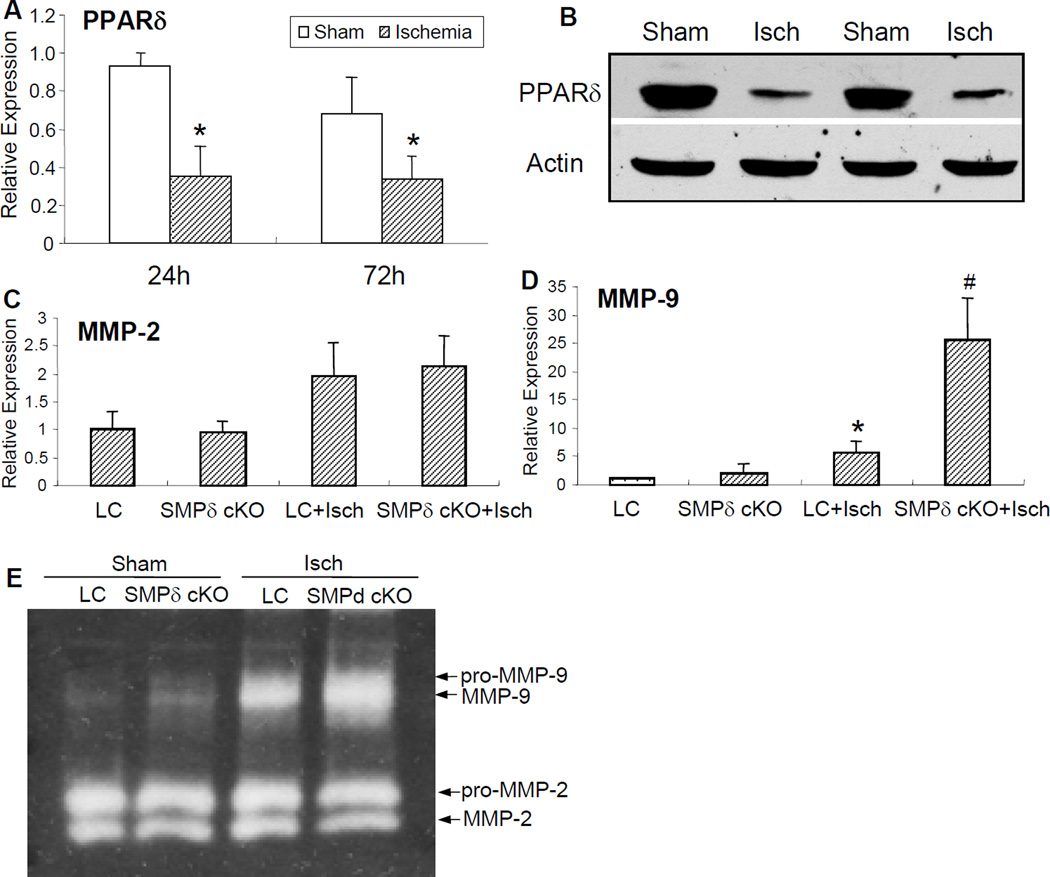
Activation of MMPs and subsequent degradation of extracellular matrix play a critical role in the pathogenesis of ischemia-induced vascular and neuronal injury 9, 10. Earlier studies have demonstrated that PPARγ is able to negatively regulate MMP activity in various tissues, including the vasculature 12, 13, 19. To explore whether MMPs function as an important link to VSMC PPARδ deletion-triggered neurological dysfunction, we employed quantitative PCR and Western blotting to evaluate their temporal profiles (Figure 2). Compared to sham experimental groups, PPARδ mRNA expression (Figure 2A) and protein levels (Figure 2B) were significantly decreased in the wild-type mouse cerebral cortex after 24 h and 72 h of MCAO. Conversely, MMP-9 mRNA (Figure 2D) and activity (Figure 2E) were induced and increased in the LC mouse cerebral cortex subjected to MCAO. Interestingly, VSMC-selective PPARδ deletion further exacerbated activation of MMP-9, but not MMP-2 activity (Figure 2C–E). These results suggest that vascular PPARδ negatively modulates MMP-9 expression in the cerebral cortex.
Figure 2.
The expression of MMPs in the brains of VSMC-selective PPARδ knockout (SMPδ cKO) mice following MCAO and 24 h reperfusion. Focal cerebral ischemia caused a time-dependent reduction of PPARδ mRNA (A) and protein (B) levels in the brains of wild-type mice 24–72 h after MCAO. Conversely, quantitative PCR data shows that MMP-9 (D), and not MMP-2 mRNA expression (C) was significantly elevated in the mouse brains of SMPδ cKO compared to littermate control (LC) mice. Concomitantly, mice with VSMC-selective PPARδ gene deletion also displayed a potentiated MMP-9 activity as determined by gelatin-based zymography in comparison with the LC mice (E). * P<0.05 vs sham group. # P<0.05 vs LC group.
PPARδ negatively regulates OGD-induced MMP-9 mRNA and activity n VSMCs
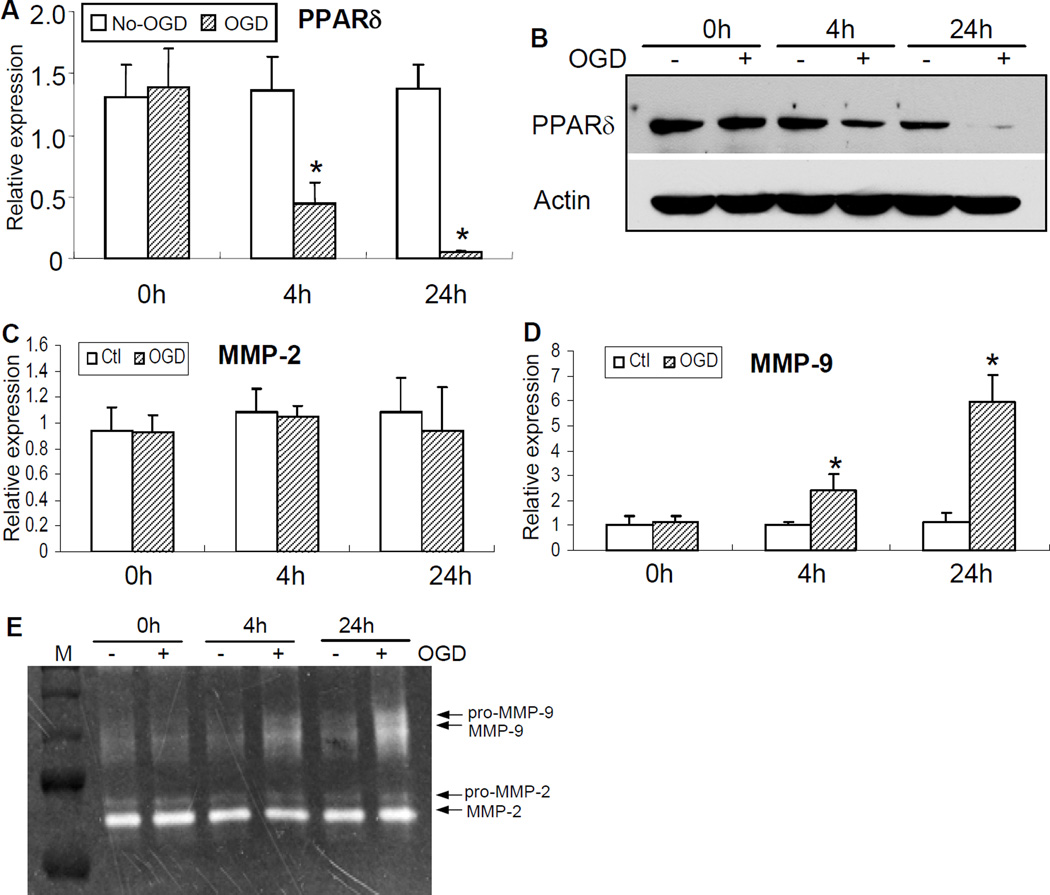
To further define the molecular mechanisms of MMPs responsible for VSMC PPARδ genetic deficiency-mediated ischemic brain injury, we examined PPARδ regulatory effects on the expression of MMPs in mouse VSMC culture. As shown in Figure 3, OGD caused a rapid reduction in PPARδ mRNA and protein expression in VSMCs, decreasing at 4 h and maintaining a lower level at 24 h after OGD onset (Figure 3A–B). In contrast to OGD-induced reduction of PPARδ levels, MMP-9 mRNA and enzymatic activity were gradually increased in VSMCs after OGD, starting at 4 h and persisting at least to 24 h after OGD stimuli (Figure 3D–E). Of interest, OGD did not significantly affect MMP-2 mRNA expression and enzymatic activity in cultured VSMCs (Figure 3C, E).
Figure 3.
The expression of PPARδ and MMP activity in mouse VSMC culture after OGD. OGD resulted in a time-dependent decrease in PPARδ mRNA expression (A) and protein levels (B), starting at 4 h post-OGD and persisting at least to 24 h after OGD. In contrast, OGD triggered an increase in MMP-9 mRNA (D) and enzymatic activity (E), initiating at 4 h post-OGD, and persisting to at least 24 h after OGD. Of note, ODG had no significant effects on MMP-2 mRNA and enzymatic activity in VSMCs (C, E). * P<0.05 vs non-OGD controls.
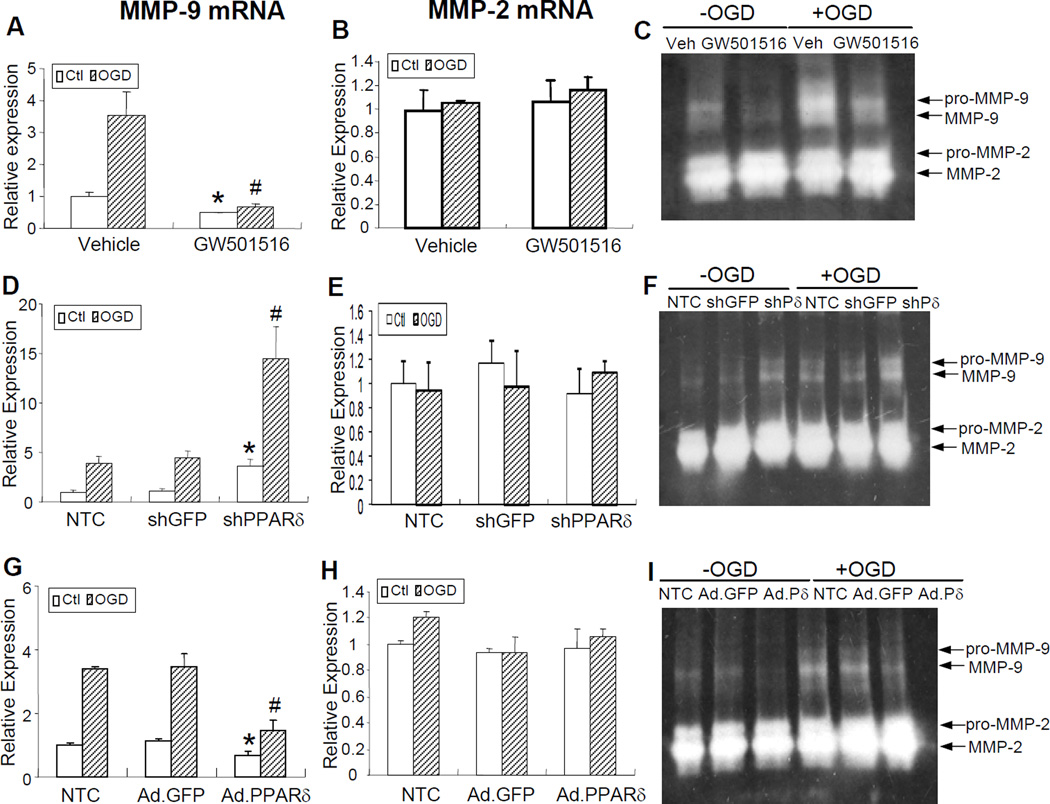
To further explore the contributory role of PPARδ in the regulation of MMP-9 activation in VSMCs after OGD exposure, gain- or loss-of-PPARδ function was achieved by infecting cells with either an adenovirus carrying PPARδ or a retrovirus carrying shPPARδ. The success of the gain-of-PPARδ function approach was demonstrated by the high percentage of infected VSMCs (Supplemental Figure III A) and significantly increased PPARδ protein levels (Supplemental Figure III B). As demonstrated in Figure 4, treatment of VSMCs with the PPARδ agonist GW 501516 at a final concentration of 1µM effectively reduced MMP-9 mRNA and enzymatic activity after 24 h of OGD (Figure 4A, C). Similarly, gain-of-PPARδ function in VSMCs by adenovirus-mediated gene transfer partially reversed OGD-induced activation of MMP-9 mRNA and activity (Figure 4G, I), whereas loss-of-PPARδ function in VSMCs by effective retrovirus-mediated gene transfer (Supplemental Figure III C) exacerbated OGD-induced activation of MMP-9 mRNA and activity compared to non-transfected control groups (Figure 4D, F). As expected, adenoviral or retroviral GFP gene transfer into VSMCs has no effect on the expression of MMPs, confirming the specific action of virus-mediated PPARδ gene upregulation or downregulation. Of note, treatment of VSMCs with GW 501516 or genetic modulation of PPARδ has no effect on MMP-2 mRNA and enzymatic activity after OGD exposure (Figure 4B, E, H).
Figure 4.
PPARδ regulation of MMP-9 levels in mouse VSMCs. PPARδ agonist GW501516 attenuated OGD-induced activation of MMP-9 mRNA as assessed by quantitative PCR (A) and MMP-9 enzymatic activity as detected by gelatin-based zymography (C). However, GW501516 treatment did not have a significant effect on MMP-2 mRNA (B). Loss-of-PPARδ function by retrovirus-mediated RNA interference exacerbated OGD-triggered activation of MMP-9 mRNA (D) and activity (F) in cultured VSMCs. However, MMP-2 mRNA was not affected by this treatment (E). Infection of VSMCs with adenoviral PPARδ (Ad.PPARδ), but not Ad.GFP effectively attenuated OGD-induced MMP-9 mRNA (G) and MMP-9 enzymatic activity (I). The same treatment did not have any effect on MMP-2 mRNA levels (H). Data are expressed as mean ± SEM. * or # P<0.05 vs vehicle or shGFP or Ad.GFP group.
Taken together, these results suggest that PPARδ activation can repress MMP-9 activity in VSMCs after OGD treatment.
PPARδ binds directly to the PPRE site in the MMP-9 promoter region and transrepresses its expression
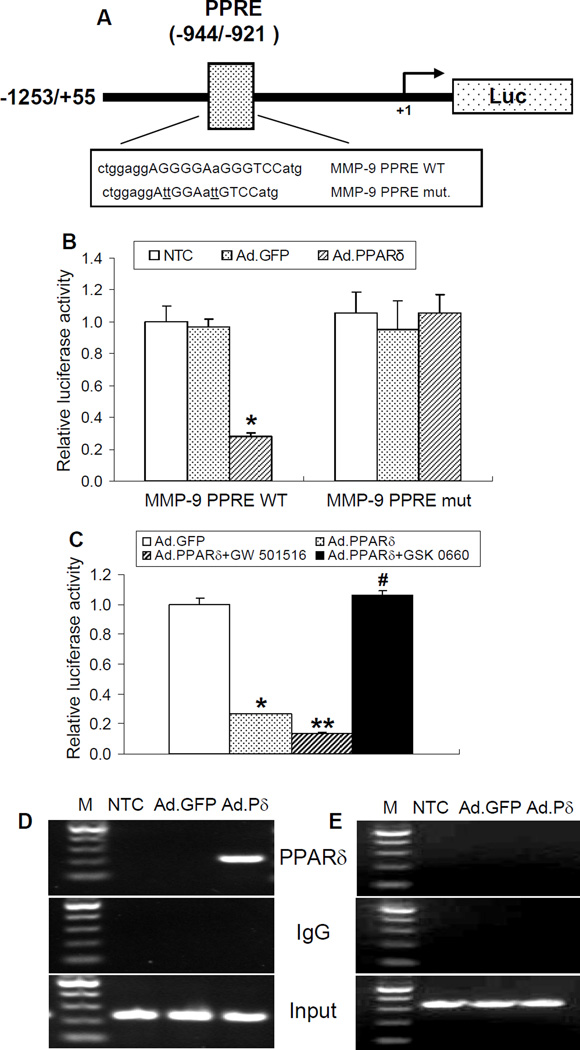
To further study whether PPARδ suppresses MMP-9 expression in a transcriptional manner, we performed a functional analysis of potential PPAR response elements (PPREs) in the mouse MMP-9 promoter. As indicated in Figure 5A, we identified a putative PPARδ binding site in the promoter of the MMP-9 gene at the location of −944 to −921 bp. To determine the functionality of this PPRE in the MMP-9 gene promoter, we cloned a 1,309 bp (−1253/+55) promoter of the mouse MMP-9 gene into a luciferase reporter vector, and the transcriptional response of the MMP-9 promoter to PPARδ was performed using a luciferase transcriptional assay in vitro. HEK 293 cells were transfected with a 1.3 kb promoter (MMP-9 PPRE WT) or a promoter with the corresponding site-directed mutation on the identified PPRE (MMP-9 PPRE mut). Cells were also infected with either an adenovirus carrying PPARδ to overexpress PPARδ, or adenoviral GFP as an infection control. As shown in Figure 5B, PPARδ overexpression significantly reduced the transcriptional activity of the MMP-9 1.3 kb promoter. Moreover, luciferase activities from the MMP-9 1.3 kb promoter driven by the mutated PPRE did not respond to PPARδ overexpression, indicating that the predicted PPRE site in the promoter region of MMP-9 is responsible for PPARδ transrepression of MMP-9 expression in vitro. Moreover, PPARδ inhibition of MMP-9 promoter activity was further enhanced by co-treatment with the PPARδ agonist GW501516. However, co-treatment with the PPARδ antagonist GSK 0660 reversed PPARδ-induced inhibition of MMP-9 promoter activity, suggesting this effect is ligand-dependent (Figure 5C). Next, chromatin domains in the MMP-9 promoter were scanned for PPARδ binding by chromatin immunoprecipitation (ChIP) analysis in VSMCs. As shown in Figure 5D, consistent with PPRE-driven transcriptional activity, adenovirus-mediated overexpression of PPARδ is detectably bound to the PPRE site located between −944 to −921 bp upstream of the transcription start site. The specificity of the ChIP assay was confirmed by using one set of primers that amplified a nonspecific region located at nucleotides −3613 to −3282 of the MMP-9 promoter, as PPARδ does not bind to this nonspecific region (Figure 5E). An isotypic IgG antibody was employed as a negative immunoprecipitation control. These data strongly suggest that in the context of chromatin, the PPRE between nucleotides −944 to −921 appears to be functional, showing binding of PPARδ to the MMP-9 promoter.
Figure 5.
Functional analysis of a PPRE site located in the mouse MMP-9 promoter. (A) Schematic representation of a luciferase reporter construct containing ~1.3 kb of the 5’-upstream sequences of the mouse MMP-9 gene. A putative PPARδ binding site was demonstrated in the promoter of MMP-9 at locations of −944/−921 bp. The translation start site is numbered as +1. (B, C) Luciferase reporter assays were performed by transfecting HEK 293 cells with a luciferase reporter vector carrying either an MMP-9 1.3 kb wild-type promoter (MMP-9 PPRE WT) or an MMP-9 promoter with a mutated PPRE (MMP-9 PPRE mut.). Transcriptional activity of MMP-9 was reduced upon overexpression of PPARδ in HEK 293 cells containing the MMP-9 wild type but not mutated promoter (B). MMP-9 transcriptional activity was further inhibited by the PPARδ agonist GW 501516 (1µM) but reversed by the PPARδ antagonist GSK 0660 (1µM) (C). (D, E) ChIP assays showing PPARδ binding to the putative PPRE site (D, −944 to −921 bp) but not a distal region (E, −3613 to −3282 bp) in the mouse MMP-9 promoter. Mouse VSMCs were infected with an adenovirus carrying GFP or PPARδ for 48 h. DNA from PPARδ antibody-immunoprecipitated chromatin and input were subjected to PCR analysis using two pairs of primers covering either a region containing the PPRE site or a distal fragment in the mouse MMP-9 promoter. Data are expressed as mean ± SD. * P<0.05 vs Ad.GFP or no transfection control (NTC) group.
Taken together, these data document that the MMP-9 gene is a novel target of PPARδ transcriptional repression.
Inhibition of MMPs in SMPδ cKO mice attenuates cerebrovascular disruption and ischemic brain injury in vivo
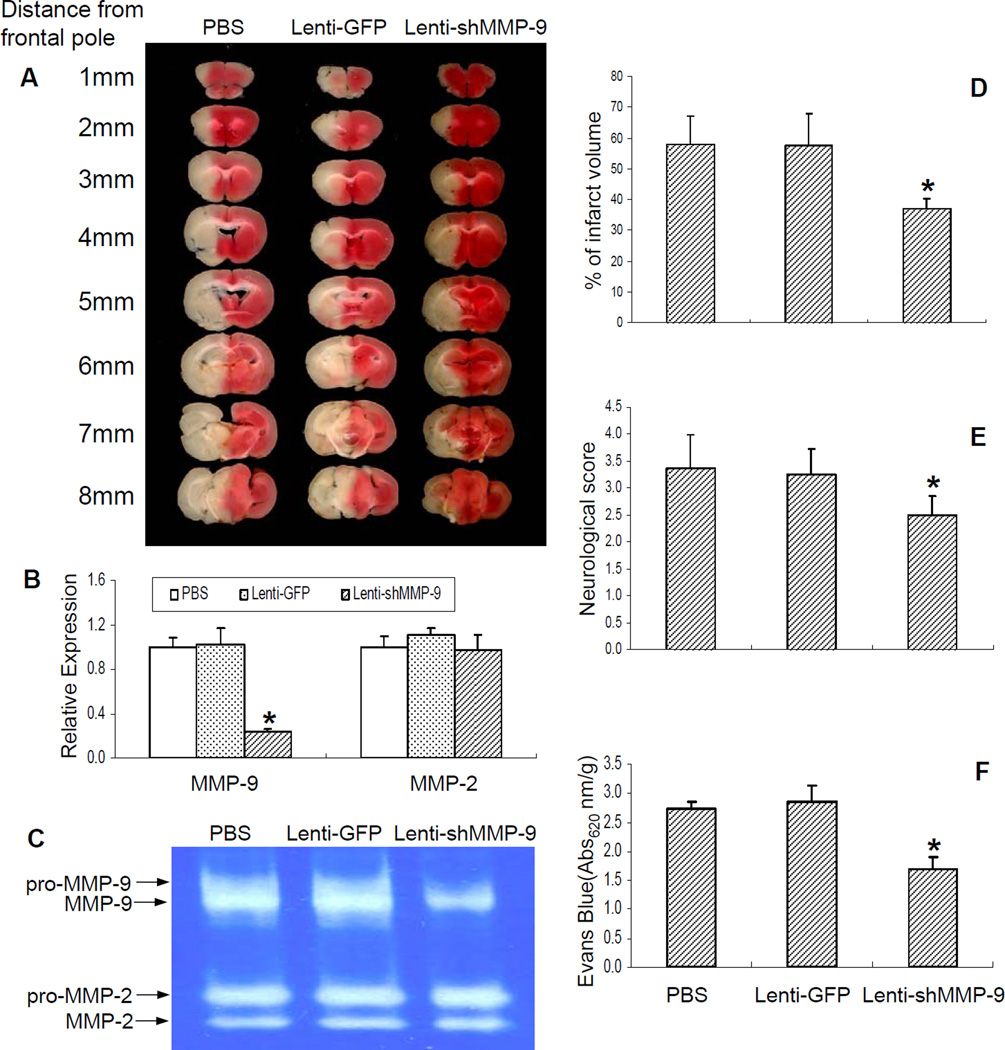
To further determine MMP-9 as a key modulator in PPARδ-mediated vaso- and neuroprotection in vivo, we stereotactically delivered a lentivirus carrying mouse shMMP-9 or GFP into the ischemic region in SMPδ cKO mice 4 weeks prior to MCAO, followed by MCAO and 24 h reperfusion. As shown in Figure 6, lentivirus-mediated cerebral MMP-9 knockdown obviously reduced ischemic brain infarction (Figures 6A, D) and neurological outcomes (Figure 6E). Moreover, this treatment also effectively improved cerebrovascular permeability in SMPδ cKO mice (Figure 6F). The specificity of lentivirus-mediated inhibition of MMP-9 in the cerebral cortex was confirmed by real-time PCR (Figure 6B) and gelatin-based zymography (Figure 6C), showing a unique reduction in MMP-9, but not MMP-2 mRNA levels and activity. These results further demonstrate that MMP-9 functions as a major downstream contributor to ischemic brain damage triggered by genetic deletion of PPARδ in VSMCs.
Figure 6.
The effect of lentivirus-mediated MMP-9 RNA interference on ischemia-induced brain infarction and cerebrovascular permeability. VSMC-selective PPARδ knockout (SMPδ cKO) mice were stereotactically injected 2 ul of a lentivirus carrying the shMMP-9 gene to ischemic regions and allowed to survive for 4 weeks. The mice were then subjected to MCAO and 24 h of reperfusion. 2% TTC-stained coronal sections were shown at different brain levels posterior to the frontal pole (A). Quantitative PCR and gelatin-based zymography were performed to measure MMP-2 and MMP-9 mRNA expression (B) and activities (C). Quantitative analysis was made on brain infarct volume (D) as well as neurological outcomes (E), and cerebrovascular permeability was determined 1 h after Evans Blue injection (F) in SMPδ cKO mice. In comparison with PBS or Lenti-GFP control groups, loss-of-MMP-9 function through lentivirus-mediated MMP-9 RNA interference significantly attenuates ischemic brain infarction (n=6–7) and improves neurological deficit (n=7–9) as well as cerebrovascular permeability (n=6). The effectiveness of this treatment was confirmed by a significant reduction of MMP-9 mRNA levels and activity. Data are expressed as mean ± SD. * p < 0.05 vs the PBS or Lenti-GFP group.
Discussion
In this study, we addressed the potential role of vascular PPARδ in ischemic brain injury. We demonstrated for the first time that VSMC-selective deletion of PPARδ led to much severer BBB breakdown, inflammatory responses and neuronal loss in mice after focal cerebral ischemia. We further identified MMP-9 as a direct downstream target of PPARδ-mediated transrepression at the transcriptional level. This inhibitory pathway is responsible for the contributory role of vascular PPARδ in the protection of the cerebral vasculature and brain after ischemic insults.
It has been recently suggested that PPARδ plays an important neuroprotective role and its agonists may be useful for the potential treatment of stroke 7, 20. For example, PPARδ-null mice showed significantly greater infarct sizes in stroke versus wild-type animals 21. However, the specific roles of vascular PPARδ in the pathogenesis of stroke have not yet been investigated in either in vitro or in vivo settings although PPARδ is extensively expressed in cerebral vessels 22, 23. Similar to PPARγ and PPARα, PPARδ displays essential regulatory roles in vascular biology as well as several pathological conditions, such as atherosclerosis and cardiovascular diseases 24–26. In vascular endothelial progenitor cells, PPARδ activation has been previously reported to stimulate proliferation and attenuate apoptosis through Akt-dependent signaling cascades 27. In addition, we have recently documented that PPARδ plays a protective role in cerebral vascular endothelial cells after ischemic insults via a microRNA-mediated apoptotic mechanism 14. Also, we have previously reported that PPARδ is expressed in VSMCs and up-regulated after vascular injury to increase VSMC proliferation 15. Conversely, Lim et al. reported that the PPARδ agonist L-165041 suppresses rat VSMC proliferation and attenuates neointima formation in the carotid artery balloon injury model 28. A possible explanation of these inconsistent results may be due to PPARδ-dependent or –independent mechanisms in VSMC proliferation. Also, recent in vivo data from others suggest an atheroprotective role of PPARδ agonists by targeting multiple pro-inflammatory pathways 24, 26. In this study, we report for the first time that PPARδ in VSMCs provides vaso- as well as neuroprotection against ischemic insults. We found that OGD induced a reduction in PPARδ expression but an increase in MMP-9 activity, and gain-of-PPARδ function by adenovirus effectively decreased OGD-induced activation of MMP-9. Moreover, we documented that selective deletion of PPARδ in VSMCs exacerbated ischemia-triggered BBB damage and postischemic inflammation, resulting in a larger infarct brain size. Thus, our data has provided the first evidence that PPARδ in VSMCs can function as a novel modulator in the regulation of ischemic vascular and brain injury.
In the current study, the mechanisms responsible for OGD-induced reduction of PPARδ in VSMCs could be complicated. MicroRNA-mediated mRNA degradation and translational repression may be possible mechanisms involved in the downregulation of PPARδ since hypoxia has been reported to dramatically alter microRNA profiles 29. For example, several recent studies have demonstrated that microRNA-27 can inhibit adipocyte differentiation 30 or promote LPS-induced inflammation in human macrophages 31 via suppressing PPARγ expression. Moreover, microRNA-122 has been recently identified as a negative regulator of the PPARα and PPARδ coactivator Smarcd1/Baf60a in hepatic metabolic control 32. Thus, further studies are needed to investigate microRNA-mediated PPARδ dysfunction after ischemic brain injury.
The vascular pathology observed in ischemic stroke suggests a pathogenic process involving the breakdown of the vascular basement membrane, leading to a compromised BBB structural integrity, edema, neuroinflammation and eventual inflammatory brain damage. Over the last two decades, cumulative data have demonstrated that the MMP family plays a critical role in the regulation of vascular base membrane structural integrity during ischemic-triggered vascular injury 33, 34. In particular, MMP-2 and -9 have received considerable attention in cerebral ischemia because of their contributory role in the proteolytic degradation of base membrane components, leading to increased BBB permeability, edema and hemorrhagic transformation 9, 10, 35. Indeed, MMP-2 and -9 have been shown to be activated widely in the ischemic regions, and inhibition of their activities through genetic or pharmacological approaches effectively protects against ischemic brain or vascular injury in rodent stroke models. For example, an earlier observation demonstrated that MMP-2 injected into the brain results in an opening of the BBB with subsequent hemorrhage around the blood vessels 36. By contrast, treatment with MMP inhibitors reduces these effects 33, 34. Further evidence that MMPs mediate BBB injury includes observations in MMP-9 knockout mice, which display reduced infarct size and less BBB damage or hemorrhagic transformation compared to wild-type mice after focal ischemia 9, 10. Consistent with these previous reports, we found that MMP-9 activity is activated in cultured VSMCs in a time-dependent manner after ODG exposure. Moreover, there is greater potentiation of MMP-9 activity in the brains of SMPδ cKO mice after focal cerebral ischemia when compared to LC mice, and this increase is also associated with a larger scale of released ischemic pro-inflammatory cytokines. Of significance, loss-of-MMP-9 function by lentivirus approach significantly reduces ischemic vascular and brain injury in SMPδ cKO mice, implying that PPARδ gene deletion-induced ischemic vascular and brain damage results from upregulated MMP-9 levels.
Recent studies have revealed that activation of another PPAR subtype, PPARγ, may directly inhibit MMP-9 expression or activity in order to execute its neuroprotective effects in ischemic stroke 19. In the vascular system, activation of the same PPAR isoform by different PPARγ agonists has also been reported to decrease MMP-9 levels, thereby reducing VSMC proliferation and migration, and effectively delaying the initiation and progression of atherosclerotic lesions, restenosis, and vessel lesions in experimental animal models 12, 13. With regard to these previous findings, we hypothesized that inhibition of MMP-9 is also responsible for VSMC PPARδ-mediated vascular and brain protection after focal cerebral ischemia observed in the present study. Indeed, we documented here for the first time that PPARδ overexpression by adenovirus, and also activation of PPARδ by the PPARδ agonist GW501516 attenuated OGD-triggered induction of MMP-9 mRNA and activity in VSMC culture. In contrast, inhibition of PPARδ by retrovirus-mediated PPARδ RNA interference effectively reversed these effects. The latter study is well confirmed by our in vivo evidence that VSMC-selective deletion of PPARδ results in a significantly higher level of MMP-9 enzymatic activity in ischemic brain regions compared to the littermate control mice. Importantly, by employing PPARδ transcriptional activity analysis and a ChIP assay, we have further identified that PPARδ may negatively regulate MMP-9 activity via a transcriptional mechanism. To our knowledge, we are the first to report that MMP-9 is under the control of PPARδ-mediated transcriptional repression in vascular cells.
In conclusion, our data clearly identify a protective action of VSMC PPARδ on cerebral ischemia-induced vascular and brain injury. We also define PPARδ-mediated transcriptional suppression of MMP-9 as a major mechanism responsible for this effect. Understanding the mechanisms of vascular PPARδ-mediated brain protection elucidated in this study may be important for uncovering the pathogenesis of cerebral ischemia. Expectedly, pharmacological activation of PPARδ may provide a novel therapy for stroke-induced vascular and neuronal damage.
Supplementary Material
Acknowledgments
Sources of Funding
This work was partially funded by the National Institutes of Health (HL75397, HL68878, and HL89544). K.J.Y. and J.Z. are supported by the American Heart Association National Scientist Development Grants 0630209N and 0835237N, respectively. M.H. is supported by a postdoctoral fellowship from the National Institutes of Health (T32 HL007853). Y.E.C. is an established investigator of the American Heart Association (0840025N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thrombosis research. 2000;98:73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa M, Zhang JH, Nanda A, Granger DN. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci. 2004;9:1339–1347. doi: 10.2741/1330. [DOI] [PubMed] [Google Scholar]
- 3.Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferatoractivated receptors: Regulation of transcriptional activities and roles in inflammation. J Steroid Biochem Mol Biol. 2003;85:267–273. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 4.Yki-Jarvinen H. Thiazolidinediones. The New England journal of medicine. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 5.Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- 6.Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- 7.Iwashita A, Muramatsu Y, Yamazaki T, Muramoto M, Kita Y, Yamazaki S, Mihara K, Moriguchi A, Matsuoka N. Neuroprotective efficacy of the peroxisome proliferators-activated receptor delta-selective agonists in vitro and in vivo. J Pharmacol Exp Ther. 2007;320:1087–1096. doi: 10.1124/jpet.106.115758. [DOI] [PubMed] [Google Scholar]
- 8.Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 9.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: Effects of gene knockout and enzyme inhibition with bb-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J. 1999;13:781–792. [PubMed] [Google Scholar]
- 12.Marx N, Schonbeck U, Lazar MA, Libby P, Plutzky J. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83:1097–1103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CS, Kwon YW, Yang HM, Kim SH, Kim TY, Hur J, Park KW, Cho HJ, Kang HJ, Park YB, Kim HS. New mechanism of rosiglitazone to reduce neointimal hyperplasia: Activation of glycogen synthase kinase-3beta followed by inhibition of mmp-9. Arterioscler Thromb Vasc Biol. 2009;29:472–479. doi: 10.1161/ATVBAHA.108.176230. [DOI] [PubMed] [Google Scholar]
- 14.Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE. Peroxisome proliferator-activated receptor delta regulation of mir-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010;30:6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Fu M, Zhu X, Xiao Y, Mou Y, Zheng H, Akinbami MA, Wang Q, Chen YE. Peroxisome proliferator-activated receptor delta is up-regulated during vascular lesion formation and promotes post-confluent cell proliferation in vascular smooth muscle cells. J Biol Chem. 2002;277:11505–11512. doi: 10.1074/jbc.M110580200. [DOI] [PubMed] [Google Scholar]
- 16.Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE. Generation of an adult smooth muscle cell-targeted cre recombinase mouse model. Arterioscler Thromb Vasc Biol. 2006;26:e23–e24. doi: 10.1161/01.ATV.0000202661.61837.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: New opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Lee KJ, Jang YH, Lee H, Yoo HS, Lee SR. Ppargamma agonist pioglitazone reduces [corrected] neuronal cell damage after transient global cerebral ischemia through matrix metalloproteinase inhibition. Eur J Neurosci. 2008;27:334–342. doi: 10.1111/j.1460-9568.2007.06007.x. [DOI] [PubMed] [Google Scholar]
- 20.Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat Med. 2004;10 Suppl:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 21.Arsenijevic D, de Bilbao F, Plamondon J, Paradis E, Vallet P, Richard D, Langhans W, Giannakopoulos P. Increased infarct size and lack of hyperphagic response after focal cerebral ischemia in peroxisome proliferator-activated receptor beta-deficient mice. J Cereb Blood Flow Metab. 2006;26:433–445. doi: 10.1038/sj.jcbfm.9600200. [DOI] [PubMed] [Google Scholar]
- 22.Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998;139:2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- 23.Woods JW, Tanen M, Figueroa DJ, Biswas C, Zycband E, Moller DE, Austin CP, Berger JP. Localization of ppardelta in murine central nervous system: Expression in oligodendrocytes and neurons. Brain Res. 2003;975:10–21. doi: 10.1016/s0006-8993(03)02515-0. [DOI] [PubMed] [Google Scholar]
- 24.Barish GD, Atkins AR, Downes M, Olson P, Chong LW, Nelson M, Zou Y, Hwang H, Kang H, Curtiss L, Evans RM, Lee CH. Ppardelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamblin M, Chang L, Fan Y, Zhang J, Chen YE. Ppars and the cardiovascular system. Antioxidants & redox signaling. 2009;11:1415–1452. doi: 10.1089/ars.2008.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, Atkins AR, Downes M, Barish GD, Evans RM, Hsueh WA, Tangirala RK. Ppardelta-mediated antiinflammatory mechanisms inhibit angiotensin ii-accelerated atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han JK, Lee HS, Yang HM, Hur J, Jun SI, Kim JY, Cho CH, Koh GY, Peters JM, Park KW, Cho HJ, Lee HY, Kang HJ, Oh BH, Park YB, Kim HS. Peroxisome proliferator-activated receptor-delta agonist enhances vasculogenesis by regulating endothelial progenitor cells through genomic and nongenomic activations of the phosphatidylinositol 3-kinase/akt pathway. Circulation. 2008;118:1021–1033. doi: 10.1161/CIRCULATIONAHA.108.777169. [DOI] [PubMed] [Google Scholar]
- 28.Lim HJ, Lee S, Park JH, Lee KS, Choi HE, Chung KS, Lee HH, Park HY. Ppar delta agonist l-165041 inhibits rat vascular smooth muscle cell proliferation and migration via inhibition of cell cycle. Atherosclerosis. 2009;202:446–454. doi: 10.1016/j.atherosclerosis.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Crosby ME, Devlin CM, Glazer PM, Calin GA, Ivan M. Emerging roles of micrornas in the molecular responses to hypoxia. Curr Pharm Des. 2009;15:3861–3866. doi: 10.2174/138161209789649367. [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, Lee YS, Kim JB. Mir-27a is a negative regulator of adipocyte differentiation via suppressing ppargamma expression. Biochem Biophys Res Commun. 392:323–328. doi: 10.1016/j.bbrc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Jennewein C, von Knethen A, Schmid T, Brune B. Microrna-27b contributes to lipopolysaccharide-mediated peroxisome proliferator-activated receptor gamma (ppargamma) mrna destabilization. J Biol Chem. 285:11846–11853. doi: 10.1074/jbc.M109.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatfield D, Le Martelot G, Vejnar CE, Gerlach D, Schaad O, Fleury-Olela F, Ruskeepaa AL, Oresic M, Esau CC, Zdobnov EM, Schibler U. Integration of microrna mir-122 in hepatic circadian gene expression. Genes Dev. 2009;23:1313–1326. doi: 10.1101/gad.1781009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and timps are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-alpha- induced gelatinase b causes delayed opening of the blood-brain barrier: An expanded therapeutic window. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- 35.Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg GA, Kornfeld M, Estrada E, Kelley RO, Liotta LA, Stetler-Stevenson WG. Timp-2 reduces proteolytic opening of blood-brain barrier by type iv collagenase. Brain Res. 1992;576:203–207. doi: 10.1016/0006-8993(92)90681-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.