Abstract
Baclofen is a gamma-aminobutyric-acid-B (GABA-B) agonist that is known to reduce the intake of some drugs of abuse. Binge eating of sugar or fat has been shown to have behavioral and neurochemical similarities to drug abuse, and may be special cases suggestive of natural addiction. In order to determine whether a treatment for drug abuse would have an effect on binge eating, and if so, which type of food intake might be affected, the present study compared the effects of baclofen on binge eating sucrose, fat, and a sweet-fat combination. Rats were maintained for 21 days on a schedule of 12-h daily access to (1) a 10% sucrose solution, (2) vegetable fat, or (3) a commercially available sweet-fat chow. A fourth group had only 2-h daily access to vegetable fat. All four experimental groups, plus a control group, had free access to water and standard rodent chow. Food intake was then measured following i.p. administration of baclofen (0, 0.6, 1.0, or 1.8 mg/kg). Results showed that while there was no effect of drug on standard chow intake of rats in any group, baclofen stimulated binge eating of sweet-fat food, suppressed binge eating of pure fat (vegetable shortening) in the group with 2-h access, and had no effect on sucrose binges. These results support previous findings of a suppressive effect of baclofen on binge eating of fat and introduce a new finding that the drug differentially affects binge eating of sucrose and a sugar-fat combination.
Keywords: animal model, GABA, bulimia, binge eating disorder, rat
Baclofen is an agonist at GABA-B receptors that can reduce the rewarding effects of substances of abuse (Brebner et al. 1999, 2000, 2005; Ranaldi and Poeggel 2002; Di Ciano and Everitt 2003; BRoberts 2005). The effect of baclofen on food intake in normal rats is generally stimulatory (Higgs and Barber 2004; Patel and Ebenezer 2008). However, baclofen can decrease feeding in diet-induced obese or diabetic (db/db) mice (Sato et al. 2007). Baclofen also attenuates binges (Buda-Levin et al. 2005) and significantly reduces conditioned responding for fat (Wojnicki et al. 2006) in rats with a history of daily binge eating of vegetable fat. Clinical findings have also shown baclofen to be effective in reducing binge eating in patients with binge eating disorder or bulimia nervosa (Broft et al. 2007).
Binge eating is defined as repeated, discrete, intermittent bouts of consuming unusually large amounts of food (American Psychiatric Association, 2000). While previous studies suggest that baclofen attenuates binge eating of fat, little is known of the drug’s effects on the intake of other macronutrients. Foods consumed during binge episodes in a clinical population, though usually high in fat, are usually also high in sugar content (Hadigan et al. 1989; Guertin and Conger 1999). Therefore, in the present study, we investigated the ability of baclofen to attenuate binges on other palatable foods, specifically a sucrose solution and a sweet-fat combination.
Methods
Diet Groups and Injection Procedures
All experiments used male Sprague-Dawley rats obtained from Taconic Farms (Germantown, NY). Rats were housed individually on a 12-h reversed light: dark cycle. All rats had free access to water and standard chow (LabDiet #5001, PMI Nutrition International, Richmond, IN; 10% fat, 20% protein, 70% carbohydrate, 3.02 kcal/g). Rats (N=50) weighing 300-400 g at the onset of the experiment were divided into five groups matched for body weight.
The three groups with 12-h access received the palatable food 4 h after the onset of the dark cycle as follows: (1) The 12-h Sweet-fat group (n=10) received 12-h daily access to sweet-fat chow (Research Diets, New Brunswick, NJ, #12451; 45% fat, 20% protein, 35% carbohydrate, 4.7 kcal/g, nutritionally complete). (2) The 12-h Vegetable Fat group (n=10) received daily access to Crisco All-Vegetable Shortening (The J.M. Smucker Co., Orrville, OH; 9.17 kcal/g). (3) A 12-h Sucrose group (n=10) received a 10% sucrose solution (0.375 kcal/mL). This 12-h access schedule is a variant of that used in our prior publications (Colantuoni et al. 2001; Colantuoni, Rada et al. 2002; Avena et al. 2006). To replicate the findings of Corwin and colleagues (Buda-Levin, Wojnicki et al. 2005), this experiment also included a 2-h Vegetable Fat group (n=10) that received daily access to Crisco from 4 to 6 h after the onset of the dark cycle. The free-access Chow group (n=10) served as a control. Body weights and intakes of palatable food and chow were measured weekly. Due to the number of rats and the design of this experiment, not all of the groups were tested together.
After 21 days of palatable-food access, drug testing began. Using a counterbalanced, experimenter-blind design, (R)-baclofen (Tocris, Ellisville, MO) diluted in 0.9% saline was injected i.p. at doses of 0.6, 1.0, and 1.8 mg/kg. Each of these doses were assigned using a uniform Latin square and had been previously tested with paradigms including 2-h access to vegetable fat (Buda-Levin et al., 2005; Wojnicki et al., 2006). On the first day of testing for each dose, half of the rats received baclofen injections, and the other half received 0.9 % saline vehicle. Injections were reversed on the second day of testing. Doses were separated by 5-day intervals.
All food was removed for 30 min after injections were administered (30 min prior to the normal palatable-food access period), then the measurements with chow and the palatable diets began, 4 h after the onset of the dark cycle.
Statistical Analysis
All intake data were converted into calories. Repeated measures ANOVAs with post-hoc Bonferonni pair-wise comparisons were used to analyze intake and bodyweights prior to testing. Mixed-factorial ANOVAs were used to analyze, across all five groups, overall main effects and interactions of baclofen injection, dose, and group for 2-h palatable intake and standard chow intakes. Within each group, two-way ANOVAs were performed to analyze main effects and interactions of dose and drug. Post-hoc one-way ANOVAs were used to analyze effects of dosage, and t-tests comparing baclofen to vehicle at each dose were used to analyze interactions of drug and dose effects. Cohen’s d values, as a measure of effect size, were calculated for these vehicle-baclofen comparisons.
Results
Intake Behavior Prior to Testing
There was a significant main effect of group in terms of palatable intake (F(3,36)=31.17, p<0.001), with the rats in the 12-h Sweet-fat group consuming more calories of palatable food during their access period than any other group (p<0.001 compared to all other groups with access to palatable food); however, there were no statistically significant differences, across the 21 days of access, in total 24-h caloric intake among any groups, including the free-access chow group. Pre-injection intake data are presented in Table 1.
Table 1.
Pre-Testing Caloric Intake (mean ± SD).
| Day 1 | Day 7 | Day 14 | Day 21 | |||||
|---|---|---|---|---|---|---|---|---|
| Group | Mean Intake (kcal) |
% Palatable Food |
Mean Intake (kcal) |
% Palatable Food |
Mean Intake (kcal) |
% Palatable Food |
Mean Intake (kcal) |
% Palatable Food |
| 12-h Sweet-fat | 93.54 ± 29.98 | 75% | 100.07 ± 21.96 | 86% | 84.60 ± 23.28 | 86% | 85.17 ± 18.88 | 89% |
| 12-h Vegetable Fat | 90.72 ± 38.41 | 26% | 86.15 ± 7.96 | 35% | 87.06 ± 9.91 | 28% | 109.93 ± 9.82 | 44% |
| 2-h Vegetable Fat | 88.53 ± 23.27 | 6% | 80.28 ± 12.70 | 20% | 90.70 ± 9.69 | 42% | 80.11 ± 9.28 | 41% |
| 12-h Sucrose | 118.41 ± 23.90 | 12% | 101.41 ± 12.27 | 26% | 93.53 ± 14.05 | 24% | 98.01 ± 12.68 | 27% |
| Ad Libitum Chow | 95.13 ± 14.07 | n/a | 84.71 ± 8.51 | n/a | 85.89 ± 8.34 | n/a | 89.30 ± 14.65 | n/a |
Over the 21-day access period prior to baclofen testing, bodyweights diverged between groups (F(4,45)=3.64, p<0.01). Further analysis showed that this effect of group was due to the higher bodyweights of rats in the 12-h Sucrose group, which came to be significantly higher than those of rats in the 2-h Vegetable Fat group over the 21 days (p<0.02). Despite differences in intake of palatable food among the experimental groups, caloric compensation in chow intake resulted in no other significant differences in body weight over the 21-day access period prior to baclofen testing.
Baclofen Attenuates Vegetable-fat Binge Eating, But Increases Sweet-fat Binge Eating with No Effect on Sucrose Binges or Standard Chow Intake
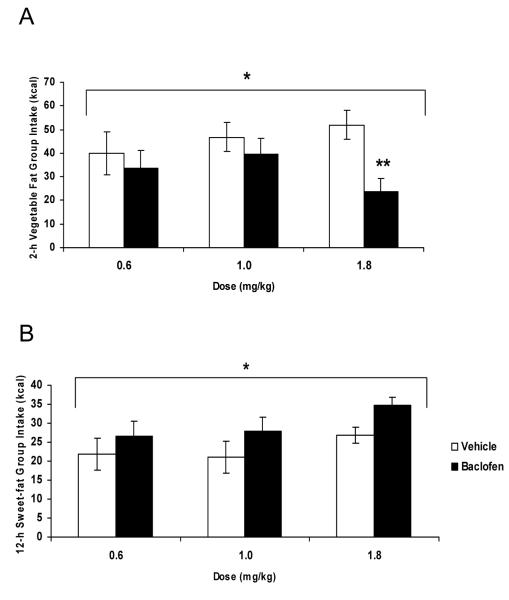
Overall, there was a significant dose x group x drug interaction (Figure 1; F(8, 88)=3.40, p<0.002). There was a significant effect of baclofen versus vehicle on the subsequent 2-h palatable chow intakes of rats in both the 12-h Sweet-fat group (F(1, 9)=8.95, p<0.02) and the 2-h Vegetable Fat group (F(1, 9)=7.74, p<0.025), with baclofen increasing intake in the 12-h Sweet-fat group, and decreasing intake in the 2-h Vegetable Fat group. There was also a significant dose x drug interaction in the palatable food intake of rats in the 2-h Vegetable Fat group (F(2, 18)=5.90, p<0.02). Post-hoc paired samples t-tests revealed that while all doses suppressed the group’s mean 2 h intake of vegetable fat, , the highest dose of baclofen (1.8 mg/kg, i.p.) significantly suppressed binge eating of vegetable fat (t(9)=−4.21, p<0.002, d= −1.53).
Figure 1.
Effect of baclofen on 2-h caloric intake (mean ± SEM). Baclofen significantly affected the intake of rats in the 2-h Vegetable Fat group (*p<0.025, A) by decreasing intake at the 1.8 mg/kg dose, compared to vehicle (**p<0.002, A). The drug increased intake in the 12-h Sweet-fat group (*p<0.025, B).
There was a significant main effect of dose (F(2, 18)=5.10, p<0.02) on the 2-h palatable food intake of rats in the 12-h Vegetable Fat group. However, post-hoc one-way ANOVA did not reveal a significant difference in intakes among doses (p=0.085), and the dose effect was likely due to the group’s larger intake following the 0.6mg/kg dose. There was no effect of drug or dose on the intakes of rats in the 12-h Sucrose group or the free-access Chow group.
There was no significant main effect of drug or dose on standard chow intake in any of the five groups.
Discussion
Baclofen differentially affected binge eating behavior. Vegetable-fat binges were suppressed in rats with daily 2-h access, sweet-fat binges were enlarged, and sucrose-solution binges were not affected. This confirms the results of Corwin and colleagues (Buda-Levin et al. 2005), in that 1.8 mg/kg baclofen (i.p.) significantly suppressed binge eating in rats with 2 h daily access to vegetable fat. Others have reported that a much higher dose (5 mg/kg) can reduce responding for sucrose (Petry and Heyman 1997).The present results suggest that baclofen has a suppressive effect on binge eating of fat and an interesting reverse effect on the intake of a sweet-fat combination.
In the present study, the observed effects of baclofen on binge eating of a sweet-fat mixture differed from the results of prior research: Wong and colleagues (2009) recently found that baclofen decreased intake of a 10% sucrose sweet-fat combination and had no effect on the intake of limitedly-available sweet-fat mixtures with higher sucrose concentrations (32%), while the present study found that baclofen augmented intake of a sweet-fat mixture that was high in sucrose (17%) and was provided on a limited access schedule. Prior research has found that baclofen, at high doses, can increase the intake of other nutritionally complete chows, like standard rodent chow (Ebenezer, 1995), and perhaps the sweet-fat intake increase we observed was due to the chow’s nutritional completeness. Conversely, there was no effect of baclofen on the standard-chow intake of rats in the present study, which would suggest that the present findings of increased intake are binge-specific.
It is not known why baclofen, at doses that suppress fat intake, increases the size of sweet-fat combination binges. These differential effects seem to be consistent, however, with findings of differences in addictive-like behavior in rats that binge eat sugar vs. fat (Avena et al., 2009). It is possible that plain fat and sweetened fat engage different GABAergic systems and that something about the addition of sugar to high-fat food, perhaps increased palatability, overcomes the binge-suppressing effects of baclofen. Further, previous studies have found that baclofen, when administered i.p., has very different effects on self-administration of substances of abuse, binge eating, and normal eating behaviors, depending on time and dose. Baclofen (1.0 mg/kg) significantly reduced responding for alcohol, but not sucrose, and a dose of 3.0 mg/kg significantly reduced responding for both (Janak and Michael Gill 2003). However, in another study, baclofen, administered at similarly high doses (2.0 and 4.0 mg/kg), increased responding for a highly-palatable mixture of sucrose, milk, and malted food powder (Ebenezer 1995).
Although the present experiment tested a systemic dose of baclofen, its effects on binge eating are presumed to be the result of agonist actions on GABAergic systems in the brain. Previous studies with drugs of abuse support this hypothesis. When injected either i.p. or locally in the nucleus accumbens or ventral tegmental area (VTA), baclofen can reduce cocaine self-administration in rats (Shoaib et al. 1998). When injected into the VTA, baclofen also reduces the reinforcing effects of heroin (Xi and Stein 1999), although this effect is not seen when baclofen is given i.p (Di Ciano and Everitt 2003).
The contrasting effects of baclofen on binge eating of fat vs. a sugar-fat combination may also be attributed to neurological changes downstream of GABA. For example, studies suggest that the neuropeptide galanin stimulates fat intake, while neuropeptide Y (NPY) stimulates carbohydrate intake (Wang et al. 1998), and galanin seems to have an inhibitory action on NPY in the hypothalamus (Parrado et al. 2007). A high-carbohydrate diet increases NPY expression, and a high-fat diet increases galanin expression (Leibowitz et al. 2004) while reducing NPY expression (Wang et al. 1998). Perhaps, then, if GABA agonism could inhibit galanin, and thus reduce pure fat intake in rats with a history of binge eating of fat, in the case of a combination of carbohydrate and fat, agonism of GABA by baclofen would block the antagonistic effects of galanin on NPY, thereby promoting intake.
Differences in food texture, levels of palatability, and baseline consumption may also have a role in the observed differential effects of baclofen on binge eating. Both oral and post-oral factors contribute to food preference (Sclafani, 2004), and each palatable diet in the present study differed in texture and may have differed in sweetness intensity. Because baclofen was peripherally injected, its effects on the different diets may have resulted from a modification at the oral level, e.g. in a palatability or texture-specific way. However, in light of findings that baclofen decreases water intake but augments intake of a malted milk powder solution (Ebenezer, 1995), it is less likely that the effect of baclofen on liquid sucrose solution consumption is related to texture.
In summary, the effects of baclofen on binge eating may be macronutrient specific. The present study replicates previous findings that baclofen suppresses binge eating of pure fat in rats with a history of 2-h fat binges (Buda-Levin et al. 2005), but also finds that the drug can increase the size of sweet-fat binges and has no effect on pure-sugar binges or standard chow intake. These results support the hypothesis that GABA-B receptors are involved in inhibiting binge eating of fat and stimulating binge eating of sugar-fat combinations. The findings may inform future clinical studies of baclofen with patients with bulimia nervosa and binge eating disorder to discern whether the drug attenuates binges dominated by fats as opposed to those dominated by sugars.
Acknowledgement
This research was supported by USPHS grant AA-12882.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: 2000. (2001) Text Revision. [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Unit 9.23C Sugar bingeing in rats. In: Crawley J, Gerfen C, Rogawski M, Sibley D, Skolnick P, Wray S, editors. Current Protocols in Neuroscience. John Wiley & Sons, Inc.; Indianapolis: 2006. pp. 9.23C.1–9.23C.6. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139(3):623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Froestl W, Andrews M, Phelan R, Roberts DC. The GABA(B) agonist CGP 44532 decreases cocaine self-administration in rats: demonstration using a progressive ratio and a discrete trials procedure. Neuropharmacology. 1999;38(11):1797–804. doi: 10.1016/s0028-3908(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology (Berl) 2000;148(3):314–21. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- Brebner K, Ahn S, Phillips AG. Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology (Berl) 2005;177(4):409–17. doi: 10.1007/s00213-004-1968-6. [DOI] [PubMed] [Google Scholar]
- Broft AI, Spanos A, Corwin RL, Mayer L, Steinglass J, Devlin MJ, et al. Baclofen for binge eating: an open-label trial. Int J Eat Disord. 2007;40(8):687–91. doi: 10.1002/eat.20434. [DOI] [PubMed] [Google Scholar]
- Buda-Levin A, Wojnicki FH, Corwin RL. Baclofen reduces fat intake under binge-type conditions. Physiol Behav. 2005;86(1-2):176–84. doi: 10.1016/j.physbeh.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patton C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10(6):478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12(16):3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. The GABA(B) receptor agonist baclofen attenuates cocaine- and heroin-seeking behavior by rats. Neuropsychopharmacology. 2003;28(3):510–8. doi: 10.1038/sj.npp.1300088. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS. Intraperitoneal administration of baclofen increases consumption of both solid and liquid diets in rats. Eur J Pharmacol. 1995;273(1-2):183–5. doi: 10.1016/0014-2999(94)00707-e. [DOI] [PubMed] [Google Scholar]
- Guertin TL, Conger AJ. Mood and forbidden foods’ influence on perceptions of binge eating. Addict Behav. 1999;24(2):175–93. doi: 10.1016/s0306-4603(98)00049-5. [DOI] [PubMed] [Google Scholar]
- Hadigan CM, Kissileff HR, Walsh BT. Patterns of food selection during meals in women with bulimia. Am J Clin Nutr. 1989;50(4):759–66. doi: 10.1093/ajcn/50.4.759. [DOI] [PubMed] [Google Scholar]
- Higgs S, Barber DJ. Effects of baclofen on feeding behaviour examined in the runway. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):405–8. doi: 10.1016/j.pnpbp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Janak PH, Michael Gill T. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30(1):1–7. doi: 10.1016/s0741-8329(03)00068-5. T. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, et al. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain Res. 2004;1008(2):168–78. doi: 10.1016/j.brainres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Parrado C, Diaz-Cabiale Z, Garcia-Coronel M, Agnati LF, Covenas R, Fuxe K, et al. Region specific galanin receptor/neuropeptide Y Y1 receptor interactions in the tel- and diencephalon of the rat. Relevance for food consumption. Neuropharmacology. 2007;52:684–692. doi: 10.1016/j.neuropharm.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Patel SM, Ebenezer IS. The effects of chronic intraperitoneal administration of the GABA B receptor agonist baclofen on food intake in rats. Eur J Pharmacol. 2008;593(1-3):68–72. doi: 10.1016/j.ejphar.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Petry NM, Heyman GM. Bidirectional modulation of sweet and bitter taste by chlordiazepoxide and Ro 15-4513: lack of effect with GABA drugs. Physiol Behav. 1997;61(1):119–26. doi: 10.1016/s0031-9384(96)00351-4. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Poeggel K. Baclofen decreases methamphetamine self-administration in rats. Neuroreport. 2002;13(9):1107–10. doi: 10.1097/00001756-200207020-00007. [DOI] [PubMed] [Google Scholar]
- Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005;86(1-2):18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Sato I, Arima H, Ozaki N, Ozaki N, Watanabe M, Goto M, et al. Peripherally administered baclofen reduced food intake and body weight in db/db as well as diet-induced obese mice. FEBS Lett. 2007;581(25):4857–64. doi: 10.1016/j.febslet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81(5):773–9. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW. The GABA-B agonist baclofen modifies cocaine self-administration in rats. Behavioural Pharmacology. 1998;9:195–206. [PubMed] [Google Scholar]
- Wang J, Akabayashi A, Dourmashkin J, Yu HJ, Alexander JT, Chae HJ, et al. Neuropeptide Y in relation to carbohydrate intake, corticosterone and dietary obesity. Brain Res. 1998;802:75–88. doi: 10.1016/s0006-8993(98)00551-4. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Roberts DC, Corwin RL. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacol Biochem Behav. 2006;84(2):197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KJ, Wojnicki FHW, Corwin RLW. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol Biochem Behav. 2009;92(3):528–536. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther. 1999;290(3):1369–74. [PubMed] [Google Scholar]