Abstract
Pseudomonas aeruginosa is an opportunistic human pathogen that preferentially infects damaged epithelial tissues. Previous studies have failed to distinguish whether the increased susceptibility of injured epithelium results from the loss of cell polarity or increased access to the basolateral surface. We have used confluent monolayers of Madin-Darby canine kidney (MDCK) cells cultured on porous filter supports for 1-3 d as a model system to investigate whether the differentiation state of a polarized model epithelium affected the response of epithelial cells to this pathogen. Confluent incompletely polarized MDCK cell monolayers (day 1) efficiently internalized apically applied P. aeruginosa via a pathway that required actin polymerization and activation of Rho-family GTPases and was accompanied by an increase in the amount of activated RhoA. In contrast, P. aeruginosa entry into highly polarized MDCK monolayers (day 3) was 10- to 100-fold less efficient and was insensitive to inhibitors of actin polymerization or of Rho-family GTPase activation. There was no activation of RhoA; instead, Cdc42-GTP levels increased significantly. Basolateral infection of highly polarized MDCK monolayers was less efficient and insensitive to Clostridium difficile Toxin B, whereas basolateral infection of incompletely polarized MDCK monolayers was more efficient and required activation of Rho-family GTPases. Together, our findings suggest that as epithelial barrier differentiates and becomes highly polarized, it becomes resistant to P. aeruginosa infection. Nevertheless, polarized epithelial cells still sense the presence of apically infecting P. aeruginosa, but they may do so through a different group of surface proteins and/or downstream signaling pathways than do incompletely polarized cells.
INTRODUCTION
The portions of the human body that contact the outside world are composed of epithelial cells and specialized cells of the immune system. Such epithelial tissues form a barrier against microbial pathogens, and the breakdown of this barrier often results in clinically apparent infection. Many pathogens have developed strategies to circumvent the barrier function of an intact epithelium. These include crossing an intact epithelial monolayer by invasion or transcytosis of epithelial cells, disrupting cell-cell contacts within the monolayer, or killing cells of the monolayer (reviewed in Kazmierczak et al., 2001a). Other pathogens usually exploit, rather than cause, disruptions in epithelial monolayers. Such disruptions may follow tissue injury secondary to inflammation or trauma, or may result from cell death or division within a monolayer.
P. aeruginosa is an opportunistic pathogen that exploits preexisting epithelial cell injury. This is apparent clinically, because P. aeruginosa infection follows burns, corneal trauma, catheter-related bladder injury, or local damage to the upper respiratory tract in mechanically ventilated patients (Salyers and Whitt, 2002). Experimentally, P. aeruginosa infection occurs preferentially at sites of epithelial injury (Yamaguchi and Yamada, 1991; Zahm et al., 1991; Tsang et al., 1994; de Bentzmann et al., 1996b). This predilection for injured epithelium has been attributed to increased levels of putative P. aeruginosa receptors on repairing cells, such as asialoGM1 (de Bentzmann et al., 1996a), or fibronectin and the integrin α5β1 (Roger et al., 1999). The loss of polarity in repairing cells may independently facilitate P. aeruginosa infection, as bacterial adhesion, internalization, and cytotoxicity increase in epithelial cells whose polarity has been pharmacologically disrupted (Fleiszig et al., 1998). Epithelial cell activation of the small GTPases Rho and Rac during wound repair (Santos et al., 1997) might also promote P. aeruginosa internalization, as we have recently shown that expression of a constitutively active RhoA allele (RhoAV14) is sufficient to increase bacterial internalization (Kazmierczak et al., 2001b).
Several host cell molecules have been identified as internalization receptors for P. aeruginosa, including the cystic fibrosis transmembrane receptor (Pier et al., 1997) and the asialoganglioside aGM1 (Hazlett et al., 1993), although their relative importance may be cell type specific. Several studies suggest that P. aeruginosa preferentially adheres to and invades the basolateral surface of polarized epithelial cells. Treatment of polarized epithelial monolayers with EGTA, which disrupts intercellular junctions, results in increased binding, cytotoxicity, or invasion (Fleiszig et al., 1997). Manipulations that disrupt polarity, such as plating cells at low density or exposure to hepatocyte growth factor, increase cytotoxicity and invasion (Fleiszig et al., 1998; Lee et al., 1999). Finally, susceptibility to invasion correlates with polarity and suggests that polarity is a protective mechanism for the host cell (Fleiszig et al., 1997; Plotkowski et al., 1999). Although these results may be explained by the restriction of P. aeruginosa receptor(s) to the basolateral surface of polarized cells, no such receptor has been identified to date.
The pathway of P. aeruginosa internalization is sensitive to cytochalasin D, an actin-depolymerizing agent, is inhibited by the tyrosine kinase inhibitors herbimicin and genistein, and may involve the tyrosine kinase src, suggesting that protein phosphorylation events accompany internalization (Fleiszig et al., 1995; Evans et al., 2002a). The components of this signal transduction cascade have not been identified, although recent evidence suggests that mitogen-activated protein kinase kinase and extracellular signal-regulated kinase kinases may be involved (Evans et al., 2002b). Some strains of P. aeruginosa trigger the activation of the acid sphingomyelinase and the release of ceramide in sphingolipid-rich rafts. Ceramide reorganizes these rafts into larger signaling platforms that are required to internalize Pseudomonas aeruginosa, induce apoptosis, and regulate cytokine response in infected cells (Grassme et al., 2003). We have recently shown that internalization is accompanied by activation of RhoA, a member of the small Rho-family GTPases involved in actin cytoskeleton and membrane rearrangements and that activation is sufficient to promote P. aeruginosa internalization by epithelial cells (Kazmierczak et al., 2001b).
Direct modulation of Rho-family GTPase activity is a strategy used by many bacterial pathogens to promote or block their internalization by host cells. P. aeruginosa strains synthesize several proteins that are injected into host cells via the bacterial type III secretion system. Two of these, ExoS and ExoT, exhibit GTPase activating protein (GAP) activity toward Rho-family GTPases in vitro (Goehring et al., 1999; Krall et al., 2000; Kazmierczak and Engel, 2002) and block bacterial internalization by both macrophage-like cell lines and epithelial cells (Frithz-Lindsten et al., 1997; Cowell et al., 2000; Garrity-Ryan et al., 2000). Point mutations that abolish the GAP activity of ExoT significantly reduce the ability of this protein to inhibit bacterial internalization, further supporting the hypothesis that RhoA activation is required for efficient bacterial internalization (Garrity-Ryan et al., 2000).
Rho-family GTPase activity is required to establish many of the features of a polarized epithelium, e.g., tight junction formation, polarized protein targeting, focal contact and adhesion formation (Braga et al., 1997; Jou et al., 1998; Kroschewski et al., 1999; Leung et al., 1999; Nobes and Hall, 1999). Because Rho-family GTPase activity is central to P. aeruginosa internalization, we investigated whether the limited ability of polarized epithelia to internalize P. aeruginosa was regulated at the level of Rho-family GTPase activity. We developed a system for examining confluent model epithelial monolayers polarized to varying extents and demonstrated that decreased internalization of P. aeruginosa by polarized cells was accompanied by the loss of a Rho-GTPase dependent uptake pathway. Polarized cells continued to respond strongly to apically infecting bacteria; however, their response shifted from RhoA activation to Cdc42 activation. Basolateral infection of polarized cells was likewise less efficient than basolateral infection of incompletely polarized cells, suggesting that the RhoA-dependent internalization pathway is down-regulated during the development of epithelial cell polarity. These findings support the idea that epithelial cells alter their responses to pathogen bacteria as a function of polarization and suggest a novel way in which epithelial cell responses to pathogens may be altered by epithelial tissue injury.
METHODS
Bacterial Strains
P. aeruginosa strains PA103pscJ::Tn5 (type III secretion deficient) and PA103ΔUΔT (type III secretion competent, ExoU-, ExoT-) have been described previously (Hauser et al., 1998; Garrity-Ryan et al., 2000). Both strains are internalized by nonprofessional phagocytes to a similar extent, a phenotype that has been ascribed to the inability of these isogenic mutants to type III secrete the effector protein ExoT (Garrity-Ryan et al., 2000). Salmonella typhimurium SL1344 and Escherichia coli MC4100 pRI203 (Invasin+) were kindly provided by Stanley Falkow (Stanford University, Stanford, CA). Plasmids expressing GST-Rhotekin binding domain (GST-TRBD) and GST-Cdc42/Rac interacting binding domain (GST-CRIB) were generously provided by Xiang-Dong Ren and Martin Schwartz (The Scripps Institute, La Jolla, CA) and Rick Cerione (Cornell University, Ithaca, NY), respectively.
Cell Culture
HeLa cells (ATCC CCL-2) and MDCK cells (type II) were cultured as described previously (Kazmierczak et al., 2001b). Before binding and internalization assays, cells were washed twice with minimal essential medium (MEM), etc. [MEM salts (Sigma-Aldrich, St. Louis, MO)/20 mM HEPES, pH 7.4/10 mM sodium bicarbonate].
Drugs and Reagents
Latrunculin A (LatA; Molecular Probes, Eugene, OR) was made up as a 2 mg/ml stock in dimethyl sulfoxide. Cells were pretreated for 30 min before bacterial infection, and LatA was present throughout the invasion assay. Clostridium difficile Toxin B (TechLab, Blacksburg, VA) was supplied at 0.38 mg/ml in phosphate-buffered saline. Cells were pretreated for 4 h before bacterial infection. We confirmed that neither LatA nor Toxin B inhibited P. aeruginosa viability at the concentrations used (our unpublished data). EDTA (Sigma-Aldrich) was made up in Hanks' Ca2+ Mg2+-free balanced salt solution (BSS) (UCSF Tissue Culture Facility, San Francisco, CA), pH 7.6. Cells were routinely pretreated for 15 min with 2.5 mM EDTA, washed twice with MEM, etc., and then infected. Anti-gp135 and anti-E-cadherin (RR1) were kindly provided by George Ojakian (SUNY Downstate, Brooklyn, NY) and Barry Gumbiner (Memorial Sloan-Kettering, New York, NY), respectively. Anti-ZO-1 (Chemicon International, Temecula, CA), anti-RhoA (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Cdc42 (BD Transduction Laboratories, Lexington, KY), anti-Rac1 (Upstate Biotechnology, Lake Placid, NY), Alexa 488-coupled secondary antibodies (Molecular Probes), and Texas-Red phalloidin (Molecular Probes) were purchased as indicated.
Binding and Internalization Assays
For all assays, single colonies of freshly plated bacteria were used to inoculate 3-ml cultures of Luria Broth (LB), which were grown overnight (14-16 h) at 37°C with agitation. Bacteria were diluted in MEM, etc., to an OD600 = 0.1 (1.5 × 108/ml) before infection unless otherwise indicated. HeLa cell assays were performed as described previously (Kazmierczak et al., 2001b). MDCK cells were plated on 12-mm Transwell filters (Corning Glassworks, Corning, NY) (0.4-μm pore size) at 1 × 106 cells/cm2, 16-18 h (“1 d”) or 3 d before assaying. For binding assays, 150 μl of bacteria was incubated with MDCK monolayers for 1 h at 37°C. Nonadherent bacteria were removed by four 5-min washes with phosphate-buffered saline. Adherent and internalized bacteria were released by incubating filters with 0.25% Triton X-100 in Hanks' Ca2+Mg2+-free BSS for 30 min at room temperature. Sterile glass beads were added to the samples, which were vortexed 2 × 10 s to ensure efficient host cell lysis and bacterial release. Adherent and internalized bacteria were enumerated by plating serial dilutions of the lysates to LB plates. Apical internalization assays were performed as described previously (Kazmierczak et al., 2001b). Briefly, infections were initiated as described for binding assays, except that bacteria were allowed to infect host cells for 2 h before washing. MEM, etc., plus amikacin (400 μg/ml) (Sigma-Aldrich) was added for a further 2 h to kill extracellular bacteria. Internalized bacteria were released by lysing MDCK cells as described above, and then enumerated by plating serial dilutions of the lysate to LB plates. For basolateral infections, cells were plated to 3-μm pore size 12-mm Transwell filters (Corning Glassworks) at 1 × 106 cells/cm2 1-3 d before assaying. Bacteria were diluted in MEM, etc., to an OD600 of ∼0.6. Filters were infected by placing them directly on top of 40-μl drops of bacterial suspension (MOI ∼25-50); 200 μl of media was added to the apical surface of the cells during the infection. After 2-h incubation with bacteria, the filters were washed, treated with antibiotic-containing medium, and lysed as for apical infections.
Lactate Dehydrogenase (LDH) Assays
LDH release was measured as described previously (Kazmierczak et al., 2001b).
Rho-GTP Determination
The procedure was adapted from Ren et al (1999). Briefly, HeLa cells were plated at 1 × 106 cells/10-cm dish 2 d before infection and assay; MDCK cells were plated at 2 × 107 cells/7-cm Transwell filter either 16-18 h or 3 d before infection (MOI ≈50) and assay. Cells were washed twice with ice-cold Tris-buffered saline, pH 7.2, plus 1 mM orthovanadate, and then lysed in 600 μl of lysis buffer (20 mM Tris-HCl, pH 8.0, 1% Triton X-100, 0.5 M NaCl, 15% glycerol, 10 mM MgCl2, 0.5 mM dithiothreitol, 1 mM orthovanadate, Complete Protease Inhibitor Cocktail, EDTA-free; Roche Diagnostics, Indianapolis, IN). Lysates were cleared by centrifugation for 5 min at 14,000 rpm at 4°C and then divided for 1) affinity precipitation with 75 μg of TRBD-GST beads (500 μl of lysate), 2) immunoblotting to determine total RhoA (20 μl of lysate), and 3) total protein concentration determination using the BCA assay kit (10 μl of lysate; Pierce Chemical, Rockford, IL). Affinity-precipitated samples and aliquots for total RhoA determination were electrophoresed on 13% SDS-PAGE gels, blotted to Immobilon P (Millipore, Bedford, MA), and incubated with anti-RhoA at 1:200 in 5% milk/Tris-buffered saline/Tween 20 for 2 h after blocking. After incubation with HRP-conjugated goat anti-mouse secondary antibody (1:2000 in 5% milk/Tris-buffered saline/Tween 20) for 1 h, blots were washed and developed with the ECL+Plus kit (Amersham Biosciences, Piscataway, NJ) according to manufacturer's instructions. Film exposed to blots was developed, scanned, and quantified using IPLab Gel H software. All samples were assayed in duplicate or triplicate; data presented are representative of three to five repetitions of each experiment.
Rac-GTP and Cdc42-GTP Determinations
CRIB-GST was prepared according to Ren et al. (1999). HeLa and MDCK cells were plated, infected, and lysed as for Rho-GTP assays. Ninety percent of the cleared lysate was precipitated with 30 μg of CRIB beads per sample; 3% of the lysate was reserved to determine total Rac1 or Cdc42 present within the lysate. Electrophoresis and Western blotting were carried out as per Rho-GTP assays, except that blots were incubated with anti-Cdc42 (1:500) or anti-Rac1 (1:1000). Samples were assayed in duplicate or triplicate; data presented are representative of three to five repetitions of each experiment.
Indirect Immunofluorescence and Confocal Microscopy
Filter grown MDCK cells were processed for staining and microscopy as described previously (Kazmierczak et al., 2001b). Primary and secondary antibodies were used as follows: anti-gp135 (1:10,000), anti-E-cadherin (RR-1, 1:1), anti-ZO-1 (1:200), Alexa 488 anti-mouse (1:500), Alexa 488 anti-rat (1:200), and Texas Red-phalloidin (1:200). Samples were imaged using a Bio-Rad MRC 1024 confocal microscope with a KrAr laser. Z-series were routinely obtained at 1.0-μm steps by using a sequential scanning program. Images were imported using NIH Image 1.67b and pseudocolored with Adobe Photoshop 5.0.
RESULTS
Entry of P. aeruginosa into HeLa Cells Is Sensitive to Inhibitors of the Actin Cytoskeleton
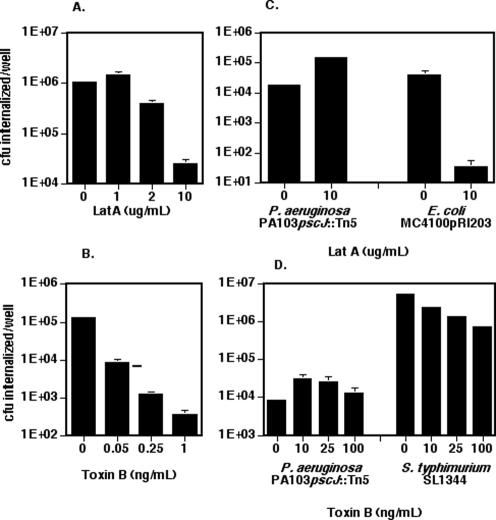
The entry of several strains of P. aeruginosa into HeLa cells is inhibited by cytochalasin D (Fleiszig et al., 1995). We confirmed that the internalization of a type III secretion mutant of PA103 (PA103pscJ::Tn5) is actin dependent by using an alternative inhibitor of actin polymerization, LatA (Lamaze et al., 1997). LatA inhibited invasion in a dose-dependent manner, up to 60-fold at 10 μg/ml (Figure 1A). The decrease in internalized bacteria was not due to either direct toxicity of LatA for P. aeruginosa or to HeLa cell damage as assayed by LDH release (our unpublished data).
Figure 1.
LatA and Toxin B have different effects on P. aeruginosa entry into nonpolarized versus polarized epithelial cells. (A and B) HeLa cells were infected with PA103::pscJTn5 (MOI 10-20) for 2 h. The number of internalized bacteria per well was determined as described in MATERIALS AND METHODS. Bars represent the mean ± SD. of triplicate samples from a representative experiment. (C and D) MDCK cells were plated on 12-mm Transwell filters at 1.5 × 106 cells/well 3 d before infection with PA103::pscJTn5, Invasin-expressing E. coli (MC4100 pRI203), or S. typhimurium (SL1344) for 2 h. Bars show the mean ± SD of triplicate samples from a representative experiment. In some instances, error bars are too small to see on the log scale used in these graphs. (A and C) Latrunculin A or DMSO was added to cells 30 min before addition of bacteria and was present for the 2 h of cocultivation with bacteria. (B and D) Cells were pretreated with Toxin B for 4 h before the addition of bacteria.
The actin cytoskeleton rearrangements that accompany the internalization of the intracellular pathogens S. typhimurium and S. flexneri are mediated by small GTPases of the Rho family (Chen et al., 1996; Tran Van Nhieu and Sansonetti, 1999; Criss et al., 2001). We tested the sensitivity of P. aeruginosa internalization to C. difficile Toxin B, which glucosylates and thereby inactivates Rho, Cdc42, and Rac GTPases (Just et al., 1995). Toxin B inhibited PA103pscJ::Tn5 internalization into HeLa cells in a dose-dependent manner (Figure 1B). Bacterial viability was not affected by Toxin B; moreover, these concentrations of Toxin B resulted in stress fiber loss and cell rounding without causing direct HeLa cell injury as measured by LDH release (our unpublished data).
Similar observations can be made for other internalized P. aeruginosa strains. Thus, internalization of PA103ΔUΔT, a mutant capable of type III secretion but carrying large deletions in the genes encoding the cytotoxin ExoU and the antiinternalization factor ExoT, is also sensitive to LatA and Toxin B at 10 μg/ml and 10 ng/ml, respectively (our unpublished data). Likewise, P. aeruginosa strains PAK and PA01 are internalized by HeLa cells via a mechanism that is inhibited by LatA and Toxin B (our unpublished data), suggesting that actin polymerization and Rho-family GTPase activity are required for P. aeruginosa internalization.
Entry of P. aeruginosa into Polarized MDCK Epithelial Cells Is Not Sensitive to Inhibitors of the Actin Cytoskeleton
Because polarized epithelial cells are the natural cell type that P. aeruginosa encounters, we tested whether LatA and Toxin B inhibit P. aeruginosa entry into polarized MDCK type II cells. Surprisingly, LatA, at doses (10 μg/ml) that caused a loss of filamentous actin staining, failed to inhibit internalization of PA103pscJ::Tn5 or PA103ΔUΔT (Figure 1C; our unpublished data). MDCK cell monolayers were nonetheless sensitive to LatA, because the actin-dependent internalization of E. coli MC4100 expressing the Yersinia enterocolitica protein Invasin was inhibited 100-fold under these conditions (Figure 1C). Likewise, P. aeruginosa invasion of polarized MDCK monolayers was not inhibited by Toxin B at concentrations up to 100 ng/ml, even though S. typhimurium invasion of these cells was inhibited 90% by Toxin B (Figure 1D). In fact, both agents slightly stimulated P. aeruginosa internalization by polarized MDCK cell monolayers. The amount of increased invasion correlated with and is likely secondary to the disruption of intercellular junctions caused by these agents (see below).
The Pathway Used by P. aeruginosa for Entry into Epithelial Cells Depends upon Their Degree of Polarization
The differential sensitivity of P. aeruginosa internalization to LatA and Toxin B seen in HeLa and in MDCK cells could be a function of cell type or of cell polarity. To distinguish between these two possibilities, we developed a system to model incompletely polarized versus polarized epithelia. MDCK cells plated at high density on permeable filters formed instant monolayers that, at 18-20 h postplating, had acquired many markers of a polarized epithelium. These included a transepithelial resistance statistically indistinguishable from that measured in monolayers 3 d after plating; restricted paracellular diffusion of tracers such as fluorescein isothiocyanate-inulin similar to that seen in 3-d-old monolayers; intact tight junctions, as seen by continuous ZO-1 staining; and correct distribution of apical and basolateral protein markers, such as gp135 and E-cadherin, respectively (our unpublished data). Between 1 and 3 d after plating, the cells continued to differentiate, increasing cell height and restricting even further the membrane distribution of E-cadherin to only the lateral membrane (our unpublished data).
Although day 1 and day 3 monolayers differed in subtle ways morphologically, their responses to P. aeruginosa were dramatically different. Day 1 confluent MDCK cell monolayers behaved much like HeLa cells, with P. aeruginosa invasion inhibited >10-fold by LatA and >50-fold by Toxin B (Figure 2). Day 3 cells, as seen previously, showed no inhibition of internalization after treatment with these agents (Figure 2), whereas their effects on day 2 monolayers were intermediate to those observed on day 1 and day 3 (our unpublished data). Internalization was not inhibited as a result of cell injury after exposure to these drugs, because LDH release was not increased by these agents (our unpublished data). Furthermore, cell loss from the filters after inhibitor treatment was <10% and could not account for the decrease in internalized bacteria, although cell retraction and condensation of the actin cytoskeleton were observed (our unpublished data).
Figure 2.
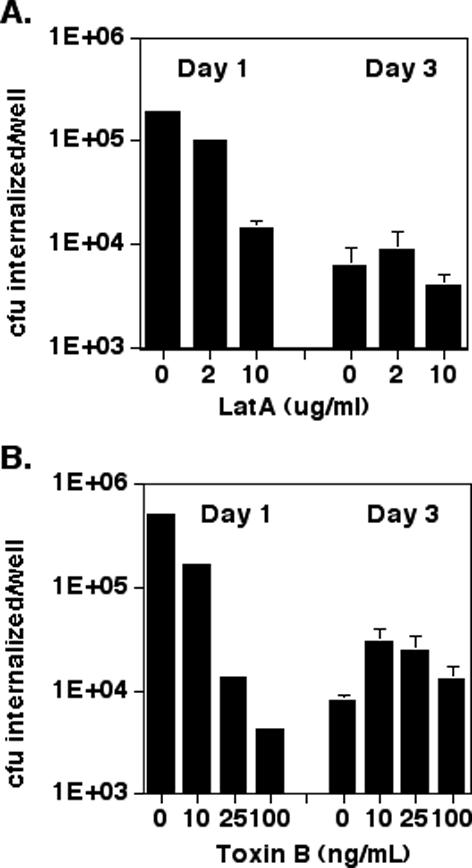
Sensitivity to inhibitors of actin polymerization is affected by cell polarity. MDCK cells were plated on 12-mm Transwell filters at 1.5 × 106 cells/well and allowed to polarize for 1 or 3 d. Cells were pretreated with LatA for 30 min (A) or Toxin B for 4 h (B) before infection with PA103::pscJTn5 at an MOI of 10-20 for 2 h. Bars show mean ± SD of triplicate samples from a representative experiment. In some instances, error bars are too small to see on the log scale used in these graphs.
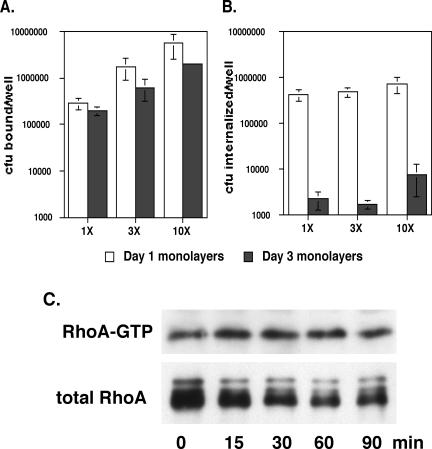
In addition to finding that day 1 and day 3 cells showed different responses to LatA and Toxin B, we also observed that P. aeruginosa internalization decreased some 50-fold between day 1 and day 3 MDCK cells (Figure 2, A and B, compare untreated cells at day 1 and day 3). This difference reflected both a decrease in bacterial adherence to the apical surfaces of day 3 cells, as well as a much greater relative decrease in the frequency with which bound bacteria were internalized (Figure 5, A and B). Microscopic examination of infected day 1 versus day 3 MDCK cells stained for internalized versus adherent bacteria also suggested that the efficiency of bacterial internalization by day 1 cells was higher than that of day 3 cells: 2 h after infection, ∼5-10% of day 1 cells contained several internalized bacteria. In contrast, day 3 cells containing internalized bacteria were extremely rare, with the majority of cell-associated bacteria being extracellular (our unpublished data). Increasing the inoculum size 3-fold or 10-fold resulted in commensurate increases in bacterial binding to the apical surfaces of both day 1 and day 3 cells (Figure 5A). However, increasing the inoculum size did not overcome the relative inability of day 3 cells to internalize P. aeruginosa, because day 3 cells infected with a 10-fold greater inoculum internalized only 1.8% as many bacteria as day 1 cells infected with the usual (“1×”) inoculum (Figure 5B).
Figure 5.
Increasing the number of bacteria bound to polarized MDCK monolayers does not lead to increased bacterial internalization nor to RhoA activation. (A and B) MDCK cells were plated to 12-mm Transwell filters at 1.5 × 106 cells/well and allowed to polarize for 1 or 3 d before infection with PA103ΔUΔT at MOIs of 10-20 (1×), 40-60 (3×), or 100-150 (10×). All determinations were carried out in quadruplicate; bars indicate the average number of adherent or internalized organisms (±SD). In some instances, error bars are too small to see on the log scale used in these graphs. (C) MDCK cells were plated to 7-cm Transwell filters at confluent density (2.0 × 107 cells/dish) and allowed to polarize for 3 d before infection with PA103ΔUΔT at an MOI of ∼200 (10×). RhoA-GTP and total RhoA were determined as described previously at various times postinfection. The gel shown is representative of four independent determinations.
Two major conclusions emerge from these experiments. First, bacterial internalization changes from a Toxin B-sensitive to a Toxin B-insensitive process in epithelial cells as they polarize. Second, the absolute amount of apical internalization markedly decreases between day 1 and day 3 MDCK monolayers, a change that cannot be ascribed to decreased bacterial binding to day 3 cells. These findings could represent either the relocalization of a Toxin B-sensitive internalization pathway to the basolateral surface and/or the loss of this pathway in polarizing cells.
Basolateral Infection of MDCK Monolayers by P. aeruginosa Decreases as Cells Become More Polarized
If P. aeruginosa internalization decreases in polarized cells because the internalization pathway is increasingly restricted to the basolateral domain of these cells, we would predict that cells infected basolaterally would retain their ability to internalize P. aeruginosa even when polarized. MDCK cells were thus plated at “confluent” density on Transwell filters with a 3-μm pore size, permitting the physical passage of P. aeruginosa through the filter to the basolateral face of the monolayer. The cells were assayed for several makers of polarity, to ensure that they formed a normal-looking monolayer. Proteins seemed to be localized correctly: gp135 and E-cadherin were restricted appropriately to the apical membrane and zona adherens, respectively (our unpublished data). Basolateral infection was established by placing filters on drops of bacterial suspension and resulted in less efficient internalization than apical inoculation. Nonetheless, polarity seemed to affect the number of bacteria internalized after basolateral infection as well, with more internalized bacteria recovered from day 1 than day 3 cells (Figure 3). Internalization was insensitive to Toxin B in day 3 cells, but inhibited 10-fold by this agent in day 1 cells. These results strongly suggest that the decrease in bacterial internalization seen with increasing polarity is not the result of restricting internalization to an inaccessible basolateral domain, but rather the consequence of down-regulating the pathway used by P. aeruginosa for internalization. This may result from down-regulation or disappearance of either the receptor used by P. aeruginosa or of more distal elements in this pathway.
Figure 3.
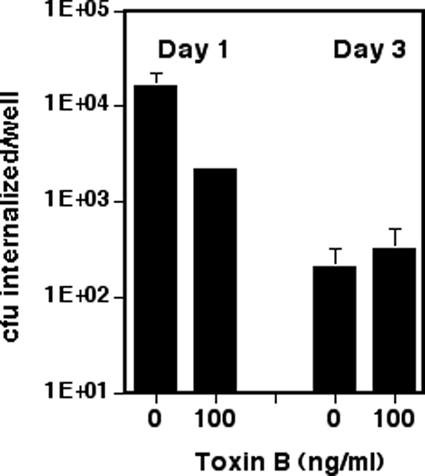
Basolateral internalization decreases with increasing MDCK polarity and becomes insensitive to Toxin B inhibition. MDCK cells were plated on 12-mm Transwell filters (3-μm pore size) at confluent density (1.5 × 106 cells/well). PA103::pscJTn5 was grown overnight in LB with shaking, diluted in MEM to A600 0.6. The filter was placed on a 40-μl drop (MOI 50) for 2 h in a humid chamber to allow infection to occur. Invasion assays were performed as described in Figure 1. The treated MDCK cells were exposed to Toxin B for 4 h before infection. Bars show mean ± SD of triplicate samples from a representative experiment. In some instances, error bars are too small to see on the log scale used in these graphs.
Basal Rho-GTPase Activity Changes Minimally during MDCK Cell Polarization
Inhibition of bacterial internalization by Toxin B in nonpolarized cells suggests that Rho-GTPases participate in bacterial internalization in this setting. If these small GTPases are less active in polarized cells compared with their nonpolarized counterparts, this might account for the relative resistance of polarized cells to bacterial internalization. We therefore assayed Rho-GTP, Rac-GTP, and Cdc42-GTP levels in day 1 versus day 3 MDCK cells by using portions of downstream effectors specific for the GTP-bound forms of these proteins as “affinity-precipitation” reagents for the activated forms of Rho (GST-TRBD) (Ren et al., 1999), or Rac and Cdc42 (GST-CRIB hPAK3) (Bagrodia et al., 1998; Benard et al., 1999). When day 1 and day 3 cells were compared, the percentage of RhoA and Rac1 in the GTP-bound form did not vary. RhoA-GTP levels were 2.32 ± 0.84% (SD) of total RhoA in day 1 cells and 2.03 ± 1.12% (SD) in day 3 cells. Rac1-GTP made up 1.55 ± 0.30% (SD) of total Rac1 in d 1 cells and 1.23 ± 0.72% (SD) in day 3 cells. However, large changes were observed in Cdc42-GTP levels, which declined approximately ninefold from day 1 to day 3 (10.1 ± 0.2% (SD) to 1.18 ± 0.04% (SD).
The Ability of PA103 to Activate Rho-Family GTPases during Internalization Is Profoundly Altered by Polarization
Basal levels of RhoA-GTP showed no change as cells became more polarized, suggesting that changes in endogenous RhoA activity, as detected by our assay, could not account for the ∼50-fold decrease in internalization we observed. However, the response of MDCK cells to bacterial challenge was altered dramatically by MDCK cell polarization. First, infection with P. aeruginosa led to RhoA activation only in day 1 cells, resulting in increased RhoA-GTP levels at 60-90 min postinfection (Figure 4A; our unpublished data). Increases in RhoA-GTP levels after bacterial infection were not seen in day 3 cells at any time point between 30 and 180 min postinfection (Figure 4B; our unpublished data). Instead, polarized MDCK cells responded to P. aeruginosa with increases in Cdc42-GTP levels 20 min after infection (Figure 4D). Infection of incompletely polarized (day 1) cells caused small decreases in Cdc42-GTP levels at 20 min postinfection, to 60-70% of uninfected controls (Figure 4C). The changes in RhoA-GTP and Cdc42-GTP levels observed in incompletely polarized MDCK cells are qualitatively similar to the responses observed in nonpolarized HeLa cells after P. aeruginosa infection. Thus, Cdc42-GTP levels measured in HeLa cells decrease to 24.8 ± 6.5% of uninfected control levels at 10 min postinfection and recover to 82.5 ± 17.8% by 40 min postinfection (our unpublished data); RhoA-GTP levels, on the other hand, increase to 277 ± 72% at 90 min postinfection and 476 ± 110% at 3 h postinfection (our unpublished data).
Figure 4.
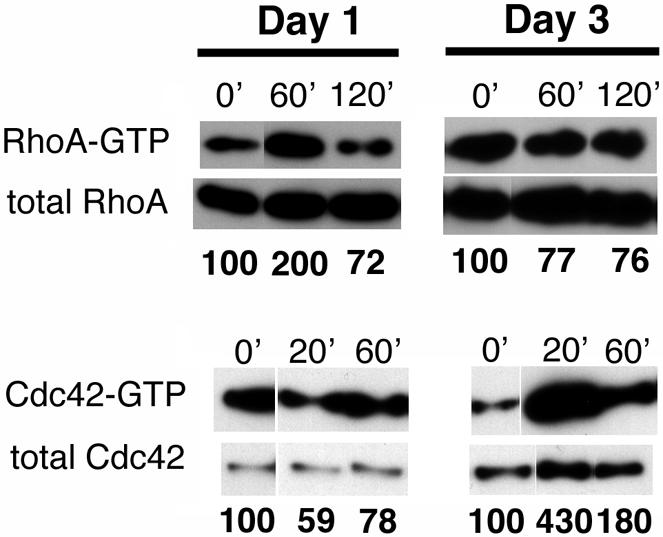
MDCK cell polarization is accompanied by a switch from RhoA activation to Cdc42 activation in response to infection. MDCK cells were plated to 7-cm Transwell filters at confluent density (2.0 × 107 cells/dish) and allowed to polarize for 1 or 3 d before infection with PA103ΔUΔT at an MOI of 20. Cells were lysed at indicated times, and aliquots of cell lysates were incubated with GST-TRBD bound to glutathione-Sepharose 4B beads (A and B) or GST-hPAK3 (C and D) as described in MATERIALS AND METHODS, allowing selective precipitation of GTP-bound RhoA or Cdc42, respectively. Both affinity-precipitated samples (GTP-bound) and aliquots of un-precipitated lysates were Western blotted with anti-RhoA (A and B) or anti-Cdc42 (C and D) antibodies. The gels shown are representative of two to four independent experiments carried out in duplicate.
P. aeruginosa adheres less well to day 3 monolayers compared with day 1 monolayers (Figure 5A); thus, it is possible that RhoA activation might be observed in day 3 monolayers if a sufficient number of bacteria could be bound to these monolayers. We thus repeated our assays using a 10-fold higher number of bacteria to infect day 3 MDCK monolayers. Although this did result in numbers of adherent bacteria that exceeded those observed for d1 monolayers infected at the usual dose (1×), we still failed to observe activation of RhoA (Figure 5C). This finding, along with the pronounced activation of Cdc42 observed in infected day 3 monolayers, argues that polarized MDCK cells continue to respond to P. aeruginosa infection albeit in a qualitatively different way than incompletely polarized (day 1) monolayers.
Disruption of Tight Junctions Increase P. aeruginosa Internalization via a Rho-independent Pathway
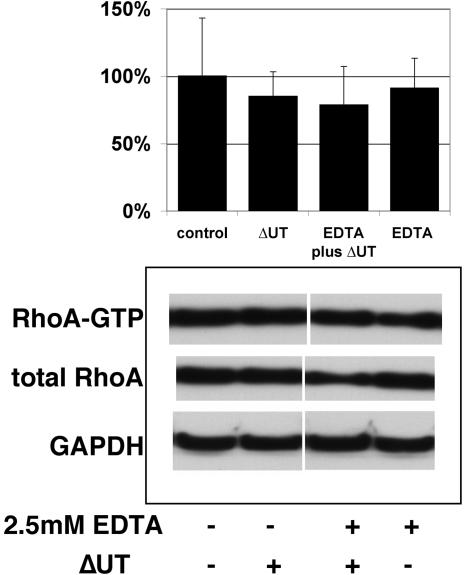
The data presented above are consistent with a model in which polarized epithelial cells down-regulate a Rho-dependent internalization pathway for apically infecting P. aeruginosa. It is possible that this pathway remains accessible at the basolateral surface of these polarized cells. Indeed, the observation that treatment of polarized epithelial cell monolayers with calcium chelators markedly increases P. aeruginosa internalization has led several investigators to conclude that P. aeruginosa preferentially interacts with polarized epithelial cells via a basolateral receptor (Fleiszig et al., 1997). Although EDTA-treated cells avidly internalize bacteria and are no longer “polarized” by virtue of having disrupted tight junctions, we hypothesized that internalization would not occur via a Rho-dependent pathway based on several observations. First, Toxin B treatment of a polarized MDCK monolayer, which efficiently disrupts tight junctions, results in increased P. aeruginosa internalization even though Rho-family GTPases are inhibited (Figure 1). Second, polarized cells treated with specific RhoA inhibitors such as C3 ADP-ribosyltransferase show disrupted tight junctions and internalize P. aeruginosa more avidly than untreated controls (Kazmierczak et al., 2001b). Last, recent studies have shown that tight junction disruption leads to RhoA down-regulation by increasing cytoplasmic pools of p120 catenin (Anastasiadis et al., 2000; Noren et al., 2000). MDCK cells treated with EDTA to disrupt tight junctions immediately before bacterial infection showed increased internalization, with 10- to 15-fold more bacteria internalized than untreated cells (our unpublished data); however, there was no detectable increase in RhoA-GTP levels in the infected EDTA-exposed cells over levels seen in uninfected cells or cells challenged with PA103ΔUΔT in the absence of EDTA (Figure 6). This observation argues that the Rho-dependent pathway present in incompletely polarized cells is not spatially restricted to the basolateral surface in polarized cells, but rather is down-regulated.
Figure 6.
Exposure of polarized MDCK cells to EDTA increases internalization without activating RhoA. MDCK cells were plated to 7-cm Transwell filters at confluent density (2.0 × 107 cells/dish) and allowed to polarize for 3 d. Immediately before infection, cells were washed with Hanks' Ca2+Mg2+-free BSS; indicated samples were treated with EDTA (2.5 mM) for 15 min. All samples were then returned to calcium and magnesium-replete tissue culture media before infection with PA103ΔUΔT for 1.5 h. Samples were lysed and total RhoA versus GTP-bound RhoA levels determined as described previously. The gel illustrates a representative experiment. Bars indicate the mean percentage of GTP-bound versus total RhoA, normalized to the uninfected/untreated control (100%); error bars show the SD for five separate determinations.
DISCUSSION
It has long been appreciated that an intact epithelial layer serves as a physical barrier to bacterial infection and penetration. Recent studies reveal that certain pathogens invade or damage cells only through interaction with basolateral receptors or targets. Because a polarized epithelium physically sequesters basolateral determinants from ingested, inhaled or aspirated bacteria, it limits the ability of pathogens to use some of their virulence factors. Our work, however, reveals a novel “defense mechanism” linked to polarity: the acquisition of a polarized phenotype alters the response of epithelial cells to an apically presented pathogen. Moreover, although it has been previously noted that different cell lines internalize P. aeruginosa to an extent that inversely correlates with their degree of polarization (Fleiszig et al., 1998; Plotkowski et al., 1999), this is the first demonstration that activation patterns of Rho-family GTPases that accompany internalization are also altered by the acquisition of polarity.
Activation of Rho-family GTPases is required for several events that lead to the establishment of polarity, including tight junction formation and membrane protein targeting. It is therefore possible that Rho-family GTPase activity may be measurably greater in incompletely polarized cells compared with fully polarized cells. When we explicitly measured GTP-bound RhoA, Rac1, and Cdc42 levels in cells during the acquisition of polarity, we found that the percentage of GTP-bound Cdc42 declined markedly over time, but that the percentage of GTP-bound RhoA and Rac1 remained constant. Thus, it is difficult to invoke decreases in basal RhoA activity as the reason for decreased P. aeruginosa internalization by polarized cells. However, epithelial cell responses to infection with P. aeruginosa change markedly with the acquisition of polarity. RhoA is strongly activated in incompletely polarized cells, which show high levels of actin-dependent Toxin B-inhibited bacterial internalization, whereas Cdc42 is inactivated. In contrast, polarized cells show minimal RhoA activation, brisk Cdc42 activation, and low levels of bacterial internalization. These patterns of Rho-GTPase activation and inactivation likely play a role in governing whether P. aeruginosa is internalized by epithelial cells, because the expression of either constitutively active RhoA (RhoAV14) or dominant negative Cdc42 (Cdc42N17) in MDCK cells strongly promotes Pseudomonas internalization (Kazmierczak et al., 2001b). The observation that polarized cells respond to invasive P. aeruginosa strains by rapidly activating Cdc42 demonstrates that the cells still sense the presence of bacteria; thus, the absence of RhoA activation in polarized cells cannot be the result of the failure of the cells to detect the presence of P. aeruginosa. Activation of Cdc42 is not accompanied by bacterial internalization, however, which is consistent with our previous observation that the expression of constitutively active Cdc42 (Cdc42V12) in MDCK cells does not promote P. aeruginosa internalization (Kazmierczak et al., 2001b). We currently cannot distinguish whether the bacteria interact with different receptors on incompletely versus completely polarized cells, or whether the same receptor engages a different downstream signaling pathway as cells become polarized. An analogous developmental change has been described in dendritic cells, which mature from highly endocytic immature cells into poorly endocytic antigen-presenting cells via a process involving down-regulation of Cdc42 activation (Garrett et al., 2000). The mechanism by which Cdc42 activation is regulated in maturing dendritic cells remains unknown.
The events occurring during MDCK II polarization are well studied. As cell-cell contacts form, transepithelial resistance rapidly increases, lipid diffusion between the apical and basolateral membranes is prohibited, and ZO-1 staining indicates the presence of a complete tight junction ring (Bacallao et al., 1989). We observe similar markers of tight junction formation in our system 18 h after plating cells at “instant monolayer” density to porous filters. Nonetheless, these cells continue to refine their segregation of apical and basolateral components, removing (by transcytosis) or degrading components present on the wrong side of the tight junction (Bacallao et al., 1989). Thus, restriction of the receptor(s) for P. aeruginosa binding and internalization may account for the failure of bacteria to activate RhoA in polarized cells. Two results, however, argue against this hypothesis. Bacterial infection established at the basolateral surface of MDCK cells remains sensitive to the degree of cell polarization, arguing that the receptor or pathway is more likely down-regulated, rather than spatially restricted in polarized cells. Second, disrupting MDCK cell tight junctions by transient exposure of polarized cells to EDTA allows bacteria access to basolateral and apical membranes and results in large increases in internalization; however, internalization occurs without detectable activation of RhoA. These findings are consistent with a model in which a RhoA-dependent uptake pathway for P. aeruginosa becomes down-regulated in polarized cells. We have not yet defined the apparently RhoA-independent mechanism by which bacterial internalization occurs in the setting of monolayer disruption.
The study of pathogen interactions with epithelial cells in vitro has been instrumental in revealing multiple mechanisms by which bacteria manipulate host cell biology. The recent use of polarized epithelial cell models to study such interactions has made it clear, however, that the signaling pathways evoked by pathogens in polarized versus nonpolarized cell types often differ. For example, Hobert et al. (2002) recently reported that polarized MDCK cells respond differently to apical S. typhimurium infection than nonpolarized cells by showing that Rac1 activity, although still necessary for actin pedestal formation in both cell types, was not required for early proinflammatory signaling leading to interleukin (IL)-8 production in MDCK cells. A similar discordance between the requirements for Rho-GTPase activation in S. typhimurium invasion of nonpolarized cells and polarized MDCK cells was demonstrated earlier by Criss et al. (2001), who found that only Rac1 was activated during, and was required for, apical invasion of MDCK cells; in contrast, Cdc42 activation seems to be required for Salmonella invasion of nonpolarized cell types. These investigators also found that apical invasion of polarized MDCK cells shows different requirements for Rho-GTPase activation than does basolateral invasion (Criss et al., 2001). Hobert et al. (2002) propose that different localization patterns of small GTPases in polarized versus nonpolarized cells may contribute to the different activation patterns observed after bacterial infection. The molecular mechanism underlying the different requirements for Rho-GTPase activity in host cell signaling pathways, however, remains obscure.
The question of how an intact epithelial tissue successfully resists infection by an opportunistic pathogen such as P. aeruginosa is of particular interest, because this virulent pathogen rarely causes clinical disease in the absence of preexisting epithelial trauma. Our approach of examining how an epithelial monolayer responds to infection as it becomes more polarized has allowed us to make the novel observation that the response of an epithelial cell to P. aeruginosa shifts from RhoA activation and Cdc42 inactivation to rapid Cdc42 activation as the cell becomes polarized. Incompletely polarized MDCK cells avidly internalize P. aeruginosa, a result that fits well with our previous observation that expression of either constitutively active RhoA (RhoAV14) or dominant negative Cdc42 (Cdc42N17) is sufficient to promote Pseudomonas internalization in polarized MDCK cells (Kazmierczak et al., 2001b).
It is particularly noteworthy that the response of polarized cells to P. aeruginosa involves Cdc42 activation, an event linked to the production of proinflammatory cytokines, such as IL-8, by epithelial cells in response to pathogenic bacteria (McCormick et al., 1995; Chen et al., 1996, 1999; Hobbie et al., 1997). Thus, part of the protection afforded by a polarized epithelium may be derived from its ability to trigger a rapid and effective immune response against pathogens. P. aeruginosa has been shown to elicit IL-8 production by respiratory epithelial cells (DiMango et al., 1995); whether disruption of this signaling pathway in the setting of epithelial trauma and loss of polarity facilitates infection by this opportunistic pathogen is currently under investigation.
Acknowledgments
We thank Stanley Falkow, Xiang-Dong Ren, Martin Schwartz, Rick Cerione, Barry Gumbiner, and George Ojakian for gifts of bacterial strains, plasmids, and antibodies, and Rick Brown for critical reading of this manuscript. This work was supported by grants from the National Institutes of Health (AI01636 to B.I.K., HL55980 to K.M., AI42806 to J.N.E.), the American Lung Association (to J.N.E.), and a Howard Hughes Medical Institute Physician Postdoctoral Fellowship (to B.I.K.). During a portion of this work, J.N.E. was a Career Investigator of the American Lung Association.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0559. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0559.
Abbreviations used: LatA, latrunculin A; LDH, lactate dehydrogenase; MOI, multiplicity of infection.
References
- Anastasiadis, P.Z., Moon, S.Y., Thoreson, M.A., Mariner, D.J., Crawford, H.C., Zheng, Y., and Reynolds, A.B. (2000). Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2, 637-644. [DOI] [PubMed] [Google Scholar]
- Bacallao, R., Antony, C., Dotti, C., Karsenti, E., Stelzer, E.H.K., and Simons, K. (1989). The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J. Cell Biol. 109, 2817-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia, S., Taylor, S.J., Jordon, K.A., Van Aelst, L., and Cerione, R.A. (1998). A novel regulator of p21-activated kinases. J. Biol. Chem. 273, 23633-23636. [DOI] [PubMed] [Google Scholar]
- Benard, V., Bohl, B.P., and Bokoch, G.M. (1999). Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274, 13198-13204. [DOI] [PubMed] [Google Scholar]
- Braga, V.M.M., Machesky, L.M., Hall, A., and Hotchin, N.A. (1997). The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 137, 1421-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., Bagrodia, S., Cerione, R., and Galán, J. (1999). Requirement of p21-activated kinase (PAK) for Salmonella typhimurium-induced nuclear responses. J. Exp. Med. 189, 1479-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L.-M., Hobbie, S., and Galan, J.E. (1996). Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274, 2115-2118. [DOI] [PubMed] [Google Scholar]
- Cowell, B.A., Chen, D.Y., Frank, D.W., Vallis, A.J., and Fleiszig, S.M.J. (2000). ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect. Immun. 68, 403-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss, A.K., Ahlgren, D.M., Jou, T.-S., McCormick, B.A., and Casanova, J.E. (2001). The GTPase Rac1 selectively regulates Salmonella invasion at the apical plasma membrane of polarized epithelial cells. J. Cell Sci. 114, 1331-1341. [DOI] [PubMed] [Google Scholar]
- de Bentzmann, S., Roger, P., Dupuit, F., Bajolet-Laudinat, O., Fuchey, C., Plotkowski, M.C., and Puchelle, E. (1996a). Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect. Immun. 64, 1582-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bentzmann, S., Roger, P., and Puchelle, E. (1996b). Pseudomonas aeruginosa adherence to remodelling respiratory epithelium. Eur. Respir. J. 10, 2145-2150. [DOI] [PubMed] [Google Scholar]
- DiMango, E., Zar, H., Bryan, R., and Prince, A. (1995). Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96, 2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, D.J., Kuo, T.C., Kwong, M., Van, R., and Fleiszig, S.M.J. (2002a). Mutation of csk, encoding the C-terminal Src kinase, reduces Pseudomonas aeruginosa internalization by mammalian cells and enhances bacterial cytotoxicity. Microb. Pathogen. 33, 135-143. [DOI] [PubMed] [Google Scholar]
- Evans, D.J., Maltseva, I.A., Wu, J., and Fleiszig, S.M.J. (2002b). Pseudomonas aeruginosa internalization by corneal epithelial cells involves MEK and ERK signal transduction proteins. FEMS Microbiol. Lett. 213, 73-79. [DOI] [PubMed] [Google Scholar]
- Fleiszig, S.M., Evans, D.J., Do, N., Vallas, V., Shin, S., and Mostov, K. (1997). Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65, 2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig, S.M., Vallas, V., Jun, C., Mok, L., Balkovetz, D., Roth, M., and Mostov, K. (1998). Susceptibility of epithelial cells to Pseudomonas aeruginosa invasion and cytotoxicity is upregulated by hepatocyte growth factor. Infect. Immun. 66, 3443-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig, S.M., Zaidi, T.S., and Pier, G.B. (1995). Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63, 4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frithz-Lindsten, E., Du, Y., Rosqvist, R., and Forsberg, A. (1997). Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol. 25, 1125-1139. [DOI] [PubMed] [Google Scholar]
- Garrett, W.S., Chen, L.-M., Kroschewski, R., Ebersold, M., Turley, S., Trombetta, S., Galan, J.E., and Mellman, I. (2000). Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102, 325-334. [DOI] [PubMed] [Google Scholar]
- Garrity-Ryan, L., Kazmierczak, B., Kowal, R., Comolli, J., Hauser, A., and Engel, J. (2000). The arginine finger domain of ExoT is required for actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68, 7100-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring, U.M., Schmidt, G., Pederson, K.J., Aktories, K., and Barbieri, J.T. (1999). The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274, 36369-36372. [DOI] [PubMed] [Google Scholar]
- Grassme, H., Jendrosser, V., Riehle, A., von Kurthy, G., Berger, J., Schwarz, H., Weller, M., Kolesnick, R., and Gulbins, E. (2003). Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 9, 322-330. [DOI] [PubMed] [Google Scholar]
- Hauser, A.R., Kang, P.J., and Engel, J. (1998). PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27, 807-818. [DOI] [PubMed] [Google Scholar]
- Hazlett, L.D., Masinick, S., Barrett, R., and Rosol, K. (1993). Evidence for asialo GM1 as a corneal glycolipid receptor for Pseudomonas aeruginosa adhesion. Infect. Immun. 61, 5164-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie, S., Chen, L., Davis, R., and Galán, J. (1997). Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159, 5550-5559. [PubMed] [Google Scholar]
- Hobert, M.E., Sands, K.A., Mrsny, R.J., and Madara, J.L. (2002). Cdc42 and Rac1 regulate late events in Salmonella typhimurium-induced interleukin-8 secretion from polarized epithelial cells. J. Biol. Chem. 277, 51025-51032. [DOI] [PubMed] [Google Scholar]
- Jou, T.-S., Schneeberger, E., and Nelson, W.J. (1998). Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 142, 101-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just, I., Selzer, J., Wilm, M., von Eichel-Streiber, C., Mann, M., and Aktories, K. (1995). Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500-503. [DOI] [PubMed] [Google Scholar]
- Kazmierczak, B., and Engel, J. (2002). Pseudomonas aeruginosa ExoT acts in vivo as a GTPase activating protein for RhoA, Rac1, and Cdc42. Infect. Immun. 70, 2198-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak, B., Mostov, K., and Engel, J. (2001a). Interaction of bacterial pathogens with polarized epithelium. Annu. Rev. Microbiol. 55, 407-435. [DOI] [PubMed] [Google Scholar]
- Kazmierczak, B.I., Jou, T.-S., Mostov, K., and Engel, J. (2001b). Rho-GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell Microbiol. 3, 85-98. [DOI] [PubMed] [Google Scholar]
- Krall, R., Schmidt, G., Aktories, K., and Barbieri, J.T. (2000). Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68, 6066-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski, R., Hall, A., and Mellman, I. (1999). Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat. Cell Biol. 1, 8-13. [DOI] [PubMed] [Google Scholar]
- Lamaze, C., Fujimoto, L., Yin, H., and Schmid, S. (1997). The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J. Biol. Chem. 272, 20332-20335. [DOI] [PubMed] [Google Scholar]
- Lee, A., Chow, D., Haus, B., Tseng, W., Evans, D., Fleiszig, S., Chandy, G., and Machen, T. (1999). Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am. J. Physiol. 277, L204-L217. [DOI] [PubMed] [Google Scholar]
- Leung, S.M., Rojas, R., Maples, C., Flynn, C., Ruiz, W.G., Jou, T.S., and Apodaca, G. (1999). Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase rhoA. Mol. Biol. Cell 10, 4369-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, B.A., Miller, S.I., Carnes, D., and Madara, J.L. (1995). Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect. Immun. 63, 2302-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C.D., and Hall, A. (1999). Rho GTPases control polarity, protrusion and adhesion during cell movement. J. Cell Biol. 144, 1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N.K., Liu, B.P., Burridge, K., and Kreft, B. (2000). p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150, 567-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier, G.B., Grout, M., and Zaidi, T.S. (1997). Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Nat. Acad. Sci. USA 94, 12088-12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkowski, M.C., de Bentzmann, S., Pereira, S.H., Zahm, J.M., Bajolet-Laudinat, O., Roger, P., and Puchelle, E. (1999). Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am. J. Respir. Cell Mol. Biol. 20, 880-890. [DOI] [PubMed] [Google Scholar]
- Ren, X.-D., Kiosses, W.B., and Schwartz, M.A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger, P., Puchelle, E., Bajolet-Laudinat, O., Tournier, J.M., Debordeaux, C., Plotkowski, M.C., Cohen, J.H., Sheppard, D., and de Bentzmann, S. (1999). Fibronectin and α5β1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur. Respir. J. 13, 1301-1309. [PubMed] [Google Scholar]
- Salyers, A.A., and Whitt, D.D. (eds.) (2002). Bacterial Pathogenesis: A Molecular Approach, Washington, DC: ASM Press.
- Santos, M.F., McCormack, S.A., Guo, Z., Okolicany, J., Zheng, Y., Johnson, L.R., and Tigyi, G. (1997). Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J. Clin. Investig. 100, 216-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu, G., and Sansonetti, P.J. (1999). Mechanism of Shigella entry into epithelial cells. Curr. Opin. Microbiol. 2, 51-55. [DOI] [PubMed] [Google Scholar]
- Tsang, K.W.T., Rutman, A., Tanaka, E., Lundt, V., Dewar, A., Cole, P.J., and Wilson, R. (1994). Interaction of Pseudomonas aeruginosa with human respiratory mucosa in vitro. Eur. Respir. J. 7, 1746-1753. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, T., and Yamada, H. (1991). Role of mechanical injury on airway surface in the pathogenesis of Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 144, 1147-1152. [DOI] [PubMed] [Google Scholar]
- Zahm, J.M., Chevillard, M., and Puchelle, E. (1991). Wound repair of human surface respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 5, 242-248. [DOI] [PubMed] [Google Scholar]