Abstract
The current study described the synthesis and the in vivo acute oral toxicity evaluations in Sprague Dawley rats. The compounds were characterized by elemental analyses, LC-MS, FTIR, 1H NMR, 13C NMR and UV-visible spectroscopy. In the acute toxicity study, a single administration of the compounds was performed orally to the rats at the single doses of 2000 mg/kg and they were then monitored for possible side effects, mortality or behavioral changes up to 14 days. The serum level of aspartate (AST), alanine aminotransferases (ALT), alkaline phosphate (ALP), triglyceride, high density lipoprotein (HDL), immunoglobulins (GAM) and the C-reactive proteins did not significantly change. The hematological indices white blood cells (WBC), haematocrit (HCT), red blood cells (RBC), mean corpuscular volume (MCV), mean corpuscular haemoglobin concentration (MCHC), and mean corpuscular hemoglobin (MCH) were within the normal range. The renal function indices examined were also within the reference range. Generally, the compounds exhibited low toxic effects as required for further in vivo therapeutic studies.
Keywords: synthesis, characterizations, acute toxicity, zinc complexes
1. Introduction
Zinc has been shown to play an important role in wound healing, proper functioning of mucosal cells, reduction of reactive oxygen species (ROS) [1] and as a cofactor for metallo-enzymes [2]. Zinc deficiency or excess can lead to many metabolic disorders such as growth retardation, decreased spermatogenesis, dysgeusia, anosmia and anemia to meat, eggs, liver and oysters. Several studies were performed to determine the mechanisms for zinc balance and the effects of zinc excess on iron metabolism [3] with much emphasis on small molecular weight metal binding proteins [4]. Despite the biological importance of zinc, the safety of its compounds in many dietary supplements has remained an issue of debate. However, the interaction of zinc ions with certain Schiff base ligands has been studied due to their relevance in bio inorganic chemistry. For example, they form carbon-nitrogen bonds [5], which make them important intermediates in a number of enzymatic reactions [6–8]. Polydentate ligands, on the other hand, have been reported to exhibit potential activities in removing the undesirable effect of metal ion by deactivating either the carcinogenic metal or the enzyme required in order to protect the cells. The activities of various ligands were reported to have increased upon coordination with the metal ions; therefore, studies on novel metal-based compounds with therapeutic potential became an area of intense investigation in biomedical and inorganic chemistry [9–12]. However, metal ions are generally toxic at a high-dose level; therefore, to study the therapeutic potential of novel metal-based compounds; the acute toxicity level must first be evaluated. Moreover, the compounds containing piperazine moiety were reported to have shown various biological activities in many studies [13,14] and, specifically, the Schiff bases derived from piperazine compounds have been described to demonstrate various biological activities; for example, anthelmintic [15], antimicrobial [16,17], acetylcholinesterase inhibition [18], melanocortin-4-receptor (MC4-R) [19,20], drug designer [21] anti-PAF [22,23], anti-HIV [24,25] and anti-obesity [26] activities. However, the literature reveals no report on their toxicity class. This, therefore, prompted the present study to synthesize, characterize and evaluate for the first time the acute oral toxicity of some novel zinc(II) complexes derived from some 1-(2-salicylaldiminoethyl) piperazine Schiff bases.
2. Result and Discussion
2.1. Chemistry
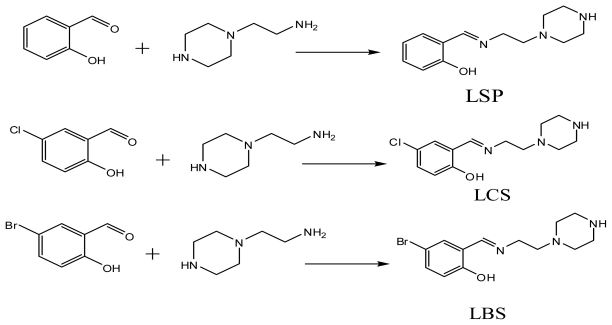
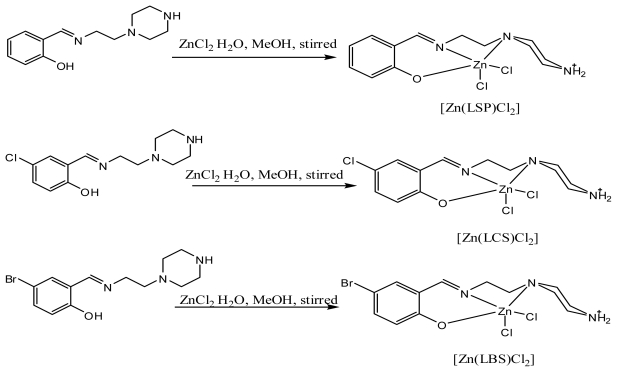
The reaction of 2-(piperazin-1-yl)ethanamine with some selected aldehydes resulted in the formation of the corresponding 1-(2-salicylaldiminoethyl)piperazines Schiff bases. The prepared Schiff bases (Scheme 1) were used to synthesize the novel complexes of zinc (II) chloride (Scheme 2). The compounds exhibited MS, NMR, IR and UV-Visible spectra consistent with the proposed structures which allowed the synthesized compounds to be recognized as 2-((2-(piperazin-1-yl)ethylimino) methyl)phenol-dichlorido-Zn-(II). [Zn(LSP)Cl2], 4-chloro-2-((2-(piperazin-1-yl)ethylimino)methyl) phenol-dichlorido-Zn-(II). [Zn(LCS)Cl2], 4-bromo-2-((2-(piperazin-1-yl)ethylimino)methyl)phenol-dichlorido-Zn-(II). [Zn(LBS)Cl2], respectively.
Scheme 1.
Reaction pathway for the Schiff bases.
Scheme 2.
Reaction pathway for zinc complexes.
The IR spectra of the complexes displayed band regions at the wavelengths of 1,628, 1,624, and 1631 cm−1 for [Zn(LSP)Cl2], [Zn(LCS)Cl2] and [Zn(LBS)Cl2], respectively, which could be due to the characteristic iminic frequency [27,28]. These bands appeared at 1636 cm−1, 1631 cm−1 and 1616 cm−1 in the spectra of the free Schiff bases of the above-mentioned complexes correspondingly. In addition, the coordination of imine nitrogen to the zinc was further ascertained by the appearance of signal at the band regions 486 cm−1, 581 cm−1 and 578 cm−1 in the spectra of the corresponding complexes due to Zn-N bond [29] which is supported by the zinc-phenolate (Zn-O) [30] signals at 569 cm−1, 645 cm−1 and 631 cm−1 respectively. The proton NMR is also consistent with the IR spectral data, where the imine-zinc coordination was observed at 7.92 ppm, 8.02 ppm and 8.05 ppm in the spectra of [Zn(LSP)Cl2], [Zn(LCS)Cl2] and [Zn(LBS)Cl2], respectively. These signals initiated from 7.28 ppm, 7.53 ppm and 7.68 ppm in the spectra of the free Schiff bases of the corresponding complexes. This supposition was supported by the 13C NMR spectra which showed imine carbon at 162.6 ppm, 164.2 ppm and 165.2 ppm respectively due to complexation. The phenolate carbon atoms also appeared at 161.5 ppm, 159.5 ppm and 158.4 ppm in the respective order of the complexes mentioned above [31]. To further elucidate the structure of the complexes, UV-visible spectra were recorded using DMSO. The spectra of the complexes exhibited two absorption band maxima each at 279 nm, 204 nm and 267 nm for [Zn(LSP)Cl2], [Zn(LCS)Cl2] and [Zn(LBS)Cl2] respectively. This could be afforded to the π-π* electronic transitions [32,33] the phenolic ring. The other absorptions noticeable to 351 nm, 362 nm and 382 nm can be due to ligand to metal charge transfer [34,35].
2.2. Acute Toxicity Study
The analysis of the toxicity level of chemical compounds is the most important step required for further biological studies [36]. The toxicity level of the zinc complexes derived from 1-(2-salicylaldiminoethyl)piperazines were evaluated at the maximum dose of 2000 mg/kg/body weight. The compounds were administered orally to the 24 h fasted rats and monitored closely after every 30 min up to 8 h of post treatment. It was observed that the compounds did not cause any gross behavioral alterations like convulsion, dizziness or respiratory distress. No mortality was recorded for the period of 14 days, which indicate that the lethal dose of the compounds is above 2000 mg/kg body weight in rats and that the compounds can be considered to be less harm at this dose.
2.3. Body and Organ Weight Changes
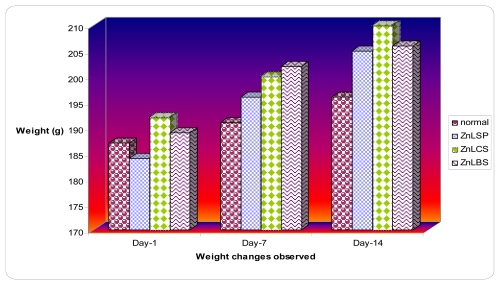
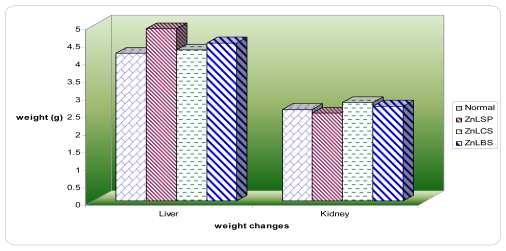
The animals treated with the zinc complexes for two weeks had manifested an increase in body weight slightly above the animals in the control group (Figure 1). The target organs such as liver and kidney of both the control and the treatment group did not exhibit any change in color or texture, and the weight of these organs was not significantly (P < 0.05) affected by the zinc complexes (Figure 2). This also demonstrated the less toxic effect [37] of the compounds.
Figure 1.
Effects of zinc complexes on the body weights.
Figure 2.
Effects of zinc complexes on the organ weights.
2.4. Effects of Zinc Complexes on the Biochemical Indices
The oral administration of the zinc complexes for two weeks did not cause any significant changes in the biochemical parameters such as renal function indices like creatinine, urea, anion gap sodium and carbon dioxide. Both the normal rats and the rats treated with zinc complexes had manifested the level of biochemical indices within the normal range (Table 1). However, the liver enzymes AST, ALT, ALP and triglyceride rose significantly in the rats that received ZnLSP, compared to the rats administered with the complexes ZnLCS and ZnLBS and the normal rats. This can be attributed to the damage in the liver cells [38] due to low toxic effect of the compounds. This is reduced in the analogue complexes that contained ring substituents in their structures, thus displaying the influence of ring substituents on the activity of the compounds [39,40]. Other liver indices like total protein count, albumin, globulins, total cholesterol, total and conjugated bilirubin did not significantly change at the dose of 2000 mg/kg/body weight (Table 2). A similar result was obtained in the acute toxicity evaluations of the free ligands in our previous study [27].
Table 1.
Effect of zinc complexes on the renal functions.
| Indices | Normal | [Zn(LSP)Cl2] | [Zn(LCS)Cl2] | [Zn(LBS)Cl2] |
|---|---|---|---|---|
| Sodium | 139.5 ± 2.6 | 139.2 ± 2.4 | 138.2 ± 2.7 | 138.5 ± 2.9 |
| Potassium | 5.05 ± 0.8 | 5.50 ± 0.6 | 4.70 ± 0.9 | 5.100 ± 0.7 |
| Chloride | 103.8 ± 1.3 | 104.6 ± 2.3 | 105.1 ± 1.2 | 102.3 ± 1.7 |
| CO2 | 23.9 ± 2.1 | 22.2 ± 2.6 | 21.3 ± 3.2 | 23.10 ± 3.3 |
| Anion gap | 17.4 ± 1.2 | 18.5 ± 0.7 | 16.5 ± 3.1 | 18.50 ± 3.5 |
| Urea | 6.10 ± 1.3 | 7.40 ± 0.6 | 7.60 ± 1.7 | 9.300 ± 2.6 |
| Creatinine | 42.5 ± 1.9 | 28.4 ± 17 | 40.8 ± 1.9 | 50.50 ± 1.9 |
Table 2.
Effects of zinc complexes on the liver functions.
| Indices | Normal | [Zn(LSP)Cl2] | [Zn(LCS)Cl2] | [Zn(LBS)Cl2] |
|---|---|---|---|---|
| Total protein | 70.5 ± 3.6 | 87.3 ± 4.9 | 75.5 ± 3.2 | 81.8 ± 2.6 |
| Albumin | 59.5 ± 2.4 | 68.6 ± 4.1 | 62.5 ± 3.2 | 69.3 ± 0.5 |
| Globulin | 59.5 ± 3.4 | 69.5 ± 4.9 | 61.9 ± 2.2 | 65.3 ± 2.4 |
| Alk. Phosphate | 59.30 ± 11.3 | 81.30 ± 10.8 | 82.3 ± 11.3 | 92.80+12.5 |
| ALT | 49.8 ± 7.2 | 61.0 ± 3.6 | 57.8 ± 3.4 | 55.3 ± 4.9 |
| AST | 259.8 ± 12.7 | 292.3 ± 10.6 | 278.5 ± 9.8 | 281.5 ± 12.6 |
| Total bilirubin | 6.25 ± 0.5 | 7.50 ± 0.7 | 6.88 ± 0.8 | 7.32 ± 0.8 |
| C.bilurubin | 3.61 ± 0.9 | 5.83 ± 1.3 | 3.85 ± 1.4 | 4.22 ± 2.1 |
| Triglyceride | 0.45 ± 0.1 | 0.60 ± 0.4 | 0.30 ± 0.05 | 0.80 ± 0.8 |
| Total cholesterol | 2.20 ± 0.3 | 3.70 ± 0.5 | 3.30 ± 0.1 | 3.6 0± 0.4 |
| HDL | 1.53 ± 0.4 | 1.47 ± 0.2 | 1.39 ± 0.4 | 1.50 ± 0.6 |
The hematological profile of the rats treated with zinc complexes did not significantly differ in the red blood cell (RBC), mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH). Furthermore, the values of the biomarkers like hematocrit (HCT), RDW and platelet in the treated rats are comparable with those in the normal rats, except the biomarker MCHC which showed inconsistent results in the treatment groups. However, for ZnLCS and ZnLBS, the values obtained are within the physiological ranges [41] for rats (Table 3). The value obtained for MCHC in the rats treated with the complex ZnLSP is below that of the normal rats and there is no interpretation from the literature for this.
Table 3.
Effects of zinc complexes on the hematological indices.
| Indices | Normal | [Zn(LSP)Cl2] | [Zn(LCS)Cl2] | [Zn(LBS)Cl2] |
|---|---|---|---|---|
| HGB | 151.3 ± 11.0 | 162.3 ± 11.8 | 158.3 ± 12.2 | 163.3 ± 13.5 |
| HCT | 0.540 ± 0.21 | 0.980 ± 0.03 | 0.830 ± 0.01 | 0.920 ± 0.04 |
| RBC | 7.90 ± 0.4 | 8.10 ± 0.3 | 8.60 ± 0.3 | 8.80 ± 0.7 |
| MCV | 65.2 ± 2.4 | 69.3 ± 1.5 | 67.9 ± 1.8 | 76.9 ± 1.8 |
| MCH | 17.9 ± 0.9 | 18.1 ± 0.3 | 18.9 ± 0.6 | 18.6 ± 0.5 |
| MCHC | 291.2 ± 2.3 | 260.5 ± 4.4 | 302.3 ± 5.6 | 269.3 ± 4.5 |
| RDW | 15.8 ± 1.4 | 17.5 ± 1.2 | 16.6 ± 1.5 | 17.9 ± 2.1 |
| WBC | 10.7 ± 3.1 | 11.3 ± 0.4 | 12.9 ± 4.4 | 12.4 ± 1.8 |
| Platelet | 654.3 ± 9.5 | 846.6 ± 8.5 | 689.3 ± 9.7 | 708.3 ± 9.4 |
The acute phase immunoglobulins’ G, A and M, the complements 3 and 4, and the level of C-reactive protein did not significantly differ between the normal, and the treated rats in both gender. This indicates that the complexes did not interfere with the immune system of the treated rats [42] (Table 4).
Table 4.
Effects of zinc complexes on the immunological indices.
| Indices | Normal | [Zn(LSP)Cl2] | [Zn(LCS)Cl2] | [Zn(LBS)Cl2] |
|---|---|---|---|---|
| ImmunoglobulinG | 933.4 ± 2.3 | 933.8 ± 4.2 | 933.5 ± 3.6 | 933.9 ± 4.8 |
| Immunoglobulin A | 97.5 ± 2.2 | 89.6 ± 3.7 | 98.7 ± 4.4 | 99.2 ± 4.3 |
| Immunoglobulin M | 43.9 ± 7.9 | 42.3 ± 3.5 | 52.7 ± 8.3 | 63.5 ± 9.6 |
| Complement 3 | 96.8 ± 2.3 | 96.2 ± 1.2 | 96.6 ±3.2 | 96.9 ±1.7 |
| Complement 4 | 29.8 ±2.1 | 52.9 ±3.2 | 57.2 ± 4.2 | 57.9 ± 4.2 |
| C-reactive Protein | 0.42 ± 0.3 | 0.34 ± 0.5 | 0.45 ± 0.2 | 0.53 ± 0.2 |
3. Experimental
3.1 Chemistry
2-(piperazin-1-yl)ethanamine, salicylaldehyde, 5-chlorosalicylaldehyde, and 5-bromosalicylaldehyde were used without further purification. Methanol, absolute ethanol, dimethylsulfoxide (DMSO) and all other solvents were of analytical grade. Spectroscopic grade DMSO-d6 was used for 1H and 13C NMR. All the chemicals used were purchased from Sigma Aldrich (Kuala Lumpur, Malaysia) and used without further purification. Mass spectra were determined using ABI 4800 Maldi TOF/TOF mass spectrophotometer (BIDMC Genomics, Proteomics and Bioinformatics Core, Boston, MA, USA) (LC-MS, ESI, 125.0 V); IR spectra was recorded at the wavelength range from 4000–400 cm−1 using a Perkin Elmer 783 spectrophotometer; NMR spectra was obtained on a ECA400 FT-NMR spectrophotometer using TMS as internal standard, UV-visible spectra was recorded on an UV-1650PC model UV-visible spectrophotometer.
3.2. Schiff Bases
The Schiff bases (LSP, LCS and LBS) were prepared according to the reported general procedure [43] described below with some modifications.
To the ethanolic solution (25 mL) of (2-piperazin-1-yl)ethanamine (2.58 g, 20 mmol), salicylaldehyde (2.44 g, 20 mmol) taken in ethanol (25 mL) was added with stirring. The resulting solution was refluxed for three hours, cooled and concentrated to give a red gel. The gel became hygroscopic solid after seven days under vacuum. The solid product is then dissolved in methanol by heating to 55 °C. While hot, few drops of diethyl ether were added and yellow solid appeared which was collected by filtration. Recrystallization was performed in ethanol-water mixture. The same procedure was followed in the preparation of LCS and LBS Schiff bases.
3.3. Complexes
3.3.1. 2-((2-(piperazin-1-yl)ethylimino)methyl)phenol-dichlorido-Zn-(II): [Zn(LSP)Cl2]
Stoichiometric amount of Zinc (II) chloride (0.14 g, 1 mmol) in methanol (25 mL), was added to an equimolar quantity of the appropriate Schiff base (1 mmol) dissolved in the same solvent (25 mL) at room temperature and followed with few drops of potassium hydroxide. A yellow precipitate was produced upon stirring. The precipitate filtered, washed with distilled water and dried in the vacuum for further analysis. The same method was applied in the synthesis of [Zn(LCS)Cl2] and [Zn(LBS)Cl2]. C13H18Cl2N3ZnO: yield; (0.15 g 40.6%). Anal. Cal. C, 66.9; H, 8.21; N, 18.01. Found: C, 65.82; H, 7.76; N, 17.97%. m/z: 369.03, 367.04, 371.01. IR (KBr disc, 4000–400 cm−1) selected bands: ν (N–H), 3442; ν (C–H) alip., 2825; ν (C=N), 1628; ν (C–C) arom., 1468; ν (C–N), 1152; ν (C–H) arom.768; ν (M–O), 569; ν (M–N), 486. 1H NMR (400 MHz, DMSO-d6) δ ppm: 7.92 (s, 1H, –C=N–); 2.66–3.45 (t, 2H, Caliph); 13C NMR (100 MHz, DMSO-d6) δ ppm: 46.5 (CH2); 34.51 (CH2); 36.5 (CH2); 39.4 (CH2); 122.8 (armC); 161.5 (CO); 162.6 (C=N); 117.6 (armC); 125.7 (armC). UV-vis (DMSO), λmax (ɛ, mol−1·L cm−1): 279 nm (2647.49, π-π*), 351 nm (2811.20, LMCT).
3.3.2. 4-chloro-2-((2-(piperazin-1-yl)ethylimino)methyl)phenol-dichlorido-Zn(II): [Zn(LCS)Cl2]
C13H17N3Cl3O2Zn: yield; (0.19 g, 47%). Anal. Cal. C, 58.31; H, 6.78; N, 15.69. Found: C, 57.97; H, 5.94; N, 15.27. m/z: 402.98, 400.98, 404.97. IR (KBr disc, 4000–400 cm−1) selected bands: ν (N–H), 3459; ν (C–H) alp., 2966; ν (C=N), 1624; ν (C-C) arom., 1466; ν (C–N), 1172; ν (C–H) arom.704; ν (M–O), 645; ν (M–N), 581. 1H NMR (400 MHz, DMSO-d6) δ ppm: 8.02 (s, 1H, –C=N–); 2.56–3.48 (t, 2H, Caliph); 13C NMR (100 MHz, DMSO-d6) δ ppm: 45.6 (s, 1 CH2); 34.8 (s, 1 CH2); 34.9 (s, 1 CH2); 123.2 (s, 1 armCH2); 159.5 (s, 1 CO); 164.2 (s, 1 C=N); 116.2 (s, 1 armCH2); 123.6 (s, 1 CH2). UV-vis (DMSO), λmax (ɛ, mol−1 L·cm−1): 204 nm (937.9, π-π*), 326 nm (3989.7, LMCT)
3.3.3. 4-bromo-2-((2-(piperazin-1-yl)ethylimino)methyl)phenol-dichlorido-Zn-(II): [Zn(LBS)Cl2]
C13H17N3BrCl2O2Zn: yield; (0.32g 71.3%). Anal. Cal. C, 34.89; H, 3.83; N, 9.39. Found: C, 34.82; H, 3.66; N, 8. 98. m/z: 446.93, 448.93, 450.92. IR (KBr disc, 4000–400 cm−1) selected bands: ν (N–H), 3448; ν (C–H) alp., 2966; ν (C=N), 1631; ν (C–C) arom., 1466; ν (C–N), 1169; ν (C–H) arom. ) 686; ν (M–O), 631; ν (M–N), 578. 1H NMR (400 MHz, DMSO-d6) δ ppm: 8.05 (s, 1H, –C=N–); 2.52–3.43 (t, 2H, Caliph); 13C NMR (100 MHz, DMSO-d6) δ ppm: 46.6 (s, 1 CH2); 34.7 (s, 1 CH2); 35.1 (s, 1 CH2); 122.1 (s, 1 CH2); 158.4 (s, 1 CO); 165.2 (s, 1 C=N); 114.4 (s, 1 CH2); 124.5 (s, 1 CH2). UV-vis (DMSO), λmax (ɛ, mol−1·L cm−1): 267 nm (1939.17, π-π*), 382 nm (1564.26, LMCT)
3.4. Animals
Adult Sprague Dawley rats of 8–9 weeks old weighed 180–200 g were obtained from Animal House, Faculty of Medicine, University of Malaya (Kuala Lumpur, Malaysia). The animals were housed in animal room at temperatures 22 ± 3 °C and 12 h dark period. After one-week acclimatization, rats were distributed into four groups of ten rats each (five males and five females, labeled as control and treated) and maintained on standard pellet food and purified drinking water. All animals received human care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institute of Health.
3.5. Acute Toxicity Test
Acute toxicity evaluations were carried out on rats according to the reported method with some modifications [44]. Sprague Dawley rats of both genders were divided into experimental and control groups (10 rats per group of five males and five females each). The study was executed at a single oral dose of 2000 mg/kg body weight, in 5 mL/kg volume. The control group was treated with distilled water. The experimental group was fasted for 24 h before the administration of the compound but allowed access to distilled water. The animals were further denied access to food for 2 h of post treatment in order to examine the possible adverse effects of the compounds such as behavioral adjustments, autonomous released of mucus, dizziness restlessness or mortality.
3.6. Statistical Analysis
The results were analyzed using one-way analysis of variance (ANOVA) and expressed as mean ± SEM. Probability values of P < 0.05 was considered statistically significant.
4. Conclusion
In conclusion, the results of this study showed that the zinc complexes derived from the Schiff bases 2-(2-(piperazin-1-yl)ethylimino)methyl)phenol, 4-chloro-2-(2-(piperazin-1-yl)ethylimino)methyl) phenol and 4-bromo- 2-(2-(piperazin-1-yl)ethylimino)methyl) phenol have fewer toxic effects based on the insignificant changes observed in the behavioral, hematological, immunological and biochemical parameters. However, a decrease in the activity of liver and some hematological indices was noted, which require further study to fully ascertain the safety of the compounds at high doses.
Acknowledgement
The author highly acknowledged the financial support given by the University of Malaya through the grants; PS358/2009C and ER009/2011A
Reference
- 1.Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 2.McCall K.A., Huang C., Fierke C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000;130:1437S–1446S. doi: 10.1093/jn/130.5.1437S. [DOI] [PubMed] [Google Scholar]
- 3.O’Neil-Cutting M.A., Bomford A., Munro H. Effect of excess dietary zinc on tissue storage of iron in rats. J. Nutr. 1981;111:1969–1979. doi: 10.1093/jn/111.11.1969. [DOI] [PubMed] [Google Scholar]
- 4.Barry C., Starcher J.G.G., Madaras J.G. Zinc Absorption and Its Relationship to Intestinal Metallothionein. J. Nutr. 1980;110:1391–1397. doi: 10.1093/jn/110.7.1391. [DOI] [PubMed] [Google Scholar]
- 5.Cruickshank P., Sheehan J.C. Gas chromatographic analysis of amino acids as N-trifluoroacetylamino acid methyl esters. Anal. Chem. 1964;36:1191–1197. [Google Scholar]
- 6.Jacobus W.E., Lehninger A.L. Creatine kinase of rat heart mitochondria. J. Biol. Chem. 1973;248:4803–4810. [PubMed] [Google Scholar]
- 7.Lehninger A.L., Vercesi A., Bababunmi E.A. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc. Nat. Acad. Sci. USA. 1978;75:1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazquez M.A., Munoz F., Donoso J., Blanco F.G. Spectroscopic study of the Schiff bases of dodecylamine with pyridoxal 5′-phosphate and 5′-deoxypyridoxal. A model for the Schiff bases of pyridoxal 5′-phosphate in biological systems. Biochem. J. 1991;279:759–767. doi: 10.1042/bj2790759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salata C.A., Youinou M.T., Burrows C.J. Preparation and structural characterization of dicopper (II) and dinickel (II) imidazolate-bridged macrocyclic Schiff base complexes. Inorg. Chem. 1991;30:3454–3461. [Google Scholar]
- 10.Dowling C., Murphy V.J., Parkin G. Bis (pyrazolylethyl) ether ligation to zinc and cobalt: Meridional vs facial coordination and the suitability of such ligands in providing a NNO donor set for modeling bioinorganic aspects of zinc chemistry. Inorg. Chem. 1996;35:2415–2420. doi: 10.1021/ic9512744. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S.M. New approaches for medicinal applications of bioinorganic chemistry. Curr. Opin. Chem. Biol. 2007;11:115–120. doi: 10.1016/j.cbpa.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Rafique S., Idrees M., Nasim A., Akbar H., Athar A. Transition metal complexes as potential therapeutic agents. Biotech. Mol. Biol. Rev. 2010;5:38–45. [Google Scholar]
- 13.Nishat N., Haq M.M., Ahamad T., Kumar V. Synthesis, spectral and antimicrobial studies of a novel macrocyclic ligand containing a piperazine moiety and its binuclear metal complexes. J. Coord. Chem. 2007;60:85–96. [Google Scholar]
- 14.Keypour H., Rezaeivala M., Valencia L., Perez-Lourido P. Synthesis and crystal structure of Mn (II) complexes with novel macrocyclic Schiff-base ligands containing piperazine moiety. Polyhedron. 2008;27:3172–3176. [Google Scholar]
- 15.Islam M.K., Miyoshi T., Yamada M., Alim M.A., Huang X., Motobu M., Tsuji N. Effect of piperazine (diethylenediamine) on the moulting, proteome expression and pyrophosphatase activity of Ascaris suum lung-stage larvae. Acta. Trop. 2006;99:208–217. doi: 10.1016/j.actatropica.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary P., Kumar R., Verma A.K., Singh D., Yadav V., Chhillar A.K., Sharma G.L., Chandra R. Synthesis and antimicrobial activity of N-alkyl and N-aryl piperazine derivatives. Bioorgan. Med. Chem. 2006;14:1819–1826. doi: 10.1016/j.bmc.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Narendra S.C.J.N., Sadashiva C.T., Kavitha C.V., Rangappa K.S. Synthesis and in vitro antimicrobial studies of medicinally important novel N-alkyl and N-sulfonyl derivatives of 1-[bis (4-fluorophenyl)-methyl] piperazine. Bioorg. Med. Chem. 2006;14:6621–6627. doi: 10.1016/j.bmc.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 18.Sadashiva C.T., Narendra S.C.J.N., Ponnappa K.C., Veerabasappa G.T., Rangappa K.S. Synthesis and efficacy of 1-[bis (4-fluorophenyl)-methyl] piperazine derivatives for acetylcholinesterase inhibition, as a stimulant of central cholinergic neurotransmission in Alzheimer’s disease. Bioorgan. Med. Chem. Lett. 2006;16:3932–3936. doi: 10.1016/j.bmcl.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Nozawa D., Okubo T., Ishii T., Takamori K., Chaki S., Okuyama S., Nakazato A. Novel piperazines: Potent melanocortin-4 receptor antagonists with anxiolytic-like activity. Bioorg. Med. Chem. 2007;15:2375–2385. doi: 10.1016/j.bmc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Chen C., Jiang W., Tucci F., Tran J.A., Fleck B.A., Hoare S.R., Joppa M., Markison S., Wen J., Chen C.W., et al. Discovery of 1-{2-[(1S)-(3-Dimethylamino-propionyl)amino-2-methylpropyl]-4-methyl-phenyl}-4-[(2R)-methyl-3-(4-chlorophenyl)-propionyl] piperazine as an Orally Active Antagonist of the Melanocortin-4 Receptor for the Potential Treatment of Cachexia. J. Med. Chem. 2007;50:5249–5252. doi: 10.1021/jm070806a. [DOI] [PubMed] [Google Scholar]
- 21.Staack R.F., Paul L.D., Springer D., Kraemer T., Maurer H.H. Cytochrome P450 dependent metabolism of the new designer drug 1-(3-trifluoromethylphenyl) piperazine (TFMPP): In vivo studies in Wistar and Dark Agouti rats as well as in vitro studies in human liver microsomes. Biochem. Pharmacol. 2004;67:235–244. doi: 10.1016/j.bcp.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Serradji N., Bensaid O., Martin M., Sallem W., Dereuddre-Bosquet N., Benmehdi H., Redeuilh C., Lamouri A., Dive G., Clayette P., et al. Structure-activity relationships in platelet-activating factor. Part 13: Synthesis and biological evaluation of piperazine derivatives with dual anti-PAF and anti-HIV-1 or pure antiretroviral activity. Bioorg. Med. Chem. 2006;14:8109–8125. doi: 10.1016/j.bmc.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Benmehdi H., Lamouri A., Serradji N., Pallois F., Heymans F. Synthesis of new trisubstituted 4-aminopiperidines as PAF-receptor antagonists. Eur. J. Org. Chem. 2008;2008:299–307. [Google Scholar]
- 24.Sallem W., Serradji N., Dereuddre-Bosquet N., Dive G., Clayette P., Heymans F. Structure-activity relationships in platelet-activating factor. Part 14: Synthesis and biological evaluation of piperazine derivatives with dual anti-PAF and anti-HIV-1 activity. Bioorg. Med. Chem. 2006;14:7999–8013. doi: 10.1016/j.bmc.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Salga M.S., Ali H.M., Abdullah M.A., Abdelwahab S.I., Hussain P.D., Hadi A.H.A. Mechanistic studies of the anti-ulcerogenic activity and acute toxicity evaluation of dichlorido-copper (II)-4-(2-5-Bromo-benzylideneamino) ethyl) piperazin-1-ium phenolate complex against ethanol-induced gastric injury in rats. Molecules. 2011;16:8654–8669. [Google Scholar]
- 26.Yu M., Lizarzaburu M., Beckmann H., Connors R., Dai K., Haller K., Li C., Liang L., Lindstrom M., Ma J., et al. Identification of piperazine-bisamide GHSR antagonists for the treatment of obesity. Bioorg. Med. Chem. Lett. 2010;20:1758–1762. doi: 10.1016/j.bmcl.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 27.Salga S.M., Ali H.M., Abdullah M.A., Abdelwahab S.I., Wai L.K., Buckle M.J.C., Sukumaran S.D., Hadi A.H.A. Synthesis, characterization, acetylcholinesterase inhibition, molecular modeling and antioxidant activities of some novel schiff bases derived from 1-(2-ketoiminoethyl) piperazines. Molecules. 2011;16:9316–9330. doi: 10.3390/molecules16119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nath M., Saini P.K., Kumar A. New di-and triorganotin (IV) complexes of tripodal Schiff base ligand containing three imidazole arms: Synthesis, structural characterization, anti-inflammatory activity and thermal studies. J. Organomet. Chem. 2010;695:1353–1362. [Google Scholar]
- 29.Yuan R., Chai Y., Liu D., Gao D., Li J., Yu R. Schiff base complexes of cobalt (II) as neutral carriers for highly selective iodide electrodes. Anal. Chem. 1993;65:2572–2575. [Google Scholar]
- 30.Holm R.H. Studies on Ni (II) complexes. I. Spectra of tricyclic schiff base complexes of Ni (II) and Cu (II) J. Am. Chem. Soc. 1960;82:5632–5636. [Google Scholar]
- 31.Cimerman Z., Galesic N., Bosner B. Structure and spectroscopic characteristics of Schiff bases of salicylaldehyde with 2,3-diaminopyridine. J. Mol. Struct. 1992;274:131–144. [Google Scholar]
- 32.Vlcek A. Mechanistic roles of metal-to-ligand charge-transfer excited states in organometallic photochemistry. Coord. Chemi. Rev. 1998;177:219–256. [Google Scholar]
- 33.Koester V.J. Interligand transmetallic charge-transfer transitions in mixed-ligand chelates. Chem. Phys. Lett. 1975;32:575–580. [Google Scholar]
- 34.Zalis S., Farrell I.R., Vlcek A., Jr The involvement of metal-to-CO charge transfer and ligand-field excited states in the spectroscopy and photochemistry of mixed-ligand metal carbonyls. A theoretical and spectroscopic study of [W (CO) 4(1,2-ethylenediamine)] and [W (CO) 4 (N,N′-bis-alkyl-1,4-diazabutadiene)] J. Am. Chem. Soc. 2003;125:4580–4592. doi: 10.1021/ja021022j. [DOI] [PubMed] [Google Scholar]
- 35.Bagus P.S., Hermann K., Bauschlicher C.W., Jr. A new analysis of charge transfer and polarization for ligand–metal bonding: Model studies of AlCO and AlNH. J. Chem. Phys. 1984;80 doi: 10.1063/1.447215.. [DOI] [Google Scholar]
- 36.Ganter B., Tugendreich S., Pearson C.I., Ayanoglu E., Baumhueter S., Bostian K.A., Brady L., Browne L.J., Calvin J.T., Day G.J., et al. Development of a large-scale chemogenomics database to improve drug candidate selection and to understand mechanisms of chemical toxicity and action. J. Biotechnol. 2005;119:219–244. doi: 10.1016/j.jbiotec.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Rozman K.K., Doull J. Dose and time as variables of toxicity. Toxicology. 2000;144:169–178. doi: 10.1016/s0300-483x(99)00204-8. [DOI] [PubMed] [Google Scholar]
- 38.Bugrim A., Nikolskaya T., Nikolsky Y. Early prediction of drug metabolism and toxicity: Systems biology approach and modeling. Drug Dis. Today. 2004;9:127–135. doi: 10.1016/S1359-6446(03)02971-4. [DOI] [PubMed] [Google Scholar]
- 39.Benigni R., Tatiana I.N., Benfenati E., Bossa C., Franke R., Helma C., Hulzebos E., Marchant C., Richard A., Woo Y.T., et al. The expanding role of predictive toxicology: An update on the (Q) SAR models for mutagens and carcinogens. J. Environ. Sci. Hea. C. 2007;25:53–97. doi: 10.1080/10590500701201828. [DOI] [PubMed] [Google Scholar]
- 40.Cronin M.T.D., Jaworska J.S., Walker J.D., Comber M.H.I., Watts C.D., Worth A.P. Use of QSARs in international decision-making frameworks to predict health effects of chemical substances. Environ. Health Persp. 2003;111:1391–1401. doi: 10.1289/ehp.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das B.K., Mukherjee S.C. Toxicity of cypermethrin in Labeo rohita fingerlings: Biochemical, enzymatic and haematological consequences. Comp. Biochem. Phys. C. 2003;134:109–121. doi: 10.1016/s1532-0456(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 42.Basketter D.A., Bremmer J.N., Buckley P., Kammuller M.E., Kawabata T., Kimber I., Loveless S.E., Magda S., Stringer D.A., Vohr H.W. Pathology considerations for, and subsequent risk assessment of, chemicals identified as immunosuppressive in routine toxicology. Food Chem. Toxicol. 1995;33:239–243. doi: 10.1016/0278-6915(94)00128-b. [DOI] [PubMed] [Google Scholar]
- 43.Mukhopadhyay S., Mandal D., Ghosh D., Goldberg I., Chaudhury M. Equilibrium studies in solution involving nickel (II) complexes of flexidentate Schiff base ligands: Isolation and structural characterization of the planar red and octahedral green species involved in the equilibrium. Inorg. Chem. 2003;42:8439–8445. doi: 10.1021/ic0346174. [DOI] [PubMed] [Google Scholar]
- 44.Salga M.S., Ali H.M., Abdulla M.A., Abdelwahab S.I. Gastroprotective activity and mechanism of novel dichlorido-zinc (II)-4-(2-5-methoxybenzylideneamino) ethyl) piperazin-1-iumphenolate complex on ethanol-induced gastric ulceration. Chem. Biol. Interact. 2011 doi: 10.1016/j.cbi.2011.11.008. in press. [DOI] [PubMed] [Google Scholar]