Abstract
The NR2E1 region on Chromosome 6q21-22 has been repeatedly linked to bipolar disorder (BP) and NR2E1 has been associated with BP, and more specifically bipolar I disorder (BPI). In addition, patient sequencing has revealed an enrichment of rare candidate-regulatory variants. Interestingly, mice carrying either spontaneous (Nr2e1frc) or targeted (Tlx−) deletions of Nr2e1 (here collectively known as Nr2e1-null) show similar neurological and behavioral anomalies, including: hypoplasia of the cerebrum, reduced neural stem cell proliferation, extreme aggression, and deficits in fear conditioning; traits that have been observed in some patients with BP. Thus, NR2E1 is a positional and functional candidate for a role in BP. However, no Nr2e1-null mice have been fully evaluated for behaviors used to model BP in rodents or pharmacological responses to drugs effective in treating BP symptoms. In this study we examine Nr2e1frc/frc mice, homozygous for the spontaneous deletion, for abnormalities in activity, learning and information processing, and cell proliferation; phenotypes that are either affected in patients with BP or commonly assessed in rodent models of BP. The effect of lithium, a drug used to treat BP, was also evaluated for its ability to attenuate Nr2e1frc/frc behavioral and neural stem cell proliferation phenotypes. We show for the first time that Nr2e1-null mice exhibit extreme hyperactivity in the open field as early as postnatal day 18 and in the home cage, deficits in open-field habituation and passive avoidance, and, surprisingly, an absence of acoustic startle. We observed a reduction in neural stem/progenitor cell proliferation in Nr2e1frc/frc mice, similar to that seen in other Nr2e1-null strains. These behavioral and cell-proliferation phenotypes were resistant to chronic-adult-lithium treatment. Thus, Nr2e1frc/frc mice exhibit behavioral traits used to model BP in rodents, but our results do not support Nr2e1frc/frc mice as pharmacological models for BP.
Keywords: Bipolar disorder, nuclear receptor, mania, habituation, pain, learning and memory, startle reactivity, mental illness, mouse behavior, Tailless/Tlx
Introduction
Although bipolar disorder (BP) is a multifactorial psychiatric disorder that is highly heritable (60–85%) (Burmeister et al., 2008), and the 6q chromosomal region has repeatedly shown evidence for genetic linkage to BP and other neurological disorders (Dick et al., 2003, Hayden & Nurnberger, 2006, Kohn & Lerer, 2005, Mcqueen et al., 2005, Middleton et al., 2004, Pato et al., 2004, Schulze et al., 2004), the causative genes for BP are just beginning to be identified (Craddock & Sklar, 2009, Martinowich et al., 2009, Ogden et al., 2004). The largest meta-analysis of BP to date, found the strongest genome-wide linkage at 6q21-22 (108.5 Mb), with the highest LOD score (4.19) specifically for bipolar I disorder (BPI) that is accompanied by mania (Mcqueen et al., 2005). One of the genes in the 6q21-22 region is nuclear receptor 2E1 (NR2E1), which encodes a brain- and eye-specific orphan nuclear receptor. Members of the nuclear receptor superfamily, encode transcription factors that have previously been implicated in disorders of human brain and behavior, including NR4A2 (Buervenich et al., 2000) and the estrogen receptor (Westberg et al., 2003). Additionally, genes known or proposed to interact with Nr2e1 (Shi et al., 2004, Stenman et al., 2003a) have been implicated in human psychiatric disorders, including PAX6 (Stober et al., 1999) and NR4A2 (Buervenich et al., 2000). A functional role for NR2E1 in BP has further been supported by a significant association, after multiple testing correction, between NR2E1 and BPI, and enrichment of rare candidate-regulatory variants in NR2E1 in BP patients (Kumar et al., 2008).
Mice lacking Nr2e1, the mammalian homolog of the DrosophilaTlx (tailless) gene, have been developed in several laboratories (aka Tlx−/−, Nr2e1frc/frc) and are generally referred to as Nr2e1-null mice. The Nr2e1frc allele, studied here, is a spontaneous deletion of all nine exons of Nr2e1 as well as its proximal promoter (Kumar et al., 2004), while two different targeted deletions of Tlx exist by removing exons two and three (Monaghan et al., 1997) and exons three, four, and five (Yu et al., 2000) by homologous recombination. Nr2e1 heterozygous mice have little to no phenotypic effects, but collectively Nr2e1-null mice have revealed this gene to be critical in the maintenance and cell fate determination of neural stem/progenitor cells (Shi et al., 2004), and when absent results in extreme aggression in mice (Young et al., 2002). The various strains of Nr2e1-null mice exhibit comparable neuroanatomical abnormalities, of particular interest are those similar to abnormalities seen in some patients with BP, including: increased lateral ventricular volume; reduced volume of the hippocampus, cerebral cortex, corpus callosum, amygdala, and cortical layers II and III; olfactory abnormality and dysfunction; reduced neurogenesis; and impairment in GABAergic interneurons (Anand & Shekhar, 2003, Brambilla et al., 2003, Goldberg & Chengappa, 2009, Kruger et al., 2006, Land & Monaghan, 2003, Mccurdy et al., 2006, Monaghan et al., 1997, Roy et al., 2004, Shi et al., 2004, Stenman et al., 2003b, Swayze et al., 1990, Tian et al., 2007, Young et al., 2002, Zhang et al., 2008). Cognitive functioning have only been examined in mice carrying targeted deletions of Nr2e1, these mice showed reduced fear conditioning, indicating abnormalities in emotion processing, a trait that has been observed in patients with BP and present in rodent models of BP (Calzavara et al., 2009, Roy et al., 2002). Furthermore, altered cell morphology and plasticity in the hippocampus has been detected in Nr2e1frc/frc mice, as well as other mouse models of BP, but have not been examined in targeted knockout mutants (Christie et al., 2006, Kvajo et al., 2008). Collectively, these neurological phenotypes, as well as linkage, association, and functional evidence, provide strong support for NR2E1 as a candidate gene for BP, especially BPI. Although the phenotypes listed above does not validate Nr2e1frc/frc mice as a model of BP, nor is BP diagnosed or defined by the phenotypes listed, however the presence of these traits provide support that Nr2e1 could play a role in the development of brain regions that might be involved in BP pathogenesis.
Despite the mounting support for NR2E1 as a candidate BP gene, Nr2e1-null mice have not been fully characterized for anomalies similar to those seen in some patients with BP, nor phenotypes commonly exhibited in rodent models of BP. Here, we examine Nr2e1frc/frc mice for abnormalities in activity level, learning, information processing, and cell proliferation in neurogenic regions. To evaluate the pharmacological validity of Nr2e1frc/frc mice as a model of BP, we tested the effect of lithium treatment on these parameters. Lithium, a mood-stabilizing drug known for its efficacy in the treatment of mania (Malhi et al., 2009) was classically used, and continues to be prescribed today, along with other medications such as valproate and olanzapine. Lithium has been shown to attenuate psychostimulus-induced hyperactivity in rodent models of mania (O’donnell K & Gould, 2007) and to promote neurogenesis in the dentate gyrus (DG) (Chen et al., 2000). Considering that Nr2e1-null neural stem/progenitor cells (NSCs) showed reduced proliferation that could be rescued by reintroducing Nr2e1in vitro (Shi et al., 2004), we tested whether lithium could attenuate the proliferative deficit in Nr2e1frc/frc mice and whether any behavioral amelioration would accompany.
Methods and Materials
Mice
The B6129F1-Nr2e1 mice used for experimental analysis were all first generation offspring resulting from mating C57BL/6J.129-Nr2e1frc (B6-Nr2e1frc/+) females (backcross generation N17-22) to 129S1/SvImJ.Cg-Nr2e1frc (129-Nr2e1frc/+) males (N15-20). The Nr2e1frc allele is a 44 kb spontaneous deletion of all 9 exons of Nr2e1 that does not affect transcription of neighboring genes (Kumar et al., 2004). In accordance with Mendelian inheritance, approximately 25% of the offspring were homozygous Nr2e1frc/frc mice and 25% were Nr2e1+/+ (Wt) littermates; the latter were used as controls. All mice were weaned at postnatal day (P)18 – 21, housed with same-sex littermates, and then individually housed by 4 weeks to avoid aggressive incidence with Nr2e1frc/frc mice and to be consistent for all mice. Mice were provided with food and water ad libitum and were provided standard care according to University of British Columbia animal care policies. Handling of all mice was minimized.
Genotyping
All mice were analyzed by two separate polymerase chain reaction (PCR) assays. Wild-type allele of Nr2e1 was amplified using oEMS1859 (5′-CTGGGCCCTGCAGATACTC-3′) and oEMS1860 (5′-GGTGGCATGATGGGTAACTC-3′), and the fierce deletion allele of Nr2e1 was detected using oEMS650 (5′-GGCGGAGGGAGCTTAAATAG-3′) and oEMS1368 (5′-GATTCATCCTATTCCACAAAGTCA-3′). Cycling conditions were as follow: 2 min at 92°C, 30 cycles of 30 s at 94°C, 30 s at 58°C, and 55 s at 72°C; and a final extension of 5 min at 72°C.
Testing procedure
All mice were tested in the pathogen-free behavior suite under reverse L/D cycle (light 23:00–11:00 h at 320 lux), at the Centre for Molecular Medicine and Therapeutics, Vancouver, Canada, as previously described (Hossain et al., 2004). The multi-room behavior suite consists of a breeding room and dedicated testing rooms, separated by corridors. The lighting in all areas was synchronized. Care was taken not to expose the mice to any inappropriate light, even during testing. When light was needed by the investigator during experiments in the dark phase, a dim red light (8 lux) was used. All adult mice tested were closely aged-matched males between the ages of 2 – 6 months, and the majority of mice used in the study were over 2 months. Naïve mice were used for each test, unless otherwise indicated. The testing chambers and equipment were thoroughly cleaned between each test subject, using Clidox (Pharmacal Research Laboratories Inc., Naugatuck, CT) and 70% ethanol.
Pup body weight and milk consumption
The body weights of 15 Wt (9 female and 6 male) and 14 Nr2e1frc/frc (5 female and 9 male) pups were measured at P0, 7, 14, and 21. Pups were individually placed on a clean plastic weigh boat and body weight was measured on a bench-top balance. The amount of milk consumption was similarly measured in a different cohort of 11 Wt and 12 Nr2e1frc/frc pups. Pups were removed from their mother and weighed, then kept separate from their mother for 2 h after which the pups were returned to their mother and given 15 min for feeding and were weighed again.
Pup open field activity
Spontaneous exploratory locomotor activity was measured on 10 Wt (8 female and 2 male) and 12 Nr2e1frc/frc (5 female and 7 male) pups at P9, 14, and 18 using a digiscan photocell-equipped automated open field apparatus 27.5 cm (L) × 27.5 cm (W) × 20.0 cm (H) with lower and upper beams at 1.5 cm and 5.5 cm from the floor, respectively (Med Associates Inc., St. Albans, VT). Each pup was placed in the center of the novel arena and allowed to explore for 3 min while the software tallied spatially identified beam breaks.
Home cage activity
Home cage activity was measured on a total of 7 Wt (3 female and 4 male) and 8 Nr2e1frc/frc (4 female and 4 male) mice during a 48-h period using identical Cage Rack Systems (San Diego Instruments, San Diego, CA). Each mouse home cage was placed in the center of a metal cage rack frame that generates a uniformly spaced 8 × 4 photobeam grid. The mice were provided with food and water ad libitum throughout the testing period and spontaneous locomotor activity was measured by counting the total number of beam breaks each hour during the 48-h period (Kopp, 2001).
Open field activity and habituation
Activity and habituation in the open field of 12 Wt and 9 Nr2e1frc/frc male mice were measured using the open field apparatus described above (Pup open field activity). Mice were introduced to the open field apparatus for three consecutive days and tested for 10 min each time. The numbers of beam breaks were recorded for all trials.
Tail suspension
Struggling during the 3 min tail suspension test was measured on 8 Wt and 4 Nr2e1frc/frc male mice using a PHM-300TSS mouse tail suspension system (Med Associates, St. Albans, VT), as previously described (Abrahams et al., 2005). The apparatus was calibrated to normalize for body weight before testing of each animal. Signals are amplified by a gain value of 4 and the struggle threshold was set at a signal of 15, meaning that only signals above the value of 15 were indicative of struggle. Percent time struggle was then calculated as time spent struggling during which force exceeded the struggle threshold (set to 15) divided by the total testing time (3 min).
Hot plate and tail flick
Thermal nociception and pain sensitivity of 8 Wt and 8 Nr2e1frc/frc male mice was measured for each mouse using the hot plate and tail flick tests, respectively, as previously described (Hossain et al., 2004). Mice were placed on the hot plate apparatus (Columbus Instruments, Columbus, OH) thermostatically set at 55.0 ± 0.5 °C. The latency of first licking or kicking of the fore or hind paw was recorded. A cut-off time of 60 s was employed to avoid tissue damage.
For the tail flick test, mice were placed in a clear restraining tube (Model 33033, Columbus Instruments, Columbus, OH) and the tail was placed freely on a level surface between two photo detector panels of the automated tail flick analgesia meter (Columbus Instruments, Columbus, OH). Immediately after a 90-s habituation period, radiant heat from a 20-V beam of light was focused on the ventral surface of the tail and the time for the mouse to flick its tail was automatically recorded by the apparatus. A 10-s cut-off time was employed to prevent tissue damage.
For both tests, the average of two consecutive trials, separated by a 1-min interval, was calculated for each animal.
Auditory brainstem response
Auditory functions of 4 Wt and 5 Nr2e1frc/frc male mice were tested using the auditory brainstem response (ABR) procedure, as previously described (Ikeda et al., 1999, Zheng et al., 1999). Briefly, the test was performed on anesthetised mice where subdermal needle electrodes were inserted at the vertex (active) and ventrolaterally to the right ear (reference) and to the left ear (ground). Specific acoustic stimuli were delivered binaurally through 1 cm plastic tubes channeled from high frequency transducers. Mice were tested with click stimuli and also with 16 kHz tone pips at varying intensity, from low to high (10–90 dB SPL). An auditory brainstem response (ABR) threshold was determined for each stimulus frequency by identifying the lowest intensity that produced a recognizable ABR pattern.
Passive avoidance
Learning and memory of 9 Wt and 6 Nr2e1frc/frc male mice was tested in the passive avoidance test using the GEMINI™ Avoidance System (San Diego Instruments, San Diego, CA). The equipment has two chambers separated by a sliding door. Mice were introduced to the first chamber in the presence of an auditory stimulus. After 30 s in the first chamber, the door separating the two chambers opened and the mouse was allowed to enter into the second chamber without the auditory stimulus. The time it took for the mouse to enter the second chamber after the door opened was recorded. The maximum time allowed to enter the second chamber was 180 s. Once the mouse entered the second chamber it received a mild electrical shock (0.2 mA lasting 2 s). The mouse was again tested 24 h later and the latency of entering the second chamber was recorded.
Acoustic startle reactivity
Acoustic startle reactivity was tested using the SR-LAB system (San Diego Instruments, San Diego, CA). Two separate groups of male mice were used: Group 1 (12 Wt, 9 Nr2e1frc/frc) and Group 2 (7 Wt, 7 Nr2e1frc/frc). After a 5-min acclimatization period, each mouse was subjected to 90 acoustic startle stimuli (10 at each of nine intensities ranging from 75 to 125 dB) in a semi-randomized sequence. The startles had a fixed duration of 50 ms and were separated by a variable inter-stimulus interval (ISI) ranging from 20 to 30 s, while the recording window was set at 100 ms. Startle response was measured at each stimulus as well as at 10 no-stimulus trials.
Lithium administration and testing procedure
3 Wt and 5 Nr2e1frc/frc male mice received lithium chloride (LiCl) diets, while 4 Wt and 4 Nr2e1frc/frc male mice received control diets. Mice on the control diet were fed with untreated purified diet with Teklad Vitamin Mix (Harlan Teklad, Madison, WI). Mice on the lithium diet were fed with 1.7 g LiCl/kg added to the untreated purified diet with Teklad Vitamin Mix (Harlan Teklad, Madison, WI) for 4 weeks, and then switched to 2.55 g LiCl/kg added to the untreated purified diet with Teklad Vitamin Mix (Harlan Teklad, Madison, WI) for 2 additional weeks, before behavior testing. These mice remained on the 2.55 g LiCl/kg of chow diet throughout the testing period. All mice were also given water ad libitum and a water bottle of 450 mM sodium chloride solution. Each mouse was subjected to behavior tests in the following order: home cage activity, open field activity and habituation, and startle reactivity. The start of each test was performed one week after the end of the previous test. Tests were performed as described in the above sections. At the end of behavior testing all animals were sacrificed and bled for serum analysis of lithium level, and brains were harvested for immunohistochemical analysis.
Serum analysis
Mice from the lithium-treatment experiment were given a lethal injection of 2,2,2-tribromoethanol in tertiary amyl alcohol (Sigma-Aldrich, St. Louis, MO) (aka avertin) and blood was collected via cardiac puncture using a 25-gauge needle. Blood samples were allowed to separate for 30 min at room temperature (RT). Samples were then centrifuged for 10 min at RT at 3000 RPM for separation of serum. The serum was then isolated and kept at −20°C until lithium levels analyses. The Department of Pathology and Laboratory Medicine at Vancouver General Hospital, blinded to the experimental conditions, analyzed serum lithium level. 0.2 mmol/L was the minimum detection limit of lithium serum assay.
Brain harvesting and immunohistochemistry
Brains of mice from the lithium-treatment experiment were dissected out intact and placed into 4% paraformaldehyde in 1× PBS at 4°C for 48 h, then transferred to a 20% sucrose solution at 4°C until saturated. Brains were then sectioned at 25 μm using the Cryo-Star HM 560 cryostat (MICROM International, Walldorf, Germany) and representative sections (every 24th) starting from the most rostral aspect of the ventricles to the most caudal aspect of the hippocampus were analyzed by immunofluorescence.
Sections were blocked with 5% normal goat serum (NGS) + 5% bovine serum albumin (BSA) in 0.1% Triton-X100 in PBS, incubated overnight at RT with rabbit anti-Ki67 polyclonal antibody (1:1000 dil, Cat. #ab15580; Abcam Inc., Cambridge, MA), and further incubated with Alexa Fluor® 594 goat anti-rabbit IgG (H+L) (Cat. #A31631; Invitrogen, Carlsbad, CA). Hoechst 33342 was used for nuclear staining for all sections. Sections were mounted onto Superfrost® Plus slides (Cat. #12-550-15; Fisher Scientific, Ottawa, ON) and coverslipped using Vectashield Hard Set™ (Cat. #H-1400; Vector Laboratories, Inc., Burlingame, CA). Images were captured on an Olympus BX61 motorized fluorescence microscope (Olympus America Inc., Center Valley, PA) and Ki67+ cells that showed overlapping Hoechst+ profiles of cell nuclei were analyzed as proliferating cells in the SVZ and DG using the ImageJ software. Hoechst+ cells were also used to trace SVZ and DG areas in all sections used for cell counting. The number of Ki67+ cells was divided by area traces (mm2) to correct for area differences between the genotypes.
Statistical analysis
All data were analyzed using STATISTICA© 6 (StatSoft, Inc., Tulsa, OK). All data were initially examined using Shapiro-Wilk test for normal distribution. Data that did not fit a normal distribution underwent non-parametric analysis, while data that were normally distributed were subjected to parametric analysis. Body weight, pup open field, and auditory brainstem response data were not normally distributed and therefore underwent non-parametric analysis (Kruskal-Wallis ANOVA). Correction for multiple comparisons for non-parametric tests was achieved by dividing the P-value with the number of comparisons made. Milk consumption, tail suspension, hot plate, tail flick, and passive avoidance data were analyzed by one-way ANOVA for genotype differences. The remaining behavioral data (i.e. open field habituation, startle reactivity) were analyzed using repeated measures ANOVA for genotype effects with time or startle intensities as repeated measures. In all repeated measures ANOVAs the Greenhouse-Geisser correction factor (ε) was used to adjust the degrees of freedom (Vasey & Thayer, 1987). Post-hoc tests with Tukey correction were performed for between- and within-subject comparisons when appropriate.
One-way ANOVA was used to analyze genotype differences in lithium serum level for mice on lithium diet. Behavioral data pertaining to the lithium experiment were analyzed using repeated measures ANOVA for effects and interactions between genotype and diet with time or startle intensities as repeated measures. The same corrections as above were performed for these analyses. Cell proliferation data were analyzed using factorial ANOVA for genotype and drug treatment. All data are reported as mean values ± standard error of the mean (SEM).
Results
Young Nr2e1frc/frc mice show early hyperactivity
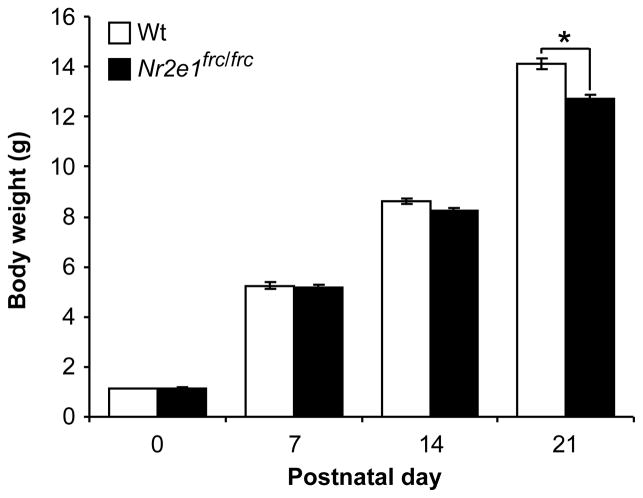
Previously, we showed that Nr2e1frc/frc pups on a C57BL/6J (B6) background failed to gain weight at the rate of their Wt littermates between postnatal weeks 2 and 3 (Young et al., 2002). For the current study, we retested this phenotype at postnatal (P) 0, 7, 14, and 21 on the B6129F1 background. When the data was analyzed for sex differences as a whole or separately for each genotype, no significant sex effect was detected at any of the postnatal days tested (P > 0.05). We further showed that regardless of sex, there were significant genotype differences at P21 (P < 0.05), but not at P0, 7, or 14 (P > 0.1). These findings indicated that B6129F1-Nr2e1frc/frc mice also failed to gain weight at the rate of their Wt siblings, and were significantly smaller by P21 Therefore, small size at wean is a stable phenotype across two genetic backgrounds.
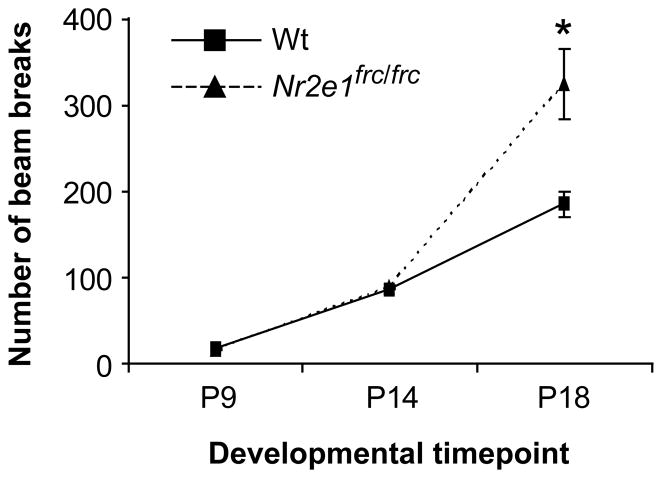
We measured milk consumption in pre-wean pups to test the hypothesis that the failure of Nr2e1frc/frcmice to gain weight normally may depend on a reduction in milk consumption. This hypothesis was not supported by the milk consumption data, where no significant differences were found between the two genotypes (Wt = 0.059 ± 0.004 g, Nr2e1frc/frc = 0.07 ± 0.01 g, P > 0.1). We then measured activity level in the same group of pre-wean pups at P9, 14, and 18 using the open field apparatus. No significant sex effect was detected at any of the postnatal days tested (P > 0.05) when the data was analyzed as a whole or separately for each genotype. We observed age-dependent increase activity level in Nr2e1frc/frc mice compared to Wt controls, as indicated by significant genotype differences at P18 (Fig. 2; P < 0.05), but not at P9 or 14 (P > 0.1). Therefore, the post-wean size reduction of Nr2e1frc/frc mice was not apparently the result of a feeding abnormality but may be a secondary effect of hyperactivity.
Figure 2.
Nr2e1frc/frc mice showed hyperactivity as early as postnatal day (P)18. A 3-min open field test showed that Nr2e1frc/frc mice were significantly more active at P18, but not at younger ages. * P < 0.01.
Adult Nr2e1frc/frc mice show hyperactivity in three behavioral tests
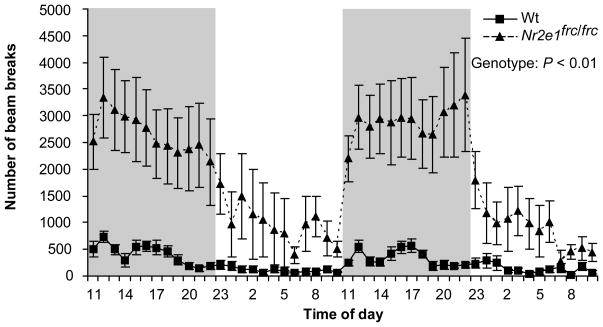
To fully characterize the extent of the hyperactivity phenotype in Nr2e1frc/frc mice we used the home cage activity monitor, a powerful and ethological test that assesses movement of mice in their home cage. This test showed that Nr2e1frc/frc mice are extremely hyperactive (Fig. 3; genotype effect F(1,11) = 10.6, P < 0.01), regardless of sex (F(1,11) = 2.22, P > 0.1). The mean number of beam breaks per hour was ~8-fold higher in Nr2e1frc/frc mice than in Wt controls for both light and dark phases (Beam breaks: Light phase: Wt = 189 ± 19.0, Nr2e1frc/frc = 1304 ± 118.9, P < 0.001; Dark phase: Wt = 313 ± 21.6, Nr2e1frc/frc = 2403 ± 148.6, P < 0.001).
Figure 3.
Nr2e1frc/frc mice showed hyperactivity in the home cage. Nr2e1frc/frc mice broke more beams than their Wt littermates over 48 h.
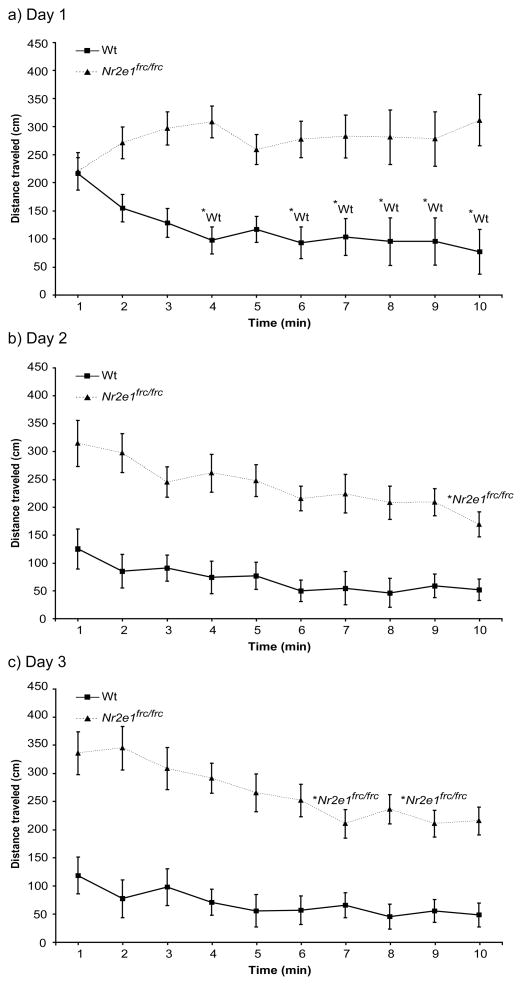
Hyperactivity in Nr2e1frc/frc mice was also seen in the open field test. Throughout the three days of open field habituation testing there was a significant effect of genotype on distance traveled (Fig. 4a–c; F(1,57) = 80.0, P < 0.001).
Figure 4.
Nr2e1frc/frc mice showed hyperactivity and habituation deficiency in the open field. Distance traveled was measured in the open field on 3 consecutive days for 10 min each day. Nr2e1frc/frc mice were significantly more active than Wt mice on all 3 days. Wt mice showed habituation on day 1 (a; solid line). Nr2e1frc/frc mice did not show habituation on day 1 (a, dotted line), but showed habituation on days 2 (b; dotted line) and 3 (c; dotted line). *Wt: P < 0.05. *Nr2e1frc/frc: P < 0.05.
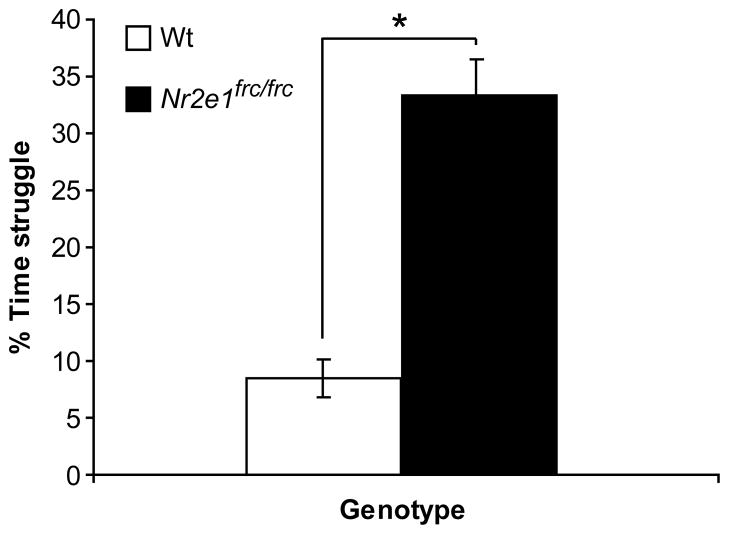
Finally, in the tail suspension test we found that Nr2e1frc/frc mice spent significantly more time struggling than Wt mice (Fig. 5; P < 0.001). This observation is consistent with a similar study testing mice lacking Nr2e1 (Abrahams et al., 2005). Therefore, increased struggle of Nr2e1frc/frc mice in the tail suspension test is a stable phenotype across studies.
Figure 5.
Nr2e1frc/frc mice struggled more during the tail suspension test. The Nr2e1frc/frc mice spent significantly more time struggling compared to their Wt controls. * P < 0.001.
Nr2e1frc/frc mice showed a deficit in two different learning and memory tasks
To characterize the behavioral manifestation of hippocampal and cortical hypoplasia, hallmarks of the Nr2e1frc/frc brain, we tested our mice for deficits in learning and memory tasks. Since Nr2e1frc/frc mice have reduced vision and showed deficits in the hidden cookie test, which could result from abnormal olfaction because of hypoplasia of olfactory bulbs (Young et al., 2002), we used two tests that do not rely primarily on visual or olfactory cues.
The ability of mice to habituate in the open field is measured by a decrease in exploratory activity over time. We demonstrate here that although Nr2e1frc/frc mice were able to habituate to the open field arena, they required significantly more time than the Wt controls. Throughout the three days of testing the two genotypes showed different activity patterns depending on the day, as shown by a significant effect of minute, day, and genotype interaction (Fig. 4a–c; F(18, 513) = 3.02, P < 0.001, ε = 0.465). More specifically, during day 1 of testing Wt mice already showed habituation by the 4th min of testing (Fig. 4a; P < 0.05), whereas Nr2e1frc/frc mice did not habituate during the 10 min on day 1 (Fig. 4a; P > 0.7). Nr2e1frc/frc mice did eventually show habituation on test days 2 and 3, at 10 (Fig. 4b; P < 0.01) and 7 (Fig. 4c; P < 0.05) min, respectively.
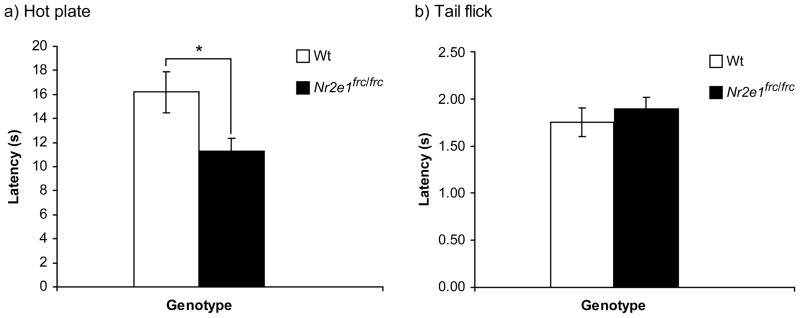
The passive avoidance test depends on the ability of the mouse to react to pain, and therefore prior to this test, we examined our mice for pain sensitivity using the hot plate and tail flick tests. Nr2e1frc/frc mice began licking their paws in significantly less time compared to Wt mice, indicating increased pain sensitivity in the hot plate test (Fig. 6a; P < 0.05). In the tail flick test there was no difference in the time required to remove the tail between Nr2e1frc/frc and Wt mice (Fig. 6b; P > 0.1). Despite the discordance in the results of these two tests we have reason to favor the finding of increased pain sensitivity when Nr2e1frc/frc mice are not restrained (see Discussion). More importantly, both tests showed the ability of Nr2e1frc/frc mice to respond to pain, thus supporting the use of the passive avoidance test.
Figure 6.
Nr2e1frc/frc mice showed increased pain sensitivity. (a) The latency to lick paws as a sign of discomfort from heat is measured in the hot plate test. Nr2e1frc/frc mice took significantly less time to lick their paws compared to the Wt controls. * P < 0.05. (b) The tail flick test was also used to test pain sensitivity in these mice; however, there was no significant difference found between the two genotypes (P > 0.1).
The standard protocol for passive avoidance testing is to use light as an adverse stimulus to encourage the animal to cross into the second chamber. However, since Nr2e1frc/frc mice have impaired vision, we decided to use sound as the adverse stimulus. We have previously tested 4-month-old Nr2e1frc/frc mice on a B6 background and showed that they have normal hearing as measured by auditory brainstem response (ABR) (Young et al., 2002). However, since our current mice are on a B6129F1 hybrid background, we retested them for ABR. Nr2e1frc/frc mice did not show any significant differences from Wt controls (Click: Wt = 50.0 ± 2.89 dB, Nr2e1frc/frc = 45.0 ± 2.74 dB, P > 0.1, 16 kHz: Wt = 22.5 ± 4.33 dB, Nr2e1frc/frc = 17.0 ± 2.00 dB, P > 0.1). Therefore, normal ABR in Nr2e1frc/frc mice is a stable phenotype across two genetic backgrounds.
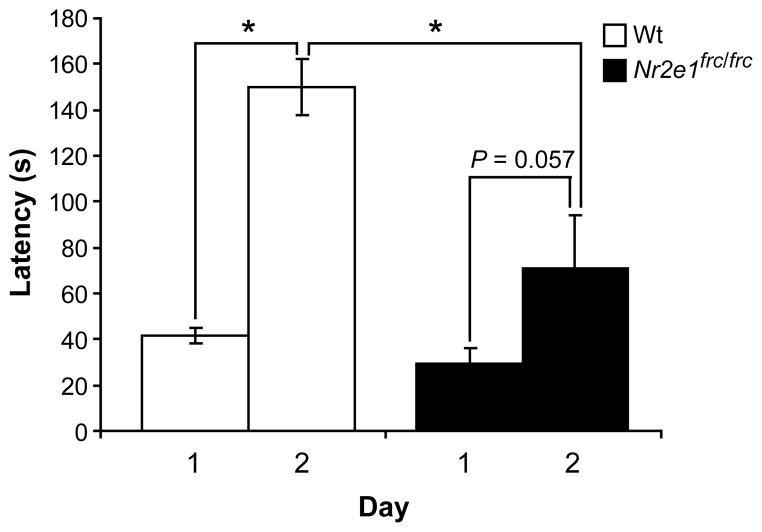
Since we confirmed that B6129F1-Nr2e1frc/frc mice are able to respond to pain and that their hearing is normal, we used sound to test these mice for passive avoidance. Wt mice demonstrated the expected learning response, showing an average >3-fold increase in latency to re-enter the second chamber upon the second exposure to the condition stimulus (Fig 7; P < 0.001). Although Nr2e1frc/frc mice also showed an increase in latency to re-enter, this change was much less than that seen in Wt mice, and did not reach statistical significance (Fig 7; P > 0.05), demonstrating that they did not perform this learning task as well as Wt mice.
Figure 7.
Nr2e1frc/frc mice showed impaired performance in the passive avoidance test. Learning is measured by the increase in latency to enter the chamber where the mouse received a mild shock the day before. Although Nr2e1frc/frc mice did show an increase in latency to enter the 2nd chamber, this was much less than that seen in Wt mice (* P < 0.001), and did not reach statistical significance (P = 0.057).
Nr2e1frc/frc mice lack startle reactivity
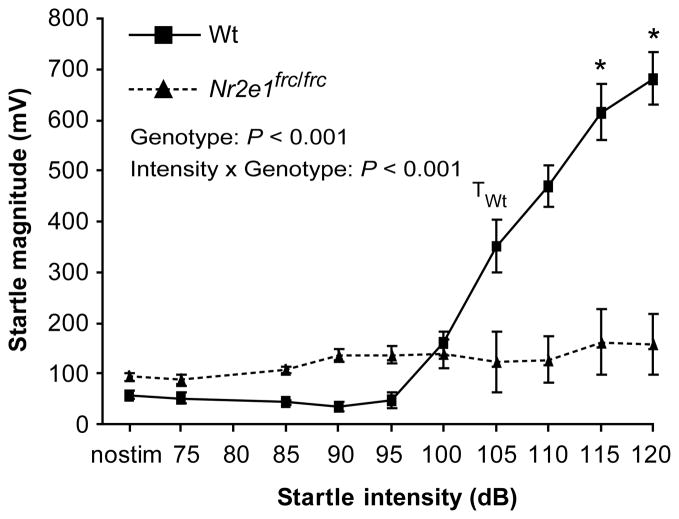
Hippocampal lesions in rodent models have been well documented to show impairments in prepulse inhibition (PPI), a measure of sensorimotor gating (Kamath et al., 2008, Pouzet et al., 1999). Prior to evaluating PPI, acoustic startle reactivity (ASR) must be tested to establish a startle threshold, as defined as the lowest startle intensity that produces a startle reaction significantly different than at the no-stimulus condition. Nr2e1frc/frc mice showed less acoustic startle reactivity than Wt controls, as shown by a significant main effect of genotype (Fig. 8; F(1,19) = 17.5, P < 0.001) and a significant interaction between intensity and genotype (F(9,171) = 29.9, P < 0.001, ε = 0.27). Post-hoc analysis indicated that the startle threshold for Wt mice was at 105 dB (P < 0.001); interestingly, there was no startle threshold for Nr2e1frc/frc mice (P > 0.05). This surprising result was confirmed with a new group of mice (data not shown). Therefore, we conclude that Nr2e1frc/frc mice show a lack of normal startle reaction. When we compared the startle magnitudes of Nr2e1frc/frc and Wt mice at the different startle intensities using post-hoc analysis, there were significant genotype differences at 115, and 120 dB (P < 0.05). Furthermore, as PPI tests are based on the startle response, PPI results for these mice would be uninformative.
Figure 8.
Nr2e1frc/frc mice showed no startle reactivity to auditory stimuli. Wt controls showed a normal pattern of increasing startle responses as startle stimuli became louder. However, Nr2e1frc/frc mice showed no increase in their startle responses at any decibel level tested. TWt, startle threshold for Wt (P < 0.001). * P < 0.05, between genotype comparison at each individual startle intensity.
Nr2e1frc/frc hyperactivity resistant to lithium treatment
Lithium chloride is the most effective drug for treatment of mania in patients with BPI, with human therapeutic plasma lithium level between 0.6–1.2 mmol/L, which can attenuate psychostimulus-induced hyperactivity (Gould et al., 2007, Gould et al., 2001) and increase neurogenesis in the dentate gyrus in rodent models (Kim et al., 2004). Using a dietary source of lithium, Wt and Nr2e1frc/frc mice showed serum lithium level that was on par with human therapeutic levels (Wt and Nr2e1frc/frc on control diet = below detection limit; Wt on lithium diet = 0.9 ± 0.1 mmol/L; Nr2e1frc/frc on lithium diet = 0.8 ± 0.1 mmol/L, no significant difference in plasma level between the two genotypes fed with lithium diet, P > 0.5).
We showed that lithium treatment was unable to alleviate the hyperactivity seen in Nr2e1frc/frc mice in the 24-h home cage activity test, as demonstrated by the significant effect of genotype (Table 1), but no significant effect of diet (Table 1), nor a significant interaction between genotype and diet (Table 1). The mean number of beam breaks in both light and dark phases was significantly higher in Nr2e1frc/frc mice compared to Wt controls, regardless of lithium treatment (Light: Wt, control diet = 78.9 ± 10.8, Wt, lithium diet = 110.2 ± 21.6, Nr2e1frc/frc control diet = 285.5 ± 46.0, Nr2e1frc/frc lithium diet = 321.4 ± 55.8; Dark: Wt, control diet = 158.5 ± 16.7, Wt, lithium diet = 197.2 ± 24.4, Nr2e1frc/frc control diet = 997.4 ± 65.1, Nr2e1frc/frc lithium diet = 1005.7 ± 79.4; for all comparisons between Wt and Nr2e1frc/frc regardless of diet P < 0.05).
Table 1.
Summary of lithium findings
| Degree of freedom | Error | F | P | ε | |
|---|---|---|---|---|---|
| a) 24-h home cage activity | |||||
| Genotype | 1 | 12 | 37.7 | < 0.001* | |
| Diet | 1 | 12 | 0.151 | 0.705 | |
| Genotype × Diet | 1 | 12 | 0.004 | 0.948 | |
| b) Open field habituation | |||||
| Genotype | 1 | 36 | 44.9 | < 0.001* | |
| Diet | 1 | 36 | 3.42 | 0.073 | |
| Genotype × Diet | 1 | 36 | 0.31 | 0.583 | |
| Minute × Day × Genotype | 18 | 324 | 1.96 | 0.011* | 0.59 |
| Minute × Day × Genotype × Diet | 18 | 324 | 0.77 | 0.542 | 0.59 |
| c) Startle reactivity | |||||
| Genotype | 1 | 14 | 5.87 | 0.017* | |
| Diet | 1 | 14 | 1.99 | 0.161 | |
| Genotype × Diet | 1 | 14 | 0.92 | 0.355 | |
| Intensity × Genotype | 9 | 126 | 9.32 | 0.002* | 0.18 |
| Intensity × Diet | 9 | 126 | 0.32 | 0.967 | 0.18 |
| Intensity × Genotype × Diet | 9 | 126 | 0.47 | 0.895 | 0.18 |
Nr2e1frc/frc mice hyperactivity in the open field test was similarly unaffected by lithium treatment, where there was a significant effect of genotype on distance traveled (Table 1) with no significant effect of diet (Table 1), and no significant interaction between genotype and diet (Table 1).
Nr2e1frc/frc open field habituation deficit is unaffected by lithium treatment
To evaluate the effect of lithium treatment on the habituation deficit in Nr2e1frc/frc mice, mice fed control and lithium diets were assayed in the open field habituation test. As before (Fig. 4), there was a significant effect of minutes, day, and genotype interaction (Table 1), indicating that Nr2e1frc/frc mice showed different activity patterns on the different test days compared to Wt controls. The lack of significant interaction between minute, day, genotype, and diet (Table 1) indicated that lithium treatment was unable to improve habituation in Nr2e1frc/frc mice. The lack of lithium effect on Nr2e1frc/frc habituation deficit was still apparent even after taking into account for activity differences (data not shown).
Lithium-treated Nr2e1frc/frc mice show no improvement in startle reactivity
The lack of startle reactivity was one of the most striking phenotypes shown in Nr2e1frc/frc mice. To assess the effect of lithium on this behavioral phenotype, Wt and Nr2e1frc/frc mice fed control and lithium diets were assayed in the startle reactivity test. Similar to our previous experiments (Fig. 8), the two genotype groups responded differently to the varying acoustic startle stimuli as evidenced by the significant interaction between intensity and genotype (Table 1). We showed that lithium treatment did not significantly correct the deficient acoustic startle response in Nr2e1frc/frc mice compared to that shown by Wt mice, as there was no significant effect of diet (Table 1), and there were no significant interactions between: genotype and diet (Table 1); intensity and diet (Table 1); nor genotype, intensity, and diet (Table 1). We were unable to perform post-hoc analysis for effect of diet as there were no significant effects or interactions involving diet. In the post-hoc analysis of intensity and genotype effect, Wt mice showed startle threshold at 110 dB (P < 0.05), while Nr2e1frc/frc mice lacked a startle threshold at any startle intensity (P > 0.05), paralleling results shown in Fig. 8. Our results demonstrated an absence of a significant lithium effect on Nr2e1frc/frc startle reactivity deficit.
Cell proliferation in subventricular zone and dentate gyrus is unaffected by lithium treatment
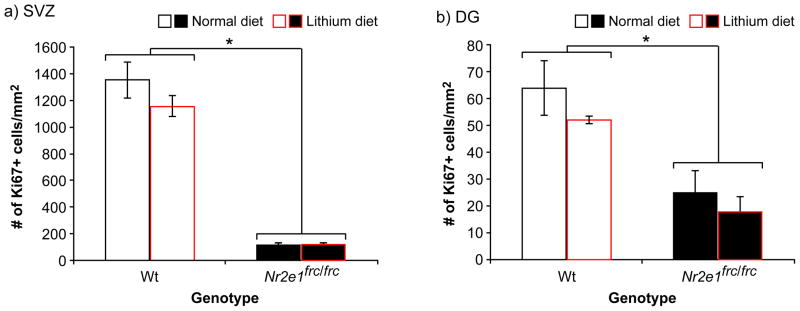
Reduced neural stem/progenitor cell proliferation has been shown in Nr2e1-knockout mice when compared to their Wt littermates (Shi et al., 2004). Here we show for the first time, using Ki67 staining of proliferating cells, a significant genotype effect for cell proliferation in the two neurogenic zones of the Nr2e1frc/frc adult brain, the subventricular zone (SVZ) and dentate gyrus (DG) (Fig. 9a & b; F(2,10) = 92.5, P < 0.001). Significant genotype effects in each region were also identified for cell proliferation (SVZ: Fig. 9a; F(1,11) = 194.5, P < 0.001; DG: Fig. 9b; F(1,11) = 18.3, P < 0.01). These results indicated that Nr2e1frc/frc mice show the same pattern of reduction in cell proliferation as other Nr2e1-knockout mice (Shi et al., 2004).
Figure 9.
Lithium treatment did not increase cell proliferation in Nr2e1frc/frc mice. There was no significant effect of diet on cell proliferation. (a) In the subventricular zone (SVZ), there were significantly less Ki67+ cells in Nr2e1frc/frc mice compared to Wt mice (* P < 0.001). (b) In the dentate gyrus (DG), there were also significantly less Ki67+ cells in Nr2e1frc/frc mice compared to Wt mice (* P < 0.01).
Since lithium has been shown to act through multiple pathways to increase neurogenesis in vivo (Jope, 1999, Kim et al., 2004, Wada et al., 2005), we analyzed its effect on cell proliferation in Nr2e1frc/frc mice. We showed that our lithium treatment was unable to alter cell proliferation in either of the two neurogenic zones (SVZ and DG), as evident by no significant effect of diet (SVZ: Fig. 9a; F(1,11) = 1.41, P > 0.5; DG: Fig. 9b; F(1,11) = 1.24, P > 0.5). We also saw no significant interaction between genotype and diet (SVZ: Fig. 9a; F(1,11) = 1.46, P > 0.5; DG: Fig. 9b; F(1,11) = 0.08, P > 0.5), suggesting that our lithium diet was unable to alter cell proliferation in the SVZ and DG of Wt and Nr2e1frc/frc mice.
Discussion
This study was the first to characterize Nr2e1frc/frc mice for a spectrum of phenotypes that have been used in the literature to model aspects of BP (Arban et al., 2005, Cao & Peng, 1993, Decker et al., 2000, Einat, 2006a, Einat, 2006b, Einat et al., 2003, El-Mallakh et al., 2003, Gessa et al., 1995, Ralph-Williams et al., 2003). In addition, it is the first to evaluate the effect of any drug treatment on Nr2e1-null mice. Results from this study showed new important behavioral phenotypes in Nr2e1frc/frc mice including extreme hyperactivity and deficits in habituation, passive avoidance, and startle reactivity. The presence of reduced cellular proliferation in the SVZ and DG was a novel finding for Nr2e1frc/frc mice, and the resistance of these behavioral and proliferative phenotypes to lithium treatment is a novel finding amongst all Nr2e1-null mice.
In the present study, the extreme hyperactivity phenotype of the Nr2e1frc/frc animals was documented in three different tests: home cage activity, tail suspension, and open field habituation. Of these tests, the tail suspension was originally chosen to evaluate depressive behavior in this study, but because of the overwhelming hyperactivity phenotype, the results were not indicative of depressive behavior. Currently, the most frequently used model of mania is psychostimulant-induced hyperactivity (Einat, 2006a, Machado-Vieira et al., 2004). Interestingly, hyperactivity seen in Nr2e1frc/frc mice was approximately 8-fold higher than basal activity level in the home cage, while administration of psychostimulant and other transgenic mice exhibited increased activity levels by approximately 2- to 5-fold over non-induced or Wt mice, respectively (Arban et al., 2005, Hiroi et al., 2005, Zhuang et al., 2001). Therefore, Nr2e1frc/frc mice show one of the most extreme hyperactivity phenotype currently documented.
Nr2e1-null mice have previously been shown to have hypoplasia of the hippocampus and decreased adult neurogenesis in the granular layer of the DG, regions important for learning and memory (Mainen & Sejnowski, 1996, Shi et al., 2004, Young et al., 2002). Our group also demonstrated that not only is the dendritic branching structure of granule cells in Nr2e1frc/frc mice reminiscent of immature neurons in the DG, the mice also lack synaptic plasticity, as demonstrated by the absence of long-term potentiation (LTP) in their dentate gyrus (Christie et al., 2006). LTP is thought by some to be an electrophysiological measure of learning and memory (Howland & Wang, 2008, Kinney et al., 2009). Collectively, learning and memory deficits are expected based on the neuroanatomical abnormalities observed in Nr2e1frc/frc mice. Furthermore, some patients with BP also show cognitive deficits, such as dysfunctions in executive function and verbal memory; however these deficits are usually less severe and differ from the typical profile seen in patients with schizophrenia (Altshuler et al., 2004, Green, 2006, Krabbendam et al., 2005). In an attempt to reveal any cognitive impairment in Nr2e1frc/frc mice, we used two distinct tests of learning and memory. Since Nr2e1frc/frc mice have reduced vision and may also have abnormal olfaction, many conventional behavioral paradigms of learning and memory were not appropriate. In particular, well-established tests of executive memory in rodents such as the Morris water, Barnes, and Y mazes could not be properly employed. Although the open field habituation and passive avoidance tests used in this study do not specifically evaluate the cognitive domains typically affected in patients with BP (Altshuler et al., 2004), these tests were chosen and designed specifically to assess hippocampal-associated learning with minimal use of visual or olfactory cues. We hypothesized that Nr2e1frc/frc mice will have deficits in hippocampal-associated learning based on (1) their hippocampal abnormalities and (2) that drugs effective in treatment of BP have shown to improve hippocampal-associated learning (Nocjar et al., 2007, Watase et al., 2007, Yan et al., 2007). The passive avoidance test was chosen as Roy et al. (2002) showed that Nr2e1 knockout mice were hyper-responsive to shock, indicating that shock was an appropriate unconditioned stimulus for inducing learning in these mice that have such extensive sensory deficits. Furthermore, both tests also provide an internal control for activity level since they consider the change in activity between the same groups of mice on different days, thus normalizing for activity levels. Nevertheless, we showed that Nr2e1frc/frc mice perform poorly on these tasks compared to Wt mice, as evident by the increased time required to habituate in the open field test and the lack of significant increase in latency to re-enter in the passive avoidance test. Yet, we cannot exclude the possibility that acquisition of environmental cues could be disrupted due to sensory deficits or the hyperactivity phenotype may interfere with the inhibition of locomotor activity in Nr2e1frc/frc mice, which contributes to their deficits in performance in these tasks. Despite these caveats, our data suggest the importance of Nr2e1 in proper brain development, without which there is a reduced performance in hippocampal-associated learning tasks.
This study was also the first to test for acoustic startle reactivity (ASR) in Nr2e1-null mice. Our novel finding of complete lack of startle was unexpected, since previously there has not been a case of hearing mice not showing ASR. ASR was done in preparation for evaluating PPI; however, we are unable to test PPI since PPI requires startle reactivity greater than movements seen at background noise and Nr2e1frc/frc mice showed no startle threshold. This result, along with normal response for the tail flick test, was surprising since our previous results, and those of others (Roy et al., 2002), led us to anticipate a hyper-responsive phenotype. However, we note that the lack of hyper-responsiveness in these instances correlates with the use of restraint, an extreme stressor in mice (Bain et al., 2004). Brain regions shown to contribute to stress-related response include the amygdala and hippocampus (Liberzon & Martis, 2006, Vermetten & Bremner, 2002). Regions suggested to be involved in modulation of ASR, include nucleus accumbens, basolateral amygdala, and prefrontal cortex (Stevenson & Gratton, 2004, Storozheva et al., 2003). All of these regions are structurally abnormal in the Nr2e1frc/frc mice and may underlie the lack of hyper-responsiveness to pain, as well as the lack of ASR. Based on the hot plate test where Nr2e1frc/frc mice were not tested under restraint and showed a significant reduction in time to lick their paws, we concluded that Nr2e1frc/frc mice had increased pain sensitivity. However, in the tail flick test, Nr2e1frc/frc mice were placed in a restrainer and, we concluded that under this stressor, the expected hyper-responsive phenotype of Nr2e1frc/frc mice was masked by the atypical stress response caused by restraint. Alternatively, the discrepancy in pain sensitivity of Nr2e1frc/frc mice in the two tests could be the result of different neurocircuitries that are activated by the different tests (Davidova et al., 2009, Fields & Heinricher, 1985, Jasmin et al., 1997, Lane et al., 2005, Morgan & Clayton, 2005).
We chose to evaluate the effect of lithium treatment on Nr2e1frc/frc mice for four reasons: (1) lithium has been shown to attenuate symptoms of mania in patients with BP (Shastry, 2005); (2) lithium reduces genetically- and amphetamine-induced hyperactivity in rodents (Gould et al., 2007, Gould et al., 2001, Yuskaitis et al.); (3) lithium has induced neural stem cell proliferation in the mouse DG both in vitro and in vivo assays (Wada et al., 2005); and (4) lithium is thought to act through multiple key neurological pathways (Jope, 1999), thus increasing the probability that lithium would effect Nr2e1frc/frc behavioral phenotypes compared to drugs with restricted modes of action.
In this study, we showed that adult lithium treatment was ineffective in attenuating any of the abnormal behavioral phenotypes observed in Nr2e1frc/frc mice including the extreme hyperactivity in the home cage, the habituation deficit in the open field test, and the lack of acoustic startle reactivity. Despite the fact that lithium can induce neurogenesis in vitro and in vivo (Kim et al., 2004) and that the introduction of Nr2e1 can rescue quiescent stem cells from Nr2e1-null brains in vitro (Shi et al., 2004), here we showed that lithium administration to adult Nr2e1frc/frc mice was unable to trigger an increase in cell proliferation in the SVZ and DG. The lack of lithium effect on Wt-cell proliferation was initially surprising given the evidence for increased hippocampal neurogenesis in normal mice treated with lithium (Chen et al., 2000, Kim et al., 2004). However, there were key experimental differences between our analysis of Ki67+ cells and studies demonstrating an increase in cell proliferation with lithium treatment. Cells analyzed by these studies are labeled with bromodeoxyuridine (BrdU) via consecutive days of injections; thereby labeling not only currently dividing cells, but their progeny as well. Our Ki67+ cell counts would more accurately mimic results from single-day BrdU injections, which would only label currently dividing cells. It has been demonstrated that under this condition, lithium treatment was unable to increase cell proliferation in Wt mice (Eom & Jope, 2009), which is consistent with our observation.
One might be tempted to speculate that with an increased number of mice we might have detected an effect of lithium on hyperactivity. However based on the literature of other genetically- and psychostimulant-induced hyperactivity in mice, lithium treatment was able to reduce the hyperactivity phenotype by at least half, if not returning activity level to that seen in wild-type controls (Gould et al., 2007, Gould et al., 2001, Yuskaitis et al.). Therefore, since Nr2e1frc/frc mice exhibit ~8-fold increase in locomotor activity compared to Wt controls, the number of mice tested in the lithium experiment would have had sufficient power to detect lithium effect given the anticipated reduction in locomotor activity. For detection of a lithium effect on startle reactivity, we had no apriori hypothesis for the number of subjects required to detect this effect since a lack of startle reactivity is a novel finding.
The development of a totally appropriate mouse model for complex disease, such as mental illness, is challenging for reasons of environmental factors, minor multiple gene effects, and appropriate pharmacological responsiveness. However, many single gene mouse models, such as Gsk3b overexpressing mice, nitric oxide synthase (NOS-III) and nNOS knockout mice, and DISC1 mutant mice (Flint & Shifman, 2008, Kato et al., 2007, Prickaerts et al., 2006, Reif et al., 2006, Tanda et al., 2009) have proven valuable as they exhibit aspects of complex disorders. We have now added Nr2e1frc/frc mice to this group. We have shown here that Nr2e1frc/frc mice demonstrate the behavioral traits of hyperactivity and deficit in habituation and learning tasks, which are commonly used in genetic models of BP.
However, since Nr2e1frc/frc mice failed to respond to the adult lithium treatment used here, they have not currently met the criteria of pharmacological validity as a model for BP (Kato et al., 2007). Given the extreme level of hyperactivity in Nr2e1frc/frc mice, the treatment regiment used in other studies, which was adopted here, may not be sufficient in reducing hyperactivity. We hypothesize for future consideration that Nr2e1frc/frc mice should be examined using higher doses or longer administration of lithium, or different combinations of mood-stabilizing and antipsychotic drugs to attenuate their behavioral phenotypes; the latter would more accurately mimic treatments regimes commonly prescribed to patients with BP. We also acknowledge that the genetic components of BP are likely to be multiple mutations of minor effect; furthermore, the phenotype of the Nr2e1 heterozygous mouse is too weak for behavioral detection (Roy et al., 2002). Therefore, we hypothesize that mice carrying subtle mutations, or patient variants, in trans across from an Nr2e1 deletion might more closely represent the human condition.
Figure 1.
Reduced body weight of Nr2e1frc/frc pups. Nr2e1frc/frc pups weighed significantly less than Wt pups by postnatal day 21. * P < 0.001.
Acknowledgments
We thank Pratibha Reebye and Allan Young for helpful discussion and Tammy Philippo, Tracey D. Weir and Veronica Yakoleff for assistance in preparing the manuscript. This work was funded by the following awards to EMS: CFI/KDF New Opportunities #3017, CFI/KDF Innovation Fund (LFG), CIHR/IHRT #43820, NAAR/Mentor-Based Fellowship (for BKYW), and a Canada Research Chair in Genetics and Behaviour #950-01-193. SMH was supported by a Jack and Doris Brown Foundation Award.
References
- Abrahams BS, Kwok MC, Trinh E, Budaghzadeh S, Hossain SM, Simpson EM. Pathological aggression in “fierce” mice corrected by human nuclear receptor 2E1. J Neurosci. 2005;25:6263–6270. doi: 10.1523/JNEUROSCI.4757-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci. 2003;985:370–388. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- Arban R, Maraia G, Brackenborough K, Winyard L, Wilson A, Gerrard P, Large C. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav Brain Res. 2005;158:123–132. doi: 10.1016/j.bbr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett. 2004;368:7–10. doi: 10.1016/j.neulet.2004.04.096. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Buervenich S, Carmine A, Arvidsson M, Xiang F, Zhang Z, Sydow O, Jonsson EG, Sedvall GC, Leonard S, Ross RG, Freedman R, Chowdari KV, Nimgaonkar VL, Perlmann T, Anvret M, Olson L. NURR1 mutations in cases of schizophrenia and manic-depressive disorder. Am J Med Genet. 2000;96:808–813. doi: 10.1002/1096-8628(20001204)96:6<808::aid-ajmg23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- Calzavara MB, Medrano WA, Levin R, Kameda SR, Andersen ML, Tufik S, Silva RH, Frussa-Filho R, Abilio VC. Neuroleptic drugs revert the contextual fear conditioning deficit presented by spontaneously hypertensive rats: a potential animal model of emotional context processing in schizophrenia? Schizophr Bull. 2009;35:748–759. doi: 10.1093/schbul/sbn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao BJ, Peng NA. Magnesium valproate attenuates hyperactivity induced by dexamphetamine-chlordiazepoxide mixture in rodents. Eur J Pharmacol. 1993;237:177–181. doi: 10.1016/0014-2999(93)90266-k. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Christie BR, Li AM, Redila VA, Booth H, Wong BK, Eadie BD, Ernst C, Simpson EM. Deletion of the nuclear receptor Nr2e1 impairs synaptic plasticity and dendritic structure in the mouse dentate gyrus. Neuroscience. 2006;137:1031–1037. doi: 10.1016/j.neuroscience.2005.08.091. [DOI] [PubMed] [Google Scholar]
- Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends Genet. 2009;25:99–105. doi: 10.1016/j.tig.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Davidova A, Schreiberova A, Kolesar D, Capkova L, Krizanova O, Lukacova N. Spinal cord transection significantly influences nNOS-IR in neuronal circuitry that underlies the tail-flick reflex activity. Cell Mol Neurobiol. 2009;29:879–886. doi: 10.1007/s10571-009-9370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S, Grider G, Cobb M, Li XP, Huff MO, El-Mallakh RS, Levy RS. Open field is more sensitive than automated activity monitor in documenting ouabain-induced hyperlocomotion in the development of an animal model for bipolar illness. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:455–462. doi: 10.1016/s0278-5846(99)00111-6. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, Moe PR, Samavedy N, El-Mallakh R, Manji H, Glitz DA, Meyer ET, Smiley C, Hahn R, Widmark C, McKinney R, Sutton L, Ballas C, Grice D, Berrettini W, Byerley W, Coryell W, DePaulo R, MacKinnon DF, Gershon ES, Kelsoe JR, McMahon FJ, McInnis M, Murphy DL, Reich T, Scheftner W, Nurnberger JI., Jr Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am J Hum Genet. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H. Establishment of a Battery of Simple Models for Facets of Bipolar Disorder: A Practical Approach to Achieve Increased Validity, Better Screening and Possible Insights into Endophenotypes of Disease. Behav Genet. 2006a doi: 10.1007/s10519-006-9093-4. [DOI] [PubMed] [Google Scholar]
- Einat H. Modelling facets of mania - new directions related to the notion of endophenotypes. J Psychopharmacol. 2006b doi: 10.1177/0269881106060241. [DOI] [PubMed] [Google Scholar]
- Einat H, Manji HK, Gould TD, Du J, Chen G. Possible involvement of the ERK signaling cascade in bipolar disorder: behavioral leads from the study of mutant mice. Drug News Perspect. 2003;16:453–463. doi: 10.1358/dnp.2003.16.7.829357. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, El-Masri MA, Huff MO, Li XP, Decker S, Levy RS. Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord. 2003;5:362–365. doi: 10.1034/j.1399-5618.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Eom TY, Jope RS. Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3alpha/beta impairs in vivo neural precursor cell proliferation. Biol Psychiatry. 2009;66:494–502. doi: 10.1016/j.biopsych.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- Flint J, Shifman S. Animal models of psychiatric disease. Curr Opin Genet Dev. 2008;18:235–240. doi: 10.1016/j.gde.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol. 1995;5(Suppl):89–93. doi: 10.1016/0924-977x(95)00023-i. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Chengappa KN. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord. 2009;11(Suppl 2):123–137. doi: 10.1111/j.1399-5618.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–1333. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium’s reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(Suppl 9):3–8. discussion 36–42. [PubMed] [Google Scholar]
- Hayden EP, Nurnberger JI., Jr Molecular genetics of bipolar disorder. Genes Brain Behav. 2006;5:85–95. doi: 10.1111/j.1601-183X.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Zhu H, Lee M, Funke B, Arai M, Itokawa M, Kucherlapati R, Morrow B, Sawamura T, Agatsuma S. A 200-kb region of human chromosome 22q11.2 confers antipsychotic-responsive behavioral abnormalities in mice. Proc Natl Acad Sci U S A. 2005;102:19132–19137. doi: 10.1073/pnas.0509635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain SM, Wong BK, Simpson EM. The dark phase improves genetic discrimination for some high throughput mouse behavioral phenotyping. Genes Brain Behav. 2004;3:167–177. doi: 10.1111/j.1601-183x.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Zheng QY, Rosenstiel P, Maddatu T, Zuberi AR, Roopenian DC, North MA, Naggert JK, Johnson KR, Nishina PM. Genetic modification of hearing in tubby mice: evidence for the existence of a major gene (moth1) which protects tubby mice from hearing loss. Hum Mol Genet. 1999;8:1761–1767. doi: 10.1093/hmg/8.9.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Carstens E, Basbaum AI. Interneurons presynaptic to rat tail-flick motoneurons as mapped by transneuronal transport of pseudorabies virus: few have long ascending collaterals. Neuroscience. 1997;76:859–876. doi: 10.1016/s0306-4522(96)00384-3. [DOI] [PubMed] [Google Scholar]
- Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry. 1999;4:117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- Kamath A, Al-Khairi I, Bhardwaj S, Srivastava LK. Enhanced alpha1 adrenergic sensitivity in sensorimotor gating deficits in neonatal ventral hippocampus-lesioned rats. Int J Neuropsychopharmacol. 2008;11:1085–1096. doi: 10.1017/S1461145708008845. [DOI] [PubMed] [Google Scholar]
- Kato T, Kubota M, Kasahara T. Animal models of bipolar disorder. Neurosci Biobehav Rev. 2007;31:832–842. doi: 10.1016/j.neubiorev.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, Son H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem. 2004;89:324–336. doi: 10.1046/j.1471-4159.2004.02329.x. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Sanchez-Alavez M, Barr AM, Criado JR, Crawley JN, Behrens MM, Henriksen SJ, Bartfai T. Impairment of memory consolidation by galanin correlates with in vivo inhibition of both LTP and CREB phosphorylation. Neurobiol Learn Mem. 2009 doi: 10.1016/j.nlm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn Y, Lerer B. Excitement and confusion on chromosome 6q: the challenges of neuropsychiatric genetics in microcosm. Mol Psychiatry. 2005;10:1062–1073. doi: 10.1038/sj.mp.4001738. [DOI] [PubMed] [Google Scholar]
- Kopp C. Locomotor activity rhythm in inbred strains of mice: implications for behavioural studies. Behav Brain Res. 2001;125:93–96. doi: 10.1016/s0166-4328(01)00289-3. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kruger S, Frasnelli J, Braunig P, Hummel T. Increased olfactory sensitivity in euthymic patients with bipolar disorder with event-related episodes compared with patients with bipolar disorder without such episodes. J Psychiatry Neurosci. 2006;31:263–270. [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, Chan KL, Wong AH, Little KQ, Rajcan-Separovic E, Abrahams BS, Simpson EM. Unexpected embryonic stem (ES) cell mutations represent a concern in gene targeting: lessons from “fierce” mice. Genesis. 2004;38:51–57. doi: 10.1002/gene.20001. [DOI] [PubMed] [Google Scholar]
- Kumar RA, McGhee KA, Leach S, Bonaguro R, Maclean A, Aguirre-Hernandez R, Abrahams BS, Coccaro EF, Hodgins S, Turecki G, Condon A, Muir WJ, Brooks-Wilson AR, Blackwood DH, Simpson EM. Initial association of NR2E1 with bipolar disorder and identification of candidate mutations in bipolar disorder, schizophrenia, and aggression through resequencing. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:880–889. doi: 10.1002/ajmg.b.30696. [DOI] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land PW, Monaghan AP. Expression of the transcription factor, tailless, is required for formation of superficial cortical layers. Cereb Cortex. 2003;13:921–931. doi: 10.1093/cercor/13.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Kapczinski F, Soares JC. Perspectives for the development of animal models of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:209–224. doi: 10.1016/j.pnpbp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Adams D, Berk M. Is lithium in a class of its own? A brief profile of its clinical use. Aust N Z J Psychiatry. 2009;43:1096–1104. doi: 10.3109/00048670903279937. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Schloesser RJ, Manji HK. Bipolar disorder: from genes to behavior pathways. J Clin Invest. 2009;119:726–736. doi: 10.1172/JCI37703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy RD, Feron F, Perry C, Chant DC, McLean D, Matigian N, Hayward NK, McGrath JJ, Mackay-Sim A. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82:163–173. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, Abou Jamra R, Albus M, Bacanu SA, Baron M, Barrett TB, Berrettini W, Blacker D, Byerley W, Cichon S, Coryell W, Craddock N, Daly MJ, Depaulo JR, Edenberg HJ, Foroud T, Gill M, Gilliam TC, Hamshere M, Jones I, Jones L, Juo SH, Kelsoe JR, Lambert D, Lange C, Lerer B, Liu J, Maier W, Mackinnon JD, McInnis MG, McMahon FJ, Murphy DL, Nothen MM, Nurnberger JI, Pato CN, Pato MT, Potash JB, Propping P, Pulver AE, Rice JP, Rietschel M, Scheftner W, Schumacher J, Segurado R, Van Steen K, Xie W, Zandi PP, Laird NM. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005;77:582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Pato MT, Gentile KL, Morley CP, Zhao X, Eisener AF, Brown A, Petryshen TL, Kirby AN, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Azevedo MH, Kennedy JL, Daly MJ, Sklar P, Pato CN. Genomewide linkage analysis of bipolar disorder by use of a high-density single-nucleotide-polymorphism (SNP) genotyping assay: a comparison with microsatellite marker assays and finding of significant linkage to chromosome 6q22. Am J Hum Genet. 2004;74:886–897. doi: 10.1086/420775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AP, Bock D, Gass P, Schwager A, Wolfer DP, Lipp HP, Schutz G. Defective limbic system in mice lacking the tailless gene. Nature. 1997;390:515–517. doi: 10.1038/37364. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC. Defensive behaviors evoked from the ventrolateral periaqueductal gray of the rat: comparison of opioid and GABA disinhibition. Behav Brain Res. 2005;164:61–66. doi: 10.1016/j.bbr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Hammonds MD, Shim SS. Chronic lithium treatment magnifies learning in rats. Neuroscience. 2007;150:774–788. doi: 10.1016/j.neuroscience.2007.09.063. [DOI] [PubMed] [Google Scholar]
- O’Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: Leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007 doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB, Kuczenski R, Niculescu AB. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry. 2004;9:1007–1029. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- Pato CN, Pato MT, Kirby A, Petryshen TL, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, Soares MJ, Ferreira CP, Lei M, Verner A, Hudson TJ, Morley CP, Kennedy JL, Azevedo MH, Daly MJ, Sklar P. Genome-wide scan in Portuguese Island families implicates multiple loci in bipolar disorder: fine mapping adds support on chromosomes 6 and 11. Am J Med Genet B Neuropsychiatr Genet. 2004;127:30–34. doi: 10.1002/ajmg.b.30001. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Feldon J, Veenman CL, Yee BK, Richmond M, Nicholas J, Rawlins P, Weiner I. The effects of hippocampal and fimbria-fornix lesions on prepulse inhibition. Behav Neurosci. 1999;113:968–981. doi: 10.1037//0735-7044.113.5.968. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, Geyer MA. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biol Psychiatry. 2003;53:352–359. doi: 10.1016/s0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- Reif A, Strobel A, Jacob CP, Herterich S, Freitag CM, Topner T, Mossner R, Fritzen S, Schmitt A, Lesch KP. A NOS-III haplotype that includes functional polymorphisms is associated with bipolar disorder. Int J Neuropsychopharmacol. 2006;9:13–20. doi: 10.1017/S1461145705005560. [DOI] [PubMed] [Google Scholar]
- Roy K, Kuznicki K, Wu Q, Sun Z, Bock D, Schutz G, Vranich N, Monaghan AP. The Tlx gene regulates the timing of neurogenesis in the cortex. J Neurosci. 2004;24:8333–8345. doi: 10.1523/JNEUROSCI.1148-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K, Thiels E, Monaghan AP. Loss of the tailless gene affects forebrain development and emotional behavior. Physiol Behav. 2002;77:595–600. doi: 10.1016/s0031-9384(02)00902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, Buervenich S, Badner JA, Steele CJ, Detera-Wadleigh SD, Dick D, Foroud T, Cox NJ, MacKinnon DF, Potash JB, Berrettini WH, Byerley W, Coryell W, DePaulo JR, Jr, Gershon ES, Kelsoe JR, McInnis MG, Murphy DL, Reich T, Scheftner W, Nurnberger JI, Jr, McMahon FJ. Loci on chromosomes 6q and 6p interact to increase susceptibility to bipolar affective disorder in the national institute of mental health genetics initiative pedigrees. Biol Psychiatry. 2004;56:18–23. doi: 10.1016/j.biopsych.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Shastry BS. Bipolar disorder: an update. Neurochem Int. 2005;46:273–279. doi: 10.1016/j.neuint.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- Stenman J, Yu RT, Evans RM, Campbell K. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 2003a;130:1113–1122. doi: 10.1242/dev.00328. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003b;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CW, Gratton A. Basolateral amygdala dopamine receptor antagonism modulates initial reactivity to but not habituation of the acoustic startle response. Behav Brain Res. 2004;153:383–387. doi: 10.1016/j.bbr.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Stober G, Syagailo YV, Okladnova O, Jungkunz G, Knapp M, Beckmann H, Lesch KP. Functional PAX-6 gene-linked polymorphic region: potential association with paranoid schizophrenia. Biol Psychiatry. 1999;45:1585–1591. doi: 10.1016/s0006-3223(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Storozheva ZI, Afanas’ev II, Proshin AT, Kudrin VS. Dynamics of intracellular dopamine contents in the rat brain during the formation of conditioned contextual fear and extinction of an acoustic startle reaction. Neurosci Behav Physiol. 2003;33:307–312. doi: 10.1023/a:1022831104116. [DOI] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Andreasen NC, Alliger RJ, Ehrhardt JC, Yuh WT. Structural brain abnormalities in bipolar affective disorder. Ventricular enlargement and focal signal hyperintensities. Arch Gen Psychiatry. 1990;47:1054–1059. doi: 10.1001/archpsyc.1990.01810230070011. [DOI] [PubMed] [Google Scholar]
- Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, Toyama K, Takao K, Miyakawa T. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian SY, Wang JF, Bezchlibnyk YB, Young LT. Immunoreactivity of 43 kDa growth-associated protein is decreased in post mortem hippocampus of bipolar disorder and schizophrenia. Neurosci Lett. 2007;411:123–127. doi: 10.1016/j.neulet.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- Wada A, Yokoo H, Yanagita T, Kobayashi H. Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sci. 2005;99:307–321. doi: 10.1254/jphs.crj05009x. [DOI] [PubMed] [Google Scholar]
- Watase K, Gatchel JR, Sun Y, Emamian E, Atkinson R, Richman R, Mizusawa H, Orr HT, Shaw C, Zoghbi HY. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med. 2007;4:e182. doi: 10.1371/journal.pmed.0040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberg L, Melke J, Landen M, Nilsson S, Baghaei F, Rosmond R, Jansson M, Holm G, Bjorntorp P, Eriksson E. Association between a dinucleotide repeat polymorphism of the estrogen receptor alpha gene and personality traits in women. Mol Psychiatry. 2003;8:118–122. doi: 10.1038/sj.mp.4001192. [DOI] [PubMed] [Google Scholar]
- Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Young KA, Berry ML, Mahaffey CL, Saionz JR, Hawes NL, Chang B, Zheng QY, Smith RS, Bronson RT, Nelson RJ, Simpson EM. Fierce: a new mouse deletion of Nr2e1; violent behaviour and ocular abnormalities are background-dependent. Behav Brain Res. 2002;132:145–158. doi: 10.1016/s0166-4328(01)00413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RT, Chiang MY, Tanabe T, Kobayashi M, Yasuda K, Evans RM, Umesono K. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proceedings of the National Academy of Sciences USA. 2000;97:2621–2625. doi: 10.1073/pnas.050566897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of Fragile X syndrome. Biochem Pharmacol. 79:632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]