Abstract
Although vaccination significantly reduces influenza severity, seasonal human influenza epidemics still cause more than 250,000 deaths annually. Vaccine efficacy is limited in high-risk populations such as infants, the elderly and immunosuppressed individuals. In the event of an influenza pandemic (such as the 2009 H1N1 pandemic), a significant delay in vaccine availability represents a significant public health concern, particularly in high-risk groups. The increasing emergence of strains resistant to the two major anti-influenza drugs, adamantanes and neuraminidase inhibitors, and the continuous circulation of avian influenza viruses with pandemic potential in poultry, strongly calls for alternative prophylactic and treatment options. In this review, we focus on passive virus neutralization strategies for the prevention and control of influenza type A viruses.
Keywords: human monoclonal antibody, immunoprophylaxis, immunotherapy, influenza A virus, neutralizing antibodies
Influenza type A viruses belong to the family Orthomyxoviridae and contain a segmented, negative-sense ssRNA genome [1]. Type A influenza viruses are further divided into subtypes based on the antigenic characteristics of the two major surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Sixteen HA subtypes and nine NA subtypes have been described so far. The lack of proofreading activity of the influenza type A virus's polymerase and the host's immune pressure lead to rapid mutations, resulting in antigenic drift and evasion of the host's immune surveillance. The segmented nature of the virus genome promotes reassortment, which can lead to novel strains with pandemic potential [2]. Humans have experienced four major influenza pandemics in the last 100 years: the Spanish flu of 1918, the Asian flu of 1957 (H2N2), the Hong Kong flu of 1968 (H3N2) and the North American flu of 2009 (H1N1) [3,4]. The rates of illness and death of these influenza pandemics were variable. The 1918 Spanish flu is reported to have claimed the lives of 50 million people, whereas the more recent 2009 H1N1 flu was characterized as an atypical mild pandemic and associated with approximately 14,000 deaths [5–8]. Global seasonal influenza can cause up to 1 billion infections annually, of which 4 million are severe and lead to more than 250,000 deaths [9]. More importantly, the continuous circulation and evolution of highly pathogenic avian H5N1 influenza viruses in poultry and other avian influenza viruses in vast geographic areas of Asia, the Middle East and parts of Africa, with the ability to cause severe disease in humans, poses a major pandemic threat [10–12].
Influenza vaccination significantly reduces the morbidity and mortality of seasonal influenza; however, its efficacy is limited in high-risk populations such as infants, the elderly and the immuno suppressed [13,14]. The lack of pre-existing immunity to pandemic/zoonotic strains, or even epidemic strains, can lead to a severe influenza disease that requires immediate antiviral treatment. Currently, two kinds of anti-influenza drugs are licensed and commercially available in the USA: adamantanes inhibitors and NA inhibitors (NAIs). Adamantanes (e.g., amantadine and rimantidine) block the proton-pump activity of the matrix protein 2 (M2) transmembrane viral protein, which leads to the inhibition of structural changes on the viral HA, the consequent failure in the fusion of the viral and endosomal membranes and the sequestration of the virus replication machinery in the endosome. The NAIs, oseltamivir (Tamiflu®, Roche, San Francisco, CA, USA) and zanamivir (Relenza®, GSK, Philadelphia, PA, USA), act mostly during virus budding, by strongly binding the NA catalytic site, inhibiting its activity and causing virus aggregation, which in turn results in less infectious particles.
Unfortunately, these drugs have a very narrow window of opportunity to be effective and must be administered within the first 48 h of onset of symptoms [15,16]. In addition, a major challenge for current antiviral drugs is that drug-resistant variants can emerge naturally or through selective pressure during treatment [15,16]. Single point mutations at either amino acids (aa) 26, 27, 30, 31 or 34 in M2 can confer resistance to adamantanes [9]. All 2009 pandemic H1N1 viruses show resistance to adamantanes owing to the presence of a S31N mutation in M2 [17]. During the 2007–2008 season, H1N1 viruses rapidly developed resistance to oseltamivir, from 12.3 to 98.5% [17], and NAI-resistant strains were also observed in the 2009 pandemic virus. The oseltamivir-resistant 2009 pandemic virus carrying the H275Y substitution was first detected in Japan, Denmark and Hong Kong during May–June 2009, and has since been identified sporadically around the world [17–21]. Oseltamivir-resistant H5N1 viruses with NA mutations (H274Y and N294S) have also been identified in infected patients during or after treatment [16]. Therefore, adequate antiviral alternatives are needed to minimize the effects and the spread of the disease. Since antibodies play a crucial role in protection against influenza infection [22], passive immunotherapy is a plausible antiviral strategy for the control of influenza disease. In this short review, we focus on passive virus neutralization strategies for the prevention and control of influenza, particularly type A influenza.
Epitopes & mechanisms for influenza virus neutralization
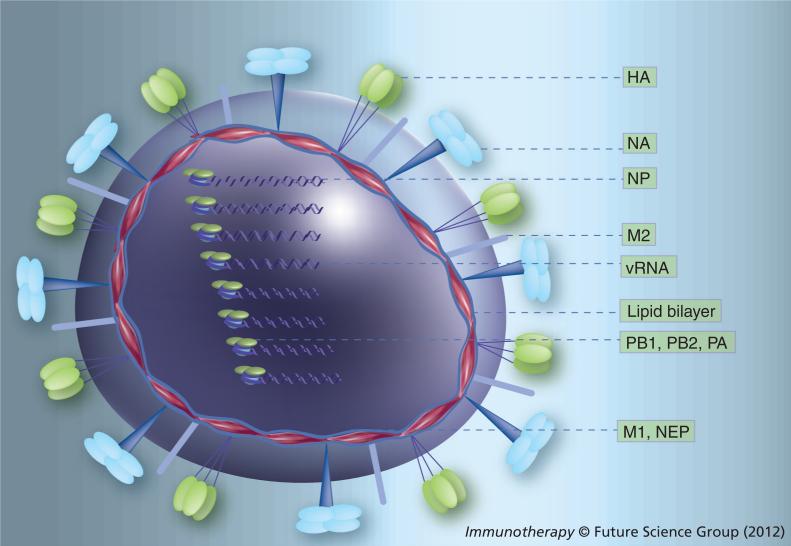
Type A influenza viruses encode at least 11 polypeptides from eight RNA segments (Figure 1). Segment 1 (2341 nucleotides [nt]) encodes the polymerase subunit PB2 (759 aa), segment 2 (2341 nt) encodes the polymerase subunit polymerase basic protein (PB1) (757 aa) and, from an alternative start site, PB1-F2 (~87–101 aa, although most human strains encode truncated PB1-F2 genes of less than 50 aa). Segment 3 (2233 nt) encodes the polymerase subunit (PA; 716 aa), segment 4 (~1766 nt, although the length varies depending on the subtype) encodes HA (~570 aa), segment 5 (1565 nt) the nucleoprotein (NP; ~504 aa) and segment 6 (~1467 nt) NA (~473 aa). Segments 7 (1023 nt) and 8 (890 nt) each encode for two polypeptides via alternative splicing: matrix protein (M); 252 aa) and M2 (97 aa), and nonstructural protein 1 (NS1; ~230 aa) and nuclear export protein (NEP; 121 aa), respectively.
Figure 1. Molecular structure of influenza A viruses.
The virus contains a lipid bilayer derived from the host plasma membrane. Two surface glycoproteins, HA and NA, are the major antigenic determinants of the virus. The virus surface also carries a few copies of an ion channel proton pump (M2). Eight vRNA segments, each one of them associated to three polymerase subunits (PB2, PB1 and PA) and several copies of NP [6], are located inside the virion, protected by a protein mesh provided by the M. In addition, the virus carries a few copies of the virus-encoded NEP. In infected cells, the virus expresses NS1 and some influenza strains express PB1-F2, derived from the second open reading frame of segment 2.
HA: Hemagglutinin; M: Matrix protein; M2: Matrix protein 2; NA: Neuraminidase; NEP: Nuclear export protein; NP: Nucleoprotein; PA: Polymerase acidic protein; PB: Polymerase basic protein; vRNA: Viral RNA.
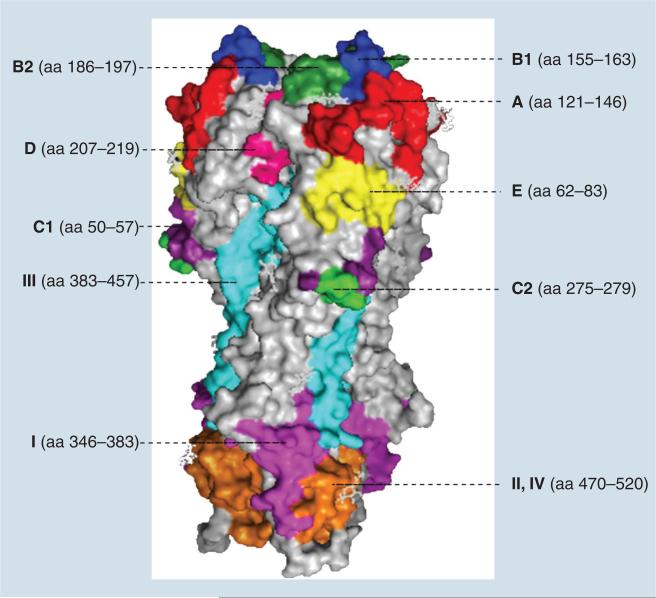
Epitopes recognized by the humoral and T-cell arms of the immune system are found in all of the influenza proteins; however, those that are the most important in terms of neutralizing activity, such as blocking the infection and limiting virus replication, are found on the HA and NA surface glycoproteins, and, to a lesser extent, on the M2 transmembrane protein (Figure 1) [23,24]. HA is present as a homotrimer and is the major virus target antigen recognized by neutralizing antibodies [25]. The HA monomer is first synthesized as a precursor molecule, HA0, then is post-translationally cleaved by trypsin-like proteases found in the lumen of the respiratory or intestinal tracts (or intracellular subtilisin-like proteases in the case of highly pathogenic strains of the H5 and H7 subtypes). The proteolytic cleavage generates two subunits, HA1 and HA2, which are subsequently linked by a disulfide bridge [25]. Each monomer is then maintained in the homotrimer by HA2 subunits via a noncovalently bound coiled structure. The HA1 subunit forms the globular head of the HA protein and contains the receptor-binding site and the dominant antigenic sites that have been mapped using monoclonal antibodies [25]. For the H3 subtype, HA1 contains seven antigenic sites labeled A, B1, B2, C1, C2, D and E, whereas antigenic sites in the H1 subtype are designated Sa, Sb, Ca1, Ca2 and Cb [26,27]. The antigenic sites A, B1, B2, C1 and C2 cover aa positions 121–146, 155–163, 186–197, 50–57 and 275–279, respectively. Site D is located spanning positions 207–219 and site E spans positions 62–83 (Figure 2) [26]. Antibodies against HA1 can efficiently block virus attachment to target cells and/or interfere with virus–host receptor interactions to neutralize the virus (Figure 3A) [28–30]. The inherent variability of the HA molecule permits constant antigenic drift and escape from the neutralizing antibody response [24,31]. This is also the reason for the reformulation of human influenza vaccines and for the need of annual revaccination [32]. In order to confer protection, the HA antigen in the vaccine must match the circulating strains in the population.
Figure 2. Structure of hemagglutinin and antigenic sites.
The illustration was prepared by using MacPymol© (DeLano Scientific, CA, USA) based on the structure of H3 hemagglutinin (Protein Data Bank accession code 1HGE). The aa forming antigenic site A were marked in red. Antigenic site B1, B2, C1, C2, D and E were marked in blue, forest green, purple, green, pink and yellow, respectively. Antigenic site I in HA2 was marked in magenta, sites II and IV were marked in orange and site III was labeled in cyan.
aa: Amino acid.
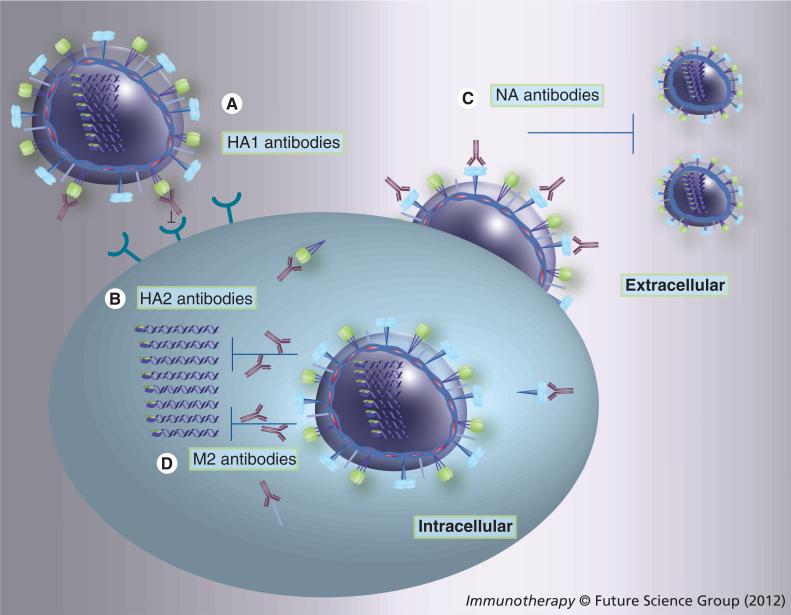
Figure 3. The action of influenza virus-neutralizing antibodies.
(A) Antibodies against HA1 can efficiently block virus attachment to target cells and/or interfere with virus–host receptor interactions to neutralize the virus. (B) Antibodies against HA2 can inhibit the fusion of viral and endosomal membranes and thus, block the release of viral ribonucleoproteins and reduce virus replication. (C) Antibodies against NA can prevent virus release, resulting in reduced virus production and decreasing the severity induced by secondary pneumococcal infection. (D) Antibodies against M2 can constrain proton transport, HA structure change during the fusion and thus, block the viral ribonucleoprotein uncoating. Antibodies such as IgG and IgA could be internalized into the cell through FcRn and pIgR, respectively, thus resulting in effective antiviral activity [118,119]. The site of action of these antibodies is not exactly known, although it could be associated the endoplasmic reticulum and/or trans-golgi apparatus.
HA: Hemagglutinin; NA: Neuraminidase.
HA2 forms a stem-like structure that mediates the anchorage of HA to the virus membrane [24,31]. In addition, HA2 contains the fusion peptide that is released by proteolytic cleavage of HA0 precursor molecule and exposed during its structural changes under low pH conditions [25,33]. The outcome of the fusion process is the release of the viral ribonucleoprotein (vRNP) complexes into the infected cell. By contrast to the HA1 subunit, the HA2 is more conserved within and among different subtypes and is less prone to antigenic drift. HA2 contains at least four antigenic sites designated antigenic sites I, II, III and IV. Based on the H3 HA structure, antigenic site I covers aa positions 346–383, antigenic sites II and IV locate in the same stretch 470–520 and antigenic site III covers positions 383–457 (Figure 2) [34]. Monoclonal antibodies against HA2 generally show broad cross-reactivity among different influenza subtypes and some of these have been shown to provide efficient cross-protection against multiple subtypes in lethal mouse models [35–48]. The cross-protective action of these antibodies is mainly mediated by inhibiting the fusion of viral and endosomal membranes and thus, blocking the release of vRNP and virus replication (Figure 3B) [34,37,38,41,49]. Although the epitopes of HA2 are less exposed, they do induce a discernible antibody response [50,51]. Recently, Wrammert et al. reported that 10% of human monoclonal antibodies recovered from plasma-blast cells from pandemic 2009 H1N1-infected patients could bind to a conserved epitope on the HA2 [36]. More recently, Ekiert et al. isolated a human neutralizing monoclonal antibody (mAb; CR8020) with broad activity against most group 2 influenza viruses [52]. At the same time, Corti et al. reported a human neutralizing mAb (FI6) that can react with all 16 HA subtypes [53]. The two monoclonal antibodies target highly conserved epitopes located in the fusion peptide of the HA2 portion. FI6 showed neutralizing activity in vitro and was also effective in vivo in protecting mice and ferrets against both group 1 and group 2 influenza viruses [53]. Therefore, the HA2 epitopes are sought out as potential targets for h eterosubtypic universal vaccines [31,34,54].
NA is the second-most abundant influenza glyco protein. NA is a type 2 glycoprotein and is present in a mushroom-like homotetrameric structure. The activity of NA is important for the release of discrete virus particles during budding from infected cells [24]. NA activity is also associated with exposure of pneumococcal receptors, increasing the possibility of post-influenza-secondary bacterial pneumonia [55–57]. Thus, although NA appears to play no role in viral attachment and/or penetration to the host cell, increasing evidence suggests that antibodies against NA can prevent virus release, resulting in reduced virus production and decreasing the severity induced by s econdary pneumococcal infection (Figure 3C) [58–61].
M2, a single type 3 viral membrane protein, is present as a homotetramer [62]. The proton channel activity of M2 is essential for release of the viral genome into the cell [15]. In M2, the extracellular N-terminal domain (eM2; a 23 aa peptide) is highly conserved among influenza A strains and, therefore, represents an attractive target for generating universal vaccines [54]. The eM2 is poorly immunogenic and antibodies are hardly detectable in humans after natural infection [63]. However, several studies have shown that antibodies against eM2 can provide protection against lethal influenza challenge in animal models [64–69], and a recent study found that more than 15% (23 out of 140) of individuals sampled had detectable antibodies against eM2 [70]. The same study also showed the rescue of a human monoclonal eM2 antibody from B cells, which elicited protection against lethal challenges with either H5N1 or H1N1 in the mouse model [70]. Although eM2 does not naturally induce a strong immune response, studies with antibodies against eM2 have shown the occurrence of escape mutants when selective pressure is applied [71]. Since eM2 antibodies do not block virus attachment to cells, their mechanism of action likely involves the constraining of proton transport activity and thus preventing structural changes on the HA needed for fusion and uncoating of vRNPs (Figure 3B), and/or antibody dependent cell-mediated cytotoxicity or complement-dependent cytotoxicity effects [64,72].
Sources of neutralizing antibodies for passive immunotherapy
The plasma from convalescent individuals, as a source of neutralizing antibodies for use in passive immunotherapy, has been used for the treatment for many virus infections, including measles virus, Ebola virus, Junín virus, CMV, respiratory syncytial virus (RSV), smallpox virus, hepatitis A and B viruses and rabies virus [73–81]. Passive immunotherapy using plasma has a long history of effectiveness and safety. So far, seven human polyclonal immunoglobulins have been approved by the US FDA for passive immunotherapy of virus infections (Table 1) [82–88]. In the case of influenza virus, convalescent plasma was reported to be beneficial in reducing clinical mortality during the 1918 Spanish influenza pandemic [89], a time in which neither antivirals, nor antibiotics were available. H5N1-infected patients treated with convalescent H5N1 plasma showed a significant reduction in virus load and eventually recovered [90]. Treatment of severe H1N1 2009 infections with a single dose of convalescent plasma could significantly reduce respiratory virus load, serum cytokine responses and mortality [91]. These reports highlight that plasma from convalescent influenza patients can be used as an effective therapeutic alternative, particularly during severe influenza infections, perhaps in combination with other antivirals or instead of other antivirals, if these are not available.
Table 1.
Therapeutic polyclonal and monoclonal antibodies against viruses.
| Target | Name | Source | Major indication | Ref. |
|---|---|---|---|---|
| RSV | Respigam† | Human polyclonal immune globulin | Prophylactic treatment of RSV in infants | [82] |
| Synagis† | Humanized monoclonal antibody | Prophylactic treatment of RSV in infants | [94] | |
| Varicella Zoster virus (chicken pox) | VZIG† VariZIG† |
Human polyclonal immune globulin | Prophylaxis against chickenpox | [83] |
| Vaccinia virus | VIG† | Human polyclonal immune globulin | Treatment of smallpox vaccine side effects | [84] |
| HBV | HepaGamB† | Human polyclonal immune globulin | Prevents HBV in patients receiving a liver transplant and in babies born to mothers infected with HBV | [85] |
| Rabies virus | HyperRab™ S/D† Imogam® Rabies-HT† |
Human polyclonal immune globulin | Postexposure prevention of rabies | [86] |
| CMV | Cytogam† | Human polyclonal immune globulin | Prophylaxis of CMV disease associated with transplantations | [87] |
| HAV | GamaSTAN S/D† |
Human polyclonal immune globulin | Postexposure prevention of hepatitis A | [88] |
| HCV | Civacir‡ | Human polyclonal immune globulin | Prevent reinfection of HCV in HCV Ag-positive liver transplant patients | [101] |
| DENV | DENV1-E105‡ DENV1-E106‡ |
Mouse monoclonal antibodies | Therapeutic activity against DENV | [102,103] |
| Influenza A virus | TCN-032‡ | Human monoclonal antibodies | Treatment of pandemic and severe seasonal influenza | [70,201] |
Available commercially.
Seeking commercial approval.
Ag: Antigen; DENV: Dengue virus; HAV: Hepatitis A virus; RSV: Respiratory syncytial virus.
Although human convalescent plasma reflects the full spectrum of the natural immune response, its production is highly dependent on blood donors, and considerable variations are expected between batches in terms of specificity, quantity and suitability [92]. The mAb technique introduced initially by Kohler and Milstein provided an answer to the challenges set by the use of convalescent plasma [93]. Monoclonal antibodies not only show high antigen specificity, but also can be produced in unlimited quantities [93]. More recently, the generation of either humanized or human monoclonal antibodies has led to an explosion in approaches using passive immunotherapy. A ‘humanized’ IgG mAb against RSV is commercially available for high-risk infants for the prevention and treatment of RSV infections [94]. In the case of influenza, neutralizing mAbs against highly pathogenic H5N1 and broadly neutralizing, subtype-independent mAbs have received considerable attention [36,39,70,95–100]. Several studies show that mAbs against the influenza virus are effective in passive protection in animal models [39,95–100]. More recently, two independent studies utilizing B cells from human blood donors in combination with antibody engineering techniques led to the identification of broadly neutralizing mAbs against epitopes in the eM2 and HA2 subunit, respectively [36,70]. In these studies, the influenza-specific antibody-secreting cells were first isolated and/or enriched from human peripheral blood mononuclear cells, and then the IgG variable regions (VH and VL) were amplified and cloned into a human IgG1 expression vector in order to generate human monoclonal antibodies. Furthermore, mice treated with these antibodies were protected against lethal challenge with highly divergent influenza subtypes, highlighting their potential for clinical passive immunotherapy [36,70]. Although the ‘humanized’ IgG mAb against RSV is the only commercial mAb approved by the FDA for prevention of a viral disease, the FDA has approved more than 30 mAbs for therapeutic use for other noninfectious diseases. Of note, mAbs against HCV, dengue virus and influenza virus are in the process of seeking approval for commercialization (Table 1) [70,101–103,201].
In addition to human convalescent plasma and mAbs against influenza, chicken IgY, which can be produced in high concentrations in egg yolk and because of its high stability, is another excellent potential resource for passive immunotherapy. Studies in animals and humans have demonstrated the passive immunoprotective effects of IgY against different pathogens [104–108]. In humans, oral administration of IgY specific to Helicobacter pylori could reduce Helicobacter infections, and antigen-specific IgY was also shown to be effective against Pseudomonas aeruginosa infections in cystic fibrosis patients [107,108]. The effects of IgY are not restricted to pathogens of the GI tract or to the oral route for entry. Treatment of chickens with anti-H5N1-specific IgY antibodies, produced in ostrich eggs, resulted in a dramatic decrease in mortality after lethal H5N1 challenge [109]. Mice treated via the intranasal route with anti-H5N1 or anti-H1N1 chicken IgY antibodies were protected against lethal challenge with H5N1 or H1N1 viruses, respectively [110]. In this same study, it was shown that IgY treatment could induce anti-IgY antibodies in mice; however, these antibodies did not interfere with the neutralizing activity of the chicken IgY [110]. It is important to note that IgY antibodies cannot trigger the human complement system or bind to the human Fc receptor and, therefore, it is unlikely that they would mediate antibody-dependent enhancement of disease or complement-mediated inflammation [111]. These studies highlight influenza virus-specific IgY, present in eggs as a natural food source for humans, as an enormous source for influenza-passive immunotherapy.
Delivery strategies for passive neutralizing influenza antibodies
Passive neutralizing antibodies can be administered via multiple routes, including the oral, intranasal, intraperitoneal, intramuscular and intravenous routes. In humans, passive immunotherapy with convalescent plasma is generally given via intramuscular and intravenous routes. In mice, however, the protective effects of either mouse- or human-derived mAbs have relied mostly on intraperitoneal administration before or after challenge with the pathogen of choice [39,95–100]. In the case of influenza, an obligatory respiratory virus in humans, administration via the respiratory route could be considered viable, as severely ill patients may be assisted by mechanical ventilation devices, which can be adjusted to administer drugs [112,113]. In mice, mAbs against H5N1 and the 2009 pandemic H1N1 virus were administered intranasally and shown to be effective in challenge studies. A single intranasal dose of anti-H5 IgA mAb either at 24, 48 or 72 h prior to H5N1 sublethal challenge resulted in no bodyweight losses, no clinical signs of disease and significantly decreased virus load in the lungs compared with mock treated controls. Administration either at 24, 48 or 72 h post-H5N1 sublethal challenge provided 100, 80 and 60% protection, respectively [99]. It was also shown that a single intranasal dose of anti-H1N1 (2009 pandemic) IgG-neutralizing mAb could provide protection against lethal influenza challenge, even when administrated 72 h prior to or postchallenge [114]. These studies show that anti-influenza neutralizing activity could be maintained for at least 72 h in the respiratory tract, suggesting that respiratory administration of mAbs could be an alternative route for the treatment of influenza. Of note, the anti-H5N1 chicken IgY described above only provided protection if it was given via the intranasal route in mice [110]. No protective effect of such IgY was observed when administered via the oral or intraperitoneal routes, even though the chicken IgY could be detected in the sera of treated mice [110]. It remains to be determined whether administration of anti-H5N1 IgY would provide significant protection in chicken.
Conclusion & future perspective
Passive immunization is an immediate preventative and therapeutic strategy for infectious diseases and has been accepted in the treatment of many virus infections such as RSV, smallpox virus, the hepatitis A and B viruses and rabies virus. This strategy would be beneficial for high-risk populations, such as immunosuppressed individuals, individuals with poor vaccine coverage and/or when other antiviral options were either not available or have failed. A Phase I study of one human mAb (TCN-032) against M2 is being evaluated for passive immunization against influenza viruses [70,201].
Perhaps the major challenge in developing a suitable immunotherapy approach against influenza is the inherent ability of the virus to constantly evolve, thus readily evading the immune system. In this regard, the identification of highly cross-reactive human mAb against the eM2 and HA2 subunit constitute major steps towards a universal immunotherapy approach. Broad human neutralizing mAbs, such as TCN-032, CR6261, CR8020 and FI6, show great thera peutic potential against influenza viruses [52,53,70,201]. If these mAbs become a viable alternative for prevention and treatment of influenza, it remains to be seen whether escape mutants can readily emerge. Even though NAIs were designed in silico, were shown to have excellent antiviral activity in vitro and deemed unlikely to promote the emergence of resistant strains, nature has shown otherwise. NAI-resistant strains have emerged for seasonal H1N1 and H3N2 strains, and readily emerged during the 2009 H1N1 pandemic. Thus, it is plausible that, once an immune reagent is used for the prevention or treatment of influenza, the virus finds a way to resist such pressure. This is the case in RSV, a human paramyxovirus that shows much less antigenic variation than influenza viruses, but for which resistant strains emerged during treatment with the commercially available humanized IgG1 mAb palivizumab [115]. An alternative to the limitation of using a single mAb would be to use a cocktail of neutralizing mAbs with specificity for various epitopes. Such a cocktail of mAbs has been used for the treatment of rabies [116]. A clinical Phase II study demonstrated the cocktail was safe and as effective as the human rabies immunoglobulin [116].
Since rapid and efficient transmission is a characteristic of influenza viruses, particularly of pandemic strains, blocking transmission could certainly curtail the disease burden of these viruses. In this regard, the work by Tsukamoto et al. [117] is worth noting, since it was observed that air filters impregnated with ostrich IgY specific to H5N1 prevented the transmission of the H5N1 virus to chickens [117], suggesting that specific influenza-neutralizing antibodies could be applied to facial masks or air-conditioning filters to prevent the population from influenza infections. Thus, immunological approaches to prevent influenza infections are not restricted to the administration of antibodies to individual subjects. Instead, alternative approaches, like those described by Tsukamoto et al. [117] could effectively prevent the spread of the disease.
Executive summary.
■ A vaccine's efficacy is limited in high-risk populations such as infants, the elderly and immunosuppressed individuals. In the event of an influenza pandemic, a significant delay in vaccine availability represents a significant public health concern. In addition to these, the increasing emergence of strains resistant to the two kinds of anti-influenza drugs licensed by the US FDA strongly calls for alternative prophylactic and treatment options.
■ Since antibodies play a crucial role in protection against influenza infection, passive immunotherapy is a plausible antiviral strategy for the control of influenza disease.
Epitopes & mechanisms for influenza virus neutralization
■ Three surface proteins, hemagglutinin (HA), neuraminidase (NA) and matrix protein 2 (M2), are major targets to induce neutralizing antibodies against the influenza virus.
■ Antibodies against HA1 can efficiently block virus attachment and/or interfere with virus–host receptor interactions in order to neutralize the virus. Antibodies against HA2 can inhibit the fusion of viral and endosomal membranes, and thus block the release of viral ribonucleoproteins and reduce viral replication.
■ Antibodies against NA can prevent viral release, resulting in reduced viral production and decreasing the severity induced by secondary pneumococcal infection. Antibodies against M2 can constrain proton transport and the structural change that occurs to HA during the fusion, and thus block the viral ribonucleoprotein uncoating.
■ As there are highly conserved epitopes in HA2 and M2, antibodies against HA2 and M2 generally show broadly neutralizing activity against different influenza virus subtypes and are considered as potential universal antibodies against influenza.
Sources of neutralizing antibodies for passive immunotherapy
■ Human convalescent plasma was reported to be beneficial in reducing clinical mortality during the 1918 Spanish influenza pandemic, in H5N1 infected patients and in severe H1N1 2009 infections.
■ The generation of monoclonal antibodies (mAbs), either humanized or human mAbs, has led to an explosion in approaches using passive immunotherapy.
■ Utilizing influenza-specific antibody-secreting cells isolated and/or enriched from human peripheral blood mononuclear cells in combination with antibody engineering techniques has led to the identification of broadly neutralizing mAbs against epitopes in the M2 extracellular N-terminal domain (eM2) and HA2 subunit.
■ Lots of mAbs against influenza viruses have been reported to be efficiently protective in animal models such as the mouse and ferret. A Phase I study of one human mAb (TCN-032) against M2 is being evaluated for passive immunization against influenza virus.
■ Chicken IgY, produced in high concentrations in egg yolk and with high stability, is another excellent potential resource for influenza passive immunotherapy. Chicken IgY against specific influenza virus subtype has been reported to be protective in the mouse and chicken models.
Delivery strategies for passive neutralizing influenza antibodies
■ Passive neutralizing antibodies can be administrated via multiple routes, including the oral, intranasal, intraperitoneal, intramuscular and intravenous routes.
■ In humans, passive immunotherapy with convalescent plasma is generally given via intramuscular and intravenous routes. In mice, the protective effects of mAbs have relied mostly on intraperitoneal administration before or after challenge.
■ In the case of influenza, an obligatory respiratory virus in humans, administration via the respiratory route could be considered viable, just like the intranasal delivery of live attenuated influenza seasonal vaccines.
■ Animal models (mouse and chicken) have shown that intranasal delivery of mAbs or chicken IgY could provide efficient protection, even when antibodies are administrated 72 h prior to or postchallenge.
Conclusion & future perspective
■ Influenza-passive immunotherapy would be beneficial for high-risk populations such as immunosuppressed individuals or individuals with poor vaccine coverage and/or when other antiviral options were either not available or have failed.
■ Perhaps the major challenge in developing a suitable immunotherapy approach against influenza is the inherent ability of the virus to constantly evolve, thus readily evading the immune system. The identification of highly cross-reactive human mAbs by antibody engineering techniques constitutes a major step towards a universal immunotherapy approach.
■ A cocktail of broad human neutralizing mAbs against the eM2 and HA2 subunit, such as TCN-032, CR6261, CR8020 and FI6, shows great therapeutic potential against influenza viruses.
■ Since rapid and efficient transmission is a characteristic of influenza viruses, particularly of pandemic strains, blocking transmission could certainly curtail the disease burden of these viruses. Specific influenza neutralizing antibodies could be applied to facial masks or air-conditioning filters to prevent the population from influenza infections.
Acknowledgments
Financial & competing interests disclosure
This work was made possible through funding by the CDC-HHS grant (1U01CI000355), NIAID-NIH grant, (R01AI052155), CSREES-USDA grant (2005–05523) and NIAID-NIH contract (HHSN266200700010C). The funders had no role in study design, data collection or analysis, the decision to publish or preparation of the manuscript.
Footnotes
Disclosure
The opinions of this manuscript are those of the authors and do not necessarily represent the views of the granting agencies.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
- 1.Lamb RA. Genes and proteins of the influenza viruses. In: Krug RM, editor. The Influenza Viruses. 1st Edition Plenum Press; NY, USA: 1989. [Google Scholar]
- 2.Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008;26(Suppl. 4):D49–D53. doi: 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster RG. Predictions for future human influenza pandemics. J. Infect. Dis. 1997;176(Suppl. 1):S14–S19. doi: 10.1086/514168. [DOI] [PubMed] [Google Scholar]
- 4.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 5.Taubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerg. Infect. Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libster R, Bugna J, Coviello S, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N. Engl. J. Med. 2010;362(1):45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 7.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson LJ, Rutter PD, Ellis BM, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:B5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard MP, Cherian T, Pervikov Y, Kieny MP. A review of vaccine research and development: human acute respiratory infections. Vaccine. 2005;23(50):5708–5724. doi: 10.1016/j.vaccine.2005.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Ghafar AN, Chotpitayasunondh T, Gao ZC, et al. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 2008;358(3):261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 11.Brown IH, Capua I, Cattoli G, et al. Continuing progress towards a unified nomenclature for the highly pathogenic H5N1 avian influenza viruses: divergence of clade 2.2 viruses. Influenza Other Respi. Viruses. 2009;3(2):59–62. doi: 10.1111/j.1750-2659.2009.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organization WH. Update: WHO-confirmed human cases of avian influenza A (H5N1) infection, November 2003–May 2008. Wkly Epidemiol. Rec. 2008;83:415–420. [PubMed] [Google Scholar]
- 13.Ambrose CS, Luke C, Coelingh K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respi. Viruses. 2008;2(6):193–202. doi: 10.1111/j.1750-2659.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17:S3–S10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 15.Davies WL, Hoffmann CE, Paulshock M, et al. Antiviral activity of 1-adamantanamine (amantadine ). Science. 1964;144(362):862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 16.Moscona A. Oseltamivir resistance – disabling our influenza defenses. N. Engl. J. Med. 2005;353(25):2633–2636. doi: 10.1056/NEJMp058291. [DOI] [PubMed] [Google Scholar]
- 17.Dharan NJ, Gubareva LV, Meyer JJ, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301(10):1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- 18.Tamura D, Mitamura K, Yamazaki M, et al. Oseltamivir-resistant influenza A viruses circulating in Japan. J. Clin. Microbiol. 2009;47(5):1424–1427. doi: 10.1128/JCM.02396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Cheung CL, Tai H, et al. Oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus, Hong Kong, China. Emerg. Infect. Dis. 2009;15(12):1970–1972. doi: 10.3201/eid1512.091057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N. Engl. J. Med. 2010;362(1):86–87. doi: 10.1056/NEJMc0910448. [DOI] [PubMed] [Google Scholar]
- 21.Baz M, Abed Y, Papenburg J, Bouhy X, Hamelin ME, Boivin G. Emergence of oseltamivir-resistant pandemic H1N1 virus during prophylaxis. N. Engl. J. Med. 2009;361(23):2296–2297. doi: 10.1056/NEJMc0910060. [DOI] [PubMed] [Google Scholar]
- 22.Gerhard W. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 23.Gerhard W, Mozdzanowska K, Zharikova D. Prospects for universal influenza virus vaccine. Emerg. Infect. Dis. 2006;12(4):569–574. doi: 10.3201/eid1204.051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Edition Lippincott-Raven Publishers; PA, USA: 2007. [Google Scholar]
- 25.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Ann. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 26.Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- 27.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 28.Knossow M, Gaudier M, Douglas A, et al. Mechanism of neutralization of influenza virus infectivity by antibodies. Virology. 2002;302(2):294–298. doi: 10.1006/viro.2002.1625. [DOI] [PubMed] [Google Scholar]
- 29.Bizebard T, Gigant B, Rigolet P, et al. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature. 1995;376(6535):92–94. doi: 10.1038/376092a0. [DOI] [PubMed] [Google Scholar]
- 30.Fleury D, Barrere B, Bizebard T, Daniels RS, Skehel JJ, Knossow M. A complex of influenza hemagglutinin with a neutralizing antibody that binds outside the virus receptor binding site. Nat. Struct. Bio. 1999;l6(6):530–534. doi: 10.1038/9299. [DOI] [PubMed] [Google Scholar]
- 31.Wang TT, Palese P. Universal epitopes of influenza virus hemagglutinins? Nat. Struct. Mol. Biol. 2009;16(3):233–234. doi: 10.1038/nsmb.1574. [DOI] [PubMed] [Google Scholar]
- 32.Lambert LC, Fauci AS. Current concepts influenza vaccines for the future. N. Engl. J. Med. 2010;363(21):2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 33.Gething MJ, Doms RW, York D, White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J. Cell Biol. 1986;102(1):11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vareckova E, Mucha V, Wharton SA, Kostolansky F. Inhibition of fusion activity of influenza A haemagglutinin mediated by HA2-specific monoclonal antibodies. Arch. Virol. 2003;148(3):469–486. doi: 10.1007/s00705-002-0932-1. [DOI] [PubMed] [Google Scholar]
- 35.Vareckova E, Mucha V, Kostolansky F, Gubareva LV, Klimov A. HA2-specific monoclonal antibodies as tools for differential recognition of influenza A virus antigenic subtypes. Virus Res. 2008;132(1–2):181–186. doi: 10.1016/j.virusres.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 36■.Wrammert J, Koutsonanos D, Li GM, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [Detailed ana lysis of plasmablast and monoclonal antibody responses induced by pandemic H1N1 infection in humans.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sui J, Hwang WC, Perez S, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009;16(3):265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza-A virus H1 and H2 strains. J. Virol. 1993;67(5):2552–2558. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Throsby M, van Den Brink E, Jongeneelen M, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE. 2008;3(12):E3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corti D, Suguitan AL, Jr, Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 2010;120(5):1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekiert DC, Bhabha G, Elsliger MA, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuno Y, Matsumoto K, Isegawa Y, Ueda S. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J. Virol. 1994;68(1):517–520. doi: 10.1128/jvi.68.1.517-520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007;25(12):1421–1434. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gocnik M, Fislova T, Sladkova T, Mucha V, Kostolansky F, Vareckova E. Antibodies specific to the HA2 glycopolypeptide of influenza A virus haemagglutinin with fusion-inhibition activity contribute to the protection of mice against lethal infection. J. Gen. Virol. 2007;88(Pt 3):951–955. doi: 10.1099/vir.0.82563-0. [DOI] [PubMed] [Google Scholar]
- 45.Prabhu N, Prabakaran M, Ho HT, et al. Monoclonal antibodies against the fusion peptide of hemagglutinin protect mice from lethal influenza A virus H5N1 infection. J. Virol. 2009;83(6):2553–2562. doi: 10.1128/JVI.02165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gocnik M, Fislova T, Mucha V, et al. Antibodies induced by the HA2 glycopolypeptide of influenza virus haemagglutinin improve recovery from influenza A virus infection. J. Gen. Virol. 2008;89(Pt 4):958–967. doi: 10.1099/vir.0.83524-0. [DOI] [PubMed] [Google Scholar]
- 47.Stanekova Z, Kiraly J, Stropkovska A, et al. Heterosubtypic protective immunity against influenza A virus induced by fusion peptide of the hemagglutinin in comparison to ectodomain of M2 protein. Acta Virol. 2011;55(1):61–67. doi: 10.4149/av_2011_01_61. [DOI] [PubMed] [Google Scholar]
- 48.Vareckova E, Cox N, Klimov A. Evaluation of the subtype specificity of monoclonal antibodies raised against H1 and H3 subtypes of human influenza A virus hemagglutinins. J. Clin. Microbiol. 2002;40(6):2220–2223. doi: 10.1128/JCM.40.6.2220-2223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbey-Martin C, Gigant B, Bizebard T, et al. An antibody that prevents the hemagglutinin low pH fusogenic transition. Virology. 2002;294(1):70–74. doi: 10.1006/viro.2001.1320. [DOI] [PubMed] [Google Scholar]
- 50.Styk B, Russ G, Polakova K. Antigenic glycopolypeptides HA1 and HA2 of influenza virus haemagglutinin. III. Reactivity with human convalescent sera. Acta Virol. 1979;23(1):1–8. [PubMed] [Google Scholar]
- 51.Kostolansky F, Mucha V, Slovakova R, Vareckova E. Natural influenza A virus infection of mice elicits strong antibody response to HA2 glycopolypeptide. Acta Virol. 2002;46(4):229–236. [PubMed] [Google Scholar]
- 52.Ekiert DC, Friesen RH, Bhabha G, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333(6044):843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53■.Corti D, Voss J, Gamblin SJ, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–856. doi: 10.1126/science.1205669. [Highlighted a single-cell culture method for screening human plasma cells to isolate a monoclonal antibody that neutralized both group 1 and group 2 influenza A viruses.] [DOI] [PubMed] [Google Scholar]
- 54.Du LY, Zhou YS, Jiang SB. Research and development of universal influenza vaccines. Microbes Infect. 2010;12(4):280–286. doi: 10.1016/j.micinf.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 2005;192(2):249–257. doi: 10.1086/430954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ja M. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powers DC, Kilbourne ED, Johansson BE. Neuraminidase-specific antibody responses to inactivated influenza virus vaccine in young and elderly adults. Clin. Diagn. Lab. Immunol. 1996;3(5):511–516. doi: 10.1128/cdli.3.5.511-516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kilbourne ED, Laver WG, Schulman JI, Webster RG. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J. Virol. 1968;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy BR, Kasel JA, Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N. Engl. J. Med. 1972;286(25):1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 60.Couch RB, Kasel JA, Gerin JL, Schulman JL, Kilbourn ED. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 1974;129(4):411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- 61.Webster RG, Reay PA, Laver WG. Protection against lethal influenza with neuraminidase. Virology. 1988;164(1):230–237. doi: 10.1016/0042-6822(88)90640-x. [DOI] [PubMed] [Google Scholar]
- 62.Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451(7178):591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng J, Zhang M, Mozdzanowska K, et al. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virol. J. 2006;3:102. doi: 10.1186/1743-422X-3-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J. Immunol. 2004;172(9):5598–5605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- 65.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 1999;5(10):1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 66.Ernst WA, Kim HJ, Tumpey TM, et al. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24(24):5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Fan J, Liang X, Horton MS, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets and rhesus monkeys. Vaccine. 2004;22(23–24):2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 68.Huleatt JW, Nakaar V, Desai P, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26(2):201–214. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 69.Liu W, Peng Z, Liu Z, Lu Y, Ding J, Chen YH. High epitope density in a single recombinant protein molecule of the extracellular domain of influenza A virus M2 protein significantly enhances protective immunity. Vaccine. 2004;23(3):366–371. doi: 10.1016/j.vaccine.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 70■.Grandea AG, Olsen OA, Cox TC, et al. Human antibodies reveal a protective epitope that is highly conserved among human and nonhuman influenza A viruses. PNAS. 2010;107(28):12658–12663. doi: 10.1073/pnas.0911806107. [Described the isolation of a panel of broadly cross-reactive monoclonal antibodies against influenza M2 extracellular N-terminal domain from human memory B cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zharikova D, Mozdzanowska K, Feng J, Zhang M, Gerhard W. Influenza type A virus escape mutants emerge in vivo in the presence of antibodies to the ectodomain of matrix protein 2. J. Virol. 2005;79(11):6644–6654. doi: 10.1128/JVI.79.11.6644-6654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 2001;166(12):7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 73.Stokes J, Jr, Maris EP, Gellis SS. Chemical, clincical and immunological studies on the products of human plasma fractionation XI. The use of concentrated normal human serum g globulin (human immune serum globulin) in the prophylaxis and treatment of measles. J. Clin. Invest. 1944;23:531–540. doi: 10.1172/JCI101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reed EC, Bowden RA, Dandliker PS, Lilleby KE, Meyers JD. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants. Ann. Intern. Med. 1988;109(10):783–788. doi: 10.7326/0003-4819-109-10-783. [DOI] [PubMed] [Google Scholar]
- 75.Whimbey E, Champlin RE, Englund JA, et al. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 1995;16(3):393–399. [PubMed] [Google Scholar]
- 76.Hopkins RJ, Lane JM. Clinical efficacy of intramuscular vaccinia immune globulin: a literature review. Clin. Infect. Dis. 2004;39:819–826. doi: 10.1086/422999. [DOI] [PubMed] [Google Scholar]
- 77.Victor JC, Monto AS, Surdina TY, et al. Hepatitis avaccine versus immune globulin for postexposure prophylaxis. N. Engl. J. Med. 2007;357:1685–1694. doi: 10.1056/NEJMoa070546. [DOI] [PubMed] [Google Scholar]
- 78.Samuel D, Muller R, Alexander G, et al. Liver-transplantation in European patients with the hepatitis-B surface-antigen. N. Engl. J. Med. 1993;329(25):1842–1847. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 79.Bahmanyar M, Fayaz A, Nour-Salehi S, Mohammadi M, Koprowski H. Successful protection of humans exposed to rabies infection: postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA. 1976;236(24):2751–2754. [PubMed] [Google Scholar]
- 80.Kudoyarova-Zubavichene NM, Sergeyev NN, Chepurnov AA, Netesov SV. Preparation and use of hyperimmune serum for prophylaxis and therapy of Ebola virus infections. J. Infect. Dis. 1999;179:S218–S223. doi: 10.1086/514294. [DOI] [PubMed] [Google Scholar]
- 81.Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: passive immunotherapy for influenza and other serious infections. Crit. Care Med. 2010;38(Suppl. 4):E66–E73. doi: 10.1097/CCM.0b013e3181d44c1e. [DOI] [PubMed] [Google Scholar]
- 82.Morris SK, Dzolganovski B, Beyene J, Sung L. A meta-ana lysis of the effect of antibody therapy for the prevention of severe respiratory syncytial virus infection. BMC Infect. Dis. 2009;9:106. doi: 10.1186/1471-2334-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.CDC MMWR Morb. Mortal. Wkly Rep. 2006;55:209–210. [A new product (VariZIG) for post exposure prophylaxis of varicella available under an investigational new drug application expanded access protocol.] [PubMed] [Google Scholar]
- 84.Wittek R. Vaccinia immune globulin: current policies, preparedness and product safety and efficacy. Int. J. Infect. Dis. 2006;10(3):193–201. doi: 10.1016/j.ijid.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Shetty K, Lukose T, Brown RS., Jr Evaluation of blood glucose levels after hepatitis B immune globulin administration utilizing two different blood glucose monitoring systems. Transplant. Proc. 2010;42(10):4123–4126. doi: 10.1016/j.transproceed.2010.09.131. [DOI] [PubMed] [Google Scholar]
- 86.Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention – United States, 2008: recommendations of the Advisory committee on immunization practices (ACIP). MMWR Recomm. Rep. 2008;57:1–28. [PubMed] [Google Scholar]
- 87.Adler SP, Nigro G. Findings and conclusions from CMV hyperimmune globulin treatment trials. J. Clin. Virol. 2009;46(Suppl. 4):S54–S57. doi: 10.1016/j.jcv.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 88.Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory committee on immunization practices (ACIP). MMWR Recomm. Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 89.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-ana lysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann. Intern. Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 90.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 2007;357(14):1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 91■.Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [Describes a prospective cohort study where patients with severe H1N1 2009 infection were treated with convalescent plasma from patients recovering from the same infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haurum JS. Recombinant polyclonal antibodies: the next generation of antibody therapeutics? Drug Discov. Today. 2006;11(13–14):655–660. doi: 10.1016/j.drudis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 93.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 94.Johnson S, Oliver C, Prince GA, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997;176(5):1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 95.Simmons CP, Bernasconi NL, Suguitan AL, et al. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4(5):E178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lim AP, Chan CE, Wong SK, Chan AH, Ooi EE, Hanson BJ. Neutralizing human monoclonal antibody against H5N1 influenza HA selected from a Fab-phage display library. Virol. J. 2008;5:130. doi: 10.1186/1743-422X-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanson BJ, Boon AC, Lim AP, Webb A, Ooi EE, Webby RJ. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir. Res. 2006;7:126. doi: 10.1186/1465-9921-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun L, Lu X, Li C, et al. Generation, characterization and epitope mapping of two neutralizing and protective human recombinant antibodies against influenza A H5N1 viruses. PLoS ONE. 2009;4(5):E5476. doi: 10.1371/journal.pone.0005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99■.Ye J, Shao H, Hickman D, et al. Intranasal delivery of an IgA monoclonal antibody effective against sublethal H5N1 influenza virus infection in mice. Clin. Vacc. Immunol. 2010;17(9):1363–1370. doi: 10.1128/CVI.00002-10. [Describes intranasal administration of an IgA neutralizing monoclonal antibody that could provide protection against sublethal H5 challenge at both prior to and postchallenge in mouse models.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kashyap AK, Steel J, Rubrum A, et al. Protection from the 2009 H1N1 pandemic influenza by an antibody from combinatorial survivor-based libraries. PLoS Pathog. 2010;6(7):E1000990. doi: 10.1371/journal.ppat.1000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uprichard SL. Hepatitis C virus experimental model systems and antiviral drug research. Virol. Sin. 2010;25(4):227–245. doi: 10.1007/s12250-010-3134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rajamanonmani R, Nkenfou C, Clancy P, et al. On a mouse monoclonal antibody that neutralizes all four dengue virus serotypes. J. Gen. Virol. 2009;90:799–809. doi: 10.1099/vir.0.006874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shrestha B, Brien JD, Sukupolvi-Petty S, et al. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog. 2010;6(4):E1000823. doi: 10.1371/journal.ppat.1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carlander D, Kollberg H, Wejaker PE, Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol. Res. 2000;21(1):1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatta H, Tsuda K, Ozeki M, et al. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res. 1997;31(4):268–274. doi: 10.1159/000262410. [DOI] [PubMed] [Google Scholar]
- 106.Suzuki H, Nomura S, Masaoka T, et al. Effect of dietary anti-Helicobacter pylori-urease immunoglobulin Y on Helicobacter pylori infection. Aliment Pharmacol. Ther. 2004;20(Suppl. 1):S185–S192. doi: 10.1111/j.1365-2036.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 107.Horie K, Horie N, Abdou AM, et al. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. J. Dairy Sci. 2004;87(12):4073–4079. doi: 10.3168/jds.S0022-0302(04)73549-3. [DOI] [PubMed] [Google Scholar]
- 108.Kollberg H, Carlander D, Olesen H, Wejaker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent pseudomonas aeruginosa infections in patients with cystic fibrosis: a Phase 1 feasibility study. Pediatr. Pulmonol. 2003;35(6):433–440. doi: 10.1002/ppul.10290. [DOI] [PubMed] [Google Scholar]
- 109.Adachi K, Handharyani E, Sari DK, et al. Development of neutralization antibodies against highly pathogenic H5N1 avian influenza virus using ostrich (Struthio camelus) yolk. Mol. Med. Report. 2008;1(2):203–209. [PubMed] [Google Scholar]
- 110■.Nguyen HH, Tumpey TM, Park HJ, et al. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS ONE. 2010;5(4):E10152. doi: 10.1371/journal.pone.0010152. [Describes intranasal administration of influenza-specific chicken IgY that could efficiently prevent infection and significantly reduce viral replication in mouse models.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Larsson A, Balow RM, Lindahl TL, Forsberg PO. Chicken antibodies: taking advantage of evolution – a review. Poult. Sci. 1993;72(10):1807–1812. doi: 10.3382/ps.0721807. [DOI] [PubMed] [Google Scholar]
- 112.Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J. Infect. Dis. 2004;57(6):236–247. [PubMed] [Google Scholar]
- 113.Weltzin R, Monath TP. Intranasal antibody prophylaxis for protection against viral disease. Clin. Microbiol. Rev. 1999;12(3):383–393. doi: 10.1128/cmr.12.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shao H, Ye J, Vincent AL, et al. A novel monoclonal antibody effective against lethal challenge with swine-lineage and 2009 pandemic H1N1 influenza viruses in mice. Virology. 2011;417:379–384. doi: 10.1016/j.virol.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adams O, Bonzel L, Kovacevic A, Mayatepek E, Hoehn T, Vogel M. Palivizumab-resistant human respiratory syncytial virus infection in infancy. Clin. Infect. Dis. 2010;51(2):185–188. doi: 10.1086/653534. [DOI] [PubMed] [Google Scholar]
- 116.Bakker AB, Python C, Kissling CJ, et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability and neutralizing activity. Vaccine. 2008;26(47):5922–5927. doi: 10.1016/j.vaccine.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 117■.Kamiyama Y, Adachi K, Handharyani E, et al. Protection from avian influenza H5N1 virus infection with antibody-impregnated filters. Virol. J. 2011;8:54. doi: 10.1186/1743-422X-8-54. [Describes specific influenza-neutralizing antibodies that could be applied to facial masks or air-conditioning filters to prevent the population from influenza infections.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshida M, Claypool SM, Wagner JS, et al. Human neonatal Fc receptor mediates transport of IgG to luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 119.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J. Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Theraclone sciences initiates Phase 1 trial of TCN-032 for influenza A. www.bioportfolio.com/news/article/809490.