Abstract
As technology expands what it is possible to accurately measure, so too the challenges faced by modern mass spectrometry applications expand. A high level of accuracy in lipid quantitation across thousands of chemical species simultaneously is demanded. While relative changes in lipid amounts with varying conditions may provide initial insights or point to novel targets, there are many questions that require determination of lipid analyte absolute quantitation. Glycerophospholipids present a significant challenge in this regard, given the headgroup diversity, large number of possible acyl chain combinations, and vast range of ionization efficiency of species. Lipidomic output is being used more often not just for profiling of the masses of species, but also for highly-targeted flux-based measurements which put additional burdens on the quantitation pipeline. These first two challenges bring into sharp focus the need for a robust lipidomics workflow including deisotoping, differentiation from background noise, use of multiple internal standards per lipid class, and the use of a scriptable environment in order to create maximum user flexibility and maintain metadata on the parameters of the data analysis as it occurs. As lipidomics technology develops and delivers more output on a larger number of analytes, so must the sophistication of statistical post-processing also continue to advance. High-dimensional data analysis methods involving clustering, lipid pathway analysis, and false discovery rate limitation are becoming standard practices in a maturing field.
Keywords: mass spectrometry, glycerophospholipids, LC-MS, quantitation, data handling, lipidomics
1. Introduction
Although the role of lipid profiling provides important initial information and fulfills a basic role in the field of metabolomics, absolute quantification is the sine qua non of analytical chemistry. To better understand processes in living cells, reliable quantitation of chemical species is necessary as mathematical modeling and comprehensive assessment of interconnections between pathways leads to more accurate predictions about responses to perturbations. A full accounting regarding most cellular processes will require measurements not only of the mass of the relevant species, but also fluxes of those species within different pools. While the available technology has advanced since being first applied in a systems biology context [1], in order for lipidomics to make the greatest possible contribution to process-based biochemical studies, lipid mass spectrometry (MS) must be up to the task of delivering both high quality absolute quantitation in snapshots taken from biological experiments and flexible tools and informative strategies for flux monitoring. Such methods for highly focused kinetic study of analytes are emerging, including the use of stable isotope labeling and novel probes. In these respects, the central needs and challenges of quantitation in lipidomics bear many commonalities with other –omics platforms and other mass spectrometry based analytical enterprises [2,3].
The glycerophospholipids include hundreds of analytes encountered in routine profiling and over a thousand species detectable from repeated fragmentation scanning from some samples. The level of complexity of the quantitative methods required is higher when more metabolites are measured due to the limitations of the necessary isotopic correction, the need to accurately assign integration windows for these lipids during LC separation, and the difficulty of appropriately using internal standards to address ionization variability across acyl chain variants.
This review addresses the specifics of glycerophospholipid analysis ranging from the issues of sample preparation, MS analysis platform choices, and data handling. Several excellent, highly technical reviews of lipidomic methodologies have been contributed over the last decade [4–15], generally having a greater focus on mass spectrometry protocols with less regard to the data handling. Only more recently has more attention been paid to methods for managing and analyzing the high-dimensional output that lipidomics generates [16–19]. There is a growing need for new strategies for statistical post-processing of lipidomic data to further address such issues as multiple hypothesis testing and pathway analysis.
Many of the details in this review also apply more generally to lipidomics on complex lipid mixtures for quantitation of other classes as well (e.g., sphingolipids, glycerolipids). A brief overview of relevant extraction and analysis protocols and various alternative MS modes of operation is provided, since certain choices make a difference in how data will subsequently be processed and how many metabolites can be reliably monitored [18]. Because electrospray ionization mass spectrometry coupled with liquid chromatography (ESI LC-MS) offers substantial advantages to the process of absolute quantitation, the focus of this article is on methods which employ LC separation as an essential step in the analysis.
2. Mass spectrometry protocols
2.1 Extractions
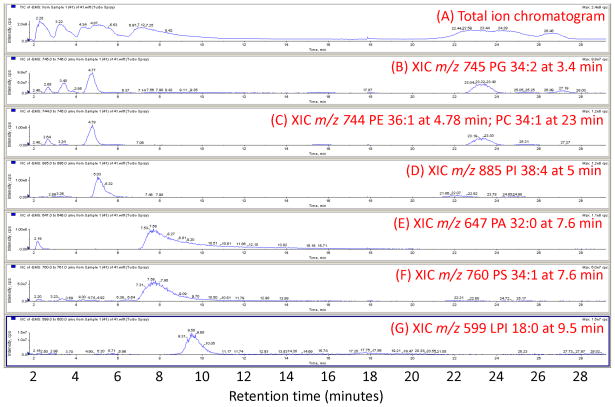
Isolation of glycerophospholipids from tissues and cell preparation is the first step in the analysis. Addition of acid to the extraction solvents (“modified” Bligh and Dyer extraction [11,12], using equal volumes of 0.1N HCl, CH3OH and CHCl3) ensures a better recovery of the anionic glycerophospholipids, such as phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidylglycerol (PG). Figure 1 shows a set of example extracted ion chromatograms (XIC), demonstrating the separation of glycerophospholipid classes by LC. In some cases the extraction proceeds from isolated subcellular fractions (membranes, nuclei), membrane domains (rafts) or other liquid systems (plasma) which are isolated in different buffer solutions. In that case, equal volumes of ice-cold 0.1N methanolic HCl and ice-cold CHCl3 are added to each fraction and extraction continues with mixing and centrifugation to separate the organic phase [20,21]. Acidification of the extraction system is important for the recovery of more GPL classes, but it should not be excessive as then the plasmalogen (vinyl ether-linked species) could be hydrolyzed. The technique outlined above is also applicable for extracting cyclic lysophosphatidic acid (cLPA) (see Figure 2).
Figure 1.
Extracted ion chromatogram from negative mode analysis of a cellular extract: (A) total ion chromatogram; (B) extracted ion chromatogram (XIC) m/z 745 corresponding to PG 34:2 at 3.4 min, and showing isotopic M+1 peak from PE 36:1 at 4.78 min and isotopic M+1 peak from PC 34:1 at 23 min; (C) XIC m/z 744 showing PE 36:1 at 4.78 min and PC 34:1 at 23 min; (D) XIC m/z 885 showing PI 38:4; (E) XIC m/z 647 showing PA 32:0 at 7.6 min; (F) XIC m/z 760 showing PS 34:1 at 7.6 min; (G) XIC m/z 599 showing lysophosphatidylinositol (LPI) 18:0 at 9.5 min.
Figure 2.
Cyclic LPA identified by MS/MS patterns indicating headgroup and acyl chain fragments.
2.2 Modes of MS operation
2.2.1 LC-MS/MS for species identification
Tandem mass spectrometry (MS/MS) is used for glycerophospholipid species structural characterization. By utilizing information dependent analysis (IDA) or a similar scheme along with class separation (LC-MS/MS), more than 1500 MS/MS spectra can be collected per injection in the m/z region characteristic for phospholipids (300–1200 m/z). An example is shown in Figure 2 for a cLPA species. Unambiguous species identification then is done by analysis of the retention time and fragmentation pattern and comparison to those acquired from chemically defined standards (Avanti Polar Lipids, Inc., Alabaster, AL) [11,12,22]. Characteristic headgroup fragments for glycerophospholipids are listed in Table 1. A large collection of MS/MS fragmentation spectra for lipids is available on the LIPID MAPS website ([23] and see http://lipidmaps.org).
Table 1.
Summary of MS/MS methods for phospholipid headgroup analysis
| Lipid Class | Precursor Ion | MS/MS Mode | Fragment |
|---|---|---|---|
| PA | [M−H]− | PIS, 153 amu | glycerol phosphate -H2O |
| PC | [M+H]+ | PIS, 184 amu | phosphocholine |
| [M+formate]− | NL, 60 amu | loss of methyl group and formate ion | |
| PE | [M−H]− | PIS, 196 amu | glycerol phospoethanolamine -H2O |
| PG | [M−H]− | PIS, 153 amu | glycerol phosphate -H2O |
| PIS, 227 amu | glycerol phospoglycerol -H2O | ||
| PI | [M−H]− | PIS, 153 amu | glycerol phosphate -H2O |
| PIS, 241 amu | cyclic inositol phosphate | ||
| PS | [M−H]− | PIS, 153 amu | glycerol phosphate -H2O |
| NL, 87 amu | serine |
2.2.2 Direct infusion MS
Direct infusion MS without prior chromatographic separation (shotgun lipidomics) is often used in conjunction with precursor ion and/or neutral loss scanning, but can be used alone to generate spectra without physical separation [9,10,24–26]. Lipid species identification is then confirmed using the resulting molecular fragments. This methodology is highly sensitive for identifying major phospholipids and can be performed on relatively small sample sizes, but is limited in terms of capturing absolute quantitation on species that are not among the most prominent. Still, reliable relative abundance information can be obtained by direct injection, and that may be satisfactory for some purposes [27–29]. For certain applications involving relatively specific classes, separations can be performed ahead of analysis (e.g., neutral lipids, polyphosphoinositides). In the case of polyphosphoinositides and other highly transient lipid species, a selective extraction can be utilized to minimize ion suppression by other phospholipids, especially phosphatidylcholine (PC), and extracts are analyzed by direct infusion [30]. Polyphosphoinositide quantitation can also be achieved by derivatization of the phosphate group and HPLC-MS analysis [31]. Nonpolar neutral lipids are extracted by a modification of the procedure described in [32]. The extraction proceeds in a mixture of isooctane/ethyl acetate (75:25) and the organic phase is collected. After solvent evaporation the lipid film is dissolved in hexane/isopropyl alcohol (4:5) and passed through a bed of Silicagel 60 to remove the polar phospholipids. The collected fraction is then submitted to solvent evaporation and the lipid film is dissolved in CH3OH:CHCl3(9:1) containing 10% 100 mM CH3COONa(aq) and subjected to direct infusion mass spectrometry essentially as described in [33]. In these instances when no isobaric influences are expected to arise between lipid classes, direct injection remains a viable method for quantitation.
2.2.3 Quantitative analysis based on multiple reaction monitoring MS
Multiple reaction monitoring (MRM) MS is a relatively new and widely used method for analysis of drug metabolites and other molecules, when individual standards are available for each one of the analytes. For that reason it is relevant for analysis of a limited number of analytes in a complex mixture (phospholipids from simple systems like yeast [34] or lipid classes with less diversity like sterols or eicosanoids [35,36].
When interested in trace level species as well as major lipids, full scan LC-MS is favorable over MRM for accurate quantitation of more species per injection. Nevertheless, many of the quantitative analysis procedures detailed below for LC-MS full scan data processing are applicable to MRM, although it should be noted the scan rate of the instrument could be a limiting factor in obtaining sufficient information on all the species of interest.
2.2.4 Full scan LC-MS for quantitation
The widespread use of ESI LC-MS has been an important advance for quantitative analysis of glycerophospholipids due to the ability to separate the various headgroup classes by their polarity. While direct infusion mass spectrometry can allow for the monitoring of many species and can employ scanning for particular fragments for a higher degree of certainty about identification, ion suppression and the lack of ability to effectively monitor for hundreds of distinct species from all the classes simultaneously has led to the use of ESI LC-MS for routine quantitation. Analysis by normal phase LC-MS [37–39] provides class separation which results in decreased ion suppression.
Usually, phosphatidylcholines (PCs) and sphingomyelins (SM) are less ionizable in negative mode than positive ion mode, while other glycerophospholipids (phosphatidic acid (PA), phosphatidylethanolamine (PE), PG, PI, PS) are readily ionizable in negative mode. By using suitable counter ions, PC and SM can be detected as formate, acetate, or chloride adducts. A method for analysis of PCs in negative mode has been developed that provides data on all glycerophospholipids within one MS run [12]. This has the effect of creating two relevant peaks for each PC (or SM) species, one which characteristically displays the loss of a methyl group and has mass [M−15] and another which is a formate adduct with mass [M+45] in negative mode. Methods for quantitation must be aware of this complication.
Reverse phase HPLC followed by MS analysis provides an approach which separates molecules by retention time based more on their fatty acyl chains than the polarity of the headgroups. As a result, the presence of molecules having different headgroups but having similar overall mass-to-charge values may interfere with the interpretation and quantitation of the results from complex mixtures, so this method provides difficulties for glycerophospholipids. However, reverse phase chromatography can be useful for analyzing other subclasses of lipids containing a smaller number of analytes of interest, such as sphingoid bases, sphingoid base 1-phosphates, and polyphosphoinositides [40–44].
3. Profiling measurements versus flux experiments
As lipidomics matures as a field, it is becoming increasingly clear that more complex questions seek not only a detailed accounting of the mass of species present in samples, but also information on the fluxes and dynamics of the lipids measured. In some cases, enzymatic reactions can be steered toward an essentially “dead-end product”. One such example is for the non-natural compound phosphatidylbutanol (PtdBuOH) which is produced as a result of butanol treatment in instances when the catalytic reaction of phospholipase D is being studied [12].
Such highly specific reaction monitoring mechanisms are often not available. More generally, an experiment may enrich cells with isotopically labeled fatty acids, glycerophospholipids, or small molecules in order to observe the incorporation of the labeled compounds into various lipid classes and pools. As discussed below in section 4.2.4 and elsewhere [19], care must be taken to deisotope the resulting spectra accounting for the fact that both natural and labeled lipids are likely to be present.
In addition to isotopically labeled lipids, alkyne derivatized fatty acids and glycerophospholipids can be utilized to monitor a vast array of signaling pathways. Alkyne-modified phospholipids can be identified and differentiated from native species in complex mixtures by formation of dicobalthexacarbonyl complexes. This reaction is specific for alkynes and is unaffected by other glycerophospholipid-related moieties. Enrichment of cells with alkyne-derivatized fatty acids or glycerophospholipids followed by solid-phase sequestration and release (see Figure 3) can greatly facilitate the unambiguous identification of lipid substrates and products. Prior results demonstrate that alkyne lipids can incorporate into cellular membranes and enter into the substrate pool for enzymes such as phospholipase D [45]. This ability to selectively purify reaction products, as well as potential binding partners, has potential for a wide range of lipidomic applications such as fatty acid incorporation and the tracking of lipase/kinase activity in a metabolomic context.
Figure 3.
Illustration of cobalt complexation method for the capture and release of alkyne labeled lipids. (top left) MS profile of cellular GPLs following alkyne labeling. (top right) Representation of a cellular extract containing natural and alkyne-derivatized lipids, with colored disks indicating various phospholipid headgroups. Complexation with cobalt and application of a specialized derivatized silica gel allows for capture of the alkyne lipids. The protocol for this process and for subsequent washes (to remove natural lipids) and oxidative release of the alkyne lipids is presented in [45]. (bottom left) Post-release MS GPL profile shows that the spectrum is dominated by the alkyne lipid species of interest, and shows how this technique can be used to selectively monitor various species and track the fluxes due to particular reactions. (bottom right) Illustration of phospholipids remaining after oxidative release from silica gel showing that only alkyne-derivatized lipids remain.
4. Issues encountered in mass spectrometry based data analysis and quantitation
Figure 4 shows a workflow diagram for processing of LC-MS full scans for quantitation starting with the decisions affecting how the samples will be treated and analyzed following through to ultimate statistical interpretation, which includes many facets common to the metabolomics fields in general. Some steps are more critical to the end result than others, and although a considerable literature exists for the common aspects of mass spectrometry data analysis such as background subtraction and the use of internal standards, the details of the issues faced along the processing still deserve to be examined in detail. Attention is drawn to certain key needs in the data analysis process that may be underappreciated, such (a) as the scriptability of the quantitative analysis data stream, (b) the need for isotopic correction, especially when the number of lipid analytes is large, and (c) the degree of flexibility and transparency of the background subtraction method.
Figure 4.
Steps for processing of MS data for glycerophospholipid quantitation.
4.1. Software packages for common data processing steps
Several software packages are available that can perform basic data processing tasks necessary for lipidomic analysis. In some cases, open source software has been designed so as not to exclude more general application to quantitative metabolomics (e.g., XCMS [46], MZmine [47]) when appropriate extractions and MS settings have been applied to the samples. Additionally, considerable work has been invested in technology to allow for reliable lipid identification alongside custom quantitation models in the form of dual parent/daughter ion scanning at programmed retention times (e.g., the commercial product MultiQuant: http://www.absciex.com). A brief review of some of the features of the aforementioned packages helps to illustrate some of the common strengths in the field of lipidomics that have emerged from several years of work on peak selection and automation, as well as motivations for further customization.
4.1.1 XCMS
XCMS is open-source and written in R, and therefore graphical support is substantial and an audit trail is built-in [46]. Support for using MS/MS libraries in conjunction with XCMS is provided; however, XCMS is not intended for quantification and has no support for applying standard curves. The method included for background subtraction is not very flexible or straightforward and relies on a smoothing function that bears assumptions about the shape of the peaks. Isotopic correction is also not built-in.
4.1.2 MZmine
MZmine is open-source and supports the use of MS/MS libraries alongside its quantitation modules, and has the advantage of being parallelizable for multiple computer processors [47]. It also has cross-platform support as it is written in Java. Isotopic correction is a built-in feature. However, while data analysis is automated, it is not scriptable. There are multiple methods included for background subtraction, but they either rely heavily on polynomial smoothing or a user-defined straight-line cutoff which is subjective.
4.1.3 MultiQuant (relies on Applied Biosystems Analyst software)
MultiQuant is commercial software and allows for the building of highly customizable standard curve scenarios. However, the program is only applicable to MRM data, not full scan LC-MS output. Although MultiQuant allows for an audit trail, it is not truly scriptable. The background subtraction algorithm included is not very flexible.
4.2 Further considerations concerning data handling
4.2.1 File processing and initial data reduction in temporal-spectral space
In order to best address the issues faced by quantitation of more than 100 glycerophospholipids from individual samples, a customized data workflow is employed. Raw MS files acquired from an Applied Biosystems 4000 Qtrap instrument in wiff format are first converted to readable format by using open source software from Institute for Systems Biology. Then, a custom Fortran program is used to extract the relevant information and construct averages of the spectra in 10-second binned intervals over 1 amu mass-to-charge (m/z) bins in a trough-to-trough manner by finding the most common trough point (e.g., xxx.65 or xxx.55) in a given file. This approach to m/z binning has several advantages. First, for relatively lower abundance species, peak shapes can have significant variability, which could result in higher variability of results when using the frequently espoused centroid method [48]. Second, the method is applicable whether the instrument is run at higher spectral resolution (to allow for distinguishing between masses of nearly isobaric species) or moderate resolution (sufficient to resolve the full width at half maximum). Third, this is also applicable to individual phospholipid classes if necessary. Lysolipids, for instance, have different typical exact mass peak locations within 1 amu bins than do other phospholipid classes. Because data can be summed on unit-Dalton bins without great loss of information for phospholipids, advanced filtering in the m/z domain is not generally required for quantitation at later stages. When analyzing glycerophospholipids, even when a search is conducted for species outside of a known list (e.g., those in the LIPID MAPS database ([23,49] and see http://lipidmaps.org), peaks encountered with high signal-to-noise (S/N) ratios still typically have masses that differ by integer multiples of Daltons within a small tolerance.
Quantitation results have been found to be only moderately sensitive to the choice of a temporal averaging interval, and the optimal window will be somewhat dependent on the scan rate of the instrument. A 5-second averaging window resulted in moderately more difficulty for the subsequent peak finding algorithm in assigning integration windows that are obviously consistent across species of a particular class. A 10-second moving window has the disadvantage of potential confusion in later interpretation, stemming from averaging in several seconds of spectrum that are not of interest for very fast eluting species. A moving average window also fails to resolve variability in the exact timing of retention time window boundaries across samples. The smoothing of peaks causes more difficulty in subsequent alignment than any functionality gained from analyzing the larger amount of data embodied in moving average spectrum than a windowed data set, and such data is also slower to process.
Starting from such 10-second, 1 amu bin averages, there are several more steps that must be undertaken before area-under-curve (AUC) calculations on a species by species basis can be carried out. Routines written in the S-plus/R data language (R is freely available at http://cran.r-project.org), can apply retention time alignment, differentiation of peaks background, and deisotoping. The logistics of these steps are described in more detail below. Software from the instrument manufacturer is insufficiently flexible to allow for all the functionality required to accomplish these three important tasks optimally. Open source software can deal with each of these aspects well, although not all packages can handle every task.
4.2.2 Alignment
Most all methods commonly used for retention time alignment are based in some fashion on correlation between spectra ([46–48], as well as several references found in [46]). In some published algorithms, the pairwise correlation of each sample s spectrum for a given m/z or a series of m/z s is used as a basis for alignment decision. For large sample sets, this is rather cumbersome. Since the retention times in lipidomic analyses are well constrained within individual classes by the observed retention times of the odd carbon standards, species of interest will be bracketed by these standards in time. This information can be used to effectively time-shift spectra within the time-m/z domain of each class without the need for pairwise spectral computations. The required alignment shifts can then be chosen to maximize the correlation of time-lag shifted images of each spectrum against one arbitrarily chosen sample from that session of MS analysis. However, the expense of computing the correlations can often be avoided altogether by using the temporal maxima of the internal standards peaks.
4.2.3 Background subtraction
Apart from reducing variation from sample to sample integrations of the same peaks, accurate background subtraction also suppresses complications faced by uncertainty about the intercept of the standard curve. The approach to background subtraction is thus fairly important, given the impact it has on the applicability of standard curves developed in a solvent background for subsequent use in analyzing cellular and non-cellular based samples. For this reason, it is useful to set high standards for the background subtraction scheme, by requiring that the intercept terms of standard curve linear regressions have no statistical difference from zero. In this way, intercepts are essentially forced through the origin after background subtraction, and this will be case for both the integration of the standard and of the species of interest. This removes reliance on any latent subtraction of noise through the intercept term.
Commonly used methods for background subtraction for general metabolomic LC-MS analysis often approach the problem from the standpoint of simultaneous peak identification and noise reduction (e.g., the CODA algorithm [50], the MEND algorithm [51], and various Gaussian-second derivative-based methods of peak picking [45,52]) and typically employ filtering or smoothing functions. However, applying such filtering implies a model of the noise and/or the peak shapes present in the original data, and this requires assumptions that may be unnecessary (e.g., the existence of vacant space in the spectrum, or an idealized shape for peaks). Especially considering the large variation in how different classes of phospholipids interact with a silica column to produce retention time tails (e.g., for PA and PS, see Figure 1), modeling the peak shapes may not be universally reliable at all. Instead, the problem can be reduced to simply finding the appropriate retention time window boundaries for a given m/z.
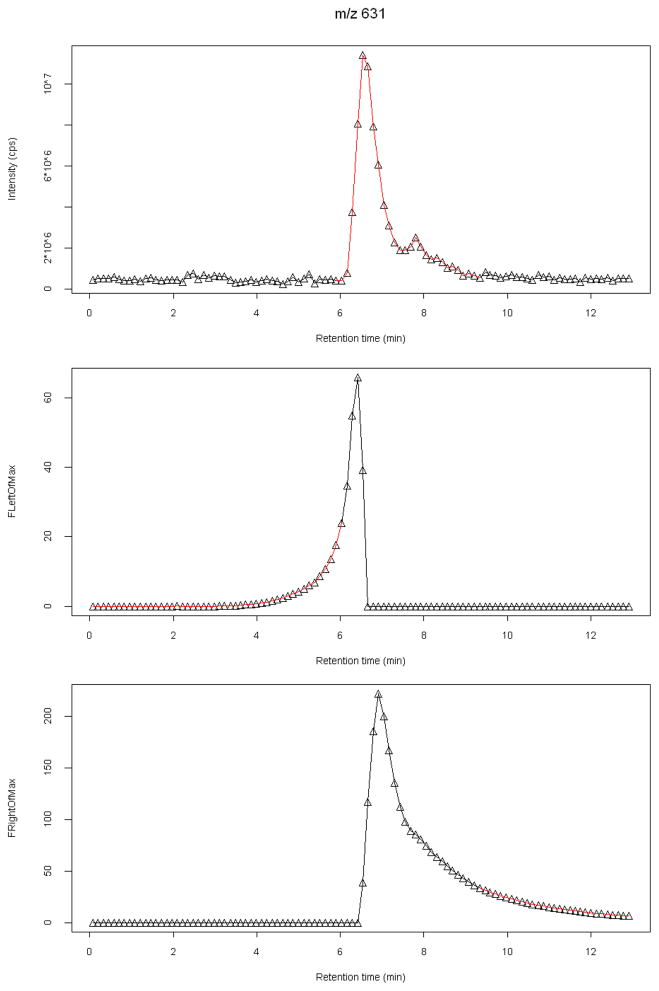
A Williams-Kloot test [53] is used to define the edges of the retention time windows. A series of tests are conducted by expanding the putative integration window in each direction of time separately from the central maximum. With each expansion, the peak is compared to the background (see example in Figure 5, middle and bottom panels), and the differences between the results obtained from successively expanded windows are evaluated by the Williams-Kloot statistic. Once a maximum in time has been identified (e.g., by filtration to be above S/N = 3 with respect to the time mean, for instance), points are added first in the positive time direction from this maximum and successive tests are performed against the entire time series with the categories of in-the-peak and not-in-the-peak as predictors. Once the usefulness of adding one more point to the peak has become indistinguishable from the addition of a background point as evaluated through the Williams-Kloot test, the window location has been defined on that side. The same is done in the time direction to the left of the maximum. The integral under the polygon including these points and bounded by a diagonal line at the bottom of the peak is used as the ion count corresponding to that feature.
Figure 5.
Demonstration of integration window selection procedure. (top) Ion counts versus retention time and resulting integration window (in red) for a PA 31:1 standard (m/z 631). (middle) Values of F-tests are shown for a region including the peak maximum and spanning successively to the right, tested against the remaining points of the extracted ion chromatogram. The area plotted in red begins where the Williams-Kloot test indicates a diminishing return and low contrast from the background (p<0.05 shown here). (bottom) Same as in middle panel, but for successive F-tests including points away from the peak maximum to the left (lower retention times), to determine the left bound of the peak.
Because the method relies on the contrast of neighboring points contributions to their assignment to being either part of or not part of a peak, no averaging of the background is required. This avoids having to model any drift in the background level that occurs from the beginning to the end of the peak of interest. Similarly, there are many m/z that contain either lipids from other classes or isotopes thereof at very different retention times (e.g., m/z 790 in negative mode as [M−1] peaks may contain peaks from both PE 40:6 and PS 36:0), and no special techniques are needed to avoid identifying these peaks as part of the background.
4.2.4 Isotopic correction
The deisotoping step, in particular, is critical given the diversity of species analyzed. We have identified over 1000 glycerophospholipids in some cellular systems we routinely analyze, and approximately 2/3 of the m/z bins in the range of 490–960 m/z contain peaks at one time or other during a LC-MS experiment. Figure 1 (panel B) demonstrates how this can manifest itself. The M, M+1, M+2, and M+3 peaks are utilized, and based on the chemical composition of lipid species previously identified by MS/MS in the same cells or in the LIPID MAPS database [23,49], the influence of isotopic peaks due to naturally occurring 13C (and 15N, 18O, etc.) is subtracted out. The M+1 and M+3 peaks are of particular importance in situations where even- and odd-numbered m/z entities can exist at similar retention times.
In situations where stable isotope labeling is used in flux studies, a similar strategy can be applied. In that case, both an isotope profile for the parent unlabeled species and the labeled species need to be created. The chromatogram then must be deconvoluted by regressing out influences of all the additional isotopes (e.g., see [54]). If only the M and M+2 isotopes are used for this purpose to simplify matters, the problem reduces to a system of two equations for two unknowns (the ion counts for the parent species at M and for the stable isotope at M from another m/z). In the event that the spectrum is considerably crowded by isotopes from various sources, a method that is likelihood-based can provide a strategy requiring few strong assumptions during deconvolution [55]. An area deserving further attention is the generation of better statistics about the uncertainty of a particular deisotoping result, which could be facilitated through a regression or likelihood approach.
4.2.5 Use of odd-carbon standards
At the heart of a usable quantitation methodology for a wide scope of phospholipids from cells or other animal preparations (tissue, plasma, body fluids) should be the use of multiple odd carbon lipid standards per class with varying degrees of unsaturation and acyl chain length for diacyl species. Spanning the acyl chain length-unsaturation range reasonably well for each class of interest is of utmost importance [56–57]. Response curves slopes for some glycerophospholipid classes (e.g., PS) are relatively invariant to these factors, but these are an exception. We typically use four (25:0, 31:1, 37:4, 43:6) such standards per class, although for some classes of glycerophospholipids, the longest or shortest standard in each group may often be less needed to cover the variety of species encountered. For lysolipids, two odd carbon standards per class are typically sufficient (13:0 and 17:1). The average of the peak integrations from the two odd standards which bound the carbon chain length of even-carbon species of interest are used for normalization. The rationale is that there are significant differences in ionization efficiency across species, even within a given class, which should be addressed by as direct of comparison to chemically similar standard(s) as possible. An approach using all four odd carbon standards in an arithmetic average showed somewhat poorer results, as did tests with only a single standard used for normalization. There was usually no statistical difference between the use of a weighted average of two bounding odd carbon standards based on carbon chain length, unsaturation level, or m/z compared to the arithmetic average. Using the above strategy, standard curves have been developed in a solvent background that have been validated by proof-of-principle spiking experiments in cells to verify that recoveries in the presence of non-solvent backgrounds are high.
For phospholipid quantitation from bacteria or lower plant and animal materials containing odd carbon fatty acid lipids, other suitable standards should be chosen that cover the span of carbon lengths and degree of unsaturation of the native lipids but are not present in the extractions. These can be selected from the large variety of synthetic standards currently available on the market.
4.2.6 Standard curve slope extrapolation to species not individually calibrated
Far more lipids of interest are monitored than ones for which standards are available. In order to model the instrument response for species with internal standards, it is attractive to try to generalize the results of standard curve generation using a multiple regression approach within each headgroup class of glycerophospholipids (against carbon chain length, number of double bonds, and presence/absence of ether linked acyl chains when applicable) similarly to the method of [33] for diacylglycerol (DAG) species. While R-squared statistics for the response curve slopes of individual species of interest are typically >0.98, the variation of response curve slopes is neither linear nor smooth for several lipid classes (e.g., PA, PE), particularly with regard to the influence of double bonds. The relationship can even be nonmonotonic. Only for PCs, where over 20 even carbon standards were available (note also that 28 DAG standards were used in [33]), was multiple regression able to predict slopes with reasonable fidelity (overall R-squared = 0.918). For this reason, a simplified approach using a variation on a nearest neighbor method is applied in which the two most closely related even carbon standard species, are used in an arithmetic average of standard curve slopes to generate interpolated/extrapolated slopes for all species encountered in quantitation.
4.2.7 Standard curves for species that generate peaks at multiple m/z bins
One complication that arises from the analysis of PC in negative ion mode is that there are dual peaks at m/z s corresponding to [M−15]− (loss of a methyl group) and [M+45]− (formate adduct) for each PC species. Care must be exercised in integrating spectra for the PC region of chromatogram. Peaks from formate adducts with [M+45] will elute later than [M−15] peaks from longer chain PCs, and ether-linked PCs will similarly have earlier elutions than their diacyl counterparts. The degree to which PC peaks appear more dominantly as formate adducts versus demethylated counterparts can be instrument-specific. A 3:1 ratio of intensities of the [M+45]:[M−15] peaks or vice versa may easily be possible. Although it is tempting to create a standard curve slope based on using information from both ions, the reliability of the resulting analysis has been found to be lower than if only one ion type is used for each quantitated species. Furthermore, longer chain species appear to have higher regression R-squared for formate adduct standard curves, while PC species with m/z < 770 amu typically have higher reliability based on [M−15] standard curves.
4.2.8 Audit capabilities through use of scripting
Reproducible data sets are essential. For this reason, it is very useful to have all user-defined settings for the calculations embedded in the command structure itself, allowing tracking of how each integration is carried out. The ease of creating an audit trail in a scripting language such as R or Matlab (http://www.mathworks.com) make these environments highly suitable for this task. By encapsulating the necessary series of commands for the data analysis in the form of a human-readable script, both the process of automation and the tracking of how the quantitation was performed are facilitated. Generally, quantitation from about 150 parent peaks from one sample are obtainable, representing over 500 lipids due to the presence of isobaric species. Hence, several thousand AUC integrations are processed per typical MS set containing 12–30 samples. Given this data load, it is extremely useful to be able to monitor any customized changes to each integration that may be necessary, such as retention time windows modifications, amid these relatively large data streams.
5. Post-processing data analysis
Subsequent to quantitation, data are normalized on a per-sample basis by weight, cell count, protein, or DNA, and this greatly controls for intersample variability. Several techniques are used to maintain a high standard of evidence with regards to statistical analysis of the resulting normalized data. First, given a large library of prior MS lipidomic spectra, a priori calculations based on expected coefficients of variation (mean/SD) demonstrate that the use of a minimum of three independent repeats, each performed in triplicate (“3×3”), provides substantial statistical power (generally β > 0.75 with α = 0.05) for cellular lipidomics experiments. In situations where singlet samples per experiment are used (e.g., when assaying animal tissue), at least n=8 animals per condition are required for similar power.
When profiling sufficiently large numbers of lipids simultaneously, methods to limit the false discovery rate are applied using family-wise error rate control (e.g., Bonferroni) or Benjamini/Hochberg-like false-discovery rate (FDR) limiting techniques [58]. Hypothesis testing is generally carried out via ANOVA or mixed-effect variants thereof to help address interaction terms between conditions that may vary between MS runs [59]. New methods for high dimensional hypothesis testing are becoming available to address the dependence between hypothesis tests across interdependent data (such as lipidomics output) simultaneously with dependence that stems from exogenous factors such as the date of the MS run [60].
Clustering of lipid changes across diverse species is often visualized by various methods, but principal components analysis (PCA) and hierarchical similarity trees are the most common tools used [17,18,29,61–64]. One point worth noting about typical PCA results is that they are usually variance based, so when absolute quantitation values are used as input, lipid species present in small proportions are not emphasized. PCA that is based on the ratios of species within lipid classes, or using decomposition of the cross-correlation matrix instead of the covariance matrix can be useful. PCA can also be used as a tool for outlier detection [18].
The LIPID MAPS suite of bioinformatic tools includes MS/MS databases spanning thousands of chemical species [23], provides m/z calculators to predict where lipids with particular acyl compositions should be found [49], and gene expression databases curated against lipid pathway diagrams which can be used in conjunction with pathway display/analysis software [16]. These resources are indispensible for routine verification of m/z identifications, to compare for consistency of MS/MS confirmations against known standards, to survey for potential novel lipids against a large database, and to investigate relationships between classes of lipids and previously documented gene regulatory responses to conditions (ligands, diet, etc.) in a targeted manner according to known metabolic pathways [16,19]. Other Clustering methods as described above can also be used to try to seek relationship between lipidomic data and other large array measurements (e.g., metabolomic or gene expression) together in an uncurated fashion. However, the balance between the number of lipid species used in the analysis and the number of other measurements can result in problematic interpretation if the lipidomic data are far outnumbered, even for results that are strictly correlation-based.
6. Conclusions
Modern methods for quantitation of lipids from complex mixtures demand several key procedures to produce high quality results. As the ability to extract more and more information from a LC-MS single spectrum has advanced into the hundreds of glycerophospholipid species, so have the data analysis challenges grown. We have identified several chief aspects among those facing analysts in the field who conduct directed quantitation, such as the ability to script, control, and provide transparency for retention time integration windows, the ability to create extrapolated standard curve slopes for species that do not have standards available, and the increasing need for strong methods for deisotoping. All of these issues will continue to grow in prominence as mass spectrometry methods are pushed to produce more reliable, higher dimensional results from complex mixtures. Absolute quantitation for lipidomics is becoming more the norm than relative quantitation as the needs for kinetic modeling and flux studies increase (in which stable isotope labeling may present significant challenges to isotopic deconvolution). All these highlighted aspects at the level of quantitation are in addition to the bioinformatic demands faced for statistical analysis of increasingly higher dimensional lipidomic output and robust hypothesis testing methodologies. We hope this review provides some context for assessing and attacking these challenges for lipidomic data analysis.
Highlights.
We review specifics of glycerophospholipid (GPL) analysis by MS and data handling.
GPL quantitation is challenging given species diversity and range of ionizability.
Lipidomics is moving beyond profiling to include flux-based measurements.
Acknowledgments
This work was supported by funding of the National Institutes of Health Large-scale collaborative initiative LIPID MAPS (U54 GM069338), NIAID HHSN272200800058C, NIEHS 1PO1ES013125, and McDonnell Foundation. We acknowledge Avanti Polar Lipids for the synthesis of high quality lipid standards, without which this work would not be possible.
Abbreviations
- MS
mass spectrometry
- LC-MS
liquid chromatography-mass spectrometry
- ESI-MS
electrospray ionization mass spectrometry
- GPL
glycerophospholipids
- AUC
area under curve
- m/z
mass to charge ratio
- XIC
extracted ion chromatogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forrester J, Milne S, Ivanova P, Brown HA. Computational lipidomics: A multiplexed analysis of dynamic changes in membrane lipid composition during signal transduction. Mol Pharm. 2004;65:813–821. doi: 10.1124/mol.65.4.813. [DOI] [PubMed] [Google Scholar]
- 2.Brown HA, Murphy RC. Working towards an exegesis for lipids in biology. Nature Chem Biol. 2009;5:602–606. doi: 10.1038/nchembio0909-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenk M. Lipidomics: New tools and applications. Cell. 2010;143:888–895. doi: 10.1016/j.cell.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Murphy RC, Fiedler J, Hevko J. Analysis of nonvolatile lipids by mass spectrometry. Chem Rev. 2001;101:479–526. doi: 10.1021/cr9900883. [DOI] [PubMed] [Google Scholar]
- 5.Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 6.Wenk M. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 7.Van Meer G. Cellular lipidomics. EMBO J. 2005;24:3159–3165. doi: 10.1038/sj.emboj.7600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson AD. Lipidomics - a global approach to lipid analysis in biological systems. J Lipid Res. 2006;47:2101–2111. doi: 10.1194/jlr.R600022-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Han X, Gross RW. Shotgun lipidomics: multi-dimensional mass spectrometric analysis of cellular lipidomes. Expert Rev Proteomics. 2005;2:253–264. doi: 10.1586/14789450.2.2.253. [DOI] [PubMed] [Google Scholar]
- 10.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of the cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 11.Milne S, Ivanova P, Forrester J, Brown HA. Lipidomics: An analysis of cellular lipids by ESI-MS. Methods. 2006;39:92–103. doi: 10.1016/j.ymeth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Ivanova P, Milne S, Byrne M, Xiang Y, Brown HA. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Meth Enyzmol. 2007;432:21–57. doi: 10.1016/S0076-6879(07)32002-8. [DOI] [PubMed] [Google Scholar]
- 13.Han X. Neurolipidomics: challenges and developments. Front Biosci. 2007;12:2601–2615. doi: 10.2741/2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanova P, Milne S, Myers D, Brown HA. Lipidomics: a mass spectrometry based, systems-level analysis of cellular lipids. Curr Opinion in Chem Bio. 2009;13:526–531. doi: 10.1016/j.cbpa.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanksby S, Mitchell T. Advances in Mass Spectrometry for Lipidomics. Annu Rev Anal Chem. 2010;3:433–65. doi: 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- 16.Fahy E, Sud M, Cotter D, Maer A, Zhou Y, Byrnes R, Subramaniam S. Bioinformatics for lipidomics. Meth Enzymol. 2007;432:245–71. doi: 10.1016/S0076-6879(07)32011-9. [DOI] [PubMed] [Google Scholar]
- 17.Orešič M. Bioinformatics and computational approaches applicable to lipidomics. Eur J Lipid Sci Technol. 2009;111:99–106. [Google Scholar]
- 18.Theodoridis G, Gika H, Wilson I. Mass Spectrometry-based holistic analytical approaches for metabolite profiling in systems biology studies. Mass Spec Rev. 2011 doi: 10.1002/mas.20306. [DOI] [PubMed] [Google Scholar]
- 19.Wheelock C, Goto S, Yetukuri L, Alexandri FD, Klukas C, Schreiber F, Orešič M. Bioinformatics strategies for the analysis of lipids. Methods Mol Biol. 2009;580:339–368. doi: 10.1007/978-1-60761-325-1_19. [DOI] [PubMed] [Google Scholar]
- 20.Andreyev A, Fahy E, Guan Z, Kelly S, Li X, McDonald J, Milne S, Myers D, Park H, Ryan A, Thompson B, Wang E, Zhao Y, Brown HA, Merrill A, Raetz CRH, Russel D, Subramaniam S, Dennis E. Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res. 2010;51:2785–2797. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quehenberger O, Armando A, Brown HA, Milne S, Myers D, Merrill A, Bandyopadhyay S, Jones K, Kelly S, Shaner R, Sullards MC, Wang E, Murphy RC, Barkley R, Leiker T, Raetz C, Guan Z, Laird G, Six D, Russell D, McDonald J, Subramaniam S, Fahy E, Dennis E. Lipidomics reveals remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanova PT, Milne SB, Brown HA. Identification of atypical ether-linked glycerophospholipid species in macrophages by mass spectrometry. J Lipid Res. 2010;51:1581–1590. doi: 10.1194/jlr.D003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sud M, Fahy E, Cotter D, Brown A, Dennis E, Glass C, Merrill A, Murphy R, Raetz C, Russell D, Subramaniam S. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007;35:D527–532. doi: 10.1093/nar/gkl838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han X, Yang J, Cheng H, Ye H, Gross R. Toward fingerprinting cellular lipidomes directly from biological samples by two-dimensional electrospray ionization mass spectrometry. Anal Biochem. 2004;330:317–331. doi: 10.1016/j.ab.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Han X, Yang K, Gross R. Microfluidics-based electrospray ionization enhances the intrasource separation of lipid classes and extends identification of individual molecular species through multidimensional mass spectrometry: development of an automated high-throughput platform for shotgun lipidomics. Rapid Commun Mass Spectrom. 2008;22:2115–2124. doi: 10.1002/rcm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahlman M, Ejsing C, Tarasov K, Perman J, Boren J, Ekroos K. High-throughput shotgun lipidomics by quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2664–2672. doi: 10.1016/j.jchromb.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Milne S, Ivanova P, Armstrong M, Myers D, Lubarda J, Shulga Y, Topham M, Brown HA, Epand R. Dramatic differences in the roles in lipid metabolism of two isoforms of diacylglycerol kinase. Biochemistry. 2008;47:9372–9379. doi: 10.1021/bi800492c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott S, Selvy P, Buck J, Cho H, Criswell T, Thomas A, Armstrong M, Arteaga C, Lindsley C, Brown HA. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nature Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rappley I, Myers D, Milne S, Ivanova P, LaVoie M, Brown HA, Selkoe D. Lipidomic profiling in mouse brain reveals differences between ages and genders, with smaller changes associated with a-synuclein genotype. J Neurochem. 2009;111:15–25. doi: 10.1111/j.1471-4159.2009.06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milne S, Ivanova P, DeCamp D, Hsueh R, Brown HA. A targeted mass spectrometric analysis of phosphatidylinositol phosphate species. J Lipid Res. 2005;46:1796–1802. doi: 10.1194/jlr.D500010-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Clark J, Anderson K, Juvin V, Smith T, Karpe F, Wakelam M, Stephens L, Hawkins P. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nature Methods. 2011;8:267–272. doi: 10.1038/nmeth.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchins P, Barkley R, Murphy RC. Separation of cellular nonpolar neutral lipids by normal-phase chromatography and analysis by electrospray ionization mass spectrometry. J Lipid Res. 2008;49:804–813. doi: 10.1194/jlr.M700521-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callender H, Forrester J, Ivanova P, Preininger A, Milne S, Brown HA. Quantification of diacylglycerol species from cellular extracts by electrospray ionization mass spectrometry using a linear regression algorithm. Anal Chem. 2007;79:263–272. doi: 10.1021/ac061083q. [DOI] [PubMed] [Google Scholar]
- 34.Shui G, Guan XL, Gopalakrishnan P, Xue Y, Goh JSY, Yang H, Wenk MR. Characterization of Substrate Preference for Slc1p and Cst26p in Saccharomyces cerevisiae Using Lipidomic Approaches and an LPAAT Activity Assay. PLoS ONE. 2010;5(8):e11956. doi: 10.1371/journal.pone.0011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald JG, Thompson BM, McCrum EC, Russell DW. Extraction and analysis of sterols in biological matrices by high performance liquid chromatography electrospray ionization mass spectrometry. Meth Enzymol. 2007;432:145–170. doi: 10.1016/S0076-6879(07)32006-5. [DOI] [PubMed] [Google Scholar]
- 36.Deems R, Buczynski MW, Bowers-Gentry R, Harkewicz R, Dennis EA. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. Meth Enzymol. 2007;432:60–82. doi: 10.1016/S0076-6879(07)32003-X. [DOI] [PubMed] [Google Scholar]
- 37.Taguchi R, Hayakawa J, Takeuchi Y, Ishida M. Two-dimensional analysis of phospholipids by capillary liquid chromatography/electrospray ionization mass spectrometry. J Mass Spectrom. 2000;35:953–966. doi: 10.1002/1096-9888(200008)35:8<953::AID-JMS23>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Houjou T, Yamatani K, Imagawa M, Shimizu T, Taguchi R. A shotgun tandem mass spectrometric analysis of phospholipids with normal-phase and/or reverse-phase liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:654–666. doi: 10.1002/rcm.1836. [DOI] [PubMed] [Google Scholar]
- 39.Hermansson M, Uphoff A, Kakela R, Somerharju P. Automated quantitative analysis of complex lipidomes by liquid chromatography/mass spectrometry. Anal Chem. 2005;77:2166–2175. doi: 10.1021/ac048489s. [DOI] [PubMed] [Google Scholar]
- 40.Ogiso H, Suzuki T, Taguchi R. Development of a reverse-phase liquid chromatography electrospray ionization mass spectrometry method for lipidomics, improving detection of phosphatidic acid and phosphatidylserine. Anal Biochem. 2008;375:124–131. doi: 10.1016/j.ab.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 41.Retra K, Bleijerveld OB, van Gestel RA, Tielens AGM, van Hellemond JJ, Brouwers JF. A simple and universal method for the separation and identification of phospholipid molecular species. Rapid Commun Mass Spectrom. 2008;22:1853–1862. doi: 10.1002/rcm.3562. [DOI] [PubMed] [Google Scholar]
- 42.Taguchi R, Houjou T, Nakanishi H, Yamazaki T, Ishida M, Imagawa M, Shimizu T. Focused lipidomics by tandem mass spectrometry. J Chromatography B. 2005;823:26–36. doi: 10.1016/j.jchromb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Ogiso H, Taguchi R. Reverse-phase LC/MS method for polyphosphoinositides analysis: changes in molecular species level during epidermal growth factor activation in A431 cells. Anal Chem. 2008;80:9226–9232. doi: 10.1021/ac801451p. [DOI] [PubMed] [Google Scholar]
- 44.Sullards MC, Allegood J, Kelly S, Wang E, Haynes C, Park H, Chen Y, Merrill A. Structure-Specific, Quantitative Methods for Analysis of Sphingolipids by Liquid Chromatography–Tandem Mass Spectrometry: “Inside-Out” Sphingolipidomics. Meth Enzymol. 2007;432:83–115. doi: 10.1016/S0076-6879(07)32004-1. [DOI] [PubMed] [Google Scholar]
- 45.Milne S, Tallman K, Serwa R, Rouzer C, Armstrong M, Marnett L, Lukehart C, Porter N, Brown HA. Capture and release of alkyne-derivatized glycerophospholipids using cobalt chemistry. Nature Chem Biol. 2010;6:205–207. doi: 10.1038/nchembio.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith C, Want E, Maille GO, Abagyan R, Siudak G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 47.Pluskal T, Castillo S, Villar-Briones A, Orešič M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katajamaa M, Oresic M. Data processing for mass spectrometry-based metabolomics. J Chromatography A. 2007;1158:318–328. doi: 10.1016/j.chroma.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35:W606–W612. doi: 10.1093/nar/gkm324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Windig W, Phalp JM, Payne A. A Noise and Background Reduction Method for Component Detection in Liquid Chromatography/Mass Spectrometry. Anal Chem. 1996;68:3602–3606. [Google Scholar]
- 51.Andreev V, Rejtar T, Chen HS, Moskovets E, Ivanov A, Karger B. A Universal Denoising and Peak Picking Algorithm for LC-MS Based on Matched Filtration in the Chromatographic Time Domain. Anal Chem. 2003;75:6314–6326. doi: 10.1021/ac0301806. [DOI] [PubMed] [Google Scholar]
- 52.Fredrickson M, Petersson P, Axelsson BO, Bylund D. An automatic peak finding method for LCMS data using Gaussian second derivative filtering. J Sep Sci. 2009;32:3906–3918. doi: 10.1002/jssc.200900395. [DOI] [PubMed] [Google Scholar]
- 53.Williams E, Kloot N. Interpolation in a series of correlated observations. Aust J Appl Sci. 1953;4:1–17. [Google Scholar]
- 54.Haimi P, Uphoff A, Hermansson M, Somerharju P. Software tools for analysis of mass spectrometric lipidome data. Anal Chem. 2006;78:8324–31. doi: 10.1021/ac061390w. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Haskins W. ICPD-A New Peak Detection Algorithm for LC/MS. BMC Genomics. 2010;11:S8. doi: 10.1186/1471-2164-11-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG. The HIV lipidome: a raft with and unusual composition. Proc Natl Acad Sci USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koivusalo M, Haimi P, Heikinheimo L, Kostiainen R, Somerharju P. Quantitative determination of phospholipid compositions by ESI-MS: effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J Lipid Res. 2001;42:663–672. [PubMed] [Google Scholar]
- 58.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journ Roy Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 59.Mercier C, Truntzer C, Pecqueur D, Gimeno J, Belz G, Roy P. Mixed-model of ANOVA for measurement reproducibility in proteomics. J Proteomics. 2009;72:974–981. doi: 10.1016/j.jprot.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Leek JT, Storey JD. A general framework for multiple testing dependence. Proc Natl Acad Sci USA. 2008;105:18718–18723. doi: 10.1073/pnas.0808709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yetukuri L, Katajamaa M, Medina-Gomez G, Seppanen-Laakso T, Vidal-Puig A, Oresic M. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst Biol. 2007;1:article 12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niemela PS, Castillo S, Sysi-Aho M, Oresic M. Bioinformatics and computational methods for lipidomics. J Chromatography B. 2009;877:2847–2854. doi: 10.1016/j.jchromb.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 63.Graessler J, Schwudke D, Schwarz PEH, Herzog R, Shevchenko A, Bornstein S. Top-Down Lipidomics Reveals Ether Lipid Deficiency in Blood Plasma of Hypertensive Patients. PloS ONE. 2009;4:e6261. doi: 10.1371/journal.pone.0006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sampaio J, Gerl M, Klose C, Ejsing C, Beug H, Simons K, Shevchenko A. Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci USA. 2011;108:1903–1907. doi: 10.1073/pnas.1019267108. [DOI] [PMC free article] [PubMed] [Google Scholar]