Abstract
Non-invasive assessment of plaque volume and composition is important for risk stratification and long-term studies of plaque stabilisation. Our aim was to evaluate dual-source computed tomography (DSCT) and colour-coded analysis in the quantification and classification of coronary atheroma. DSCT and virtual histology intravascular ultrasound (IVUS-VH) were prospectively performed in 14 patients. 22 lesions were compared in terms of plaque volume, maximal per cent vessel stenosis and percentages of fatty, fibrous or calcified components. Plaque characterisation was performed with software that automatically segments luminal or outer vessel boundaries and uses CT attenuation for a colour-coded plaque analysis. Good correlation was found for per cent vessel stenosis in DSCT (53 ± 13%) and IVUS (51 ± 14%; r2 = 0.70). Mean volumes for entire plaque and non-calcified atheroma were 68.5 ± 33 mm3 and 56.7 ± 30 mm3, respectively, in DSCT and 60.8 ± 29 mm3 and 55.8 ± 26 mm3, respectively, in IVUS. Mean percentages of fatty, fibrous or calcified components were 28.2 ± 6%, 53.2 ± 9% and 18.7 ± 13%, respectively, in DSCT and 29.9 ± 5%, 55.3 ± 12% and 14.4 ± 9%, respectively, in IVUS-VH. Significant overestimation was present for the entire plaque and the volume of calcified plaque (p = 0.03; p = 0.0004). Although good correlation with IVUS was obtained for the entire plaque (r2 = 0.76) and non-calcified plaque volume (r2 = 0.84), correlation proved very poor and insignificant for percentage plaque composition. Interclass correlation coefficients for non-calcified plaque volume and percentages of fatty, fibrous or calcified components were 0.99, 0.99, 0.95 and 0.98, respectively, and intraclass coefficients were 0.98, 0.93, 0.98 and 0.99, respectively. We found that using Hounsfield unit-based analysis, DSCT allows for accurate quantification of non-calcified plaque. Although percentage plaque composition proves highly reproducible, it is not correlated with IVUS-VH.
Rupture of unstable coronary plaque is known to be the primary cause of acute coronary syndrome. The vulnerability of atherosclerotic plaque has thereby been linked to a distinct pathological composition, notably a large lipid core covered by a thin fibrous cap [1–3]. Invasive coronary angiography has traditionally been employed to assess the extent of coronary artery disease and has been proven to be the clinical standard for quantification of stenosis. However, owing to the phenomenon of coronary artery remodelling, a significant amount of plaque may be found in non-stenotic coronary segments [4–6]. Intravascular ultrasound (IVUS) is now accepted as the standard of reference for detection of non-stenotic atheroma and provides additional information on plaque composition [7–9]. Recently, spectral analysis of IVUS radiofrequency or so-called virtual histology IVUS (IVUS-VH) has been introduced and enables differentiation of the four primary plaque components (necrotic core, fatty fibrous, fibrous, and calcified) with good accuracy [10–12].
However, the invasive nature of IVUS, its restriction to proximal and medial segments, very high costs and a considerable rate of complications have prevented widespread use of the procedure and fuelled the quest for non-invasive methods, including multislice computed tomography (MSCT). In fact, the potential of CT to quantify and classify both luminal and extraluminal plaque has been demonstrated in a variety of previous studies [13–19]. However, until now, the ability of CT to accurately characterise non-calcified plaque has been restricted by limited image quality and the need to visually or manually determine the boundaries of plaque components.
Only recently, a new dual-source CT system (DSCT) equipped with two tubes and corresponding detectors in a 90° geometry has been designed that provides temporal resolution of approximately one-quarter of its 330 ms gantry rotation time [20]. Likewise, dedicated software is now available that allows for more sophisticated assessment of non-calcified plaque through definition of a set of Hounsfield unit (HU) ranges and subsequent colour mapping. Using the same software, outer wall and luminal boundaries may now be automatically segmented. Such an approach holds the promise of providing a more accurate and reproducible characterisation of non-calcified plaque. To our knowledge, the benefit of automated CT-based virtual histology has not been evaluated in comparison with spectrum analysis of IVUS radiofrequency.
Therefore, the primary aim of our study was to assess DSCT and automated colour-coded analysis in the quantification and classification of non-calcified plaque, using IVUS-VH as the standard of reference.
Methods and materials
Study population
Our study protocol was approved by the local ethics committee, with radiation dose information having been supplied to that committee. All patients provided informed consent for participation in the study after having been informed of radiation dose information.
From September 2006 to June 2007 we studied 120 patients who were scheduled to undergo conventional coronary angiography because of suspected coronary artery disease (CAD) or suspected progression of known CAD. All DSCT studies were performed the day prior to conventional angiography. Exclusion criteria were renal insufficiency (serum creatinine >1.5 mg dl−1), hyperthyroidism (basal thyroid-stimulating hormone (TSH) <0.03 µl l−1 in combination with elevated thyroid hormone levels in the peripheral blood), known allergic reaction against iodinated contrast media or inability to follow breath-hold commands.
In 14 of these patients (11 men and 3 women; 66 ± 7 years), IVUS was performed in at least one vessel either to evaluate the extent of in-stent restenosis (n = 11) or to assess for plaque volume and composition in vessels without angiographic evidence of significant stenosis (n = 3). In this 14-patient collective, 22 atherosclerotic lesions were selected for further investigation. Plaques within stents or without unequivocal fiduciary markers were excluded from the analysis.
Grey-scale and IVUS-VH imaging protocol and data analysis
All IVUS studies were evaluated by a single, experienced observer blinded to the results of DSCT. IVUS-VH data were acquired with a dedicated IVUS-VH console (Volcano Corporation, Rancho Cordova, CA). A phased-array, 20-MHz, 3.2-Fr IVUS catheter (Eagle Eye, Volcano Corporation) was placed distally to the lesion of interest. Motorized pull-back was performed with a pull-back rate of 0.5 mm s−1. The pull-back was stopped as soon as the IVUS catheter reached the guiding catheter. During the pull-back, a grey-scale IVUS was recorded and raw radiofrequency (RF) data were captured at the top of the R wave. IVUS-VH data were transferred to an offline workstation and reconstructed using a commercial software tool (pcVH 2.1 software, Volcano Corporation). Thereby, the lumen and the media–adventitia interface were defined by semiautomatic contour detection. For every frame, morphometric parameters were expressed as cross-sectional area. Plaque area was defined as plaque plus media and was calculated as vessel area minus lumen area. Per cent vessel stenosis was calculated as [(vessel area minus lumen area)/vessel area] × 100, and maximal per cent vessel stenosis was determined on the frame with the smallest lumen cross-sectional area. Total plaque volume per lesion was calculated according to the Simpson’s rule.
Although for description of morphometric data, plaque was defined as plaque plus media, the media was not included for calculation of compositional parameters. According to the radiofrequency signal processing, areas or volumes of the four characteristic plaque components were automatically determined for every recorded frame and for the entire imaged segment. The necrotic core was displayed in red, the fatty fibrous plaque in light green, the fibrous plaque in dark green and the calcified plaque in white. For comparison with DSCT, IVUS-VH fatty fibrous and necrotic core components were summed up as fatty plaque.
CT coronary angiography
All CT scans were performed on a dual-source CT scanner (Somatom Definition, Siemens Medical Solutions, Forchheim, Germany). Prior to acquisition of the topogram, patients received a single dose of 0.8 mg of glycerol trinitrate. For contrast-enhanced scans, vessel opacification was achieved through automated injection by a power injector (CT2™, Medtron, Saarbrücken, Germany) of 80 ml of iomeprol (Imeron® 400, Altana, Konstanz, Germany) at a flow rate of 5 ml s−1 plus a 60-ml chaser bolus. Estimation of individual circulation time was based on the test bolus technique, using a 20-ml bolus and dynamic evaluation software (Dyn Eva™, Syngo®, Siemens, Forchheim, Germany).
Collimation was 32 × 0.6 mm, slice acquisition 64 × 0.6 mm using the z-flying focal spot technique, gantry rotation time 330 ms, pitch 0.20–0.43 adapted to heart rate, tube voltage 120 kV and maximum tube current 400 mAs per rotation. For dose reduction, prospective tube current modulation was applied. Thus, at heart rates below 60 beats per min (bpm), full tube current was applied from 60% to 70%, at 60–70 bpm from 50% to 80%, and at heart rates above 70 bpm from 30% to 80% of the cardiac cycle. For data reconstruction, a single-segment reconstruction algorithm was applied that used the data of a quarter-rotation of both detectors for image reconstruction.
An initial reconstruction window was based on the results of a test series which was obtained in a transverse plane at the level of segment 2 and which displayed reconstruction window offsets by 5% of the entire cardiac cycle. In case of motion artefacts in the initial reconstruction, further reconstructions were obtained in 5% increments of the cardiac cycle until all individual arteries could be visualised at optimal image quality.
Effective slice thickness was 0.75 mm with a reconstruction increment of 0.4 mm. Data sets were filtered with a medium-soft convolution kernel (B26f).
DSCT image analysis
CT data were referred to an offline workstation (Vitrea 2, Version 4; Vital Images, Minnetonka, MN) and assessed using the SUREPlaque™ software for vessel or plaque analysis. The software is based on curved multiplanar reconstructions; thin-slab maximum intensity projections and three-dimensional (3-D) volume renderings were used for additional orientation.
Analysis was performed by an experienced reader (reader 1), who was blinded to the results of IVUS. Accurate matching of corresponding lesions was ensured by referring to lesion length and fiduciary markers (stents, side branches or characteristic calcifications) which had previously been defined in longitudinal reconstructed IVUS data. Target lesions were identified on curved multiplanar reconstructions by fixing their proximal or distal boundaries according to the marker and subsequent definition of lesion length. A maximal measurement discrepancy of 5% was accepted with regard to segment length.
Luminal and outer wall boundaries were automatically segmented by the software and volumes were calculated for the entire plaque as well as its fatty, fibrous or calcified components. The HU value settings for the various plaque components were −150 to 60 for lipid plaque, 61–149 for fibrous plaque and 150–1300 for calcium. Corresponding colours in the colour-coding overlay were red for fatty plaque, blue for fibrous plaque and yellow for calcified plaque. Maximal per cent vessel stenosis was determined by dividing luminal area by vessel area at the narrowest point of the lesion. For assessment of interobserver reproducibility of CT parameters, all plaque measurements were repeated by a second independent and blinded reader (reader 2). For assessment of intraobserver variability, plaque measurements were repeated by reader 1 following an 8-week interval.
Statistical analysis
Interclass correlation coefficients for entire plaque volume, non-calcified plaque volume as well as percentage of fatty, fibrous or calcified components were 0.99, 0.99, 0.95, 0.98 and 0.99, respectively, and intraclass coefficients were 0.98, 0.98, 0.93, 0.98 and 0.99, respectively. (Imaging examples are provided in Figure 3.)
Figure 3.
Virtual plaque histology in dual-source CT (DSCT) and intravascular ultrasound (IVUS). In a curved planar reconstruction (a), the arrow indicates a non-calcified plaque in segment 6 of the left anterior descending coronary artery. (b) Software automatically performs segmentation of luminal and outer wall vessel boundaries. Contrast the standard axial CT image (c) and colour-coded overlay with the grey-scale IVUS (e) and IVUS-VH (f). Despite a satisfactory match of plaque components, IVUS-VH excels at higher spatial resolution and reveals a greater level of complexity.
Statistical analysis was performed with JMP, GraphPad Prism and SPSS software (JMP version 6, SAS Institute, Cary, NC; GraphPad Prism version 4.00, GraphPad Software, San Diego, CA; SPSS version 15, SPSS Inc., Chicago, IL). A p-value of <0.05 indicated statistical significance. Continuous variables are expressed as the mean ± standard deviation, and comparisons between volumes and compositional percentages were performed using the t-test for paired observations. Bland–Altman analysis is used to display the bias and confidence interval (CI) between measurements, and the correlation between DSCT and IVUS is assessed by calculation of the Pearson’s correlation coefficient. Interobserver reliability was assessed with two-way, random, single-measure, intraclass correlation coefficient. Intraobserver reliability was assessed with a one-way, random, two-measure, intraclass correlation coefficient.
Results
DSCT and IVUS were successfully performed in all patients without complications. Mean heart rate was 64 ± 8 bpm (range 50–78 bpm), and good image quality was obtained in all CT studies. Plaque-containing segments, as defined on IVUS, were unequivocally identified on CT curved multiplanar reconstructions through fiduciary markers and segment length. The distribution of lesions was three lesions in the left main artery, eight lesions in the left anterior descending artery (n = 4 in segment 6; n = 3 in segment 7; n = 1 in segment 8), six lesions in the left circumflex artery (n = 5 in segment 11; n = 1 in segment 12) and three lesions in the right coronary artery (n = 2 in segment 1; n = 1 in segment 1).
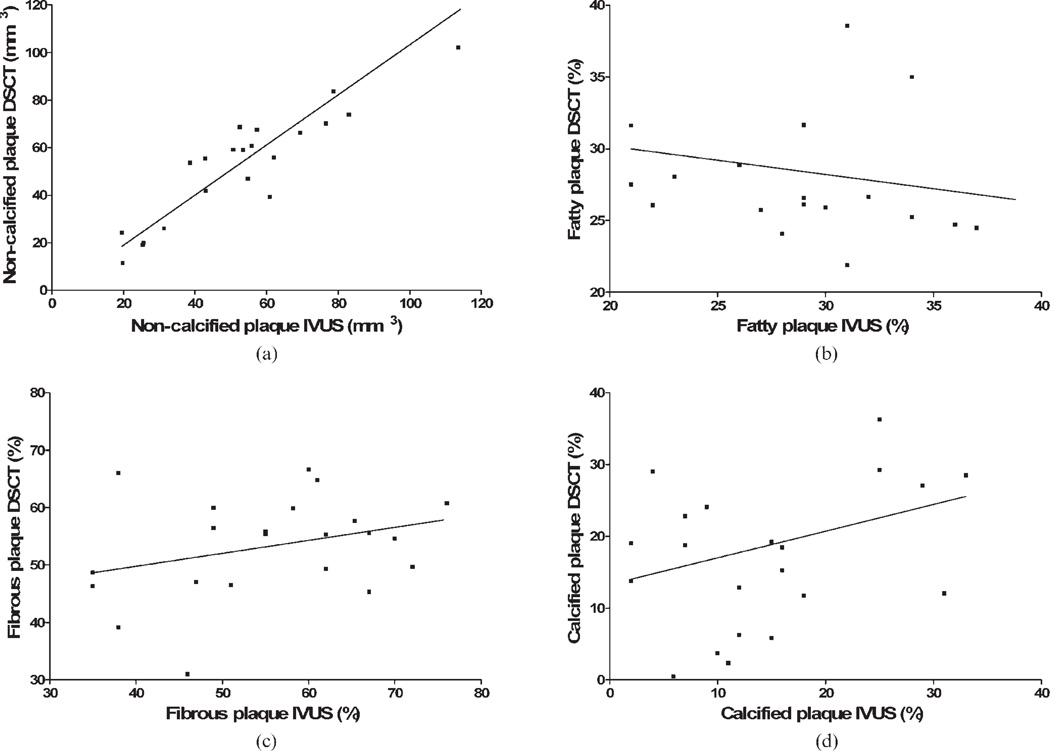
The results of the morphometric and compositional plaque analyses are provided in Table 1 and Table 2. Although DSCT significantly overestimated total plaque volume (p = 0.03) and absolute volume of calcification (p = 0.0004), there was a good concordance for non-calcified plaque volume (Figure 1). When comparing DSCT to IVUS-VH, a good match of mean percentages was, likewise, observed for fatty, fibrous or calcified plaque components (Figure 1). Good intermodality correlation was obtained for the entire plaque and non-calcified plaque volume (Table 1; Figure 2). By contrast, correlation between HU-based analysis in DSCT and IVUS-VH was very poor and not significant for percentages of plaque composition (Table 2; Figure 2). Mean per cent vessel stenosis as determined by CT (52.9 ± 13.9%) was not significantly different from IVUS (51.3 ± 14.3%; p = 0.35) and showed a close correlation (r2 = 0.70).
Table 1.
Morphometric plaque characterisation in DSCT and IVUS
| DSCT | IVUS | |
|---|---|---|
| Entire plaque burden (mm3) | 68.5 ± 32.9; p = 0.03 7.7; CI −23 to 38 r2 = 0.76 |
60.8 ± 29.0 |
| Non-calcified plaque burden (mm3) | 56.7 ± 29.8; p = 0.7 0.9; CI −21.7 to 23.5 r2 = 0.84 |
55.8 ± 26.1 |
| Percent vessel stenosis | 52.9 ± 13.9; p = 0.3 1.6; CI −14 to 17 r2 = 0.70 |
51.3 ± 14.3 |
Plaque volume refers to vessel volume minus lumen volume and is thus defined as plaque plus media. Non-calcified plaque volume is plaque volume minus calcified plaque volume. Per cent vessel stenosis refers to [(vessel area minus lumen area)/vessel area] × 100. Values are mean ± SD. p-Values refer to comparison with IVUS. The deviation of means is expressed by bias and confidence interval values, and the degree of correlation is given in r2 values.
DSCT, dual-source CT system; IVUS, intravascular ultrasound; SD, standard deviation.
Table 2.
Compositional plaque characterization in DSCT and IVUS-VH
| DSCT | IVUS-VH | |
|---|---|---|
| Fatty component (%) | 28.2 ± 6.1; p = 0.3 −1.7; CI −19 to 15 r2 = 0.03 |
29.9 ± 5.2 |
| Fibrous component (%) | 53.2 ± 8.7; p = 0.4 −2.1; CI −27 to 23 r2 = 0.10 |
55.3 ± 12.2 |
| Calcified component (%) | 18.7 ± 12.5; p = 0.1 4.1; CI −22 to 30 r2 = 0.07 |
14.4 ± 9.1 |
Percentages of composition refer to the plaque plus media volume in DSCT and to plaque excluding the media in IVUS-VH. For comparison with DSCT, IVUS-VH fatty fibrous and necrotic core components are summed up as fatty plaque component. Values are mean ± SD. p-Values refer to comparison with IVUS-VH. The deviation of means is expressed by bias and confidence interval values, and the degree of correlation is given in r2 values.
DSCT, dual-source CT system; IVUS-VH, virtual histology intravascular ultrasound; SD, standard deviation.
Figure 1.
(a) Bland–Altman analysis comparing non-calcified plaque volume in dual-source CT (DSCT) and intravascular ultrasound (IVUS). (b–d) Comparison of percentage plaque composition between DSCT and IVUS-VH. The x-axis denotes the average of DSCT and IVUS, and the point of intersection with the y-axis indicates the bias of DSCT. Dotted lines show one standard deviation of the bias.
Figure 2.
(a) Correlation of non-calcified plaque volume in dual-source CT (DSCT) and intravascular ultrasound (IVUS). (b–d) Correlation of percentage plaque composition in DSCT with IVUS-VH.
Discussion
Multiple studies have proved significant differences between CT density units for hypoechoic, hyperechoic and calcified lesions, suggesting that HUs are reflecting plaque composition [15, 19, 21–24]. Yet, until now, analysis of CT density within atherosclerotic lesions has been limited by partial volume and motion artefacts. Hence, in most studies, CT attenuation has not primarily been employed in the assessment of plaque morphology but has only retrospectively been obtained from visually detected plaque components. With the advent of DSCT, coronary arteries can be visualised with reduced motion artefacts. Also, new software has been designed which automatically segments vessel boundaries and quantifies different plaque components by various HU thresholds. In this way, colour maps of plaque composition are provided, just like virtual histology in IVUS radiofrequency.
This is the first study to assess both DSCT and colour-coded analysis with automatic wall segmentation in the analysis of non-calcified plaque. Our primary findings were twofold. First, we found a good concordance between the morphometric parameters in both modalities. Most notably, quantification of non-calcified plaque was closely correlated and showed a high level of reproducibility in DSCT. The same was true for per cent vessel stenosis. By contrast, the correlation of percentage plaque composition turned out to be very poor and insignificant.
The correlation between plaque volume in MSCT and IVUS has shown a considerable discrepancy in recent literature. In fact, the coefficients for plaque as assessed in terms of cross-sectional area range from r = 0.55 to r2 = 0.82 [16, 17, 25]. The degree of correlation as found in our study is in agreement with results from Achenbach et al or Leber et al [13, 14], who used manual segmentation for plaque volumetry. It is also in line with findings from Sun et al [26], who were the first to compare HU-based plaque analysis in 64-slice CT with IVUS. However, although Leber and Achenbach report a considerable underestimation of atheroma, HU-based assessment in Sun et al’s [26] and our study was associated with significant overestimation of total plaque volume [13, 14]. Overestimation of plaque has also been described with manual segmentation and non-volumetric quantification of plaque [17, 25, 27].
These discrepancies in plaque quantification ultimately derive from two variables: different amounts of calcified plaque and a variant delineation of the luminal and outer wall boundaries. Relative overestimation of heavily calcified plaque appears inevitable when using IVUS as the standard of reference. Although IVUS has much greater sensitivity for micro-calcifications, a considerable amount of eccentric calcification is missed owing to the phenomenon of acoustic shadowing [28]. In fact, overestimation of entire plaque volume in our study was due to a discrepant quantification of the calcified component; no significant bias was observed for the non-calcified component.
Until now, the segmentation of vessel edges has been done by visual assessment and manual contour drawing. Hence, varying amounts of lumen or adventitia may be included in the CT plaque analysis. This frequently accounts for discrepant biases in comparison with IVUS. In our investigation, good concordance of non-calcified plaque volume suggests that the automated wall segmentation algorithm used includes only negligible amounts of adventitia and closely matches the lumen and media–adventitia interfaces of IVUS.
In contrast to the morphometric parameters of atheroma, we found only poor or insignificant correlation with regard to the relative plaque composition. A first and obvious reason may be that compositional data in IVUS-VH refer to plaque tissue only, thus excluding the media. With DSCT composition being derived from the total plaque volume, a systematic distortion of results may be assumed. However, despite including the media in our CT analysis, there was a close match of mean compositional percentages and no systematic bias for any plaque component. Poor correlation of relative compositional data may thus primarily be related to the complex plaque morphology. This complex plaque morphology features lipid, fibrous and calcified components, not only side by side, but more often interlaced with each other. Certainly, spatial resolution of CT is still insufficient to break down such a level of complexity, and partial volume artefacts continue to impair accurate assessment of plaque composition.
On the other hand, it should be noted that IVUS-VH is subject to methodological limitations, too. In support of this, we repeatedly found IVUS-VH to project necrotic core areas into regions of hard plaque-induced acoustic shadow. Notably, in a recent comparison between in vivo IVUS-VH and histology, similar observations were highlighted and correlation of any non-calcified plaque component, likewise, turned out to be insignificant [29]. In addition, in terms of reproducibility, IVUS-VH has been found to show relative differences of up to 10% in compositional data [30]. Thus, misinterpretation in our reference method may have played a role in the poor correlation of percentage plaque composition.
In this context, it is important to note that CT attenuation of fatty fibrous plaque and the necrotic core are most probably not significantly different and, thus, the two tissue types turn out to be inseparable in CT colour-coded images. In addition to the individual components, we therefore used the sum of fatty fibrous plaque and the necrotic core in IVUS-VH for comparison with low-density plaque in DSCT. Certainly, the inability to distinguish the necrotic core represents a considerable weakness of MSCT. However, its general capacity to discriminate and reproducibly quantify fibrous and fatty components may in future be used to establish a CT-based definition of the vulnerable atheroma.
The difficulty in defining the luminal and outer wall boundaries has previously been emphasised and accounts for a high degree of interobserver variability [14]. Hence, a major advantage of the software used in our study is its capacity for automatic vessel edge segmentation. Since no manual editing of vessel boundaries was required, we were able to achieve an excellent inter- and intraobserver concordance for both morphometric and compositional parameters. In non-invasive, serial follow-up of patients receiving lipid-lowering medication, such high interobserver reliability is a key prerequisite for accurate monitoring of therapy response.
In the quest for non-invasive identification of the vulnerable plaque, CT is competing with other imaging techniques, most notably MRI and positron emission tomography (PET). Owing to its excellent soft-tissue contrast, the former has great potential in the evaluation of compositional plaque features [31, 32]. However, at present MRI lacks sufficient spatial resolution for robust coronary plaque imaging, and most studies are based on data from relatively large human vessels. PET may localise the vulnerable plaque by assessing the uptake of fluorodeoxyglucose in macrophages of the inflamed atheroma [33, 34]. In a recent animal study, the uptake of the tracer was correlated with the degree of inflammation and peaked with plaque disruption [35]. With use of fusion PET/CT, limited spatial resolution of PET may be compensated by CT data on morphology, and information from both methods may ultimately complement each other in reaching a reliable definition of the vulnerable plaque.
The small number of observations is an obvious limitation to our pilot study. Since IVUS-VH was performed in proximal segments only, vessel diameters were relatively large. The same was true for average plaque volumes. Reproducibility of our results in more distal segments and with smaller plaques needs to be confirmed by further analysis. The HU cut-offs used to differentiate the various plaque components have been chosen in accordance with recent literature or the default settings of the software. However, different thresholds will yield discrepant results, and systematic screening should be done to find the optimal cut-offs. The potential to use two X-ray tubes with different voltages and, thereby, further characterise plaque composition has not been assessed because the dual-energy mode of the scanner works only at an inferior temporal resolution.
In summary, first experience with DSCT and HU-based plaque analysis and vessel wall segmentation demonstrates the potential for accurate and reproducible quantification of non-calcified plaque in vivo. Percentage plaque composition, likewise, turns out to be highly reproducible but does not significantly correlate with IVUS-VH. Our findings justify larger trials on longitudinal monitoring of plaque stabilisation. However, a CT-based definition of the vulnerable plaque remains problematic until further improvement on spatial resolution.
References
- 1.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 2.Kragel AH, Reddy SG, Wittes JT, Roberts WC. Morphometric analysis of the composition of atherosclerotic plaques in the four major epicardial coronary arteries in acute myocardial infarction and in sudden coronary death. Circulation. 1989;80:1747–1756. doi: 10.1161/01.cir.80.6.1747. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 4.Mintz GS, Painter JA, Pichard AD, Kent KM, Satler LF, Popma JJ, et al. Atherosclerosis in angiographically “normal” coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. 1995;25:1479–1485. doi: 10.1016/0735-1097(95)00088-l. [DOI] [PubMed] [Google Scholar]
- 5.Nissen SE, Gurley JC, Grines CL, Booth DC, McClure R, Berk M, et al. Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation. 1991;84:1087–1099. doi: 10.1161/01.cir.84.3.1087. [DOI] [PubMed] [Google Scholar]
- 6.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–2342. doi: 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura RA, Edwards WD, Warnes CA, Reeder GS, Holmes DR, Jr, Tajik AJ, et al. Intravascular ultrasound imaging: in vitro validation and pathologic correlation. J Am Coll Cardiol. 1990;16:145–154. doi: 10.1016/0735-1097(90)90472-2. [DOI] [PubMed] [Google Scholar]
- 8.Potkin BN, Bartorelli AL, Gessert JM, Neville RF, Almagor Y, Roberts WC, et al. Coronary artery imaging with intravascular high-frequency ultrasound. Circulation. 1990;81:1575–1585. doi: 10.1161/01.cir.81.5.1575. [DOI] [PubMed] [Google Scholar]
- 9.Yock PG, Linker DT. Intravascular ultrasound. Looking below the surface of vascular disease. Circulation. 1990;81:1715–1718. doi: 10.1161/01.cir.81.5.1715. [DOI] [PubMed] [Google Scholar]
- 10.Moore MP, Spencer T, Salter DM, Kearney PP, Shaw TR, Starkey IR, et al. Characterisation of coronary atherosclerotic morphology by spectral analysis of radiofrequency signal: in vitro intravascular ultrasound study with histological and radiological validation. Heart. 1998;79:459–467. doi: 10.1136/hrt.79.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 12.Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 13.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 14.Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672–677. doi: 10.1016/j.jacc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 15.Leber AW, Knez A, Becker A, Becker C, von Ziegler F, Nikolaou K, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43:1241–1247. doi: 10.1016/j.jacc.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 16.Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–154. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Moselewski F, Ropers D, Pohle K, Hoffmann U, Ferencik M, Chan RC, et al. Comparison of measurement of cross-sectional coronary atherosclerotic plaque and vessel areas by 16-slice multidetector computed tomography versus intravascular ultrasound. Am J Cardiol. 2004;94:1294–1297. doi: 10.1016/j.amjcard.2004.07.117. [DOI] [PubMed] [Google Scholar]
- 18.Schoenhagen P, Tuzcu EM, Stillman AE, Moliterno DJ, Halliburton SS, Kuzmiak SA, et al. Non-invasive assessment of plaque morphology and remodeling in mildly stenotic coronary segments: comparison of 16-slice computed tomography and intravascular ultrasound. Coron Artery Dis. 2003;14:459–462. doi: 10.1097/00019501-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder S, Kopp AF, Baumbach A, Meisner C, Kuettner A, Georg C, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 2001;37:1430–1435. doi: 10.1016/s0735-1097(01)01115-9. [DOI] [PubMed] [Google Scholar]
- 20.Flohr TG, McCollough CH, Bruder H, Petersilka M, Gruber K, Suss C, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16:256–268. doi: 10.1007/s00330-005-2919-2. [DOI] [PubMed] [Google Scholar]
- 21.Carrascosa PM, Capunay CM, Garcia-Merletti P, Carrascosa J, Garcia MF. Characterization of coronary atherosclerotic plaques by multidetector computed tomography. Am J Cardiol. 2006;97:598–602. doi: 10.1016/j.amjcard.2005.09.096. [DOI] [PubMed] [Google Scholar]
- 22.Iriart X, Brunot S, Coste P, Montaudon M, Dos-Santos P, Leroux L, et al. Early characterization of atherosclerotic coronary plaques with multidetector computed tomography in patients with acute coronary syndrome: a comparative study with intravascular ultrasound. Eur Radiol. 2007;17:2581–2588. doi: 10.1007/s00330-007-0665-3. [DOI] [PubMed] [Google Scholar]
- 23.Pohle K, Achenbach S, Macneill B, Ropers D, Ferencik M, Moselewski F, et al. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: comparison to IVUS. Atherosclerosis. 2007;190:174–180. doi: 10.1016/j.atherosclerosis.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Schmid M, Pflederer T, Jang IK, Ropers D, Sei K, Daniel WG, et al. Relationship between degree of remodeling and CT attenuation of plaque in coronary atherosclerotic lesions: an in-vivo analysis by multi-detector computed tomography. Atherosclerosis. 2008;197:457–464. doi: 10.1016/j.atherosclerosis.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Hara T, Yamada S, Hayashi T, Ikeda Y, Yamashiro K, Mizutani K, et al. Accuracy of nonstenotic coronary atherosclerosis assessment by multi-detector computed tomography. Circ J. 2007;71:911–914. doi: 10.1253/circj.71.911. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Zhang Z, Lu B, Yu W, Yang Y, Zhou Y, et al. Identification and quantification of coronary atherosclerotic plaques: a comparison of 64-MDCT and intravascular ultrasound. AJR Am J Roentgenol. 2008;190:748–754. doi: 10.2214/AJR.07.2763. [DOI] [PubMed] [Google Scholar]
- 27.Bruining N, Roelandt JR, Palumbo A, La Grutta L, Cademartiri F, de Feijter PJ, et al. Reproducible coronary plaque quantification by multislice computed tomography. Catheter Cardiovasc Interv. 2007;69:857–865. doi: 10.1002/ccd.21067. [DOI] [PubMed] [Google Scholar]
- 28.Gutfinger DE, Leung CY, Hiro T, Maheswaran B, Nakamura S, Detrano R, et al. In vitro atherosclerotic plaque and calcium quantitation by intravascular ultrasound and electron-beam computed tomography. Am Heart J. 1996;131:899–906. doi: 10.1016/s0002-8703(96)90171-4. [DOI] [PubMed] [Google Scholar]
- 29.Granada JF, Wallace-Bradley D, Win HK, Alviar CL, Builes A, Lev EI, et al. In vivo plaque characterization using intravascular ultrasound-virtual histology in a porcine model of complex coronary lesions. Arterioscler Thromb Vasc Biol. 2007;27:387–393. doi: 10.1161/01.ATV.0000253907.51681.0e. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Granillo GA, Vaina S, Garcia-Garcia HM, Valgimigli M, Duckers E, van Geuns RJ, et al. Reproducibility of intravascular ultrasound radiofrequency data analysis: implications for the design of longitudinal studies. Int J Cardiovasc Imaging. 2006;22:621–631. doi: 10.1007/s10554-006-9080-0. [DOI] [PubMed] [Google Scholar]
- 31.Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS, et al. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64–77. doi: 10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 32.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–2056. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 33.Davies JR, Rudd JH, Weissberg PL, Narula J. Radionuclide imaging for the detection of inflammation in vulnerable plaques. J Am Coll Cardiol. 2006;47:C57–C68. doi: 10.1016/j.jacc.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 34.Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Aziz K, Berger K, Claycombe K, Huang R, Patel R, Abela GS. Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation. 2008;117:2061–2070. doi: 10.1161/CIRCULATIONAHA.106.652313. [DOI] [PubMed] [Google Scholar]