Abstract
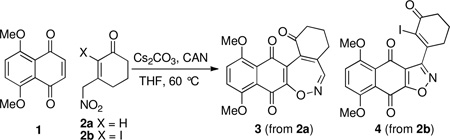
In the course of studies directed toward the synthesis of dideoxy lomaiviticinone, 3-(nitromethyl)cyclohexenones 2a (X = H) and 2b (X =I) were prepared. The corresponding enolates were reacted with naphthazarin (1) and unexpectedly afforded 1,2-oxazepine 3 and isoxazole 4, respectively. Rationale for their formation is proposed.
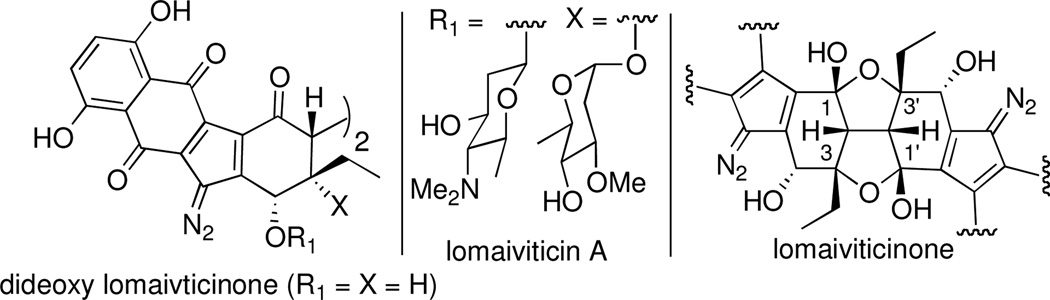
The diazofluorene antitumor antibiotics lomaiviticins A and B1 have attracted considerable attention from the synthetic community due to their molecular complexity, potent cell cytotoxicity and scarcity in nature.2,3 Lomaiviticinone, the aglycone common to lomaiviticin A and B, was recently prepared by an 11-step synthesis reported by Herzon and co-workers.4 As expected lomaiviticinone prepared by this route was isolated as a rigid polycyclic ring system formed by closure of the C3/C3’ tertiary alcohols onto the neighboring C1/C1’ keto groups (Figure 1). In anticipation of DNA cleavage studies, and simplified synthetic obstacles, we considered it advantageous to access C3/C3’ dideoxy lomaiviticinone, an aglycone with free-rotation about the C2-C2’ carbon-carbon bond as found in lomaiviticin A, the more abundant and studied of the two dimeric diazofluorene natural products.
Figure 1.
Structure of lomaiviticin A, lomaiviticinone and dideoxy lomaiviticinone.
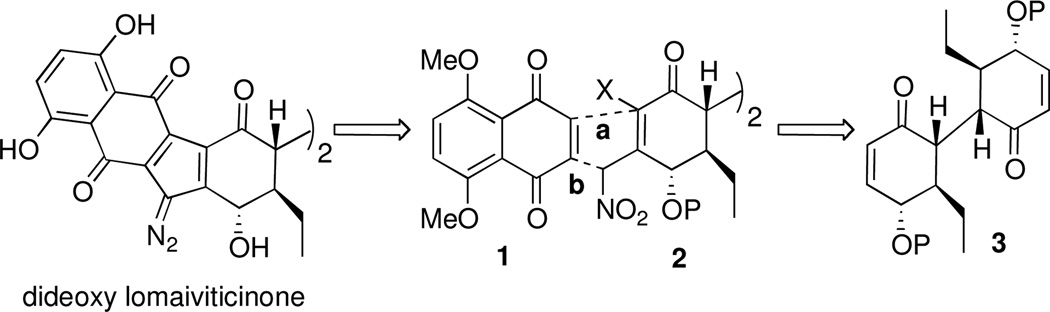
In 2008 we reported on the synthesis of the C2-symmetric core of dideoxy lomaiviticinone (3) starting from (−)-quinic acid.3c We planned to advance bis-enone 3 to dideoxy lomaiviticinone starting with conversion of 3 to nitromethylcyclohexenone 2 (X = H or halogen), with the nitro group serving the purposes of methylene activation and as a progenitor to the central diazo group (Figure 2). Given the symmetry of quinone 1, annulation between 1 and nitro activated cyclohexenone 2 could proceed by one of two orders of bond formation (a versus b). Herein we describe model studies in anticipation of Michael addition of 2 to quinone 1 (bond b). Our investigations started from cyclohexenone led us to discover novel oxidative nitronate mediated [5+2] and [3+2] quinone annulations.
Figure 2.
Synthetic strategy leading to dideoxy lomaiviticinone by way of quinone annulation.
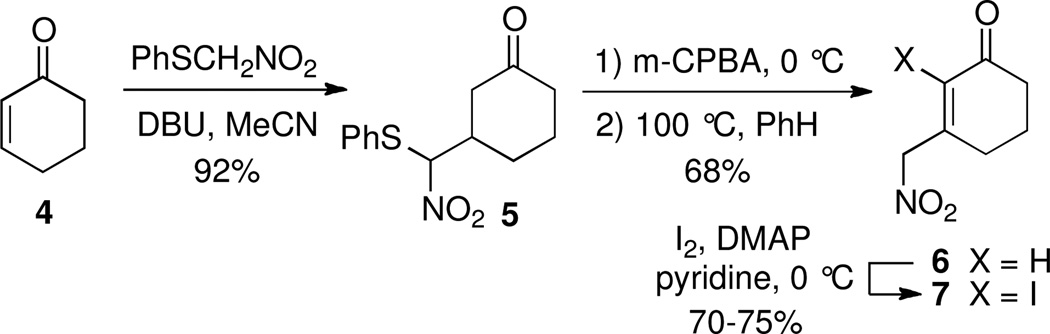
Our studies began with an efficient two-step conversion of 2-cyclohexenone (4) to 3-(nitromethyl)cyclohexenone starting with the addition of the conjugate base of (phenylthio)nitromethane to 4.5,6 The resulting Michael adduct (5) was then oxidized with m-CPBA to the corresponding sulfoxides and immediately heated in refluxing benzene to provide 2-(nitromethyl)cyclohexenone 6. The alpha carbon of enone 6 was then iodinated7 in anticipation of an intramolecular Heck reaction (bond a formation) following formation of bond b (Figure 2). Surprisingly, this proved to be the first example of using (phenylthio)nitromethane to introduce a nitromethyl group at the beta position of an enone. Historically, (phenylthio)nitromethane has been used primarily in carbonyl additions, alkylations, dipole additions and ring expansion reactions.6a,8
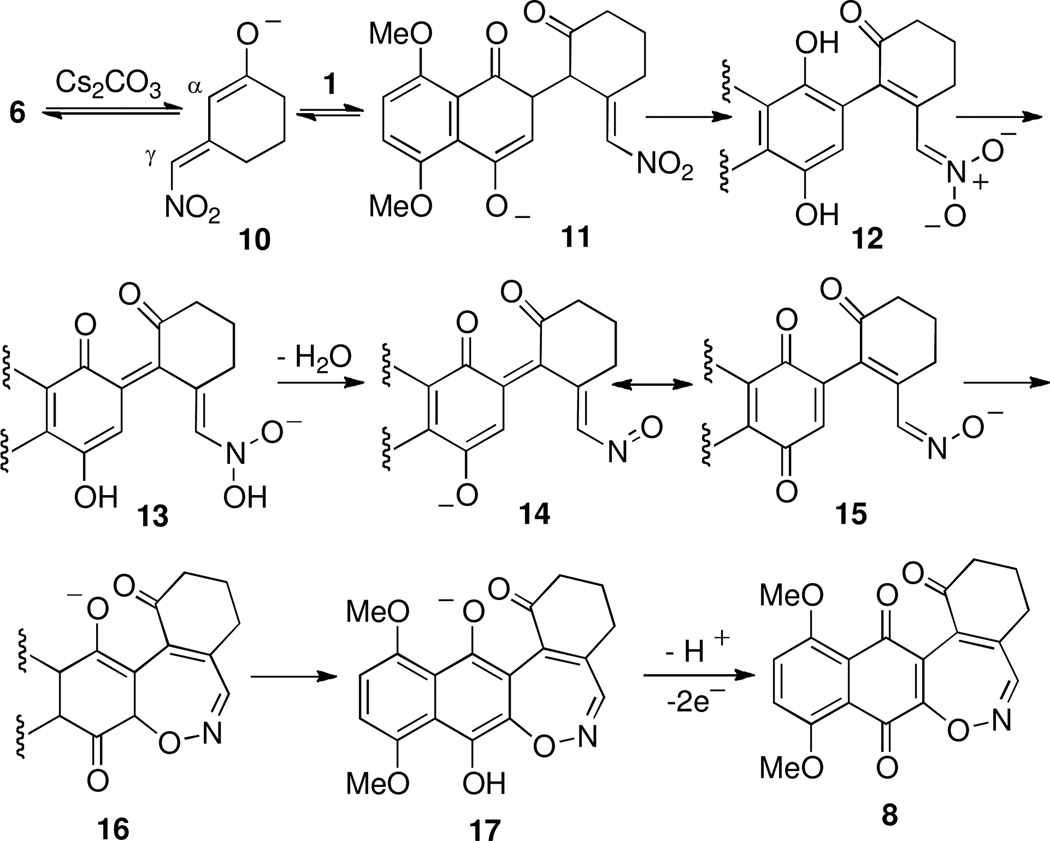
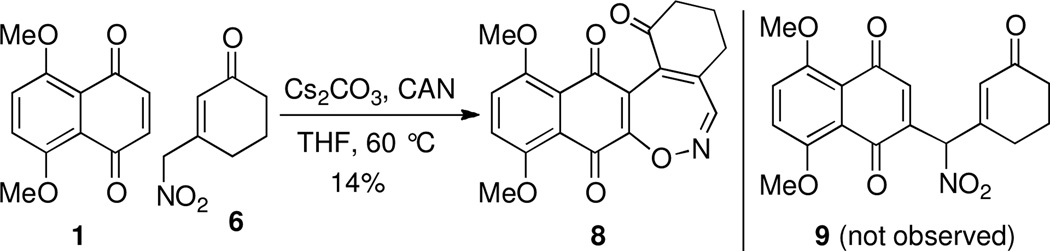
The Michael oxidative addition of enolates to quinones is often times complicated by secondary reactions and electron transfer mediated processes.9 Nonetheless, we chose to explore the addition of the conjugate base of 3-(nitromethyl)cyclohexenone (6) to naphthazarin 110 under oxidative conditions aimed to deliver adduct 9. After screening a large number of reaction conditions including varying pH and base we eventually isolated an adduct of enone 6 and quinone 1 which, surprisingly, proved not to be 9 but instead [5+2] adduct 1,2-oxazepine 8, albeit isolated in only 14% yield. The structural assignment of 8 was based on extensive NMR and high-resolution mass spectral analysis. Presented in Scheme 4 is a tentative mechanisim for the formation of 8 starting with the addition of 10 to quinone 1. Tautomerization accompanied by proton transfer results in conversion of 11 to hydroquinone 12, poised for quinone methide formation (13). Loss of a molecule of water then leads to nitroso 14, equivalent to oxime anion 15 by electron delocalization. Finally, cyclization followed by terminal oxidation accounts for production of 1,2-oxazepine 8.
Scheme 4.
Base catalyzed oxidative addition of nitrocyclohexenone 7 to quinone 1.
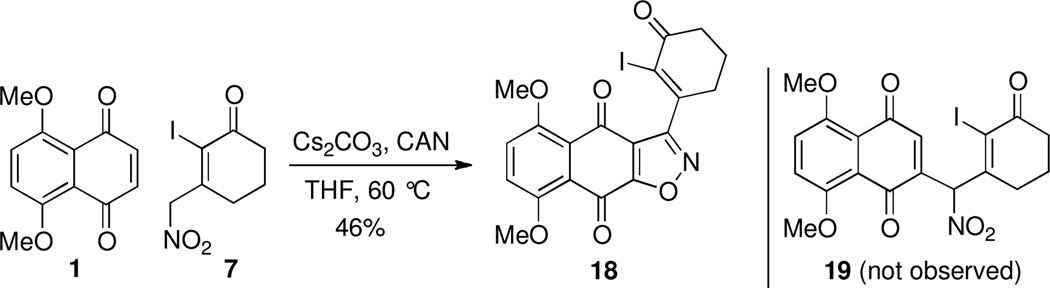
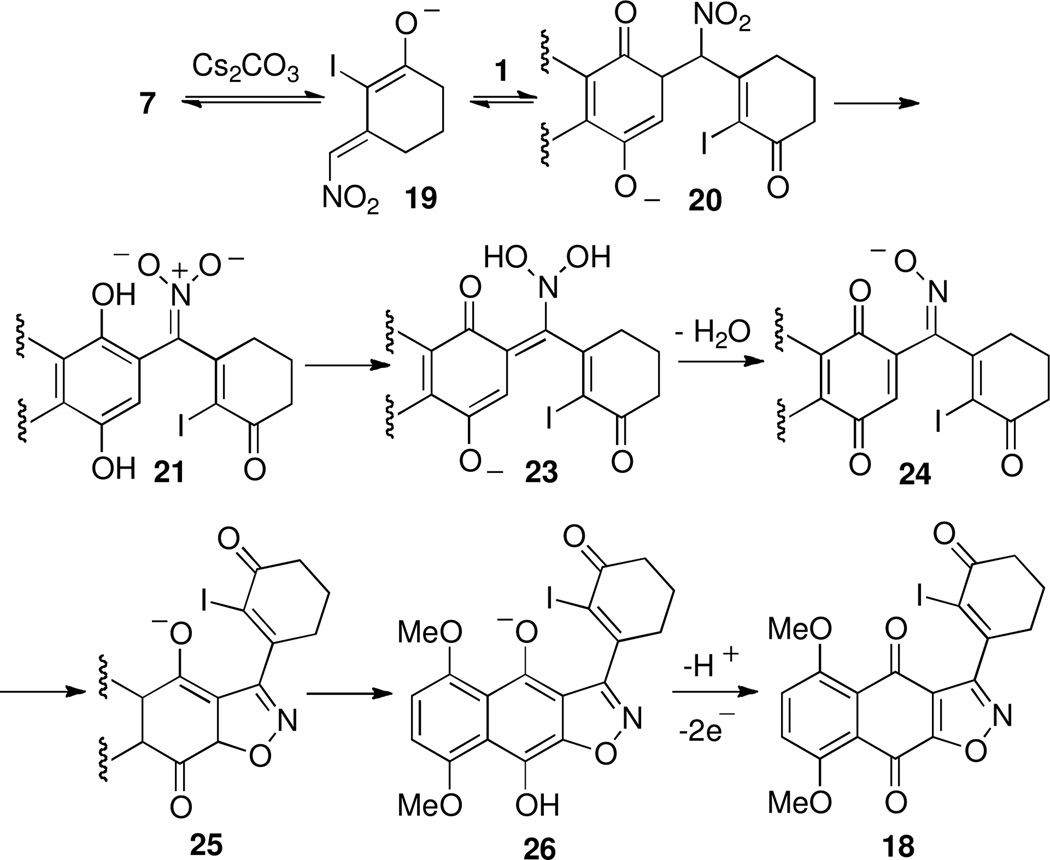
Examination of the reaction pathway leading to undesired quinone 8 (Scheme 3) suggested the desired carbon-carbon bond formation (bond b, Scheme 1) could be directed by blocking the α-carbon of dienolate 10 by a halogen atom (cf. 7, Scheme 5). In this case we anticipated base-catalyzed addition of 7 to naphthazarin 1 under oxidative conditions would afford adduct 19 appropriately functionalized for an intramolecular Heck reaction as demonstrated by Herzon’s group.4 In the event, our plan was once again thwarted leading to a 46% yield of isoxazole quinone 18 without 19 being observed. In this case the desired carbon-carbon bond formation (19+1Π20) was followed by undesired reorganization of oxidation state (21Π24) followed by oxidative cyclization (24Π18).
Scheme 3.
Proposed base-catalyzed oxidative addition of 6 to quinone 1 leading to 8.
Scheme 1.
Preparation 3-(nitromethyl)cyclohexenones 6 and 7.
Scheme 5.
Proposed base-catalyzed oxidative addition of 7 to quinone 1 leading to 16.
Our failure to effect base-promoted annulation between either cyclohexenone 6 or 7 and quinone 1 can be ascribed to the incompatibility of the nitronate and hydroquinone conjugated systems 12 and 21. A potential solution to this incompatibility is reduction of the nitro group to a protected amine. This possibility and other approaches to dideoxy lomaiviticinone are under investigation and will be reported in due course.
Supplementary Material
Scheme 2.
Base catalyzed oxidative addition of nitrocyclohexenone 6 to quinone 1.
Acknowledgment
This work was supported by the National Institutes of Health (CA 059515).
Footnotes
Supporting Information Available Experimental procedures and full spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.He H, Ding W, Bernan V, Richardson A, Ireland C, Greenstein M, Ellestad G, Carter G. J. Am. Chem. Soc. 2001;123:5362–5363. doi: 10.1021/ja010129o. [DOI] [PubMed] [Google Scholar]
- 2.For a comprehensive review of the lomaiviticins and kinamycins, see: Herzon SB, Woo CM. Nat. Prod. Rep. 2012;29:87–118. doi: 10.1039/c1np00052g.
- 3.Synthetic studies: Nicolaou KC, Denton RM, Lenzen A, Edmonds DJ, Li A, Milburn RR, Harrison ST. Angew. Chem. Int. Ed. 2006;45:2076–2081. doi: 10.1002/anie.200504466. Krygowski ES, Murphy-Benenato K, Shair MD. Angew. Chem. Int. Ed. 2008;47:1680–1684. doi: 10.1002/anie.200704830. Zhang W, Baranczak A, Sulikowski GA. Org. Lett. 2008;10:1939–1941. doi: 10.1021/ol800460a. Nicolaou KC, Nold AL, Li H. Angew. Chem. Int. Ed. 2009;48:5860–5863. doi: 10.1002/anie.200902509. Gholap SL, Woo CM, Ravikumar PC, Herzon SB. Org. Lett. 2009;11:4322–4325. doi: 10.1021/ol901710b. Lee HG, Ahn JY, Lee AS, Shair MD. Chem-Eur. J. 2010;16:13058–13062. doi: 10.1002/chem.201002157. Morris WJ, Shair MD. Org. Lett. 2008;11:9–12. doi: 10.1021/ol8022006. Morris WJ, Shair MD. Tetrahedron Lett. 2010;51:4310–4312. doi: 10.1016/j.tetlet.2010.06.028.
- 4.(a) Herzon SB, Lu L, Woo CM, Gholap SL. J. Am. Chem. Soc. 2011;133:7260–7263. doi: 10.1021/ja200034b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Herzon SB. Synlett. 2011:2105–2114. [Google Scholar]
- 5.(a) Barrett A, Graboski G, Russell M. J. Org. Chem. 1985;50:2603–2605. [Google Scholar]; (b) Bordwell F, Bartmess J. J. Org. Chem. 1978;43:3101–3107. [Google Scholar]
- 6.(a) Boivin J, Chauvet C, Zard SZ. Tetrahedron Lett. 1992;33:4913–4916. [Google Scholar]; (b) Ballini R, Bosica G, Fiorini D, Palmieri A, Petrini M. Chem. Rev. 2005;105:933–971. doi: 10.1021/cr040602r. [DOI] [PubMed] [Google Scholar]
- 7.Johnson CR, Adams JP, Braun MP, Senanayake CBW, Wovkulich PM, Uskokovic MR. Tetrahedron Lett. 1992;33:917–918. [Google Scholar]
- 8.(a) Ashwell M, Jackson RFW, Kirk JM. Tetrahedron. 1990;46:7429–7442. [Google Scholar]; (b) Kim S, Park JH. Chem. Lett. 1988:1323–1324. [Google Scholar]; (c) Ashwell M, Jackson RFW. J. Chem. Soc. Chem. Commun. 1988:282–283. [Google Scholar]; (d) Barrett AGM, Graboski GG, Russell MA. J. Org. Chem. 1986;51:1012–1015. [Google Scholar]; (e) Miyashita M, Kumazawa T, Yoshikoshi A. J. Chem. Soc. Chem. Commun. 1978:362–363. [Google Scholar]
- 9.(a) Chuang C-P, Wu Y-L, Jiang M-C. Tetrahedron. 1999;55:11229–11236. [Google Scholar]; (b) Chen H-L, Lin C-Y, Cheng Y-C, Tsai A-I, Chuang C-P. Synthesis. 2005:977–985. [Google Scholar]; (c) Tseng C-C, Wu Y-L, Chuang C-P. Tetrahedron. 2002;58:7625–7633. [Google Scholar]; (d) Saraswathy VG, Sankararaman S. J. Org. Chem. 1995;60:5024–5028. [Google Scholar]
- 10.Huot R, Brassard P. Can. J. Chem. 1974;52:838–842. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.