Abstract
The anticancer agent 3-aminopyridine-2-carboxaldehyde thiosemicarbazone is a ribonucleotide reductase inhibitor. It inactivates ribonucleotide reductase by disrupting an iron-stabilized radical in ribonucleotide reductase's small subunits, M2 and M2b (p53R2). Unfortunately, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone also alters iron II (Fe2+) in hemoglobin. This creates Fe3+ methemoglobin that does not deliver oxygen. Fe2+ in hemoglobin normally auto-oxidizes to inactive Fe3+ methemoglobin at a rate of nearly 3% per day and this is counterbalanced by a reductase system that normally limits methemoglobin concentrations to less than 1% of hemoglobin. This balance may be perturbed by symptomatic toxicity levels during 3-aminopyridine-2-carboxaldehyde thiosemicarbazone therapy. Indications of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone sequelae attributable to methemoglobinemia include resting dyspnea, headaches and altered cognition. Management of methemoglobinemia includes supplemental oxygen, ascorbate and, most importantly, intravenously administered methylene blue as a therapeutic antidote.
Keywords: methemoglobinemia, ribonucleotide reductase, triapine
Ribonucleotide reductase (RNR) provides cells with deoxyribonucleoside triphosphates demanded for DNA synthesis and repair [1–5]. RNR consists of two large M1 subunits and either two small M2 [6] or M2b (p53R2) [7,8] subunits. The small subunits house an iron-stabilized free radical that shuttles to and from the enzyme's active site in the large subunit [9]. Well-known inhibitors of RNR include hydroxyurea [10], which annihilates the radical, and gemcitabine [11], which becomes a cytidine diphosphate analog that covalently destroys RNR's substrate binding site [12]. These and other RNR inhibitors in cancer therapy were recently reviewed [13,14].
The RNR inhibitor 3-aminopyridine-2-carboxyaldehyde thiosemicarbazone (3-AP; NSC #663249) holds promise as an anticancer agent [15,16]. Timed after DNA damage (e.g., damage promulgated by ionizing radiation), 3-AP's cell death-provoking effect may be due to a cell's protracted inability to supply on-the-spot deoxyribonucleoside triphosphates, which are needed for DNA damage repair [2,3]. This idea led to anticancer clinical trials that tested RNR inhibition by 3-AP when administered alone compared with coadministration with DNA-damaging chemotherapy or radiation [15–20]. A major dose-limiting toxicity in early clinical trials was symptomatic dyspnea due to treatment-related methemoglobinemia. Methemoglobinemia is a reversible condition in which greater than 15% of a patient's hemoglobin is incapable of carrying oxygen because its iron is oxidized. Because 3-AP efficacy presumably depends on its disruption of the iron-stabilized tyrosyl free-radical site of RNR's small subunit (M2 or M2b), its hemoglobin iron toxicity may be inseparable from its efficacy. In this review, we discuss methemoglobin metabolism, the pharmacodynamics of RNR inhibitor methemoglobinemia and its treatment.
Hemoglobin & methemoglobin metabolism
Hemoglobin in erythrocytes carries oxygen in reversible association with iron in a reduced, ferrous Fe2+ state. Oxygenated Fe2+ hemoglobin iron oxidizes to Fe3+ methemoglobin and superoxide at a rate of approximately 3% per day. Methemoglobin returns to hemoglobin by action of cytochrome b5 reductase and cytochrome b5 (upper path in Figure 1) [21]. This pathway accounts for 94% of the conversion of methemoglobin to hemoglobin [22] and normally maintains methemoglobin levels below 1% of total hemoglobin. Dyspnea is observed when methemoglobin blood levels reach 25% [23].
Figure 1. Recycling of methemoglobin to hemoglobin.
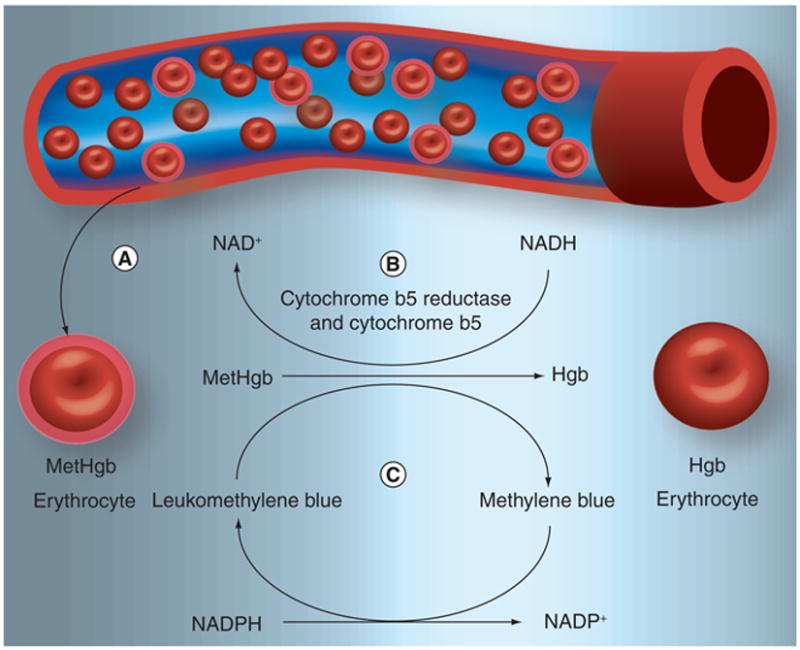
Normal erythrocyte Hgb carries oxygen in a reversible association with reduced or ferrous iron (Fe2+). Oxygenated Fe2+ Hgb oxidizes to Fe3+ MetHgb and superoxide at a rate of approximately 3% per day. (A) In the presence of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP), 3-AP interacts with the Fe2+ of Hgb in order to form 3-AP-Fe3+, which, in effect, creates MetHgb. (B) MetHgb may be reduced to Hgb by a combination of cytochrome b5 reductase and cytochrome b5. (C) The MetHgb antidote, methylene blue, can also facilitate this reaction. Hgb: Hemoglobin; MetHgb: Methemoglobin.
The mechanism of RNR inhibition by 3-AP is via inactivation of the tyrosyl free radical within the M2 or M2b (p53R2) small subunits [24,25], which in effect, is a molecular interaction of an Fe2+–3-AP chelate and of oxygen generating local reactive oxygen species capable of annihilating the nearby tyrosyl free radical. In a similar manner, an Fe2+–3-AP chelate impairs methemoglobin–hemoglobin cycling (Table 1) wherein 3-AP-induced methemoglobinemia occurs in 23% of the treated patients on clinical trials [15,20]. Other RNR inhibitors do not cause methemoglobinemia because their mechanisms of action are different: hydroxyurea, as a one-electron reductant, disrupts the free radical in RNR M2 and M2b subunits but does not associate directly with molecular iron; and gemcitabine blocks RNR's M1 subunit but does not interact with iron in the M2 and M2b subunits. In contrast to 3-AP, chemicals, such as parabactin and desferrioxamine, chelate intracellular iron pools. By creating low intracellular iron levels, these chemicals interfere with activation and reactivation of iron moieties in RNR after spontaneous loss of iron from the native enzyme [26].
Table 1.
Methemoglobinemia induced by 3-aminopyridine-2-carboxaldehyde thiosemicarbazone.
| Number of patients | 3-AP dose (mg/m2) | Infusion length (h) | Reaction | Mean pulse O2 saturation (%) | Mean peak methemoglobin (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 105 | 2 | Dyspnea, hypertension | 75 | 12 | [20] |
|
| ||||||
| 2 | 105 | 4 | Dyspnea, pallor | 88 | 11 | |
|
| ||||||
| 6 | 25 | 2 | None | 96 | 1 | [15] |
|
| ||||||
| 4 | 50 | 2 | None | 94 | 6 | |
3-AP: 3-aminopyridine-2-carboxaldehyde thiosemicarbazone.
Pharmacodynamics of 3-AP methemoglobinemia
Two clinical trials monitored methemoglobin after 3-AP infusion (Table 1). In the first Phase I dose-escalation clinical trial in patients with advanced solid cancers [20], 3-AP was administered intravenously over 2–4 h at dose levels of 105, 140 or 185 mg/m2 on days 1, 8 and 15 of each 28-day cycle. In this study, gemcitabine was also given over a 30-min intravenous infusion 1–4 h after 3-AP infusion at a dose level of 0–1000 mg/m2. Of 26 patients, a total of six manifested dyspnea at approximately the time of 3-AP infusion. In three patients, methemoglobin levels were determined and were 12, 12 and 11%. A suggested association of 3-AP-related dyspnea was claimed.
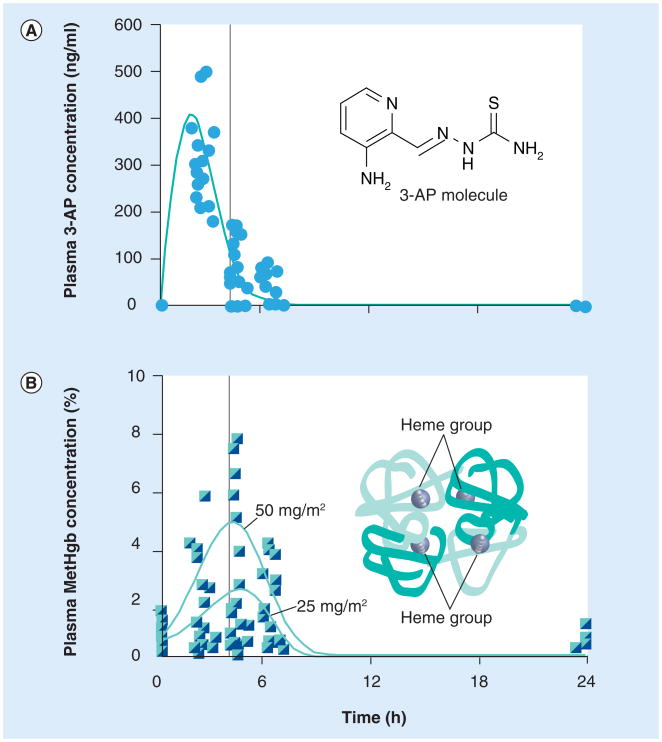
Because of this, a second Phase I dose-escalation clinical trial in patients with advanced-stage cervical cancer tracked serum levels of 3-AP and methemoglobin [15]. In this trial, 3-AP (25 or 50 mg/m2) was given as a three-times weekly intravenous 2-h infusion during once-weekly cisplatin (40 mg/m2) and daily radiation. Here, 3-AP serum concentrations were measured in heparinized blood samples by high-pressure liquid chromatography tandem mass spectrometry with parallel online turbulent flow extraction and positive ion selected reaction monitoring [15]. Samples were taken on day 1 and day 10 of treatment before, and at 2, 4, 6 and 24 h after the start of the 2-h 3-AP infusion (Figure 2). For procedural detail, serum concentrations of 3-AP were determined according to a modified internally standardized high-pressure liquid chromatography UV procedure provided by the manufacturer [JD Talton, Nanotherapeutics, Inc., FL, USA, Pers. Comm.]. Linear calibration curves of 3-AP:internal standard peak height ratios versus 3-AP concentration were established over a concentration range of 0.02–10 μg/ml of 3-AP in serum for each analyzed batch of serum specimens. Quality-control specimens at low (0.05 μg/ml), medium (0.8 μg/ml), and high (8.5 μg/ml) concentrations of 3-AP were determined concurrently with each analytical specimen batch. Permissible control ranges were ± 20, ± 15 and ± 15% of nominal concentration for the low-, medium- and high-concentration quality-control specimens, respectively. Typical within-day precision for replicate determinations of the low-, medium- and high-concentration quality-control specimens was 6–21, 5–8 and 2–6%, respectively (relative standard deviation [SD], n = 6 within-day replicates, 3 days of results compared). Interday precision for six replicate determinations of the low-, medium-and high-quality-control specimens during method validation studies was 21, 7 and 5%, respectively (relative SD, n = 3). Corresponding time-point serum methemoglobin concentrations were determined by direct spectrophotometry with a verified detection range of 0–100% [27,28]. Methemoglobin specimens were drawn into heparinized arterial blood-gas syringes on wet ice and measured within 10 min of procurement.
Figure 2. 3-aminopyridine-2-carboxaldehyde thiosemicarbazone pharmacokinetics and methemoglobin pharmacodynamics.
Plasma concentration of 3-AP was determined in ten women with cervical cancer undergoing 3-AP radiochemotherapy. (A) Peak 3-AP concentration was observed at the end of a 2-h infusion. (B) Peak methemoglobin levels were detected 2 h after discontinuation of 3-AP infusion. (A & B) A vertical bar denotes the 4-h time point after the start of infusion.
3-AP: 3-aminopyridine-2-carboxaldehyde thiosemicarbazone; MetHgb: Methemoglobin.
Patient 3-AP and methemoglobinemia levels were measured in blood samples collected for pharmacokinetic evaluation from ten patients with advanced-stage cervical cancer. A total of 20 treatment courses (ten on day 1 and ten on day 10) were evaluated for each of two dose levels of 3-AP (25 mg/m2 [n = 6] or 50 mg/m2 [n = 4]) studied. Maximal plasma concentrations (Cmax) of 3-AP were measured at the termination of the 2-h intravenous 3-AP infusion. The mean 3-AP peak plasma concentration was 262 ng/ml (SD = 51 ng/ml) at the 25 mg/m2 dose level and 560 ng/ml (SD = 59 ng/ml) at the 50 mg/m2 dose level. The mean elimination half-life was 2 h, with no change in elimination half-life between day 1 and day 10 for either the 25 mg/m2 (p = 0.18) or 50 mg/m2 (p = 0.35) dose levels. The plasma concentration of 3-AP 6 h after the start of the 2-h infusion fell to 2% Cmax (25 mg/m2) and 13% Cmax (50 mg/m2). The corresponding peak methemoglobin level was 1% (SD = 0.6%) at the 3-AP 25 mg/m2 dose level and was 6% (SD = 2.8%) at the 3-AP 50 mg/m2 dose level.
Although patient numbers were small in the two clinical trials performed, these two trials suggest that higher doses of 3-AP lead to decreased mean oxygen saturation and higher peak methemoglobin levels (Table 1). For example, the peak methemoglobin levels recorded in one of these studies follow peak serum 3-AP levels by a 2 h delay (Figure 2). The elevated methemoglobin levels usually resolved within 2 h of the peak (Figure 2). Repeated 3-AP dosing had no substantial additive effect (3-AP at 25 mg/m2: day 1 mean [SD] = 1% [0.6% ], day 10 mean [SD] = 0.9% [0.4% ]; 3-AP at 50 mg/m2: day 1 mean [SD] = 6% [2.8%] day 10 mean [SD] = 4.7% [2.2% ]), suggesting that mechanisms responsible for recycling methemoglobin were not irreversibly impaired.
For clinical and interpretative context, chemically-induced methemoglobinemia occurs most commonly from inhalation or tactile exposure to oxidizing chemical agents. Agents known to induce methemoglobinemia up to 15% after prolonged exposure, include acetonitrile (e.g., nail varnish remover), anesthetics (e.g., lidocaine, prilocaine), aniline dyes, chlorates (e.g., matches, explosives, weed killers), naphthalene (e.g., moth balls), volatile nitrites, phenazopyridine (e.g., pyridium), quinones (e.g., chloroquine and primaquine), sulfonamides (e.g., sulfamethoxazole) and dapsone. Mechanisms of methemoglobin induction involve direct oxidizing effects on the hemoglobin or generation of oxygen and peroxide free-radicals capable of oxidizing hemoglobin.
Clinical signs & symptoms of methemoglobinemia
Relative to hemoglobin, the oxygen dissociation curve of methemoglobin is left-shifted [29]. This means that methemoglobin binds tighter to oxygen, and thus, less oxygen is released for tissue perfusion. While healthy individuals have few symptoms when methemoglobin constitutes up to 15% of total hemoglobin, concentrations of 20–30% may lead to symptomatic dyspnea, headache and cognitive changes. Patients with anemia, pulmonary disease, abnormal hemoglobins and the elderly may have symptoms at lower methemoglobin levels. When methemoglobins rise above 30%, adverse sequelae may become more severe and lead to cardiac dysrhythmias, seizures, coma and, if devastating organ function ensues, patient death.
Inhibitors of RNR, such as 3-AP, are likely to induce low-level (+1–+10%) methemoglobinemia in all patients administered with the drug. 3-AP may also promote metabolic acidosis and hypoxia independent of its iron-chelating properties. Treating physicians and staff should be aware of these phenomena, and should consider implementing an antidote therapy (e.g., methylene blue) when patients self-report symptoms such as shortness of breath, headache or altered cognition.
Methylene blue as an antidote for RNR inhibitor-induced methemoglobinemia
Three antidotes for 3-AP-induced methemoglobinemia include methylene blue, ascorbate, and glutathione. Methylene blue infusion has been advocated for treatment of symptomatic methemoglobinemia owing to the dye's ability to donate an electron for nonenzymatic reduction of methemoglobin. In this reaction, NADPH methemoglobin reductase converts the oxidized form of the dye (methylene blue) to the reduced form (leukomethylene blue) using the cofactor NADPH (Figure 2). The reduced dye biochemically reduces Fe3+ iron in methemoglobin to Fe2+ hemoglobin, with the dye being recycled. Of note, oximeter readings of blood oxygen saturation may be obscured by methylene blue [30]. Also, NADPH methemoglobin reductase accounts for 6% of restoration of hemoglobin from methemoglobin and is a distinct enzyme from its counterpart NADH-cytochrome b5 reductase. Chemical restoration of hemoglobin from methemoglobin by ascorbate and glutathione are beyond the scope of this review [31].
It is important for treating physicians to recognize that patients receiving 3-AP, who have a known glucose-6-phosphate dehydrogenase (G6PD) deficiency, are more susceptible to cellular oxidative stress and related adverse events. Patients with G6PD deficiency are unable to produce the NADPH needed to maintain glutathione levels. Since the methylene blue antidote system requires NADPH as a cofactor in the reaction, methylene blue will be ineffective in these individuals. Indeed, methylene blue administration to patients with G6PD deficiency could actually worsen methemoglobinemia due to depletion of cellular reserves of NADPH. Under comorbid conditions of profound cell hemolysis resulting from chemotherapeutics, erythrocyte enzymes that are needed to reduce methylene blue are released and the antidote is ineffective. If methylene blue cannot be reduced, it can act as an oxidizing agent converting hemoglobin to methemoglobin, thus exacerbating the condition it is intended to treat.
Expert commentary & conclusion
Encouraging early results of 3-AP radiochemotherapy in the management of cervical cancer [15,16] raises the possibility of future cancer therapy clinical trials, utilizing RNR inhibitors paired with DNA-damaging therapies. RNR inhibitors, such as 3-AP, are associated with excellent targeted disease control, but are also linked to dose-limiting methemoglobinemia. Further study of the manipulation of hemoglobin–methemoglobin metabolism is warranted prior to widespread clinical use of RNR inhibitors in anticancer management strategies. The present literature on intravenous 3-AP in cervical cancer management suggests that the biologically effective 25-mg/m2 3-AP dose level is safe and not associated with burdensome treatment-related methemoglobinemia and dyspnea. Our findings in this study confirm this sentiment. Finally, while it is important to investigate the manipulation of iron in erythrocyte hemoglobin during intravenous administration of 3-AP, it is also equally critical to evaluate the effects of oral administration of 3-AP on cellular iron. Both excitement and caution are appropriate in interpreting available properties of 3-AP for cancer therapies.
Future perspective
Clinical trials incorporating RNR inhibitor radiochemotherapy are eagerly awaited over the next 5 years. Understanding of iron-chelating RNR inhibitors, such as 3-AP, and their resultant anticipated low levels of methemoglobinemia are critical to the gradual acceptance of this combined approach to anticancer management. RNR inhibitors, such as 3-AP, seemingly disrupt iron at the free radical site of RNR's small subunit (M2 or M2b), making its hemoglobin iron toxicity inseparable from its efficacy. It is hoped that pharmacodynamic results from clinical trials of 3-AP radiochemotherapy will, at least in part, evaluate the methemoglobin-inducing effect of oral 3-AP, as this form of the drug may be expected to be more widely used in the worldwide management of cervical cancer due to its logistical advantage of convenient cost-effective administration.
Executive summary.
Ribonucleotide reductase rate-limits the conversion of ribonucleotide to deoxyribonucleotides
▪ To catalyze this reaction, an iron-stabilized free radical in the small subunit of ribonucleotide reductase shuttles to and from the enzyme's large subunit active site.
3-aminopyridine-2-carboxaldehyde thiosemicarbazone interacts with iron II
▪ 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP)–Fe2+ interactions disrupt a free radical in the small subunits of ribonucleotide reductase to inactivate the enzyme.
3-AP disrupts hemoglobin & methemoglobin metabolism
▪ 3-AP also interacts with Fe2+ in hemoglobin, interrupting recycling of spontaneously generated methemoglobin (Fe3+) back to Fe2+ hemoglobin.
Anticancer inhibitors of ribonucleotide reductase manifest methemoglobinemia in human clinical trials
▪ Shortness of breath, headaches and altered cognition are observed with these anticancer agents.
▪ Methemoglobinemia may be managed by supplemental oxygen, ascorbate and, most importantly, intravenously administered methylene blue as a therapeutic antidote.
Footnotes
Financial & competing interests disclosure: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research: The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Reichard P. Interactions between deoxyribonucleotides and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- 2.Kunos C, Chiu S, Pink J, Kinsella T. Modulating radiation resistance by inhibiting ribonucleotide reductase in cancers with virally or mutationally silenced p53 protein. Radiat Res. 2009;172(6):666–676. doi: 10.1667/RR1858.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunos C, Radivoyevitch T, Pink J, et al. Ribonucleotide reductase inhibition enhances chemoradiosensitivity of human cervical cancers. Radiat Res. 2010;174(5):574–581. doi: 10.1667/RR2273.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunos C, Colussi V, Pink J, Radivoyevitch T, Oleinick N. Radiosensitization of human cervical cancer cells by inhibiting ribonucleotide reductase: enhanced radiation response at low dose rates. Int J Radiat Oncol Biol Phys. 2011;80(4):1198–1204. doi: 10.1016/j.ijrobp.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunos C, Ferris G, Pyatka N, Pink J, Radivoyevitch T. Deoxynucleoside salvage facilitates DNA repair during ribonucleotide reductase blockade in human cervical cancers. Radiat Res. 2011;176(4):425–433. doi: 10.1667/rr2556.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Arakawa H, Yamaguchi T, et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404(6773):42–49. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- 8.Guittet O, Hakansson P, Voevodskaya N, et al. Mammalian p53R2 protein forms an active ribonucleotide reductase in vitro with the R1 protein, which is expressed both in resting cells in response to DNA damage and in proliferating cells. J Biol Chem. 2001;276:40647–40651. doi: 10.1074/jbc.M106088200. [DOI] [PubMed] [Google Scholar]
- 9.Eklund H, Uhlin U, Farnegardh M, Logan DT, Nordlund P. Structure and function of the radical enzyme ribonucleotide reductase. Prog Biophys Mol Biol. 2001;77(3):177–268. doi: 10.1016/s0079-6107(01)00014-1. [DOI] [PubMed] [Google Scholar]
- 10.Yarbro JW. Mechanism of action of hydroxyurea. Semin Oncol. 1992;19(3 Suppl. 9):1–10. [PubMed] [Google Scholar]
- 11.Heinemann V, Xu YZ, Chubb S, et al. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2′,2′-difluorodeoxycytidine. Mol Pharmacol. 1990;38(4):567–572. [PubMed] [Google Scholar]
- 12.Wang J, Lohman G, Stubbe J. Mechanism of inactivation of human ribonucleotide reductase with p53R2 by gemcitabine 5′-diphosphate. Biochemistry. 2009;48(49):11612–11621. doi: 10.1021/bi901588z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence TS. Radiation sensitizers and targeted therapies Oncology. 2003;17(12 Suppl. 13):23–28. [PubMed] [Google Scholar]
- 14.Shao J, Zhou B, Chu B, Yen Y. Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets. 2006;6(5):409–431. doi: 10.2174/156800906777723949. [DOI] [PubMed] [Google Scholar]
- ▪▪15.Kunos C, Waggoner S, Von Gruenigen V, et al. Phase I trial of intravenous 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in combination with pelvic radiation therapy and weekly cisplatin chemotherapy for locally advanced cervical cancer. Clin Cancer Res. 2010;16(4):1298–1306. doi: 10.1158/1078-0432.CCR-09-2469. Biologically effective dose of ribonucleotide reductase inhibitor produces substantial anticancer clinical benefit without undue methemoglobinemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunos C, Waggoner S, Zanotti K, et al. Phase II trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical and vaginal cancer. J Clin Oncol. 2011;29(Suppl) doi: 10.1158/1078-0432.CCR-09-2469. Abstract 5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feun L, Modiano M, Lee K, et al. Phase I and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chemother Pharmacol. 2002;50(3):223–229. doi: 10.1007/s00280-002-0480-0. [DOI] [PubMed] [Google Scholar]
- 18.Murren J, Modiano M, Clairmont C, et al. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin Cancer Res. 2003;9(11):4092–4100. [PubMed] [Google Scholar]
- 19.Wadler S, Makower D, Clairmont C, Lambert P, Fehn K, Sznol M. Phase I and pharmacokinetic study of the ribonucleotide reductase inhibitor, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, administered by 96-hour intravenous continuous infusion. J Clin Oncol. 2004;22(9):1553–1563. doi: 10.1200/JCO.2004.07.158. [DOI] [PubMed] [Google Scholar]
- ▪▪20.Yen Y, Margolin K, Doroshow J, et al. A Phase I trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother Pharmacol. 2004;54(4):331–342. doi: 10.1007/s00280-004-0821-2. Dual combination of ribonucleotide reductase inhibitors induces substantial methemoglobinemia. [DOI] [PubMed] [Google Scholar]
- 21.do Nascimento TS, Pereira RO, de Mello HL, Costa J. Methemoglobinemia: from diagnosis to treatment. Rev Bras Anestesiol. 2008;58(6):651–664. doi: 10.1590/s0034-70942008000600011. [DOI] [PubMed] [Google Scholar]
- 22.Bulbarelli A, Valentini A, DeSilvestris M, Cappellini M, Borgese N. An erythroid-specific transcript generates the soluble form of NADH-cytochrome b5 reductase in humans. Blood. 1998;92:310–319. [PubMed] [Google Scholar]
- 23.Umbeit J. Methemoglobin – it's not just blue: a concise review. Am J Hematol. 2007;82:134–144. doi: 10.1002/ajh.20738. [DOI] [PubMed] [Google Scholar]
- 24.Chaston TB, Lovejoy DB, Watts RN, Richardson DR. Examination of the antiproliferative activity of iron chelators: multiple cellular targets and the different mechanism of action of triapine compared with desferrioxamine and the potent pyridoxal isonicotinoyl hydrazone analogue 311. Clin Cancer Res. 2003;9(1):402–414. [PubMed] [Google Scholar]
- 25.Popović-Bijelić A, Kowol CR, Lind ME, et al. Ribonucleotide reductase inhibition by metal complexes of triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone): a combined experimental and theoretical study. J Inorg Biochem. 2011;105(11):1422–1431. doi: 10.1016/j.jinorgbio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyholm S, Mann GJ, Johansson AG, Bergeron RJ, Gräslund A, Thelander L. Role of ribonucleotide reductase in inhibition of mammalian cell growth by potent iron chelators. J Biol Chem. 1993;268(35):26200–26205. [PubMed] [Google Scholar]
- 27.Dennis R, Valeri C. Measuring percent oxygen saturation of hemoglobin, percent carboxyhemoglobin and methemoglobin, and concentrations of total hemoglobin and oxygen in blood of man, dog, and baboon. Clin Chem. 1980;26(9):1304–1308. [PubMed] [Google Scholar]
- 28.Copeland B, Dyer P, Pesce A. Hemoglobin by first derivative spectrophotometry: extent of hemolysis in plasma and serum collected in vacuum container devices. Ann Clin Lab Sci. 1989;19(5):383–388. [PubMed] [Google Scholar]
- ▪29.Mansouri A, Lurie AA. Concise review: methemoglobinemia. Am J Hematol. 1993;42(1):7–12. doi: 10.1002/ajh.2830420104. Reviews methemoglobinemia management strategies. [DOI] [PubMed] [Google Scholar]
- 30.Kessler M, Eide T, Humayun B. Spurious pulse oximeter desaturation with methylene blue injection. Anesthesiology. 1986;65:435–436. doi: 10.1097/00000542-198610000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe E. Methaemoglobinaemia. Clin Haematol. 1981;10:99–122. [PubMed] [Google Scholar]