Abstract
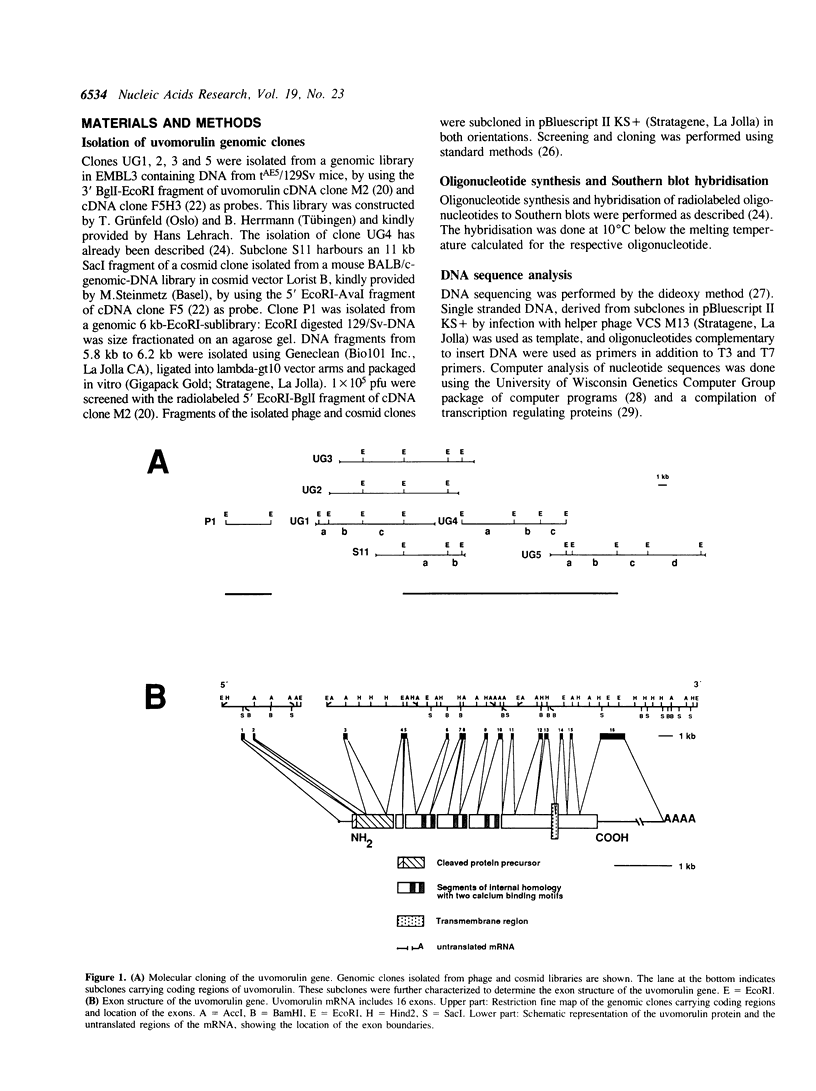
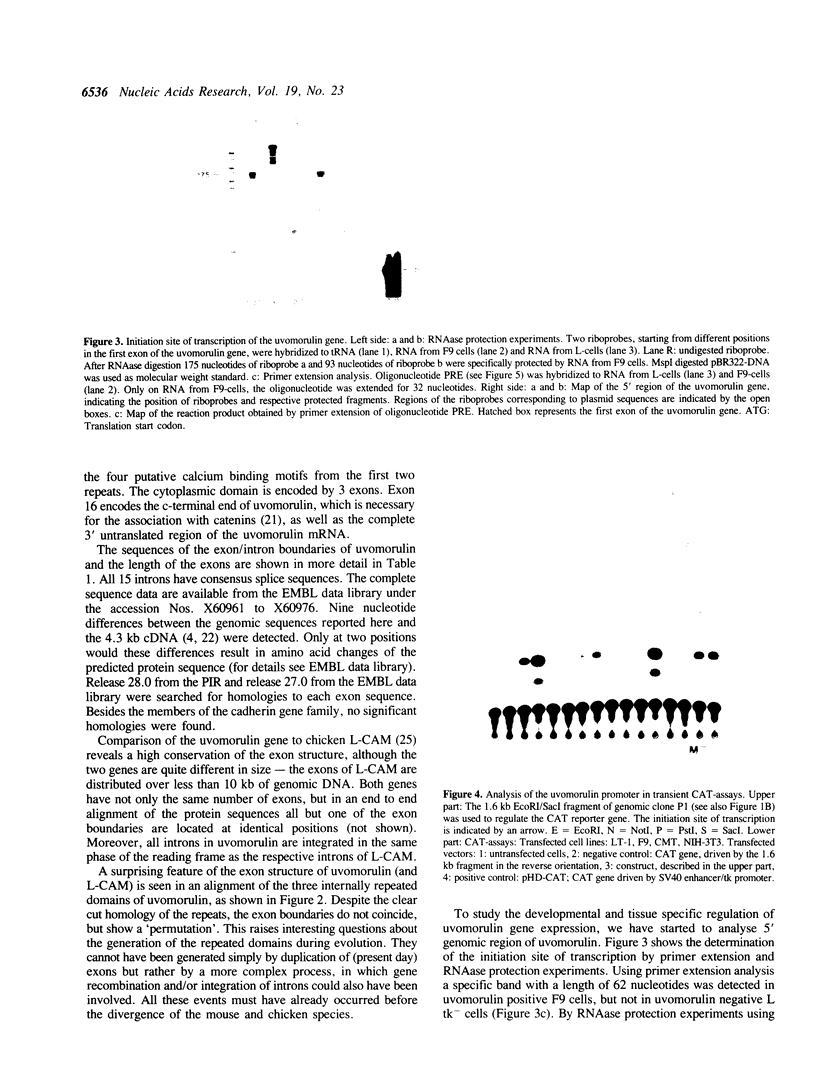
We have recently shown that the Ca2+ dependent cell adhesion molecule uvomorulin is encoded by a single gene, localized on mouse chromosome 8. Here we describe the organization of the uvomorulin gene and give an initial characterization of the uvomorulin promoter. Uvomorulin is encoded by 16 exons, which are distributed over a region of more than 40 kb genomic DNA. The exon structure of the genes for uvomorulin and its chicken homologue L-CAM are nearly identical and thus highly conserved. The relationship between the exon structure and the structure of the uvomorulin protein is analysed. The initiation site of transcription of the uvomorulin gene is located 127 bp upstream of the translation start site in a GC-rich region with no TATA-box, but with a GC-box in position -48 and a CCAAT-box starting at position -65 with respect to the transcription start site. 1.6 kb of the uvomorulin promoter (-1492 to + 92) confer cell type specific promoter activity to the CAT reporter gene. Homologies to known cis acting elements of other promoters are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly A., Le Page C., Rauch M., Milgrom E. Sequence-specific DNA binding of the progesterone receptor to the uteroglobin gene: effects of hormone, antihormone and receptor phosphorylation. EMBO J. 1986 Dec 1;5(12):3235–3241. doi: 10.1002/j.1460-2075.1986.tb04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Birchmeier W., Goodman S. L., Imhof B. A. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol. 1985 Oct;101(4):1307–1315. doi: 10.1083/jcb.101.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Mareel M. M., Van Roy F. M., Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989 Jun;108(6):2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C. Exons and the evolution of proteins. Int Rev Cytol. 1985;93:149–185. doi: 10.1016/s0074-7696(08)61374-1. [DOI] [PubMed] [Google Scholar]
- Blake C. C. Exons and the evolution of proteins. Int Rev Cytol. 1985;93:149–185. doi: 10.1016/s0074-7696(08)61374-1. [DOI] [PubMed] [Google Scholar]
- Blaschuk O. W., Farookhi R. Estradiol stimulates cadherin expression in rat granulosa cells. Dev Biol. 1989 Dec;136(2):564–567. doi: 10.1016/0012-1606(89)90283-2. [DOI] [PubMed] [Google Scholar]
- Boller K., Vestweber D., Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985 Jan;100(1):327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J. Retroposons--seeds of evolution. Science. 1991 Feb 15;251(4995):753–753. doi: 10.1126/science.1990437. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Choi Y. S., Sehgal R., McCrea P., Gumbiner B. A cadherin-like protein in eggs and cleaving embryos of Xenopus laevis is expressed in oocytes in response to progesterone. J Cell Biol. 1990 May;110(5):1575–1582. doi: 10.1083/jcb.110.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Richa J., Solter D., Knudsen K., Buck C. A. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983 Sep;34(2):455–466. doi: 10.1016/0092-8674(83)90379-3. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion and the molecular processes of morphogenesis. Annu Rev Biochem. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- Eistetter H. R., Adolph S., Ringwald M., Simon-Chazottes D., Schuh R., Guénet J. L., Kemler R. Chromosomal mapping of the structural gene coding for the mouse cell adhesion molecule uvomorulin. Proc Natl Acad Sci U S A. 1988 May;85(10):3489–3493. doi: 10.1073/pnas.85.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks L. M., Hemmings V. J. A cell line from an induced carcinoma of mouse rectum. J Pathol. 1978 Jan;124(1):35–38. doi: 10.1002/path.1711240108. [DOI] [PubMed] [Google Scholar]
- Gallin W. J., Sorkin B. C., Edelman G. M., Cunningham B. A. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J. M., Flanagan J. R., Recker D., Max E. E. Identification and partial purification of a protein binding to the human immunoglobulin kappa enhancer kappa E2 site. Nucleic Acids Res. 1988 Jun 10;16(11):4967–4988. doi: 10.1093/nar/16.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., DeSimone D., Geiger B. Expression of a novel cadherin (EP-cadherin) in unfertilized eggs and early Xenopus embryos. Development. 1991 Feb;111(2):315–325. doi: 10.1242/dev.111.2.315. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Kedes L. Identification of multiple proteins that interact with functional regions of the human cardiac alpha-actin promoter. Mol Cell Biol. 1989 Aug;9(8):3269–3283. doi: 10.1128/mcb.9.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Nose A., Nagafuchi A., Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J Cell Biol. 1988 Mar;106(3):873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil F., Morello D., Babinet C., Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980 Oct;21(3):927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Pastan I. Molecular cloning and characterization of a human DNA binding factor that represses transcription. Cell. 1989 Dec 1;59(5):815–825. doi: 10.1016/0092-8674(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Kemler R., Babinet C., Eisen H., Jacob F. Surface antigen in early differentiation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4449–4452. doi: 10.1073/pnas.74.10.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R., Ozawa M., Ringwald M. Calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1989 Oct;1(5):892–897. doi: 10.1016/0955-0674(89)90055-0. [DOI] [PubMed] [Google Scholar]
- Koch P. J., Walsh M. J., Schmelz M., Goldschmidt M. D., Zimbelmann R., Franke W. W. Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol. 1990 Oct;53(1):1–12. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A., Spurr N., Goodfellow P. N., Kemler R. Characterization and chromosomal localization of the gene encoding the human cell adhesion molecule uvomorulin. Differentiation. 1988 Jun;38(1):67–71. doi: 10.1111/j.1432-0436.1988.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Müller G., Ruppert S., Schmid E., Schütz G. Functional analysis of alternatively spliced tyrosinase gene transcripts. EMBO J. 1988 Sep;7(9):2723–2730. doi: 10.1002/j.1460-2075.1988.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Avner P., Gaillard J., Guenet J. L., Jakob H., Jacob F. Cell lines derived from teratocarcinomas. Cancer Res. 1976 Nov;36(11 Pt 2):4224–4231. [PubMed] [Google Scholar]
- Nicolas J. F., Jakob H., Jacob F. Metabolic cooperation between mouse embryonal carcinoma cells and their differentiated derivatives. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3292–3296. doi: 10.1073/pnas.75.7.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. J., Trachsel H. The mouse protein synthesis initiation factor 4A gene family includes two related functional genes which are differentially expressed. EMBO J. 1988 Jul;7(7):2097–2105. doi: 10.1002/j.1460-2075.1988.tb03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988 Sep 23;54(7):993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Isolation of placental cadherin cDNA: identification of a novel gene family of cell-cell adhesion molecules. EMBO J. 1987 Dec 1;6(12):3655–3661. doi: 10.1002/j.1460-2075.1987.tb02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Engel J., Kemler R. Single amino acid substitutions in one Ca2+ binding site of uvomorulin abolish the adhesive function. Cell. 1990 Nov 30;63(5):1033–1038. doi: 10.1016/0092-8674(90)90506-a. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Ringwald M., Schuh R., Vestweber D., Eistetter H., Lottspeich F., Engel J., Dölz R., Jähnig F., Epplen J., Mayer S. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Ben-Levy R. Multiple nuclear proteins in liver cells are bound to hepatitis B virus enhancer element and its upstream sequences. EMBO J. 1987 Jul;6(7):1913–1920. doi: 10.1002/j.1460-2075.1987.tb02451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin B. C., Hemperly J. J., Edelman G. M., Cunningham B. A. Structure of the gene for the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7617–7621. doi: 10.1073/pnas.85.20.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Gossler A., Boller K., Kemler R. Expression and distribution of cell adhesion molecule uvomorulin in mouse preimplantation embryos. Dev Biol. 1987 Dec;124(2):451–456. doi: 10.1016/0012-1606(87)90498-2. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]