Abstract
Background
We established multiple UM-SCC (University of Michigan Squamous Cell Carcinoma) cell lines. With time, these have been distributed to other labs all over the world. Recent scientific discussions have noted the need to confirm the origin and identity of cell lines in grant proposals and journal articles. We genotyped the UM-SCC cell lines in our collection to confirm their unique identity.
Design
Early passage UM-SCC cell lines were genotyped and photographed.
Results
Thus far, 73 unique head and neck UM-SCC cell lines (from 65 donors including 21 lines from 17 females) were genotyped. In 7 cases separate cell lines were established from the same donor.
Conclusions
These results will be posted on the U of M Head and Neck SPORE Tissue Core website for other investigators to confirm that the UM-SCC cells used in their laboratories have the correct features. Publications using UM-SCC cell lines should confirm the genotype.
Introduction
Head and neck squamous cell cancers (HNSCC) account for 11,170 deaths annually in the United States and nearly 250,000 deaths annually world wide. HNSCC cell lines developed from patients with cancers of various sites in the head and neck region(1) have been distributed to a wide-array of institutions to study this disease. The UM-SCC cell lines developed at the University of Michigan have been among the most widely used because many specific characteristics are known, such as relative radiation sensitivity(2), p53 mutation status(3), karyotype(4), antigen expression(5, 6), cisplatin sensitivity(3), as well as integrin expression and activation(7–12) that make these useful tools for other investigators. Until now these cell lines have not undergone extensive genetic fingerprinting analysis; which makes it difficult to readily confirm the identity of the individual cell lines.
Cell line identity can be derived from several different methods including sequencing of DNA polymorphisms(13), karyotyping (14), sequencing of hypervariable mitochondrial sequences(15), and some groups have even suggested TP53 sequencing(16) since the gene is frequently mutated in human cancers(17, 18). Unfortunately, these methods are limited by the time it takes to produce meaningful results, the expense of each protocol, and/or the value of the data. For example, TP53 sequencing cannot be used to distinguish the identity of cell lines when the gene is wild type. Because of this, cross-contamination has become a frequent problem for researchers. For example, 45/252 (18%) novel cell lines collected in the German Cell Line Bank were found to have non-unique genotypes(19). Thus, many researchers have concluded that there is a need for a rapid and standardized universal method for cell line identification(20–23).
Despite the realization that genetic verification is a necessary component of cell line research, until recently, cell line genotyping was not reliable because some transformed tissue cultures have defective mismatch repair pathways, which lead to increased microsatellite instability(24) and prevent reliable genotyping. Microsatellites are short tandem repeat (STR) loci that are highly polymorphic repetitive DNA sequence elements 2–7 nucleotides in length(25, 26). These STR loci are distributed throughout the human genome and alleles of STR loci can be differentiated by the number of repeat sequence (2–7 nucleotides long) copies located at each locus(27). Because PCR-based methods can be used to amplify STR loci, researchers have used radioactive, silver stain or fluorescence-based methods to detect STR loci length following separation of the different alleles by electrophoresis. Many of these loci are made up of dinucleotides repeats that are susceptible to instability and polymerase slippage during PCR amplification(28). The advent of commercially available assays based on amplifying tetranucleotide STR sequences, which have greater intrinsic stability than dinucleotide repeats, provide a much more reliable means of genetic identification(28, 29). As such, STR profiling has become a common reference for most commercially available cell lines(23). Here, we present genotyping data obtained with 10 common tetranucleotide repeat sequences on 73 of the most commonly used UM-SCC head and neck cell lines.
Materials and Methods
Cell Culture
All of the UM-SCC cell lines were established from head and neck cancer patients who gave written informed consent in studies reviewed and approved by the University of Michigan Medical School Institutional Review Board. Current and early passage human UM-SCC cell lines established at the University of Michigan (1–3, 5, 30) were retrieved from liquid nitrogen storage. Cell lines were grown in complete Dulbecco’s Modified Eagle’s Medium (cDMEM) containing 2 mM L-glutamine, 1% nonessential amino acids, 1% Penicillin-Streptomycin (Invitrogen, Carlsbad, CA) and 10% fetal bovine serum, in a humidified atmosphere of 5% CO2 at 37°C. All cell lines were tested for mycoplasma, using the MycoAlert Detection Kit (Cambrex, Rockland, ME). Contaminated cultures were treated with Plasmocin according to the manufacturer’s protocol, and testing was repeated at monthly intervals.
Genomic DNA Purification
Exponentially growing (60–80% confluence) cells were trypsinized and washed in PBS. Cell pellets were flash frozen at −80°C, resuspended in 500μL of 0.1M Tris pH 8.0, 0.1M EDTA (ethylene diamine tetraacetic acid), 0.4M NaCl, 1% SDS (sodium dodecylsulfate) and 0.3mg/mL Proteinase K (NEB, Ipswich, MA and incubated overnight at 55°C). Following incubation, 500μL of phenol/chloroform pH 6.7 was added (Fisher Scientific, Pittsburg, PA), and the dissolved cells were centrifuged for 10 minutes at 1700×g. The upper phase containing the DNA was transferred to a new tube with 150μL of 7.5M ammonium acetate and 800μL of 100% ethanol. The precipitated DNA was pelleted by centrifugation for 2 minutes at 1700×g. DNA pellets were washed with 70% ethanol, air dried, and resuspended in HPLC grade H2O.
Analysis of Genetic Loci
DNA samples were diluted to 0.10ng/μl and analyzed in the University of Michigan DNA sequencing Core using the Profiler Plus PCR (polymerase chain reaction) Amplification Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. The 9 loci D3S1358, D5S818, D7S820, D8S1179, D13S317, D18S51, D21S11, FGA, vWA and the amelogenin locus were analyzed and compared to ladder control samples.
Results
Genetic Profiling of UM-SCC cell lines
For each of the genotyped cell lines now represented in the U of M Head and Neck SPORE cell line bank, the UM-SCC cell line number, the donor gender, the anatomic tumor site (specimen site and primary tumor location), the passage number of the genotyped cell line that was included in the SPORE tissue core freezer, and the alleles for each of the following microsatellite loci: AMEL, D3S1358, D5S818, D7S820, D8S1179, D13S317, D18S51, D21S11, FGA, and vWA are given in Table 1. The amelogenin locus on the X and Y chromosomes is used for gender identification, however, since some cell lines and even normal cells from older male donors lose the Y chromosome(4, 31–33), an AMEL-X genotype does not confirm that the donor is female. However, presence of a Y signal was only observed in HNSCC cell lines derived from male donors. Of the 65 patient donors, 17 of 65 were females (26%).
Table 1. Genotyping results for 73 UM-SCC cell lines.
Patients with heterozygous alleles for each locus have two numbers corresponding to the different alleles. Where only a single allele is listed, either the patient had homozygous alleles at the given locus, an allele was not amplified (false negative), or was lost from tumor chromosome instability cloned out in the process of cell line establishment. In all cases, the lowest passage culture of UM-SCC cell lines available was used for analysis. Genotypes of 73 UM-SCC cell lines established from 65 donors. For each cell line the donor sex, the specimen site, the primary tumor location, the passage number of the cells genotyped for this study, and the alleles at 10 different tetranucleotide short tandem repeat loci (AMEL, D31358, vWA, FGA, D8S1179, D21S51, D18S51, D5S818, D13S317, and D7S820) are shown. Abbreviations: UM-SCC, University of Michigan Squamous Cell Carcinoma cell line series; M, male; F, female; BOT, base of tongue.
| CELL LINE | Gender | Specimen Site | Primary Location | Passage | AMEL | D3S1358 | vWA | FGA | D8S1179 | D21S11 | D18S51 | D5S818 | D13S317 | D7S820 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UM-SCC-1 | Male | floor of mouth | floor of mouth | 4 | X, Y | 18 | 15, 18 | 18, 22 | 13, 16 | 27 | 18 | 10, 13 | 8, 11 | 9, 12 |
| UM-SCC-2 | Female | alveolar ridge | alveolar ridge | 6 | X | 16 | 16 | 19 | 12, 14 | 30, 33.2 | 18 | 12 | 11, 13 | 8, 12 |
| UM-SCC-3 | Female | lymph node | nasal | 6 | X | 17 | 17 | 20, 27 | 14 | 31.2 | 12, 15 | 11, 13 | 11, 13 | 9, 10 |
| UM-SCC-4 | Female | BOT | tonsillar pillar | 7 | X | 17 | 17,18 | 26 | 13, 15 | 28 | 16 | 11 | 12 | 9,10 |
| UM-SCC-5 | Male | Supraglottis | Supraglottis | 20 | X | 17 | 18 | 25 | 11, 13 | 31 | 12,16 | 13 | 8,11 | 9 |
| UM-SCC-6 | Male | BOT | BOT | 22 | X, Y | 15 | 15, 16 | 23 | 11, 14 | 28 | 11, 19 | 12 | 13 | 10, 11 |
| UM-SCC-7 | Male | alveolus | alveolus | 23 | X, Y | 15 | 16 | 20, 22 | 12, 15 | 33.2 | 14, 15 | 11, 12 | 10 | 8, 12 |
| UM-SCC-8 | Female | alveolus | alveolus | 9 | X | 14, 16 | 16 | 22, 23 | 10, 13 | 29, 31.2 | 13, 15 | 11, 12 | 12 | 10, 11 |
| UM-SCC-9 | Female | ant. tongue | ant. tongue | 3 | X | 14 | 17, 18 | 22 | 9, 13 | 30, 31.2 | 13, 15 | 12 | 11, 14 | 8, 11 |
| UM-SCC-10A | Male | true vocal cord | true vocal cord | 80 | X | 17 | 19 | 22, 26 | 13, 14 | 30 | 15 | 12, 13 | 9 | 9 |
| UM-SCC-10B | Male | lymph node | larynx | 24 | X | 17 | 19 | 22 | 13,14 | 30 | 15 | 12,13 | 9 | 9 |
| UM-SCC-11A | Male | epiglottis | epiglottis | 10 | X | 16 | 16, 17, 18 | 19, 24 | 12, 15 | 28 | 16 | 11 | 14 | 11 |
| UM-SCC-11B | Male | supraglottic larynx | supraglottic larynx | 38 | X | 16 | 16, 17, 18 | 19 | 15 | 28 | 16 | 11 | 14 | 11 |
| UM-SCC-12 | Male | larynx | larynx | 77 | X | 15 | 16 | 23, 25 | 14, 15 | 29 | 12, 16 | 12 | 13 | 10, 11 |
| UM-SCC-13 | Male | esophagus | larynx | 21 | X | 16,18 | 17 | 19,24 | 12, 14 | 27,28 | 14, 16 | 13 | 12 | 8,12 |
| UM-SCC-14A | Female | floor of mouth | floor of mouth | 32 | X | 15 | 14, 18 | 20, 21 | 8, 13 | 29 | 15 | 11, 14 | 12 | 9, 10 |
| UM-SCC-14B | Female | floor of mouth | floor of mouth | 8 | X | 15 | 14, 18 | 20, 21 | 8, 13 | 29 | 15 | 11, 14 | 12 | 9, 10 |
| UM-SCC-14C | Female | floor of mouth | floor of mouth | 5 | X | 15 | 14, 18 | 20, 21 | 8, 13 | 29 | 15 | 11, 14 | 12 | 9, 10 |
| UM-SCC-15* | Male | hypopharynx | hypopharynx | 3 | X | 18 | 17 | 23 | 13, 14 | 31, 31.2 | 14 | 12 | 12 | 9 |
| UM-SCC-16 | Female | larynx | larynx | 2 | X | 14 | 15, 17 | 21, 24 | 10, 14 | 28, 29.2 | 15 | 10, 12 | 8, 10 | 10, 12 |
| UM-SCC-17A | Female | supraglottis | supraglottis | 22 | X | 15, 18 | 14, 17 | 20, 22 | 12, 13 | 28 | 17, 22 | 11 | 11, 13 | 13 |
| UM-SCC-17B | Female | soft tissue-neck | supraglottis | 33 | X | 15, 18 | 14, 17 | 20, 22 | 12, 13 | 28 | 22 | 11 | 11, 13 | 13 |
| UM-SCC-18 | Male | BOT | BOT | 25 | X | 13, 14 | 15, 19 | 19, 20 | 14, 15 | 29, 30 | 14 | 11 | 9, 12 | 9 |
| UM-SCC-19 | Male | BOT | BOT | 4 | X, Y | 14 | 16, 19 | 24 | 10, 12 | 28, 30 | 10, 16 | 11 | 11 | 9 |
| UM-SCC-20* | Male | neck node | larynx | 2 | X, Y | 14, 15 | 16, 18 | 18, 22.2 | 12, 15 | 28, 30.2 | 13, 15 | 11, 12 | 8, 10 | 9, 10 |
| UM-SCC-21A | Male | ethmoid sinus | skin of nose | 56 | X, Y | 15 | 16, 18 | 21 | 13, 14 | 29, 32.2 | 14 | 11, 12 | 8, 9 | 10, 11 |
| UM-SCC-22A | Female | hypopharynx | hypopharynx | 16 | X | 16 | 15, 18 | 22, 24 | 11, 13 | 28 | 18 | 12 | 8, 12 | 8, 9 |
| UM-SCC-22B | Female | neck metastasis | hypopharynx | 18 | X | 16 | 15, 18 | 22, 24 | 11, 13 | 28 | 18 | 12 | 8, 12 | 8, 9 |
| UM-SCC-23 | Female | larynx | larynx | 47 | X | 17 | 17 | 20 | 10, 15 | 29 | 10 | 11, 12 | 8 | 8, 13 |
| UM-SCC-24 | Male | larynx | true vocal cord | 5 | X, Y | 17 | 16, 17 | 18, 25 | 13 | 32.2 | 15 | 13 | 11, 12 | 9 |
| UM-SCC-25 | Male | neck | larynx | 41 | X, Y | 16 | 19 | 21 | 13, 14 | 27 | 19 | 12 | 11 | 12, 13 |
| UM-SCC-26 | Male | neck | BOT | 10 | X, Y | 16 | 16, 17 | 21, 24 | 13, 15 | 32.2 | 14 | 10, 11 | 11 | 7, 12 |
| UM-SCC-27 | Male | neck | ant. tongue | 4 | X, Y | 14 | 17, 18 | 19 | 12, 13 | 29, 32.2 | 14, 16 | 12, 13 | 8? | 8, 11 |
| UM-SCC-28 | Female | true vocal cord | true vocal cord | 5 | X | 15 | 18, 19 | 23, 25 | 13, 15 | 30, 32.2 | 14 | 12 | 12 | 11 |
| UM-SCC-29 | Male | alveolus | alveolus | 18 | X | 14 | 18, 19 | 23, 25 | 15 | 28 | 15 | 11 | 8, 11 | 8, 10 |
| UM-SCC-30 | Male | pyriform sinus | pyriform sinus | 15 | X | 16 | 16, 17 | 21 | 14, 15 | 29 | 14 | 12 | 13 | 9, 10 |
| UM-SCC-31 | Male | tonsil | tonsil | 9 | X | 16 | 17, 18 | 24 | 14 | 30 | 13, 18 | 12 | 10, 12 | 8 |
| UM-SCC-33 | Male | neck | maxillary sinus | 16 | X | 17 | 14, 18 | 19 | 12 | 31 | 18 | 10 | 11 | 10 |
| UM-SCC-34 | Male | tonsillar pillar | tonsillar pillar | 11 | X, Y | 15 | 15, 18 | 23 | 9, 14 | 30, 31 | 13 | 12, 13 | 12, 13 | 10, 12 |
| UM-SCC-35 | Male | tonsillar fossa | tonsillar fossa | 8 | X, Y | 17 | 15, 18 | 18.2, 26 | 11, 15, 16 | 36 | 15 | 13 | 11 | 9, 11 |
| UM-SCC-36 | Male | false vocal cord | false vocal cord | 11 | X, Y | 17 | 17, 18 | 21 | 12, 13 | 30 | 20 | 11 | 11, 12 | 9, 11 |
| UM-SCC-37 | Male | vallecula | vallecula | 12 | X | 15 | 18, 19 | 20, 22 | 11, 14 | 30 | 14, 17 | 11, 12 | 10 | 9, 11 |
| UM-SCC-38 | Male | tonsillar pillar | tonsillar pillar | 28 | X, Y | 18 | 17, 18 | 28 | 14, 15 | 27, 29 | 19 | 12 | 10, 11 | 10 |
| UM-SCC-39 | Male | pyriform sinus | pyriform sinus | 7 | X, Y | 16 | 17, 18 | 24 | 14 | 32.2 | 16, 17 | 11 | 13 | 10, 11 |
| UM-SCC-40 | Male | esophagus | esophagus | 10 | X | 14 | 14, 16 | 21, 22 | 13 | 28, 30 | 12 | 10 | 10 | 8. 10 |
| UM-SCC-41 | Male | arytenoid | arytenoid | 9 | X | 16 | 18 | 25 | 14 | 29 | 18 | 12 | 11 | 10, 12 |
| UM-SCC-42 | Male | neck | pyriform sinus | 7 | X | 16 | 18 | 19, 20 | 10, 14 | 29, 30 | 12 | 11, 12 | 13 | 11, 12 |
| UM-SCC-43 | Male | palate | palate | 10 | X, Y | 18 | 14, 15 | 20, 22 | 13, 15 | 32.2 | 14, 16 | 11 | 11, 13 | 10, 12 |
| UM-SCC-44 | Male | neck | retromolar trigone | 13 | X | 17 | 16, 17 | 24 | 13 | 30 | 12, 13 | 12 | 12 | 9, 13 |
| UM-SCC-45 | Female | neck | floor of mouth | 6 | X | 17 | 14, 16 | 21 | 11, 14 | 28, 30 | 19 | 12 | 10 | 9, 10 |
| UM-SCC-46 | Female | larynx | larynx | 3 | X | 15 | 14, 18 | 21 | 13, 15 | 30, 31.2 | 19 | 10, 11 | 10 | 12, 14 |
| UM-SCC-47 | Male | lateral tongue | lateral tongue | 29 | X, Y | 15 | 18 | 23, 25 | 15 | 29, 30 | 18 | 11, 12 | 8, 11 | 11 |
| UM-SCC-48 | Male | neck | retromolar trigone | 5 | X | 14 | 17 | 18, 21 | 13, 14 | 29, 31.2 | 15 | 12, 13 | 12 | 8, 11 |
| UM-SCC-49 | Male | lateral tongue | lateral tongue | 1 | X | 15 | 16, 20 | 23, 26 | 12 | 27 | 15 | 11 | 12, 13 | 8, 9 |
| UM-SCC-50 | Male | BOT | BOT | 7 | X | 15 | 16, 18 | 21, 22 | 13, 15 | 29 | 17 | 9, 12 | 11 | 10, 12 |
| UM-SCC-51 | Male | floor of mouth | floor of mouth | 12 | X | 16 | 14, 15 | 20, 21 | 9, 12 | 29 | 14 | 12 | 11 | 13 |
| UM-SCC-52 | Female | supraglottic larynx | supraglottic larynx | 10 | X | 16 | 16, 18, 19 | 19 | 13, 16, 17 | 30 | 12 | 13 | 13 | 9, 12 |
| UM-SCC-53 | Male | tonsil | pyriform sinus | 9 | X | 14 | 17 | 24 | 12, 15 | 27, 30 | 14, 21 | 12 | 8 | 10 |
| UM-SCC-54 | Male | larynx | true vocal cord | 43 | X | 16 | 17 | 17 | 14 | 29 | 16, 18 | 10, 12 | 12 | 10, 11 |
| UM-SCC-55 | Male | tonsil | retromolar trigone | 12 | X, Y | 17 | 17 | 22, 24 | 12, 14 | 30, 31 | 16 | 12, 13 | 8, 13 | 10 |
| UM-SCC-56 | Male | penis | penis | 11 | X, Y | 17 | 14,16 | 22,25 | 14 | 28,31 | 14 | 11,12 | 8,14 | 8,10 |
| UM-SCC-57 | Male | supraglottic larynx | supraglottic larynx | 6 | X, Y | 15 | 16, 17 | 23, 24 | 12 | 29 | 17, 18 | 11, 12 | 9, 12 | 9, 10 |
| UM-SCC-58 | Female | supraglottic larynx | supraglottic larynx | 8 | X | 14 | 15, 16, 17 | 22, 25 | 13, 15 | 30, 32.2 | 13 | 11 | 9, 13 | 9, 11 |
| UM-SCC-59 | Female | lateral tongue | lateral tongue | 11 | X | 14 | 15 | 22 | 13, 14 | 28, 29 | 12 | 10 | 10 | 11, 12 |
| UM-SCC-60 | Male | hypopharynx | hypopharynx | 15 | X, Y | 16 | 14, 15 | 19, 26 | 11 | 32 | 12 | 11 | 14 | 9, 10 |
| UM-SCC-69 | Male | hard palate | hard palate | 17 | X, Y | 17, 18 | 16, 17 | 20, 24 | 10, 14 | 30, 32.2 | 15, 16 | 11, 12 | 11 | 11, 13 |
| UM-SCC-70 | Male | larynx | larynx | 8 | X | 14 | 16, 17 | 20 | 14 | 30 | 18 | 11 | 11 | 12 |
| UM-SCC-73B | Male | neck | tongue | 9 | X | 16 | 16, 19 | 21 | 13, 15 | 30 | 12, 14 | 12 | 11 | 8, 9 |
| UM-SCC-74A | Male | BOT | BOT | 14 | X | 15, 16 | 15, 16 | 21, 26 | 12, 13 | 30, 34.2 | 17 | 12 | 12 | 11 |
| UM-SCC-74B | Male | intraoral | larynx | 4 | X | 15, 16 | 15, 16 | 21, 26 | 12, 13 | 30, 34.2 | 17 | 12 | 12 | 11 |
| UM-SCC-80 | Male | hypopharynx | hypopharynx | 12 | X, Y | 17 | 14,17 | 22 | 13 | 31.2 | 24 | 9 | 11 | 10 |
| UM-SCC-81A | Male | L false vocal cord | larynx | 7 | X | 15, 17 | 17 | 22 | 10 | 33.2 | 14, 19 | 11, 12 | 9 | 9, 11 |
| UM-SCC-81B | Male | tonsillar pillar | tonsil | 18 | X, Y | 17 | 17 | 20,22 | 10,13 | 29 | 14 | 11,12 | 9,11 | 9,11 |
| UM-SCC-92 | Female | lateral tongue | lateral tongue | 16 | X | 15 | 17, 20 | 19, 21 | 10, 13 | 29, 31 | 14, 15 | 11 | 12, 14 | 11, 12 |
In several cases it was possible to derive more than one cell line from the same donor(1). In some cases, these were from different sites during the same procedure (UM-SCC-17A from the endolarynx, UM-SCC-17B from tumor extending outside the thyroid cartilage(34); UM-SCC-22A from the primary site, UM-SCC-22B from a lymph node metastasis), or from different surgical procedures (UM-SCC-10A from the larynx at the time of laryngectomy and UM-SCC-10B from a submental lymph node metastasis 10 months later; UM-SCC-11A pretreatment biopsy, UM-SCC-11B post chemotherapy surgery; UM-SCC-14A wide local excision after excisional biopsy, UM-SCC-14B recurrence after surgery and radiation, UM-SCC-14C skin metastasis after chemotherapy; UM-SCC-74A surgical resection after chemotherapy and radiation, UM-SCC-74B second surgery for persistent cancer; UM-SCC-81A laryngeal primary, UM-SCC-81B, tonsil primary)(1). With a few exceptions the lines from the same donor exhibited the same genetic profile.
Losses of single alleles at individual loci were fairly common in the cell lines. This pattern of allelic loss is consistent with prior karyotype studies(4, 34–36) and loss of heterozygosity studies with these cell lines that revealed frequent losses of individual chromosome arms(37–39). In some cases we noted loss of an allele in one but not both of the cell lines derived from the same donor. For example, in UM-SCC-17A and -17B, allele 17 at D18S51 was lost in UM-SCC-17B but not in UM-SCC-17A. UM-SCC-81A and -81B are perhaps the most unlike each other of all of the paired sets. These cell lines were considered to be from two separate primary tumors of the same donor that arose 5 years apart; one from the larynx and the second from the tonsil. In this pair there were differences at 7 loci, although the genotype of each is consistent with the same donor origin of the cell lines. The cell lines share at least one allele at each locus with one exception. At AMEL UM-SCC-81A but not -81B lost the Y chromosome signal. At D3S1358, UM-SCC-81A has allele 15, -81B does not; at FGA -81B has allele 20, -81A does not; at D8S1779 -81B has 13, -81A does not; at D18S51 81A has 19, -81B does not; at D13S317 81B has 11, -81A does not. The most interesting difference was at D21S11 where -81A has 33.2 whereas 81B has 29. We suspect that the donor’s normal complement was allele 29, and 33.2 at this locus, but each tumor lost a different allele.
Genetic drift over time in cultured cell lines has been raised as a major concern for scientists using established cell lines. We had previously assessed the karyotype of cultured SCC cell lines over numerous passages and found remarkable stability(34). In the present study comparison of allelic patterns in three different cell lines taken at low passage and greater than 50 passages revealed no changes in the distribution of alleles, suggesting stability at each locus (Table 2). However, in high passage UM-SCC-1, allele amplicons for AMEL-Y and FGA-22 were lost and, in high passage UM-SCC-22A, one wVA-15 allele was lost.
Table 2. Genotyping results following long-term cell culture.
Genotyping results for three UM-SCC cell lines at high and low passages demonstrate that alleles may be lost due as a result of in vitro evolution of the population.
| CELL LINE | Passage | AMEL | D3S1358 | vWA | FGA | D8S1179 | D21S11 | D18S51 | D5S818 | D13S317 | D7S820 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UM-SCC-1 | >150 | X | 18 | 15, 18 | 18 | 16 | 27 | 18 | 10, 13 | 8, 11 | 9, 12 |
| UM-SCC-2 | 62 | X | 16 | 16 | 19 | 12, 14 | 30, 33.2 | 18 | 12 | 11, 13 | 8, 12 |
| UM-SCC-22A | 138 | X | 16 | 18 | 22, 24 | 11, 13 | 28 | 18 | 12 | 8, 12 | 8, 9 |
To further characterize the ability of this assay to discriminate genotypes between cancer cell lines and normal human fibroblasts, we genotyped short-term cultured fibroblasts from the donors of UM-SCC-11, -26 and -42 and then we compared the results to the genotypes of the cancer cell lines. As shown in Table 3, many of the alleles that were lost during either malignant transformation of cell culture were present in fibroblast line. For example, UM-SCC-11A has 7 loci that appear to have either homozygous or lost alleles and UM-SCC-11B appears to have 9 loci with only a single marker. However, genotyping of the donor fibroblast line revealed that only D21S11 has a single allele. Thus, only this allele is potentially homozygous or lost during culture. Analysis of the fibroblast data reveals that the UM-SCC cancer cell lines occasionally gain or lose a single allele at various loci. For example, in both UM-SCC-26 and UM-SCC-42, four alleles are lost at four different loci in each cancer cell line as compared to the donor fibroblast line.
Table 3. Genotyping results of 3 fibroblast and cancer cell lines from matched donors.
For the UM-SCC-11, -26, and -42 cell lines, we were able to grow and genotype fibroblasts from the donor. In each case, the matched genotypes are shown. Donors with heterozygous alleles for each locus have two numbers corresponding to different alleles. Where only a single allele is listed, either the patient had homozygous alleles at the given locus, an allele was not amplified (false negative), or was lost from tumor chromosome instability cloned out in the process of cell line establishment.
| Passage | AMEL | D3S1358 | vWA | FGA | D8S1179 | D21S11 | D18S51 | D5S818 | D13S317 | D7S820 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UM-SCC-11 Fibroblasts | 1 | X, Y | 14, 16 | 16, 17 | 19, 24 | 12, 15 | 28 | 16, 18 | 11, 12 | 12, 14 | 10, 11 |
| UM-SCC-11A | 10 | X | 16 | 16, 17, 18 | 19, 24 | 12, 15 | 28 | 16 | 11 | 14 | 11 |
| UM-SCC-11B | 38 | X | 16 | 16, 17, 18 | 19 | 15 | 28 | 16 | 11 | 14 | 11 |
| UM-SCC-26 Fibroblasts | 2 | X, Y | 16, 17 | 16, 17 | 21, 24 | 13, 15 | 31, 32.2 | 14, 18 | 10, 11 | 11, 12 | 7, 12 |
| UM-SCC-26 | 10 | X, Y | 16 | 16, 17 | 21, 24 | 13, 15 | 32.2 | 14 | 10, 11 | 11 | 7, 12 |
| UM-SCC-42 Fibroblasts | 7 | X, Y | 15, 16 | 18 | 19, 20 | 10, 14 | 29, 30 | 12, 15 | 11, 12 | 12, 13 | 11, 12 |
| UM-SCC-42 | 7 | X | 16 | 18 | 19, 20 | 10, 14 | 29, 30 | 12 | 11, 12 | 13 | 11, 12 |
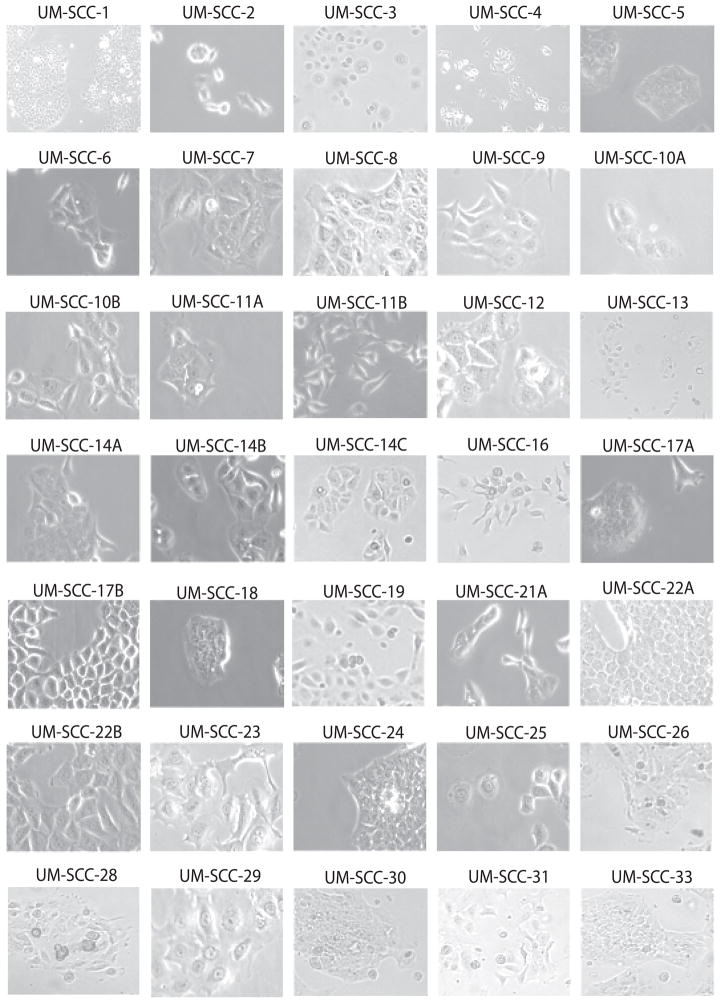
Representative photomicrographs of UM-SCC cell lines are shown in Figure 1 to illustrate the various in vitro morphologies typically exhibited by individual cell lines. Additional photographs of UM-SCC cell lines are also reported in two book chapters for comparison(40, 41). Note that changes occur with increasing cell density in some cell lines. For example, UM-SCC-5 and UM-SCC-17A grow as tightly packed colonies. UM-SCC-17B has a similar morphology to UM-SCC-17A, but the cells are less inclined to pack tightly especially shortly after passage. UM-SCC-74A and -74B are from a patient who was previously treated with chemotherapy and radiation and the cells in both cultures have undergone epithelial-mesenchymal transition, giving the culture a fibroblastoid appearance. This is consistent with the sarcomatoid morphology sometimes observed in tissue samples from recurrent SCC after radiation.
Figure 1. Representative photomicrographs.
University of Michigan squamous cell carcinoma (UM-SCC) cell lines were cultured for 24 hours before photographs were captured under either a 10x (UM-SCC-1) or 40x objective lens (remaining cell lines). In all cases, genotyped cell lines were imaged.
It was not possible to retrieve viable isolates for some of the original UM-SCC- cell lines from liquid nitrogen storage. However, we did genotype the DNA from these non-viable cells so that if others have healthy cultures of the UM-SCC cell lines no longer available in our bank, the correct genotype of the original cell line is provided. Such examples (UM-SCC-15, -20 and -27) are marked in Table 1 with an asterisk. We discovered several examples of mislabeled cell lines within our own bank. However, for each of the mislabeled cell lines, we retrieved early passage vials from our bank and found unique genotypes for each cell line. These were expanded and used to repopulate the tissue core bank.
Discussion
A lack of vigilance in cell acquisition and identity testing has plagued scientific studies and publications since the inception of cell line methods(42–47). In the 1970s and 80s examples of inter and intraspecies cross contamination of human cell lines was documented by Nelson-Rees(43, 45). Data produced from cross-contaminated heterogeneous populations of cells, or incorrectly identified cell lines that might be from a different tumor type or even the wrong species leads to incorrect conclusions, experimental results that are not representative of a particular tumor or tissue type, confusion in the literature and a general mistrust of data produced with cell lines. In 2004, for example, one study reported that 9% of 483 researchers used cultures containing HeLa contaminants.(48) Additionally, we performed a simple literature search for scientific papers that compared parental MCF-7 cells with an adriamycin resistant cell line thought to be derived from MCF-7 called and MCF-7/Adr. This search revealed 187 different papers, some of which have gone on to propose the use of novel chemotherapeutics in specific patient populations. However, it has recently been shown that MCF-7/Adr is actually an ovarian carcinoma cell line(49) meaning that most of the data analysis between two cell lines is completely invalid.
Despite the critical nature of correctly identified cell lines as model systems, it has been difficult to get funding for cell line characterization, leaving researchers who realize the importance of the problem in the dark. The problem of contaminated cell lines has been addressed previously by one of us(40) as well as in more recent editorial articles in Science and several other journals.(20–22) A recent paper(16) examined reports in the literature of the TP53 mutational status from different investigators who studied the cells that are included in the NCI panel of 60 representative human tumor cell lines. The authors reported finding discrepancies in the reported TP53 mutation status for 13/60 (22%) cell lines included this important repository. Their findings suggest that different version of the cell lines are being used in various laboratories and that they may not be the cell line the investigators think they are using.
Because it is necessary to reliably genotype cells that have been cultured in independent laboratories for multiple years, several studies have focused on the reproducibility of microsatellite genotyping by studying long-term microsatellite stability. For example, Masters et al.(23) analyzed HeLa cells that had been cultured independently by different labs over several years and found both gains and losses of alleles. However, only a few alleles were altered in each case and, because of the consistency between the other alleles, the cell lines were still able to be identified as HeLa with very high probability. Likewise, the group analyzed the genotypes of cell lines derived by in vitro selection by long term exposure to chemotherapy, and found that the differences between the STR loci were no greater than those between HeLa cells that had been independently cultured(23). Despite the fact that cell lines can be identified after long periods of independent culturing, phenotypic differences arise in different laboratories because cell lines evolve in vitro, likely leading to the increased growth potential. As such, cell lines should be periodically refreshed from the low passage stocks.
With the intense demand for the UM-SCC head and neck cancer cell lines from colleagues around the world, and a desire to insure that results from multiple labs could be compared, we took advantage of the availability of rapid, low cost, highly polymorphic microsatellite analysis to genotype our entire University of Michigan cell line panel. Like others before us, we were chagrined to find that over time mistakes had been made and mislabeling of cell lines had occurred even within our own cell line bank. Since ours is a laboratory that stresses good principles of tissue culture, this example shows how easily mistakes can be made and perpetuated in cell culture studies. Table 1 from this paper and representative photographs of each of our genetically characterized cell lines will be posted on the University of Michigan Head and Neck Cancer SPORE web page for easy access for other investigators who have these lines in their laboratory.
Supplementary Material
Acknowledgments
This work was supported by the NIH through the University of Michigan Cancer Specialized Programs of Research Excellence Grant P50 CA97248, the University of Michigan Comprehensive Cancer Center, National Cancer Institute Core Grant P30 CA46592, NIH National Institute on Deafness and Other Communication Disorders grant P30 DC05188, and NIDCR DE13346 (TEC), NIH NCI CA83087 (CRB).
References
- 1.Lansford C, Grenman R, Bier H, Somers KD, Kim S-Y, Whiteside TL, Clayman GL, Welkoborsky HJ, Carey TE. Head and Neck Cancers. In: Masters JaPB., editor. Human Cell Culture Vol II Cancer Cell Lines Part 2. Norwell MA: Kluwer Academic Publishers; 1999. pp. 185–256. [Google Scholar]
- 2.Grenman R, Carey TE, McClatchey KD, et al. In vitro radiation resistance among cell lines established from patients with squamous cell carcinoma of the head and neck. Cancer. 1991;67(11):2741–7. doi: 10.1002/1097-0142(19910601)67:11<2741::aid-cncr2820671105>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Bradford CR, Zhu S, Ogawa H, et al. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck. 2003;25(8):654–61. doi: 10.1002/hed.10274. [DOI] [PubMed] [Google Scholar]
- 4.Van Dyke DL, Worsham MJ, Benninger MS, et al. Recurrent cytogenetic abnormalities in squamous cell carcinomas of the head and neck region. Genes Chromosomes Cancer. 1994;9(3):192–206. doi: 10.1002/gcc.2870090308. [DOI] [PubMed] [Google Scholar]
- 5.Kimmel KA, Carey TE. Altered expression in squamous carcinoma cells of an orientation restricted epithelial antigen detected by monoclonal antibody A9. Cancer Res. 1986;46(7):3614–23. [PubMed] [Google Scholar]
- 6.Kimmel KA, Carey TE, Judd WJ, McClatchey KD. Monoclonal antibody (G10) to a common antigen of human squamous cell carcinoma: binding of the antibody to the H type 2 blood group determinant. J Natl Cancer Inst. 1986;76(1):9–19. [PubMed] [Google Scholar]
- 7.Carey TE, Laurikainen L, Nair TS, et al. Regulation of expression and phosphorylation of A9/alpha 6 beta 4 integrin in normal and neoplastic keratinocytes. J Natl Cancer Inst Monogr. 1992;(13):75–86. [PubMed] [Google Scholar]
- 8.Carey TE, Nair TS, Chern C, et al. Blood group antigens and integrins as biomarkers in head and neck cancer: is aberrant tyrosine phosphorylation the cause of altered alpha 6 beta 4 integrin expression? J Cell Biochem Suppl. 1993;17F:223–32. doi: 10.1002/jcb.240531033. [DOI] [PubMed] [Google Scholar]
- 9.Liebert M, Wedemeyer G, Stein JA, et al. The monoclonal antibody BQ16 identifies the alpha 6 beta 4 integrin on bladder cancer. Hybridoma. 1993;12(1):67–80. doi: 10.1089/hyb.1993.12.67. [DOI] [PubMed] [Google Scholar]
- 10.Van Waes C, Carey TE. Overexpression of the A9 antigen/alpha 6 beta 4 integrin in head and neck cancer. Otolaryngol Clin North Am. 1992;25(5):1117–39. [PubMed] [Google Scholar]
- 11.Van Waes C, Kozarsky KF, Warren AB, et al. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin alpha 6 beta 4. Cancer Res. 1991;51(9):2395–402. [PubMed] [Google Scholar]
- 12.Van Waes C, Surh DM, Chen Z, et al. Increase in suprabasilar integrin adhesion molecule expression in human epidermal neoplasms accompanies increased proliferation occurring with immortalization and tumor progression. Cancer Res. 1995;55(22):5434–44. [PubMed] [Google Scholar]
- 13.Gilbert DA, Reid YA, Gail MH, et al. Application of DNA fingerprints for cell-line individualization. Am J Hum Genet. 1990;47(3):499–514. [PMC free article] [PubMed] [Google Scholar]
- 14.MacLeod RA, Spitzer D, Bar-Am I, et al. Karyotypic dissection of Hodgkin’s disease cell lines reveals ectopic subtelomeres and ribosomal DNA at sites of multiple jumping translocations and genomic amplification. Leukemia. 2000;14(10):1803–14. doi: 10.1038/sj.leu.2401894. [DOI] [PubMed] [Google Scholar]
- 15.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23(2):147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 16.Berglind H, Pawitan Y, Kato S, Ishioka C, Soussi T. Analysis of p53 mutation status in human cancer cell lines: a paradigm for cell line cross-contamination. Cancer Biol Ther. 2008;7(5) doi: 10.4161/cbt.7.5.5712. [DOI] [PubMed] [Google Scholar]
- 17.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54(18):4855–78. [PubMed] [Google Scholar]
- 18.Hollstein M, Rice K, Greenblatt MS, et al. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22(17):3551–5. [PMC free article] [PubMed] [Google Scholar]
- 19.MacLeod RA, Dirks WG, Matsuo Y, Kaufmann M, Milch H, Drexler HG. Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int J Cancer. 1999;83(4):555–63. doi: 10.1002/(sici)1097-0215(19991112)83:4<555::aid-ijc19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee R. Cell biology. A lonely crusade. Science. 2007;315(5814):930. doi: 10.1126/science.315.5814.930. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee R. Cell biology. When 60 lines don’t add up. Science. 2007;315(5814):929. doi: 10.1126/science.315.5814.929. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee R. Cell biology. Cases of mistaken identity. Science. 2007;315(5814):928–31. doi: 10.1126/science.315.5814.928. [DOI] [PubMed] [Google Scholar]
- 23.Masters JR, Thomson JA, Daly-Burns B, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A. 2001;98(14):8012–7. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y, Tao H, Kim JJ, et al. Alterations of DNA mismatch repair proteins and microsatellite instability levels in gastric cancer cell lines. Lab Invest. 2004;84(7):915–22. doi: 10.1038/labinvest.3700117. [DOI] [PubMed] [Google Scholar]
- 25.Edwards A, Hammond HA, Jin L, Caskey CT, Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992;12(2):241–53. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- 26.Warne D, Watkins C, Bodfish P, Nyberg K, Spurr NK. Tetranucleotide repeat polymorphism at the human beta-actin related pseudogene 2 (ACTBP2) detected using the polymerase chain reaction. Nucleic Acids Res. 1991;19(24):6980. doi: 10.1093/nar/19.24.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura Y, Leppert M, O’Connell P, et al. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987;235(4796):1616–22. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- 28.Schlotterer C, Tautz D. Slippage synthesis of simple sequence DNA. Nucleic Acids Res. 1992;20(2):211–5. doi: 10.1093/nar/20.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MP. Exploring molecular biology. An older surgeon looks at a new universe. Arch Surg. 1995;130(8):811–6. doi: 10.1001/archsurg.1995.01430080013001. [DOI] [PubMed] [Google Scholar]
- 30.Krause CJ, Carey TE, Ott RW, Hurbis C, McClatchey KD, Regezi JA. Human squamous cell carcinoma. Establishment and characterization of new permanent cell lines. Arch Otolaryngol. 1981;107(11):703–10. doi: 10.1001/archotol.1981.00790470051012. [DOI] [PubMed] [Google Scholar]
- 31.Bukvic N, Gentile M, Susca F, et al. Sex chromosome loss, micronuclei, sister chromatid exchange and aging: a study including 16 centenarians. Mutat Res. 2001;498(1–2):159–67. doi: 10.1016/s1383-5718(01)00279-0. [DOI] [PubMed] [Google Scholar]
- 32.Guttenbach M, Schakowski R, Schmid M. Aneuploidy and ageing: sex chromosome exclusion into micronuclei. Hum Genet. 1994;94(3):295–8. doi: 10.1007/BF00208287. [DOI] [PubMed] [Google Scholar]
- 33.Hunter S, Gramlich T, Abbott K, Varma V. Y chromosome loss in esophageal carcinoma: an in situ hybridization study. Genes Chromosomes Cancer. 1993;8(3):172–7. doi: 10.1002/gcc.2870080306. [DOI] [PubMed] [Google Scholar]
- 34.Carey TE, Van Dyke DL, Worsham MJ, et al. Characterization of human laryngeal primary and metastatic squamous cell carcinoma cell lines UM-SCC-17A and UM-SCC-17B. Cancer Res. 1989;49(21):6098–107. [PubMed] [Google Scholar]
- 35.Worsham MJ, Benninger MJ, Zarbo RJ, Carey TE, Van Dyke DL. Deletion 9p22-pter and loss of Y as primary chromosome abnormalities in a squamous cell carcinoma of the vocal cord. Genes Chromosomes Cancer. 1993;6(1):58–60. doi: 10.1002/gcc.2870060111. [DOI] [PubMed] [Google Scholar]
- 36.Worsham MJ, Wolman SR, Carey TE, Zarbo RJ, Benninger MS, Van Dyke DL. Common clonal origin of synchronous primary head and neck squamous cell carcinomas: analysis by tumor karyotypes and fluorescence in situ hybridization. Hum Pathol. 1995;26(3):251–61. doi: 10.1016/0046-8177(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 37.Frank CJ, McClatchey KD, Devaney KO, Carey TE. Evidence that loss of chromosome 18q is associated with tumor progression. Cancer Res. 1997;57(5):824–7. [PubMed] [Google Scholar]
- 38.Jones JW, Raval JR, Beals TF, et al. Frequent loss of heterozygosity on chromosome arm 18q in squamous cell carcinomas. Identification of 2 regions of loss--18q11.1-q12.3 and 18q21. 1-q23. Arch Otolaryngol Head Neck Surg. 1997;123(6):610–4. doi: 10.1001/archotol.1997.01900060052009. [DOI] [PubMed] [Google Scholar]
- 39.Takebayashi S, Ogawa T, Jung KY, et al. Identification of new minimally lost regions on 18q in head and neck squamous cell carcinoma. Cancer Res. 2000;60(13):3397–403. [PubMed] [Google Scholar]
- 40.Carey TE. Establishment of Epidermoid Carcinoma Cell Lines. In: Wittes RE, editor. Head and Neck Cancer. New York: John Wiley & Sons; 1985. pp. 287–314. [Google Scholar]
- 41.Carey TE. Head and Neck Tumor Cell Lines. In: Hay RJ, Park J-G, Gazdar A, editors. Atlas of Human Tumor Cell lines. New York: Academic Press Inc; 1994. pp. 79–120. [Google Scholar]
- 42.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953;97(5):695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson-Rees WA, Daniels DW, Flandermeyer RR. Cross-contamination of cells in culture. Science. 1981;212(4493):446–52. doi: 10.1126/science.6451928. [DOI] [PubMed] [Google Scholar]
- 44.Nelson-Rees WA, Flandermeyer RR, Daniels DW. T-1 cells are HeLa and not of normal human kidney origin. Science. 1980;209(4457):719–20. doi: 10.1126/science.7394535. [DOI] [PubMed] [Google Scholar]
- 45.Nelson-Rees WA, Flandermeyer RR. Inter- and intraspecies contamination of human breast tumor cell lines HBC and BrCa5 and other cell cultures. Science. 1977;195(4284):1343–4. doi: 10.1126/science.557237. [DOI] [PubMed] [Google Scholar]
- 46.Gartler SM. Genetic markers as tracers in cell culture. Natl Cancer Inst Monogr. 1967;26:167–95. [PubMed] [Google Scholar]
- 47.Gartler SM. Apparent Hela cell contamination of human heteroploid cell lines. Nature. 1968;217(5130):750–1. doi: 10.1038/217750a0. [DOI] [PubMed] [Google Scholar]
- 48.Buehring GC, Eby EA, Eby MJ. Cell line cross-contamination: how aware are Mammalian cell culturists of the problem and how to monitor it? In Vitro Cell Dev Biol Anim. 2004;40(7):211–5. doi: 10.1290/1543-706X(2004)40<211:CLCHAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Liscovitch M, Ravid D. A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (redesignated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett. 2007;245(1–2):350–2. doi: 10.1016/j.canlet.2006.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.