Abstract
The event related potential (ERP) effect of mismatch negativity (MMN) was the first electrophysiological probe to evaluate cognitive processing (change detection) in newborn infants. Initial studies of MMN predicted clinical utility for this measure in identification of infants at risk for developmental cognitive deficits. These predictions have not been realized. We hypothesized that in sleeping newborn infants, measures derived from wavelet assessment of power in the MMN paradigm would be more robust markers of the brain's response to stimulus change than the ERP-derived MMN. Consistent with this premise, we found increased power in response to unpredictable and infrequent tones compared to frequent tones. These increases were present at multiple locations on the scalp over a range of latencies and frequencies and occurred even in the absence of an ERP-derived MMN. There were two predominant effects. First, theta band power was elevated at middle and late latencies (200 to 600 ms), suggesting that neocortical theta rhythms that subserve working memory in adults are present at birth. Second, late latency (500 ms) increased power to the unpredictable and infrequent tones was observed in the beta and gamma bands suggesting that oscillations involved in adult cognition are also present in the neonate. These findings support the expectation that frequency-dependent measures, such as wavelet power, will improve the prospects for a clinically useful test of cortical function early in the postnatal period.
Introduction
Twenty years ago, the discovery that newborn infants demonstrate an event related potential (ERP) effect called mismatch negativity (MMN) provided the first known electroencephalographic probe of a cognitive process present at birth (Alho, Sainio, Sajaniemi, Reinikainen, & Naatanen, 1990). MMN provides a quantitative measure of pre-conscious change detection (Friedman, Cycowicz, & Gaeta, 2001). The ability to automatically detect sudden environmental changes underpins the orienting response, a behavioral phenotype crucial for survival that is widely conserved across animal phyla (Sokolov, 1990). Moreover, for humans, automatic auditory change detection is crucial for speech perception; indeed, various theories have proposed low-level phonological deficits as causal factors in language disorders (D. V. Bishop, 2007). In the auditory MMN paradigm, a frequently occurring sound is intermixed with an infrequent sound, usually in a random sequence, while electroencephalogram (EEG) activity is recorded. For each sound, the time-locked EEG recording is averaged over many repetitions to produce the ERP. MMN is the difference between ERPs obtained for the two sounds. MMN is generally thought to reflect sensory memory rather than simply adaptation to the more frequent sound (Naatanen, Jacobsen, & Winkler, 2005; Naatanen, Paavilainen, Rinne, & Alho, 2007) (but for an alternative hypothesis see (May & Tiitinen, 2010)). MMN originates in the auditory cortex and discrete frontal regions (Alho, Huotilainen, & Naatanen, 1995), and can be detected during sleep in infants (Cheour, Leppanen, & Kraus, 2000).
Adult studies have shown that the MMN can have clinical utility e.g. predicting coma recovery and indexing cognitive decline in disease and aging (Duncan, et al., 2009). Although there may be qualitative differences between adult and infant processes of change detection, when first discovered in infants MMN was considered a promising candidate for a clinical test of newborn cortical function (Alho, et al., 1990). In the ensuing decades, however, the clinical potential for early identification of risk for subsequent cognitive deficits has not been realized, in part because the MMN is less reliably detected and has more variable morphology in infants than in adults (Fellman & Huotilainen, 2006). Further, methodological differences in recording and analysis (D. V. Bishop, 2007), weak stimulus contrast (Kushnerenko, et al., 2007) and the range of gestational age at testing (P. H. Leppanen, et al., 2004) may have contributed to inconsistent results across laboratories (reviewed in He at al. (He, Hotson, & Trainor, 2007)).
For change detection in newborns to make the transition from a research phenomenon to a clinical tool, several conditions must ultimately be met. First, clinical and analytic strategies must be developed to consistently detect the cortical response in healthy low-risk newborns. Second, universally accepted norms must be defined for the cortical response for “normal” infants across a range of post-conceptional ages. Ultimately, carefully designed prospective studies will then be needed to describe the association between an absent or abnormal newborn cortical response and indices of subsequent cognitive development. Progress in meeting these conditions for the ERP-derived MMN has been hindered by the variable morphology and latency and inconsistency within and across individual subjects (D. V. Bishop, 2007; He, et al., 2007). The lack of a consistent MMN in newborns may indicate insufficient validity for risk assessment, in which case the MMN paradigm would have limited clinical utility. An alternate possibility is that current measurement techniques are not sufficiently sensitive to consistently detect the infant's cortical response to the MMN paradigm. This report focuses on one promising candidate for a more sensitive index, namely, frequency dependent measures of power.
EEG spectral power is a frequency-dependent measure of voltage amplitude on the scalp resulting from oscillations of neuronal membrane potentials. These rhythms reflect increases or decreases in an individual neuron's probability of firing at specific times during an oscillation (or nested set of oscillations). Oscillatory processes provide mechanisms for organizing neuronal activity at the population level and play a role in many aspects of ERP morphology (Makeig, et al., 2002). Assessment of the modulation of EEG spectral power has the potential to provide a sensitive measure of cortical function during the MMN paradigm because oscillatory processes are correlated with diverse cognitive functions. Moreover, specific cognitive functions are differentially correlated with oscillatory activity in particular frequency bands (Buzsaki & Draguhn, 2004). For example, successful memory formation is associated with greater theta band (4 to 8 Hz) power in temporal and parietal cortex (Sederberg, Kahana, Howard, Donner, & Madsen, 2003), while perception of faces is correlated with gamma band (> 30 Hz) synchronization between frontal and parietal scalp regions (Rodriguez, et al., 1999). Thus, we hypothesized that wavelet measures of power, which quantify oscillatory activity in response to a stimulus across both time and frequency domains, would provide a more sensitive measure of a cortical response to stimulus change than the MMN derived from infant ERPs, which do not differentiate spectral information.
It is important to note that in an ERP experiment, both ERP and power effects are not necessarily due to cortical oscillations. ERPs arise from activity that is phase-locked to the time of stimulation (i.e. has the same phase at a given latency across stimulus trials). However the extent to which ERPs are due to stimulus-induced phase-resetting of endogenous rhythms versus exogenous signal transduction and integration is an ongoing topic of research (Sauseng, et al., 2007). One hypothesis is that short latency ERP features (e.g. brain stem auditory evoked response) have more to do with exogenous influences while longer latency features may have more to do with endogenous rhythms. Attempts to delineate power effects due to one mechanism from those due to the other, by for example removing the ERP from single trial data prior to spectral estimation, are complicated by trial-to-trial variability in exogenous signals (Truccolo, Ding, Knuth, Nakamura, & Bressler, 2002). In contrast, estimating spectral power without removing the ERP leaves open the possibility that modulations in power reflect the spectral signature of a transient exogenous signal rather than amplitude modulation of an endogenous rhythm (Sauseng, et al., 2007). In this study, we employ the latter approach, estimating spectral power without removing the ERP, and discuss the relative contribution of the two putative mechanisms in our findings. Since previous newborn MMN studies found larger amplitude ERPs for infrequent compared to frequent sounds, we further hypothesized that the infrequent tone response would show greater power at lower frequencies, where ERP power is concentrated.
These hypotheses motivated the present study of power in the MMN paradigm in full term newborns. High-density EEG recordings obtained during an MMN paradigm were used to derive two measures of cortical activity during sleep: conventional ERP responses and wavelet power.
Materials and Methods
Participants
The study was approved by the Institutional Review Boards at the New York State Psychiatric Institute and Columbia University College of Physicians and Surgeons. The subjects were full term infants tested within two days of birth in the well-baby nursery of the Morgan Stanley Children's Hospital of New York Presbyterian at the Columbia University Medical Center after obtaining informed parental consent. Thirty-two sleeping infants were enrolled in the study group. Data from 21 of the neonates (13 female) passed the stringent data quality requirements outlined below and were analyzed for this report. The most common reason subjects failed to have sufficient usable data was movement artifact. Gestational ages ranged from 36.9 to 40.6 weeks with mean of 38.9 weeks and standard deviation 1.1 weeks.
Procedures
Data were recorded with an Electrical Geodesics Inc. system using 128 electrode nets, allowing no more than 50K ohm impedance at any electrode. Electrodes were referenced to the vertex electrode (Cz) during recording. Data were sampled at 1000 Hz and hardware filtered below 400 Hz and above 0.01 Hz. Presentation software on a separate computer delivered sound stimuli to open air headphone speakers ~80 dB at the infant ear and sent stimulus marks to the EEG system. Stimuli were a random sequence of 100 ms long tone complexes with fundamental frequencies of 750 Hz and 1000 Hz, with probabilities of 0.84 and 0.16, respectively. The time between tone onsets was 768 ms with random jitter of ~15 ms.
Data processing
After recording, a 1600 point linear phase (FIR) software notch filter (4 Hz wide notches with 60 dB falloff within 2Hz of the notch edges) for 60 Hz and its harmonics up to 360 Hz was applied. To avoid edge effects in the analysis window, recordings were segmented into 2304 ms epochs that included three stimuli, with the middle stimulus being either the frequent or infrequent tone but the first and third stimuli always the frequent tone. Frequent tone epochs were downsampled to match the number of infrequent tone epochs. Data in the outer ring of electrodes were excluded as those tend to have the most artifact (Srinivasan, Nunez, & Silberstein, 1998). Data in the remaining electrodes were rejected for an epoch if any of the following criteria were met: absolute sample-to-sample change greater than 25 microvolts; absolute value greater than 100 microvolts; spectral slope between 20 and 200 Hz greater than -0.1 (to detect muscle artifact). Epochs with more than 16 percent of channels matching one of the above criteria were rejected. For the remaining epochs, eye movements were detected using a bipolar montage of smoothed (40-point boxcar) raw data from eleven channels near the eyes and epochs with suspected eye movements were rejected (criteria were four or more good bipolar channels with an absolute first derivative greater than 0.5 microvolts per sample). If fewer than 100 epochs for each condition remained after pre-processing, the subject was excluded from further analysis. After preprocessing, data were referenced to the average over all good electrodes at each time point in order to characterize topography in an unbiased fashion (Junghofer, Elbert, Tucker, & Braun, 1999). For ERPs, an averaged-mastoids reference was used as well in order to facilitate comparisons with previous literature.
MMN effects
ERPs were computed as the mean voltage across epochs from 100 ms pre-stimulus to 768 ms post-stimulus after removing the mean voltage in the 100 ms pre-stimulus interval (“baseline correction”). To test for presence of an MMN response, a Repeated Measures Analysis of Variance (ANOVA) was conducted with stimulus type (infrequent vs. frequent tone), electrode site, and time as within-subjects factors and ERP amplitude as the dependent measure. Greenhouse-Geisser corrections were used for tests of within-subjects effects. This analysis was conducted separately with the data referenced to an average mastoid reference and to an average reference. Mean ERP amplitudes over 100 ms intervals were used for 6 sequential intervals spanning 50 to 650 ms post-stimulus. Electrode sites were 6 locations in fronto-central regions of the International 10-20 system (F3, F4, Fz, C3, C4, Cz). ERPs at each of those locations were spatially smoothed (averaged) over the given electrode and its nearest neighbor electrodes.
For depiction of the full topography of MMN effects, independent sample t-tests were conducted at each time point (ms) comparing mean voltage for infrequent vs. frequent tones. The difference of the group-averaged ERPs (infrequent minus frequent tone) was then plotted at all electrode locations with asterisks identifying time points of significance at the p < .05 level.
Wavelet power effects
A time-frequency decomposition of power was performed using wavelet transforms. To avoid edge effects at low frequencies, a broader temporal window (768 pre-stimulus to 1536 post-stimulus) was used, but analysis was restricted to the same window as the ERP. For each trial, wavelet power was computed using a Morlet wavelet of 6 cycles at 10 scales per octave using programs modified from Torrence and Compo (Torrence & Compo, 1998) and power was then averaged across trials. Wavelet power was then log transformed. Wavelet power was not baseline corrected; the interstimulus interval, 768 ms, precluded estimation of a baseline for lower frequencies. For wavelet power, the aim was to identify the spatial, time and frequency parameters which show the most promise for accurate detection of an cortical response. To that end we used both spatial and time-frequency averages of power to explore the full 4 dimensional results. The spatial average emphasizes global effects that have a widespread spatial extent, while the time-frequency averages highlight local effects that occur over several frequencies and time points. T-tests were used to compare power for infrequent vs. frequent tone trials. T-tests were performed for every point in the time-frequency plane for spatially-averaged power. For power averaged over discrete time-frequency areas, t-tests were performed at each electrode location. Multiple comparisons were not formally controlled for due to the exploratory nature of this study, but subsequent interpretation was limited to results where non-random clustering occurred (cf. (Hsiao, Wu, Ho, & Lin, 2009)) either spatially or within the time-frequency plane.
Comparisons
To illustrate the relative sensitivities of the two measures of cortical activity, ERP and wavelet power results were compared at the same six electrode locations used in the ANOVA. To more globally test the relative sensitivities of the two measures in an unbiased fashion, sign tests were performed at each electrode location. For the MMN, the sign of the mean ERP difference in each of the 6 ANOVA intervals was used. For wavelet power, the sign of the mean log power difference within discrete time-frequency areas was used. Finally, for those power effects with the lowest p-values, histograms of individual subject results were used to show the distribution across subjects.
Results
Wavelet power differences between infrequent and frequent tone trials
The first set of analyses compared wavelet power during the infrequent vs. frequent tone trials. Since the results for wavelet power are four-dimensional (2D scalp location × time × frequency), visualization is challenging, but an attempt is made to summarize these results concisely in Figures 1 and 2. In Figure 1, differences in log power (infrequent tone response minus frequent tone response) were averaged over all electrodes, and the resulting time-frequency plane is displayed, with the difference shown in panel 1A and p-values shown in panel 1B. In a complementary fashion, in Figure 2 the average log power difference over fine-grained time-frequency areas was calculated and the resulting spatial topography displayed, with each topography plot shown in the corresponding time-frequency area. Together, Figures 1 and 2 show that increased power in response to the infrequent tone is apparent at lower frequencies but also occurs in the beta and gamma bands, with fewer effects present at intermediate frequency bands. Furthermore, both time-frequency areas (Figure 1) and spatial regions (Figure 2) with significant effects occurred in contiguous clusters, making it highly unlikely that such significance was due to chance (Hsiao, et al., 2009). Below we discuss the two power effects with the highest degree of clustering.
Figure 1.
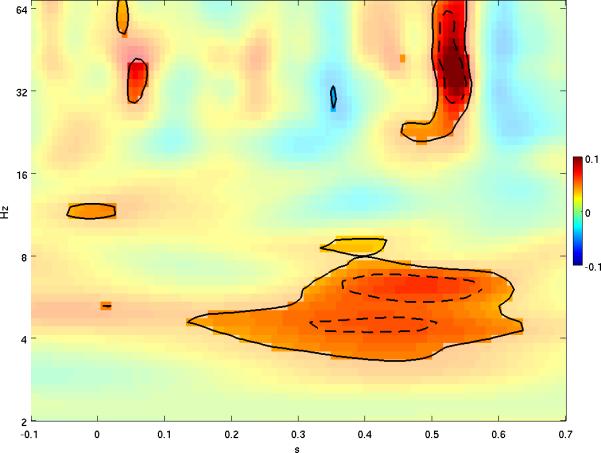
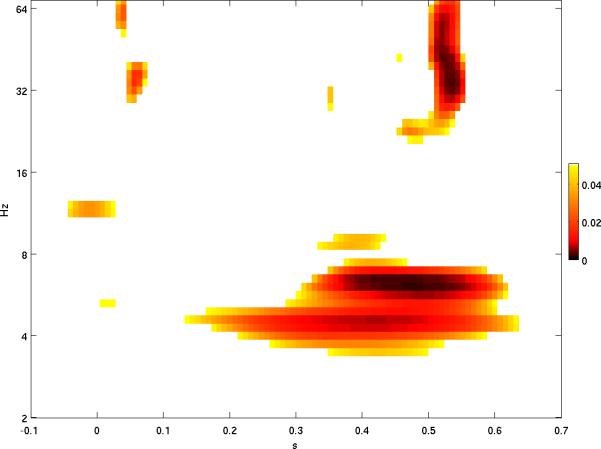
A. Mean over all electrodes of log power difference (response to infrequent minus response to frequent tones) shown by color, with red denoting greater positive difference (i.e. log power higher for infrequent than frequent tones) and blue denoting greater negative difference (i.e. log power higher for frequent tones). In A, significant differences are shown with full transparency and contours are drawn for p = 0.05 and 0.01 (solid and dashed, respectively). P-values are shown in B.
Figure 2.
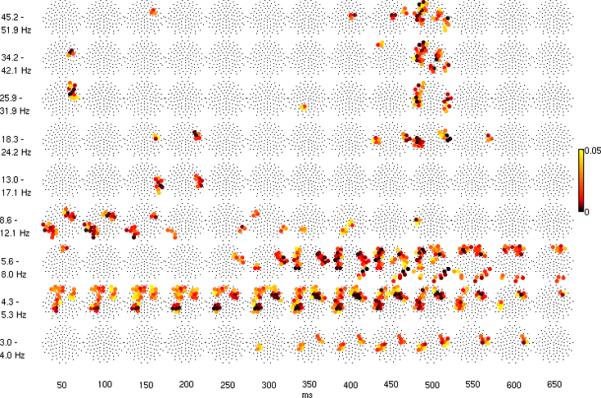
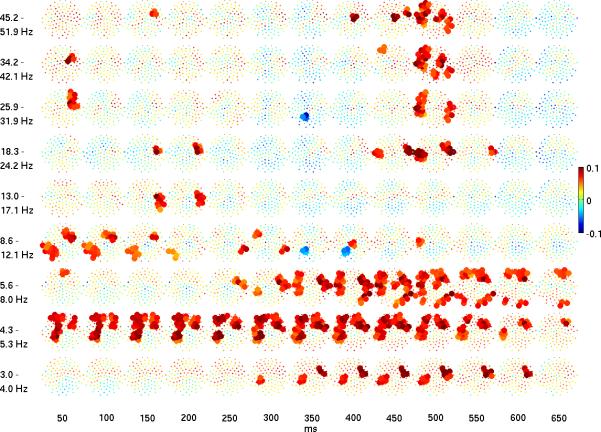
A. Mean over time-frequency areas of log power difference (response to infrequent tones minus response to frequent tones) at each electrode, shown in topography plots that are planar projections of electrode locations (nose at the top). The time-frequency areas have a frequency width that varies as log 2 times the center frequency and a temporal width fixed at 100 ms, with 50% overlap. In A, difference in log power is shown with the same color scale as Figure 1A and significant differences (p < 0.05) are shown with larger ball size. P-values are shown in B.
Middle and late latency theta power
Greater power for the infrequent tone occurred in the delta band at 3 to 4 Hz at middle latencies of 300 to 500 ms. In the theta band at 4 to 8 Hz, increased power occurred over a broader time interval, from 200 to 600 ms. Time-frequency areas of elevated delta and theta power had similar spatial topographies, with relatively circumscribed right frontal, left frontal, and left parietal-temporal regions. In the upper theta band elevated power also occurred in bilateral temporal regions and right occipital regions. Greater power in the alpha band at early latencies was also observed, limited to right frontal and left parieto-occipital regions. Considered together, Figures 1 and 2 suggest elevated theta power at middle and late latencies (200 to 600 ms) was the most robust lower frequency effect.
Late latency beta and gamma power
In the beta and gamma bands, greater power occurred early (< 150 ms) and late (450 - 550 ms) but not at middle latencies. The topography of the early high frequency effect was limited to a right frontal region. In contrast, the late high frequency effect in the gamma band at approximately 500 ms was more widespread, overlapping many of the frontal, parietal and temporal regions where effects also were seen at the lower frequencies. In the beta band, the effect was limited over left and right temporal regions. Considered together, Figures 1 and 2 suggest the most robust higher frequency effect was a transient increase in power in the beta and gamma bands at approximately 500 ms in response to the deviant stimulus (Figures 1 and 2). In fact, for the spatially averaged results (Figure 1), the maximum log power difference occurred in the gamma band exclusively at this latency.
Recently, it has been shown that much of what may appear as gamma band power in scalp EEG may actually be due to microsaccadic eye movements (Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008). Unlike saccades, which can be identified in single trial data, microsaccades have lower amplitudes and greater high frequency content. Thus, in an ERP experiment, if microsaccades are correlated with one condition more than another, a spurious gamma band effect could result. It is unlikely that microsaccades account for the high frequency effect reported here for two reasons. First, the effect was not maximal in fronto-polar regions where microsaccades are most pronounced (Figure 2). Second, while the spectrum of microsaccadic power extends to well above 100 Hz, the gamma band effect in the current study was absent at frequencies above 80 Hz (Supplementary Figure S2).
MMN differences between infrequent and frequent tone trials
In order to facilitate comparisons both with prior newborn ERP-derived MMN studies and with the wavelet power analysis, ERPs were computed using two different references: an averaged-mastoid reference (typically used in prior ERP studies) and the average reference (used in the power analysis). To illustrate the MMN effect, the difference of ERP waveforms (infrequent minus frequent tone) at all electrode locations for the averaged-mastoids reference is shown in Figure 3, with asterisks identifying times of significance (a corresponding plot for the average reference is shown is Supplementary Figure S1). At the mid-latencies where the MMN effect typically is found, the only significant difference was at a single left-central electrode using the averaged-mastoids reference (and a single right temporal location using the average reference). At frontal and central locations where a MMN effect was expected at mid-latencies, there was a non-significant trend toward a MMN effect (for the average reference (Supplementary Figure S1), the trend was less pronounced). However, significant differences were observed at longer latencies (> 500 ms) over posterior central and parietal areas, especially in the right hemisphere.
Figure 3.
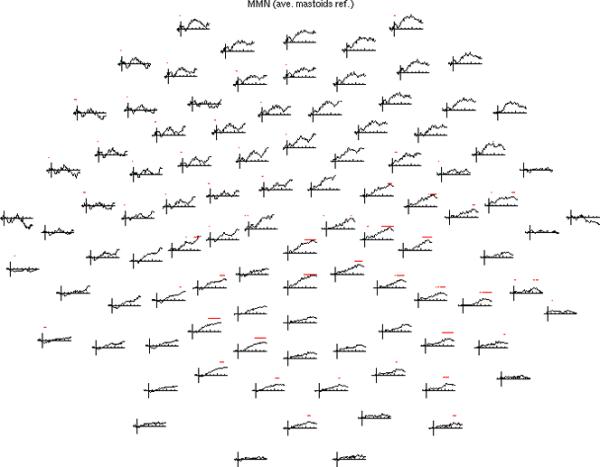
Topography of the MMN (infrequent tone ERP minus frequent), shown in a planar projection of electrode locations with nose at the top. ERPs used an averaged-mastoids reference. Time axes are from -100 ms to 768 ms relative to stimulus onset. Vertical lines at t = 0 extend from -1 to +1 microvolt. Red asterisks denote time points where the difference was significant (p < 0.05). A significant difference at mid-latencies below 450 ms only occurred at one left central Electrode.
Statistical tests (ANOVA) employed by previous studies to detect an MMN effect in frontal and central locations were performed (Fellman, et al., 2004; P.H. Leppanen, Eklund, & Lyytinen, 1997). Using an average mastoid reference, a Repeated Measures ANOVA with stimulus type, electrode site, and time as within-subjects factors, found that there was no main effect for stimulus type, F (1,18) = 1.91, p = 0.18, indicating that the ERPs in response to standard vs. deviant stimuli did not differ significantly. There also were no interactions between stimulus type and electrode site, F (1.94, 34.97) = 0.63, p = 0.54, or between stimulus type and time window, F (1.83, 32.99) = 1.23, p = 0.30. Similar results were obtained using an average reference for the ERPs.
Interpretation of wavelet power
Because we applied the wavelet transform to single trial data and averaged power across trials, our power analysis captured two types of cortical activity: first, the purely phase-locked activity responsible for the ERP (whether due to an exogenous signal or phase-resetting of endogenous rhythms); and second, both non phase-locked exogenous signals and non phase-locked modulations of endogenous oscillatory amplitudes. It is probable that the theta power effect reported here was most likely due to modulation of endogenous theta rhythms. This can be seen comparing the power and the ERP results. When spatially averaged over the entire electrode array in Figure 1, the theta effect occurs from 200 to 600 ms, with greatest power difference and highest significance from 300 to 500 ms. ERPs cannot be spatially averaged over such widespread regions because of polarity reversals. However, over all electrode locations (see below, Figure 3 and Supplementary Figure S1), no ERP effects were seen from 300 to 450 ms. In contrast, as seen in Figure 2 at the same latencies, increased theta power occurred bilaterally in widespread frontal, central, and temporal regions and in left parietal and right parieto-occipital regions. Another indication that the theta power effect reflected modulation of endogenous rhythms is the fact that no increase was observed when wavelet power was computed from the averaged waveform rather than single trials (not shown).
Comparison of ERP and power effects
To illustrate the sensitivity of wavelet power compared to ERPs and to highlight trends that occurred in the ERPs, Figure 4 compares power and ERP effects at the six electrode locations used in the ANOVA. An MMN effect has been reported in newborn infants at these electrode sites (Ceponiene, et al., 2002; P.H. Leppanen, et al., 1997). At each location, the upper panels display ERPs +/- standard error for the infrequent (red) and frequent (blue) tones. In the topmost panels an averaged-mastoid reference was used and in the second row an average reference was used. Time points where ERP effects occurred using independent sample t-tests (p < 0.05) are marked with asterisks. The lower panels display the difference in log power for the same locations in a format identical to that used in Figure 1A. Peak ERP amplitudes occurred at approximately 300 ms, with amplitudes for the infrequent tone trending larger than amplitudes for the frequent tone; however, ERP differences were not significant at middle latencies at any of the six locations. ERP effects at longer latencies (> 600 ms) at C4 and Cz occurred for the averaged-mastoid reference. In contrast, middle latency power effects in the theta band occurred at all six locations, while the high frequency power effect at approximately 500 ms seen in Figures 1 and 2 occurred in four of the six locations.
Figure 4.
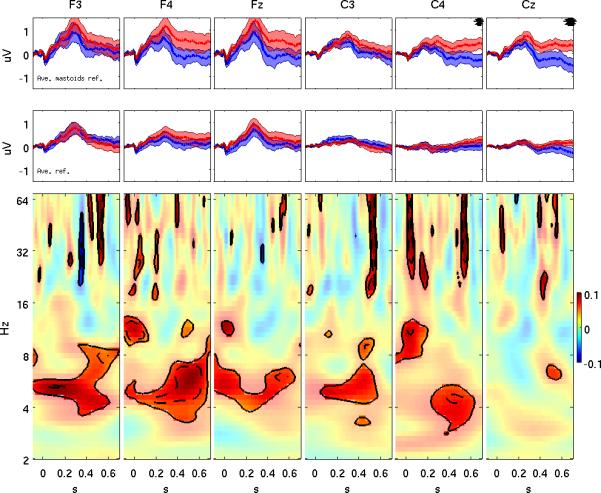
Top panels: Group mean (+/- standard error) ERPs for the frequent (blue) and infrequent (red) tones referenced to averaged-mastoids for six electrode locations of the International 10-20 system. Asterisks denote time points where the difference between ERPs reached significance (p < 0.05). Middle panels: Same as top panels except using average reference. Bottom panels: Differences in log power (infrequent minus frequent) at the same locations over both time and frequency in the same format as Figure 1A. Significant differences are highlighted using contour lines of p-values (solid p = 0.05, dashed p = 0.01) and full transparency of the color scale. Note the absence of an MMN effect (i.e. any significant difference at middle latencies) in the upper two rows in contrast to many significant wavelet power effects in the third row.
To compare the relative sensitivities of the two measures more globally, sign tests were performed at each electrode location. For the MMN, sign tests were conducted on the sign of the mean ERP difference in each of the 6 time intervals used in the ANOVA. No electrodes showed a significant MMN sign effect, except for six unclustered electrodes during the latest interval (550 to 650 ms). In contrast, for wavelet power, sign tests yielded significant effects for mid-late latency theta power (N=37 electrodes), late latency beta power (N=23 electrodes), and late latency gamma power (N=27 electrodes), in each case exhibiting spatial clustering. The sensitivity of these three measures at the electrode location where they were most robust is further illustrated in Figure 5 which provides histograms of individual subject results. The sensitivity of the mid-latency theta power effect is 90%.
Figure 5.
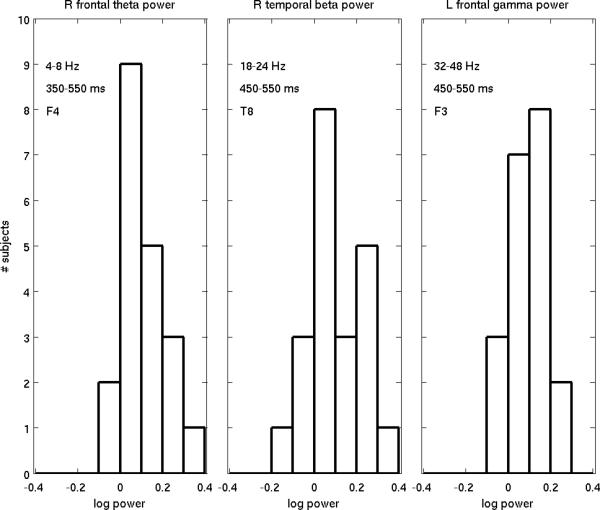
Histograms of individual subject results for mid-late latency theta power, late latency beta and late latency gamma power at the electrode location where they were most significant.
Discussion
The current study assessed two indices of cortical function – ERPs and wavelet power – in sleeping full term newborn infants during an MMN paradigm. The ERP-derived MMN at birth has been observed inconsistently using standard methods (He, et al., 2007). We proposed that wavelet power might yield a more robust measure of cortical response in the MMN paradigm because it incorporates the frequency domain and doesn’t require the response to be phase locked to the stimulus. Indeed, wavelet power analysis showed consistently increased power in response to deviant stimuli (infrequent tones) versus standard stimuli (frequent tones) across multiple scalp locations, latencies and frequencies. The most pronounced observed effects were increased theta power at middle to late latencies and increased beta and gamma power at late latencies. In contrast, ERP measures failed to detect a difference between the average waveforms for frequent vs. infrequent tones at middle latencies. There were, however, some late latency waveform differences noted, particularly in central and posterior scalp locations. In direct comparisons of ERP and wavelet power results at six frontal and central scalp locations, the ERP did not yield significant MMN effects at any of the locations, while wavelet power analysis consistently showed increases in theta power at these same sites. This pattern of results suggests greater sensitivity of wavelet power in detecting cortical responses in the MMN paradigm. Each of these results will be discussed in turn, along with their limitations and implications.
Middle and late latency theta power
Neocortical theta oscillations, especially in temporal regions, have been thought to be involved in memory processes, perhaps via coupling to hippocampal theta rhythms (Buzsaki, 2006). A seminal study using electrocorticography (ECoG) in children and adolescents showed that increased theta band power in right frontal and temporal areas during a word memory task was correlated with successful recall (Sederberg, et al., 2003). Moreover, in a MMN study with adults, Funtemilla et al. (Fuentemilla, Marco-Pallares, Munte, & Grau, 2008) found bilateral increases of theta power in frontal regions in response to the deviant stimulus, but with greater magnitude over right than left frontal hemisphere. In a recent MMN study using adult MEG, Hsiao et al. (Hsiao, et al., 2009) reported increased theta power for the deviant stimulus from 50 to 300 ms in right temporal regions, with a trend toward higher power in left temporal regions. A recent developmental MMN study (D.V. Bishop, Hardiman, & Barry, 2011) with children and adults reported an MMN theta power effect in adults but not in 7-12 or 13 to 16 year old children. However, there were numerous methodological differences between their study and ours. Our finding of increased theta power for the deviant stimulus is the first such demonstration of which we are aware in infant studies, and is convergent with results for adults and older children.
Interestingly, mid-latency theta power effects were often absent over the midline, in spite of ERP trends toward mid-latency differences along the midline (Figure 3 and Supplementary Figure S1). This may reflect regional variations in the degree to which single-trial power receives contributions from phase-locked versus non phase-locked activity. Fuentemilla et al. (Fuentemilla, et al., 2008) concluded in their study of adults that theta power in frontal regions was predominantly due to exogenous power ,while in temporal regions theta power was predominantly due to phase-locked activity stemming from phase re-setting. We report here a theta power effect that cannot be wholly attributed to phase-locked activity. These findings suggest the possibility that neocortical theta rhythms subserving memory are present at birth.
Late latency beta and gamma power
In adults, gamma oscillations have been associated with diverse cognitive processes, including awareness, conscious perception, feature binding in object perception, and bottom-up attention capture (Buschman & Miller, 2007; Fries, 2009). In contrast, gamma band activity in ERP experiments in infants is largely unexplored. Indeed, many ERP studies low-pass filter data at 20 to 30 Hz prior to deriving averaged waveforms, so any phase-locked higher frequency activity is lost in the ERPs. One previous study has reported a gamma band effect in a MMN paradigm in newborn infants. Stefanics et al. (Stefanics, et al., 2007) found a burst of gamma power from 140 to 270 ms in response to the infrequent tone. In our results at the same latencies, there was a trend toward higher gamma power for the infrequent tone. Methodological differences in the stimulus paradigm and the interval between time onsets preclude direct comparisons between Stefanics et al. and the current study. However, these two results provide converging evidence for an association of increased beta/gamma activity with the infrequent tone. Taken together, these results suggest that beta/gamma band activity is present at birth in a manner that correlates with a fundamental cognitive process. Finally, it is of note that as seen in Figure 1A there appears to be a trend for gamma band power at a theta rate. Given the highly rhythmic nature of the stimulus presentation rate (~ 1.3 Hz), it is intriguing to speculate that an endogenous delta rhythm entrained to the sound stream, with the result that theta power became phase-locked to delta phase, and gamma power phase locked to theta phase, along the lines suggested by Lakatos et al. (Lakatos, Karmos, Mehta, Ulbert, & Schroeder, 2008). We intend to test this hypothesis in a subsequent study.
ERP responses
Consistent with previous studies, this study found the morphology of the newborn auditory ERP to be a broad positive potential peak between 200 and 400 ms (blue curves in the upper panels of Figure 4). The response to the infrequent stimulus was characterized by a trend toward an increase in this broad peak, nearly 50% larger at Fz (red curves in Figure 4). Some central and posterior locations showed a tendency for the potential to the infrequent tone to remain elevated above pre-stimulus levels at latencies beyond 450 ms (Figure 3). There is research that suggests a late latency effect may be an index of cognitive processes other than stimulus change detection (Ceponiene, et al., 2002). In agreement with previous studies (Ceponiene, et al., 2002; Fellman & Huotilainen, 2006), we consider the MMN effect in newborns to be a waveform difference at middle latencies between auditory ERPs in fronto-central areas. Using both repeated measures ANOVA and independent sample t-tests with two referencing schemes, we found no significant MMN. Note that the trends in the ERP results reported here are consistent with much of the previous literature (Fellman, et al., 2004; Kushnerenko, et al., 2007; P.H. Leppanen, et al., 1997); however, a similar middle latency result with opposite polarity (i.e. a negative potential with larger amplitude for the infrequent stimulus) has been reported (Alho, et al., 1990; Ceponiene, et al., 2002; Cheour, et al., 1999). These discrepancies in the literature of MMN in newborns are somewhat puzzling because they are not likely to be simply the result of different references for the electrical potential (He, et al., 2007). Other methodological differences across studies (e.g. rate of stimulus presentation) could contribute to these discrepancies (He, et al., 2007), but one possibility suggested by the seminal study of Leppanen et al. (P. H. Leppanen, et al., 2004) is that variation in mean gestational age is an important factor. They found that younger gestational ages were associated with negative auditory ERPs, while more mature newborns showed positive ERPs. This shift in ERP polarity during the perinatal period may have precluded use of the MMN in newborns as a clinical tool in describing individual differences. The Leppanen et al. finding suggested to us that a quadratic measure (e.g. power) may be a better index of change detection in newborns because group effects will not be dampened when the mean includes subjects with opposing ERP polarities.
Sensitivity of wavelet power versus ERPs
As hypothesized, we found differences in wavelet power values were more sensitive measures of change detection than differences in ERPs. This is illustrated in Figure 4, showing wavelet power and ERP comparisons at specific frontal and central locations. At each location with a significant power effect, only trends appeared in the ERPs. In fact, we noted many locations, such as C3 and F3, where in spite of negligible or absent MMN trends in the ERPs, significant theta power MMN effects were apparent. Furthermore, considering effects at all electrode locations (Figure 3 and Supplementary Figure S1), though late latency ERP effects occurred in some regions, as at C4 and Cz, there was no MMN effect at any location except at one left central electrode (averaged-mastoids reference) and one right temporal electrode (averaged reference). In marked contrast, increased theta power occurred at MMN latencies bilaterally in widespread frontal, central, and temporal regions and in left parietal and right parieto-occipital regions (Figure 2).
While robust power effects were observed at the group level in the present study, to develop these results into a clinical tool it will be necessary to define normative parameters for these effects and determine the sensitivity of detecting them within individual subjects. The highest sensitivity of these effects in the present preliminary study was 90%. In the current study, we were constrained to a very narrow window of opportunity for each subject in the newborn nursery in which to obtain sufficient continuous uninterrupted sleep to conduct the paradigm. In future studies, to further examine the potential of this measure as a clinical tool, it will be important to implement strategies to optimize the data quality obtained from each individual infant, including being flexible in the timing of the assessment to best fit the infant's sleep schedule and using a mesh cap to hold the EEG net in place to minimize movement artifact. Additionally, as noted above, multiple comparisons were not formally controlled for in this exploratory analysis, so it will be important to replicate the observed mid to late latency theta and late latency beta and gamma effects in future studies.
Conclusions and Implications
Mismatch negativity was found to occur in newborn infants 20 years ago. The discovery provided the first electrocortical probe of a cognitive process (stimulus change detection) present at birth. Predictions that MMN would be clinically useful in early identification of risk for subsequent cognitive deficits have not yet been realized. We suggest that wavelet power may provide a more sensitive marker of the brain's response to stimulus change than ERP-derived MMN, at least in newborn infants. This improved sensitivity may simply reflect the fact that power is blind to the polarity of the electrical potential whereas ERPs are not. Alternatively, it may be because single trial power captures phase-locked as well as non phase-locked activity, with the latter perhaps integral to the change detection process itself. Measures of oscillatory processes such as wavelet power may improve the prospects for a clinically useful test of cortical function at birth. The results reported here suggest a fruitful line of research should focus on determination of the most replicable features of wavelet power at the individual level.
Supplementary Material
Acknowledgements
We thank David Friedman and Michael Myers for helpful comments. This research was supported by the Sackler Institute of Developmental Psychobiology at Columbia University and by National Institutes of Health Grants R37 HD032774 (to W.P.F.), T32 MH018264 (to A.R.T.), and K25-NS052230 (to J.R.I.).
The project described was also supported by Grant Number UL1 RR024156 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from NIH Roadmap website.
References
- Alho K, Huotilainen M, Naatanen R. Are memory traces for simple and complex sounds located in different regions of auditory cortex? Recent MEG studies. Electroencephalogr Clin Neurophysiol Suppl. 1995;44:197–203. [PubMed] [Google Scholar]
- Alho K, Sainio K, Sajaniemi N, Reinikainen K, Naatanen R. Event-related brain potential of human newborns to pitch change of an acoustic stimulus. Electroencephalogr Clin Neurophysiol. 1990;77(2):151–155. doi: 10.1016/0168-5597(90)90031-8. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychol Bull. 2007;133(4):651–672. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Hardiman MJ, Barry JG. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Dev Sci. 2011;14(2):402–416. doi: 10.1111/j.1467-7687.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. Oxford University Press; New York: 2006. [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Kushnerenko E, Fellman V, Renlund M, Suominen K, Naatanen R. Event-related potential features indexing central auditory discrimination by newborns. Brain Res Cogn Brain Res. 2002;13(1):101–113. doi: 10.1016/s0926-6410(01)00093-3. [DOI] [PubMed] [Google Scholar]
- Cheour M, Ceponiene R, Hukki J, Haapanen ML, Naatanen R, Alho K. Brain dysfunction in neonates with cleft palate revealed by the mismatch negativity. Clin Neurophysiol. 1999;110(2):324–328. doi: 10.1016/s1388-2457(98)00005-4. [DOI] [PubMed] [Google Scholar]
- Cheour M, Leppanen PH, Kraus N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clin Neurophysiol. 2000;111(1):4–16. doi: 10.1016/s1388-2457(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009;120(11):1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Fellman V, Huotilainen M. Cortical auditory event-related potentials in newborn infants. Semin Fetal Neonatal Med. 2006;11(6):452–458. doi: 10.1016/j.siny.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Fellman V, Kushnerenko E, Mikkola K, Ceponiene R, Leipala J, Naatanen R. Atypical auditory event-related potentials in preterm infants during the first year of life: a possible sign of cognitive dysfunction? Pediatr Res. 2004;56(2):291–297. doi: 10.1203/01.PDR.0000132750.97066.B9. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neurosci Biobehav Rev. 2001;25(4):355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Marco-Pallares J, Munte TF, Grau C. Theta EEG oscillatory activity and auditory change detection. Brain Res. 2008;1220:93–101. doi: 10.1016/j.brainres.2007.07.079. [DOI] [PubMed] [Google Scholar]
- He C, Hotson L, Trainor LJ. Mismatch responses to pitch changes in early infancy. J Cogn Neurosci. 2007;19(5):878–892. doi: 10.1162/jocn.2007.19.5.878. [DOI] [PubMed] [Google Scholar]
- Hsiao FJ, Wu ZA, Ho LT, Lin YY. Theta oscillation during auditory change detection: An MEG study. Biol Psychol. 2009;81(1):58–66. doi: 10.1016/j.biopsycho.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clin Neurophysiol. 1999;110(6):1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Winkler I, Horvath J, Naatanen R, Pavlov I, Fellman V, et al. Processing acoustic change and novelty in newborn infants. Eur J Neurosci. 2007;26(1):265–274. doi: 10.1111/j.1460-9568.2007.05628.x. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320(5872):110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Leppanen PH, Eklund K, Lyytinen H. Event-related brain potentials to change in rapidly presented acoustic stimuli in newborns. Developmental Neuropsychology. 1997;13(2):175–204. [Google Scholar]
- Leppanen PH, Guttorm TK, Pihko E, Takkinen S, Eklund KM, Lyytinen H. Maturational effects on newborn ERPs measured in the mismatch negativity paradigm. Exp Neurol. 2004;190(Suppl 1):S91–101. doi: 10.1016/j.expneurol.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295(5555):690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- May PJ, Tiitinen H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology. 2010;47(1):66–122. doi: 10.1111/j.1469-8986.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Jacobsen T, Winkler I. Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology. 2005;42(1):25–32. doi: 10.1111/j.1469-8986.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007;118(12):2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception's shadow: long-distance synchronization of human brain activity. Nature. 1999;397(6718):430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146(4):1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23(34):10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. The orienting response, and future directions of its development. Pavlov ianJournal of Biological Science. 1990;25(3):142–150. doi: 10.1007/BF02974268. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Trans Biomed Eng. 1998;45(7):814–826. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Stefanics G, Haden G, Huotilainen M, Balazs L, Sziller I, Beke A, et al. Auditory temporal grouping in newborn infants. Psychophysiology. 2007;44(5):697–702. doi: 10.1111/j.1469-8986.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998;79:61–78. [Google Scholar]
- Truccolo WA, Ding M, Knuth KH, Nakamura R, Bressler SL. Trial-to-trial variability of cortical evoked responses: implications for the analysis of functional connectivity. Clin Neurophysiol. 2002;113(2):206–226. doi: 10.1016/s1388-2457(01)00739-8. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.