Abstract
Among the key properties that distinguish adult mammalian stem cells from their more differentiated progeny is the ability of stem cells to remain in a quiescent state for prolonged periods of time1,2. However, the molecular pathways for the maintenance of stem cell quiescence remain elusive. Using adult muscle stem cells (“satellite cells” (SCs)) as a model system, we show that the microRNA (miRNA) pathway is essential for the maintenance of the quiescent state. SCs lacking a functional miRNA pathway spontaneously exit quiescence and enter the cell cycle. We identified quiescence-specific miRNAs in the SC lineage by microarray analysis. Among these, microRNA-489 (miR-489) is highly expressed in quiescent SCs and quickly down-regulated during SC activation. Further analysis revealed that miR-489 functions as a regulator of SC quiescence by post-transcriptionally suppressing the oncogene DEK, a protein that localizes to the more differentiated daughter cell during asymmetric division of SCs and promotes the transient proliferative expansion of myogenic progenitors. Our results provide the first evidence of the miRNA pathway in general, and a specific miRNA, miR-489, in actively maintaining the quiescent state of an adult stem cell population.
Keywords: satellite cell, DEK, microRNA-489, myogenesis, stem cell quiescence
The miRNA pathway has been shown to be essential for stem cell pluripotency, proliferation, and differentiation3,4. To understand whether adult quiescent stem cells are under active post-transcriptional control by miRNAs, we conditionally ablated the miRNA processing enzyme Dicer in adult muscle stem cells, or “satellite cells” (SCs), using a mouse strain that both expresses a SC-specific, Tamoxifen (Tmx)-inducible Cre/loxP system5 (Supplementary Fig. 1) and is homozygous for a floxed Dicer allele6 and a Cre-dependent YFP reporter7. Six days after the first Tmx injection to this conditional knockout (cKO) strain, Dicer protein and miRNA levels were significantly down-regulated in YFP+ve SCs (Supplementary Fig. 2, 3). Strikingly, in cKO mice we detected YFP+ve SCs that had spontaneously exited quiescence and entered the cell cycle (Fig. 1a, b). In control mice, less than 1% of YFP+ve SCs were Ki67+ve at this time. These observations suggest that an intact miRNA pathway is essential for the maintenance of SC quiescence. Deletion of Dicer also led to apoptosis of proliferating SC progeny (Fig. 1c, d; Supplementary Fig. 4). Together, these experiments demonstrate the essential role of miRNAs in the maintenance of SC quiescence and survival of proliferating myogenic progenitors.
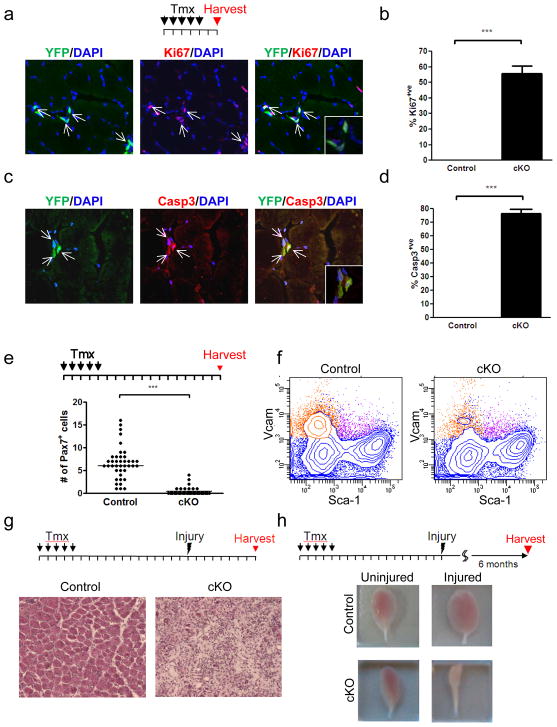
Figure 1. The miRNA pathway is essential for maintenance of SC quiescence and survival of activated SCs.
a, The tamoxifen (Tmx) injection scheme for conditional Dicer gene inactivation is shown above. Each tick represents one day. Six days after the first injection, Ki67/YFP double positive SCs were found (arrows). b, Quantitation of the percentage of YFP+ve cells that were Ki67+ve in control and cKO animals. (***P<0.001) c, Six days after the first Tmx injection, muscles from control or cKO mice were analyzed for apoptosis by staining for cleaved Caspase3. d, Quantitation of the percentage of YFP+ve cells that were Casp3+ve in control and cKO animals. (***P<0.001) e, SC numbers were quantified on freshly isolated single fibers from control and cKO mice two weeks after Tmx injections. (***P<0.001) f, SC numbers were quantified by FACS analyses of mononuclear cells from hindlimb muscles of control and cKO mice. SCs are shown in orange in these representative FACS plots (See Fig. S4). In 4 replicates, SC percentage in total mononuclear cells in cKO muscles was dramatically reduced (0.7%) when compared to control muscles (3.0%). g, Tibialis anterior (TA) muscles from control or cKO mice were injured two weeks after Tmx injection and cryosections were stained with H&E seven days after injury. h, TA muscles from control or cKO mice were injured two weeks after Tmx injection and harvested six months post injury. Severe muscle loss was observed in injured muscles from cKO mice only, shown in comparison to the contralateral, uninjured muscle.
To assess the impact of miRNA pathway disruption on SC homeostasis, we quantified SC number using single fiber explants and mononuclear cells isolated from uninjured muscles of cKO mice two weeks after Tmx injections. We observed a dramatic reduction in SC number in the absence of Dicer (Fig. 1e, f). To confirm the functional loss of SCs, hindlimb muscles of Tmx-injected cKO animals were injured to induce SC-mediated regeneration. Seven days after injury, virtually no regenerated fibers were observed in the cKO mice, indicating severely impaired regeneration (Fig. 1g). Further analysis six months after injury revealed a dramatic reduction in the mass of injured muscles compared to the contralateral, uninjured muscles. In comparison, control mice exhibited a hypertrophic response after muscle injury (Fig. 1h). Consistent with the finding that adult muscle SCs have a low turnover rate8, uninjured muscle appeared to be grossly normal six months after disruption of the Dicer gene (Supplementary Fig. 5a). However, the loss of SCs resulted in mild muscle fiber atrophy in cKO animals over time (Supplementary Fig. 5b).
As the disruption of Dicer caused SCs to break quiescence and enter the cell cycle, we were interested in defining the role of specific miRNAs in maintaining the quiescent state. Quantitative RT-PCR-based (qRT-PCR) miRNA microarray analysis of highly purified QSCs and activated SCs (ASCs) (Supplementary Fig. 6) revealed that 351 miRNAs were differentially regulated during SC activation (Supplementary Table 1). Of these, 22 were highly expressed in the quiescent state and markedly down-regulated upon SC activation (Fig. 2a). Among the 22 quiescence-specific miRNAs, we focused on miR-489 because it is broadly conserved among species9 and because it resides in intron 4 of the gene encoding Calcitonin Receptor (CT-R) (Supplementary Fig. 7a), a gene that is highly expressed in QSCs (Supplementary Fig. 7b, c) and has been previously shown to regulate SC quiescence10. Previous reports have suggested that intronic miRNAs co-express with host genes to co-regulate similar pathways11. The quiescence-specific expression of miR-489 and CT-R was verified by qRT-PCR analysis (Fig. 2b, c). To determine if miR-489 is specifically expressed in QSCs, we performed qRT-PCR analysis of isolated SCs and other mononuclear cell populations from uninjured muscle. As expected from the expression pattern of CT-R (Supplementary Fig. 7c), miR-489 was highly enriched in QSCs relative to multinucleate muscle fibers or other mononuclear cells in the muscle (Fig. 2d, e).
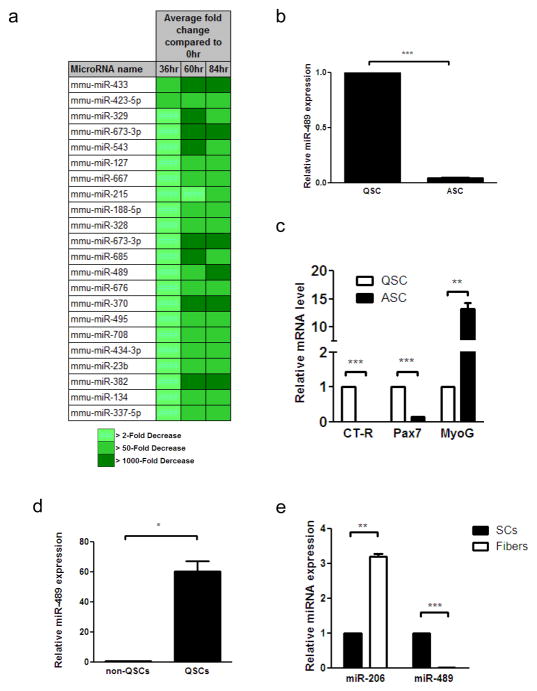
Figure 2. miRNA expression in purified QSCs and ASCs.
a, miRNA expression profiling during SC activation using qRT-PCR based miRNA arrays. QSCs from uninjured muscles and ASCs from injured muscles at indicated time points were isolated by FACS (Supplementary Fig. 6). QSC-specific miRNAs are shown. Complete dataset is shown in Supplementary Table 1. b, qRT-PCR analysis of miR-489 transcript in QSCs and ASCs. Expression levels were normalized to snoRNA420. (***P<0.001) c, qRT-PCR analysis of CT-R, Pax7 and Myogenin mRNA. Expression levels were normalized to GAPDH. (***P<0.001; **P<0.01) d, qRT-PCR analysis of miR-489 transcript in QSCs and all other mononuclear cells in hindlimb muscles. Expression levels were normalized to snoRNA420. (*P<0.05) e, qRT-PCR analysis of miR-206 and miR-489 transcript in QSCs and single fiber explants. Expression levels were normalized to snoRNA420. (***P<0.001; **P<0.01)
To test if a sustained expression of miR-489 could lead to an impairment of muscle regeneration by suppressing SC activation, a miR-489 expression plasmid was electroporated into hindlimb muscles in vivo. qRT-PCR analysis revealed a high level of miR-489 expression in TA muscles electroporated with miR-489 plasmid compared to controls (Supplementary Fig. 8b). Six days after electroporation, control muscles exhibited normal regeneration whereas muscles expressing miR-489 exhibited a severe defect in regeneration (Fig. 3a; Supplementary Fig. 8a).
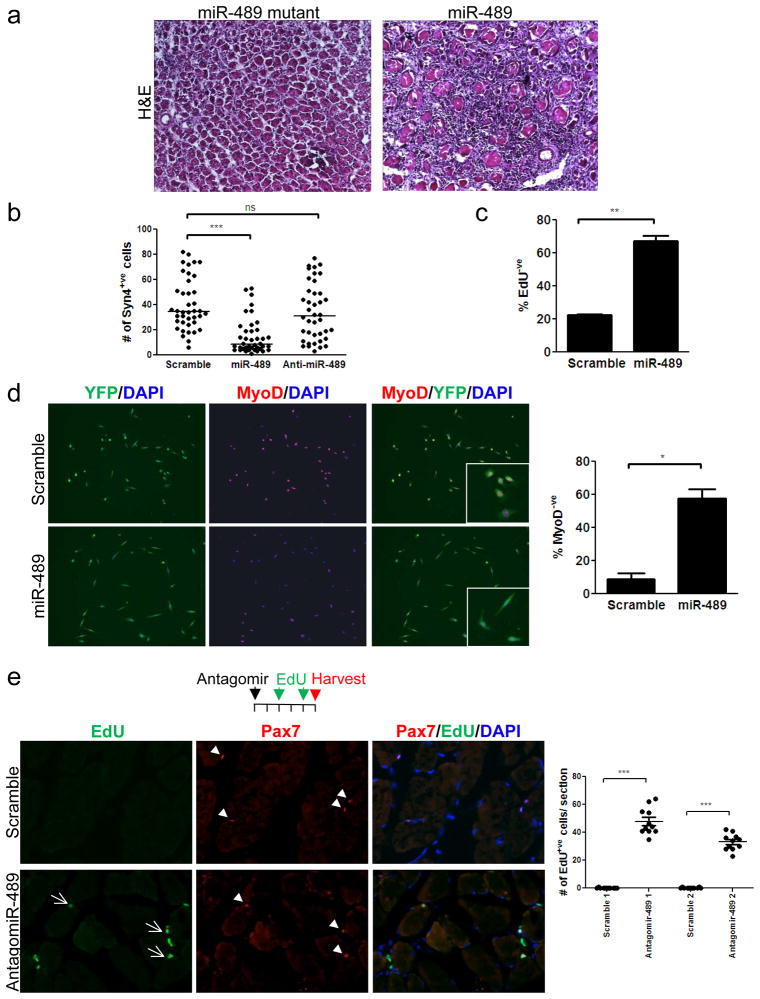
Figure 3. miRNA-489 regulates SC quiescence.
a, Hindlimb muscles were electroporated with either a mutant or control CMV-miR-489 expression plasmid. Muscles were harvested 6 days later and H&E staining was performed on cryosections. Representative image of 3 independent experiments is shown. b, miR-489 or anti-miR-489 was overexpressed in fiber-associated SCs. Three days after transfection, the number of Syndecan-4+ve (Syn4+ve) SC progeny was quantified. (***P<0.001; ns: not significant) c, In studies as in panel b, EdU was added to the medium at the time of miR-489 (or control) transfection and the percentage of Syn4+ve cells that were EdU−ve was determined after three days. (**P<0.01) d, (Left) FACS-sorted QSCs from Pax7CreER/+; ROSAeYFP/+ mice were plated and transfected with miR-489 and analyzed for MyoD expression 48 hours later. (Right) Quantitation of the percentage of YFP+ve cells that were MyoD−ve. (*P<0.05) e, (Left) SC activation in vivo, as determined by EdU incorporation, was assessed in muscles in which miR-489 was inhibited by the systemic injection of a cholesterol-conjugated anti-miR-489 oligonucleotide (“antagomir”) or a scrambled antagomir. Pax7+ve/EdU+ve (arrows) and Pax7+ve cells (arrowheads) are highlighted. (Right) Quantitation of the number of EdU+ve cells on cryosections. Two representative replicates of 4 independent experiments are shown. (***P<0.001)
To test the hypothesis that overexpression of miR-489 suppresses muscle regeneration by maintaining SC quiescence and suppressing activation, we overexpressed miR-489 or anti-miR-489 in fiber-associated QSCs ex vivo. Using Syndecan-4 as a SC marker on fiber explants12–13, we quantified the number of SCs on fibers three days after transfection. SCs treated with anti-miR-489 exhibited similar proliferative activity as control SCs, whereas SCs treated with miR-489 exhibited markedly reduced proliferation (and no evidence of apoptosis) (Fig. 3b). Furthermore, fewer than 50% of the cells treated with miR-489 progressed through a single round of cell division over the course of the experiment as determined by 5-ethynyl-2′-deoxyuridine (EdU) labeling (Fig. 3c). To further test that miR-489 regulates SC quiescence in a cell autonomous manner, we used MyoD expression as an indicator of SC activation14 and quantified the percentage of SCs expressing MyoD 48 hours after miR-489 transfection. Consistent with the fiber explant experiment, miR-489 suppressed SC activation (Fig. 3d). Together, these experiments demonstrate that miR-489 regulates SC quiescence in a cell-autonomous manner and that overexpression of a single miRNA is sufficient to prolong the quiescent state and delay QSC activation, resulting in an impairment of regeneration in vivo.
We next tested whether inhibition of miR-489 could result in the spontaneous activation of QSCs, which rarely divide in the absence of any activating stimuli8,. Cholesterol-conjugated “antagomirs” 15 specifically targeting miR-489 or control scrambled antagomirs were delivered systemically to adult mice. Four days after a single antagomir injection, miR-489 transcript levels decreased precipitously (Supplementary Fig. 9). In contrast to the control mice injected with scramble antagomirs, mice injected with anti-miR-489 antagomir exhibited spontaneous activation of QSCs which incoproated EdU (Fig. 3e). Strikingly, inhibition of one quiescence-specific miRNA, miR-489, was sufficient to induce QSCs to break quiescence and progress through the cell cycle in uninjured muscle.
The observation that inhibition of miR-489 induced SC activation and proliferation prompted us to hypothesize that miR-489 functions to suppress one or more key regulators of proliferation, thereby maintaining the quiescent state. To this end, we used the bioinformatics tool TargetScan to search for miR-489 target genes that contain putative miR-489 target sites in their 3′ untranslated regions (3′UTR)9. Among the 86 targets predicted by TargetScan, the transcript with the highest context score16 was the oncogene DEK (Supplementary Fig. 10), which has been shown to be induced in tumor cells and to regulate cell proliferation and mRNA splicing17,18. We analyzed the temporal expression of DEK mRNA and protein during SC activation. Using Pax7 as a marker of QSCs and MyoD as a marker of ASCs19–20, we found that DEK protein was not expressed in QSCs but strongly up-regulated following SC activation both in fiber explant studies ex vivo and in regeneration studies in vivo (Fig. 4a, Supplementary Fig. 11a, b, c). Likewise, DEK mRNA levels were higher in ASCs compared to QSCs (Supplementary Fig. 11d).
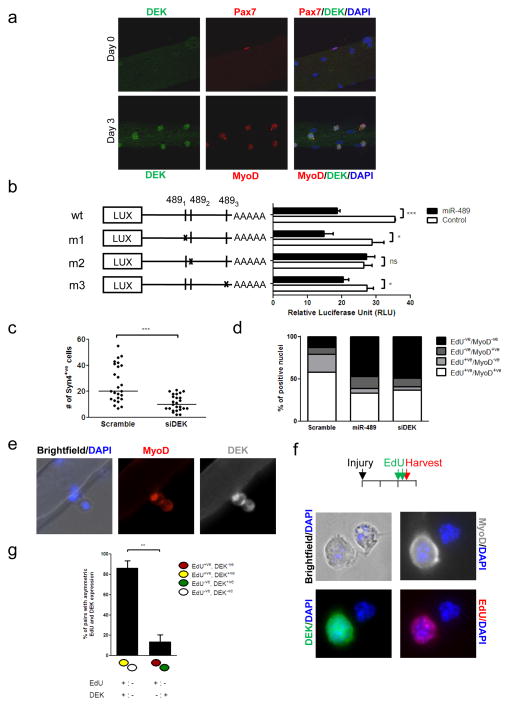
Figure 4. Targeting of DEK mRNA by miR-489 and regulation of cell fate decision of SC progeny by DEK.
a, Co-localization of DEK and myogenic markers in fiber-associated SCs. Fiber explants were fixed immediately after isolation (Day 0) or cultured for three days in suspension and stained for expression of Pax7 and DEK or MyoD and DEK, as indicated. Nuclei were stained with DAPI. b, CMV-miR-489 was co-transfected into 293T cells with wild-type (wt) or mutant DEK 3′UTR constructs inserted after the stop codon of a luciferase gene. DEK 3′UTR carries 3 putative miR-489 target sites (putative pairing as shown in Supplementary Fig. 10). Schematics of wt and mutant constructs (m1, m2 or m3) are shown along with the relative luciferase activities associated with each construct. (***P<0.001; *P<0.05; ns: not significant) c, SCs in fiber explants were transfected with DEK siRNA, cultured for 3 days, and the SC progeny were quantified by Syn4 staining (n=3). (***P<0.001) d, FACS-purified QSCs were plated and transfected with miR-489 or DEK siRNA for 48 hours. At the time of transfection, EdU was added to the medium. Cells were stained for EdU incorporation and MyoD expression. Bar graphs exhibit the proportion of cells expressing each marker under each condition. e, DEK asymmetrically localizes to one daughter after cell division. Fiber-associated SCs were cultured for 48 hours and stained for expression of MyoD and DEK. Nuclei were stained with DAPI. f, The timeline for injury, EdU injections, and collection of cells is shown above. Cells were stained for EdU incorporation to reveal non-random template strand segregation and for MyoD expression to reveal divergent cell fates. DEK co-segregates almost exclusively with the newly synthesized template strands. Images show a representative example of a cell pair exhibiting divergent cell fates with asymmetric segregation of template strands. g, Quantitative analysis of concordant and discordant asymmetries of DEK and EdU in asymmetric SC divisions in studies as in panel (f). (**P<0.001)
DEK protein was down-regulated when QSCs or myoblasts were transfected with miR-489 (Supplementary Fig. 12), suggesting that DEK is a direct target of miR-489. To test this directly, wild-type and mutant versions of the 3′UTR of DEK were cloned downstream of a luciferase reporter, and these reporter constructs were co-transfected with a miR-489 expression construct into 293T cells. The wild-type DEK 3′UTR was effectively down-regulated by miR-489 (Fig. 4b). Although TargetScan analysis revealed three potential target sites for miR-489, a single site (m2) was sufficient to account for the suppression of reporter expression by miR-489 (Fig. 4b).
We next examined the role of DEK in SC quiescence and activation using a loss-of-function approach. DEK knock-down reduced SC proliferation (Fig. 4c) and prevented SC activation to the same degree as miR-489 overexpression (Fig. 4d). The ability of DEK knockdown to phenocopy the effect of miR-489 overexpression suggests a central role of DEK in regulating SC exit from quiescence. To understand whether miR-489 overexpression suppresses proliferation by regulating DEK expression, we overexpressed miR-489 or miR-489 mutant along with a DEK cDNA construct that lacks its 3′UTR in proliferating myoblasts. While miR-489 overexpression alone reduced cell proliferation, overexpression of DEK significantly increased cell proliferation independent of the expression of miR-489 or miR-489 mutant (Supplementary Fig. 13). Together, these experiments suggest that DEK is an important target of miR-489 involved in the regulation of SC quiescence and activation.
Although DEK was highly induced upon SC activation, consistent with its role in proliferative expansion of the transit-amplifying myogenic progenitors, it was absent in self-renewed SCs following muscle injury in vivo (Supplementary Fig. 11c). We thus studied SC self-renewal in fiber explants ex vivo, in which the asymmetric expression of MyoD by daughter cells heralds a divergent cell fate where the MyoD+ve daughter progresses along the myogenic lineage and the MyoD−ve daughter renews the SC population 19. Intriguingly, in such pairs, we observed asymmetric DEK expression, where DEK expression coincided with MyoD expression in the same daughter cell (Fig. 4e). This co-localization suggests that the DEK+ve daughter is destined for proliferative amplification as a progenitor and that the DEK−ve daughter is destined for self-renewal. To test whether the process of self-renewal is associated with the absence of DEK, we examined cells undergoing asymmetric division by analyzing non-random chromosome segregation, a process that we and others have previously shown to distinguish the differentiating progenitor from the self-renewing stem cell21–22. Consistent with the MyoD asymmetry, we found that DEK was absent in the daughter cell inheriting chromosomes bearing older template DNA strands, an inheritance pattern characteristic of the self-renewing cell, whereas DEK was expressed in the daughter cell destined for proliferative amplification and differentiation (Fig. 4f, g; Supplementary Fig. 14).
The finding that DEK is a key target of miR-489 in maintaining quiescence provides insight into the molecular pathways that regulate the quiescent state. These data demonstrate that the molecular regulation of quiescence is dependent upon the expression of specific miRNAs and integrated in the signaling network that regulates divergent fates of stem cell progeny during asymmetric cell division.
Method Summary
Single fiber explants
EDL muscles were excised and digested in Collagenase II (500 unit /ml in Ham’s F10 medium) as previously described23. Fibers were then washed extensively and cultured in medium containing Ham’s F10, 10% horse serum and 0.05% chick embryo extract. Every 24 hours, 50% of the medium was replaced with Ham’s F10 medium with 20% FBS. EDL fibers were cultured in suspension. Fixed fibers were stained and the number of satellite cells was quantified on a per fiber basis.
Satellite cell isolation and FACS
Hindlimb muscles were dissected and dissociated to yield a muscle suspension and digested with Collagenase II (500 unit/ml; invitrogen) in Ham’s F10 medium with 10% horse serum (Invitrogen) for 90 minutes. Digested fiber suspension were washed and further digested with Collagenase II (100unit /ml) and Dispase (2 unit/ml; invitrogen) for 30 minutes. Digested fiber suspension were triturated and washed further to yield a mononuclear cell suspension for cell surface staining for FACS. Mononuclear cells were stained with Vcam-biotin (clone 429; BD Bioscience), CD31-APC (clone MEC 13.3; BD Bioscience), CD45-APC (clone 30-F11; BD Bioscience) and Sca-1-Pacific-Blue (clone D7; Biolegend) at 1:75. Streptavidin-PE-cy7 was used to amplify the Vcam signal (BD Biosciences, 1:75). Cell sorting was performed using a BD FACSAria II or BD FACSAria III cell sorter equipped with 488 nm, 633 nm and 405 nm lasers. The machine was carefully optimized for purity and viability, and sorted cells were subjected to FACS analysis right after sorting to ensure purity. A small fraction of sorted cells was plated and stained for Pax7 and MyoD to assess the purity of the sorted population purity.
Methods
Animals
C57BL/6, ROSAeYFP/eYFP and DcrloxP/loxP were obtained from Jackson Laboratory6–7. Pax7CreER Cre mouse was kindly provided by Charles Keller at the Oregon Health and Science University. Tamoxifen injection for Cre recombinase activation was performed as described previously5. Unless indicated, all control animals used in this study carried the genotype Pax7+/+; DcrloxP/loxP; ROSA26+/+ and all cKO animals carried the genotype Pax7CreER/+; DcrloxP/loxP; ROSA26+/+. In Fig. 1A, B, C, D and Fig. S3, control and cKO animals refer to mice that carry the genotypes Pax7CreER/+; Dcr+/+; ROSA26eYFP/eYFP and Pax7CreER/+; DcrloxP/loxP; ROSA26eYFP/eYFP, respectively. To control for tamoxifen injection toxicity, we injected all mice with tamoxifen. Mice were housed and maintained in the Veterinary Medical Unit at Veterans Affairs Palo Alto Health Care Systems. Animal protocols were approved by the Administrative Panel on Laboratory Animal Care of Stanford University.
Satellite cell isolation and FACS
Hindlimb muscles were dissected and dissociated to yield a fragmented muscle suspension using gentleMACS dissociator (Miltenyl Biotec). The muscle suspension was then digested with Collagenase II (500 unit/ml; Invitrogen) in Ham’s F10 medium containing 10% horse serum (Invitrogen) for 90 minutes. Fragmented myofibers were washed and further digested in Collagenase II (100 unit/ml) and Dispase (2 unit/ml; Invitrogen) for 30 minutes. Digested fiber suspensions were triturated and washed to yield a mononuclear cell suspension. Mononuclear cells were stained with Vcam-biotin (clone 429; BD Bioscience), CD31-APC (clone MEC 13.3; BD Bioscience), CD45-APC (clone 30-F11; BD Bioscience) and Sca-1-Pacific-Blue (clone D7; Biolegend) at 1:75. Streptavidin-PE-cy7 was used to amplify the Vcam signal (BD Biosciences, 1:75). Cell sorting was performed using a BD FACSAria II or BD FACSAria III cell sorter equipped with 488 nm, 633 nm and 405 nm lasers. The machine was carefully optimized for purity and viability, and sorted cells were subjected to FACS analysis right after sorting to ensure purity. A small fraction of sorted cells was plated and stained for Pax7 and MyoD to assess the purity of the sorted population purity.
Injections and electroporation
Mice were anesthetized using isoflurane through a nose cone. Muscle injury was induced by injecting 1–2 μl of 1.2% BaCl2 into approximately 25 sites in the lower hindlimb muscles. Electroporation of plasmid DNA into the TA muscle was performed as described previously24 using a two-needle electrode array at a setting of five pulses of 50 ms duration at 150 V/cm. Antagomir molecules were tail-vein injected into 8 week old mice at a dose of 8 mg/kg body weight.
Antagomir synthesis
PAGE purified RNAs were synthesized with modifications (Dharmacon). Sequences of single stranded RNAs used in this study are as follows (m represents 2′OMe-modified nucleotides; * represents phosphorothioate backbone at given position; ‘Chl’ represents cholesterol linked through a hydroxyprolinol linkage):
Antagomir-489 5′mG*mC*mUmGmCmCmAmUmAmUmAmUmGmUmGmGmUmGmUmC*mA* mU*mU*3′-Chl
Scramble 5′mU*mU*mUmCmUmAmAmUmCmAmAmGmGmGmUmCmUmGmUmG*mG* mC*mU*3′-Chl
Histology and immunohistochemisty
For H&E staining, TA muscles were dissected and directly frozen in O.C.T. (Tissue-Tek). For immunohistology, TA muscles were fixed for 5 hours using 0.5% EM grade paraformaldehyde and subsequently transferred to 20% sucrose overnight. Muscles were then frozen in O.C.T., cryosectioned at 6μm thickness and stained using an M.O.M kit (Vectorlabs) or Zenon labeling kit (Invitrogen) according to manufacturers’ instructions.
miRNA and siRNA transfections
Approximately 40 fibers were placed in each well of a 6 well plate containing 1 ml of Ham’s F10, 10% horse serum, 0.5% chicken embryo extract (US Biological). 100nM of miR-489 or anti-miR-489 synthetic molecules (Ambion) were transfected into either freshly isolated single fiber explants or C2C12 cells using Lipofectamine 2000 (Invitrogen). Cells were harvested for western blot at 48 hours after transfection. Control (Cyclophilin B) and DEK siRNAs were dissolved and diluted as suggested by the manufacturer (Dharmacon). Lipofectamine 2000 was used for the transfection of DEK siRNA according to the manufacturer’s instruction (Invitrogen).
Single fiber explants
EDL muscles were excised and digested in Collagenase II (500 unit /ml in Ham’s F10 medium) as previously described23. Fibers were then washed extensively and cultured in medium containing Ham’s F10, 10% horse serum and 0.05% chick embryo extract. Every 24 hours, 50% of the medium was replaced with Ham’s F10 medium with 20% FBS. EDL fibers were cultured in suspension. Fixed fibers were stained and the number of satellite cells was quantified on a per fiber basis.
RT-PCR and miRNA microarray
Total RNA was isolated using Trizol (Invitrogen). For individual RT-PCR, Taqman probes were used for detecting miR-17, miR-27b, miR-206, miR-489, sno420, GAPDH, CT-R, Pax7 and MyoG mRNA expression (Applied Biosystems). For miRNA microarrays, reverse transcription and amplification was performed as described by the manufacturer (Applied Biosystems). Diluted cDNAs were loaded onto the Taqman Array Rodent MicroRNA A+B Cards Set v2.0 and qRT-PCR analysis was performed using an ABI 7900HT Fast Real-Time PCR System. miRNA gene expression was normalized to U6 snRNA. Relative quantitation of miRNA gene expression was performed using the delta delta CT method25. Data is available at NCBI Gene Expression Omnibus under the accession number GSE26780.
DNA cloning and luciferase assay
A 300 bp genomic fragment flanking pre-miR-489 was cloned from mouse genomic DNA with the 5′ primer CCCCATGAGGGCAGAAACCAT and the 3′ primer TTATGATGCAACAAATATAT. The fragment was then sub-cloned into pGEM-T-Easy (Promega) and inserted into pcDNA3.1 plasmid to generate CMV-miR-489 plasmid. To generate the miR-489 mutant plasmid, four point mutations were introduced into the miR-489 plasmid using the following primers:
489_m1-5′ primer: CTGCAGTGGCAGCTTGGTTTTCATATCTGTAATGATACTTTCTAAAGTCTTCC AG; 3′ primer: CTGGAAGACTTTAGAAAGTATCATTACAGATATGAAAACCAAGCTGCCACT GCAG.
489_m2-5′ primer: CTTTCTAAAGTCTTCCAGAATAACACTACAGATATGGAAGCTAAACTGTTAC ATGGAACAAC; 3′ primer GTTGTTCCATGTAACAGTTTAGCTTCCATATCTGTAGTGTTATTCTGGAAGAC TTTAGAAAG.
These inserts (CMV-miR-489 and CMV-miR489-mutant) were then subcloned into pMR-Zsgreen1 to generate plasmids containing a ZsGreen reporter.
The DEK 3′UTR was cloned by amplifying the region of the DEK 3′UTR that contains miR-489 binding sites from mouse genomic DNA using the 5′ primer AAGTGACAGATGTTATTTTT and the 3′primer AACATTGATTTATTCTTTAT. The DEK UTR luciferase construct was generated by inserting this fragment into pMIR-report plasmid (Ambion). DEK mutants (m1, m2 and m3) were generated using the QuikChange II site directed mutagenesis kit (Stratagene). For each putative miR-489 site, two point mutants were introduced to the seed sequence using the following primers:
m1-5′ primer: GTTCTGCTTTGCCCTCAAAGTATAATCAATGTGGTTGTG; 3′ primer: CACAACCACATTGATTATACTTTGAGGGCAAAGCAGAAC.
m2-5′ primer: GTCATCAATGTGGTTGTGTTAACTCTAAGTATAATAGAAATTTTATAATGAGG ; 3′ primer: CCTCATTATAAAATTTCTATTATACTTAGAGTTAACACAACCACATTGATGAC
m3-5′ primer: GTTGGCCTTTAAGCAATTTATAATAAATCTTCACAATAAAGAATAAATC; 3′ primer: GATTTATTCTTTATTGTGAAGATTTATTATAAATTGCTTAAAGGCCAAC.
Luciferase assays were performed by seeding 5×105 cells per well in 6 well plates. Cells were then transfected with 0.25 μg of 3′DEK UTR constructs, 0.75 μg of the miR-489 expression construct and 50 ng of the pRL-TK Renilla luciferase control vector. Cells were transfected using FuGENE 6 according to the manufacturer’s instructions. 48 hours after transfection, cells were lysed and luciferase activities were measured using the Dual Luciferase Assay System (Promega) with a 20/20n luminometer (Turner Biosystems).
The mouse pCMV-Sport6 DEK plasmid was purchased from Open Biosystems. The pCMV-Sport6 DEKdeltaUTR construct was made by excising the DEK 3′UTR using restriction enzymes Bgl II and Not I and re-ligated to generate a DEK expression plasmid without its 3′UTR.
Template strand analysis
Analysis of non-random template strand segregation was performed as described with several modifications21. Briefly, muscles of 8 week old mice were injured as described and 200 μg of 5-ethynyl-2′-deoxyuridine (EdU; Invitrogen) were injected intraperitoneally 48 and 52 hours post-injury. Satellite cells were then sorted using the scheme as described and plated on poly-L-lysine-treated chamber slides (BD Biosciences) coated with ECM gel (Sigma) diluted at 1:100. To facilitate the analysis of sister cell pairs, sorted cells were plated at very low density (~10 cells/mm2). After allowing cells to adhere for 1 hour, cultures were treated with cytochalasin D (5 μM; Sigma) to prevent cytokinesis. Cells were fixed and stained using the Click-iT EdU Imaging Kit (Invitrogen) and antibodies recognizing DEK or MyoD. Sister cell pairs were identified as two nuclei with less than one cell diameter apart with contiguous cytoplasm evident by brightfield microscopy. Between 200 and 250 cell pairs were scored per experiment and all experiments were performed in triplicate.
Western blot analysis
Muscle tissues and cells were extracted in lysis buffer (50 mM Tris-HCl, pH 7.5, 0.5% SDS, 20 μg/ml aprotinin, 20 μg/ml leupeptin, 10 μg/ml phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, and 1 mM dithiothreitol). Protein extracts were electrophoresed on 4–15% polyacrylamide gradient gels and then transferred to nitrocellulose membranes. The membranes were incubated in blocking buffer (PBS and 5% milk) prior to overnight incubation with primary antibodies. After incubation with corresponding fluorescent secondary antibodies (Invitrogen), the membranes were analyzed using the Odyssey imaging system (LI-COR). GAPDH or actin was used as a loading control.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software). Unless noted, all error bars represent standard error of the mean (SEM).
Immunofluorescence and antibodies
Immunofluorescence was performed using a Zeiss Observer Z1 fluorescent microscope (Zeiss) equipped with a Hamamatsu Orca-ER camera or a Zeiss confocal system LSM710 (Zeiss). Data acquisition and fiber diameter measurements were performed using Improvision Volocity software (Perkin Elmer) or Zeiss LSM ZEN software (Zeiss).
The following antibodies were used in this study:
Pax7 (DSHB, 1:100)
Ki67 (Abcam, 1:100 and BD Bioscience, 1:50)
Laminin (Sigma, 1:1000)
Cleaved Caspase3 (Cell signaling, 1:100)
MyoD (Dako, 1:1000)
GFP (Invitrogen, 1:250 and Abcam, 1:250)
DEK (Proteintech Group, 1:2000)
Syndecan-4 (gift from Bradley Olwin, 1:1000).
Supplementary Material
Acknowledgments
We thank the members of the Rando lab for comments and discussions. We thank Dr. Bradley Olwin (University of Colorado at Boulder) for providing the Syndecan-4 antibody. This work was supported by the Glenn Foundation for Medical Research and by grants from the NIH (P01 AG036695, R37 AG23806 (MERIT Award), R01 AG23806, and DP1 OD000392 (an NIH Director’s Pioneer Award)) and the Department of Veterans Affairs (Merit Review) to T.A.R.
Footnotes
Contributions
T.H.C. and T.A.R. conceived the study. T.H.C., N.L.Q., G.W.C., L.L. and T.A.R. designed the experiments. T.H.C, B.Y. and L.L. performed all FACS analyses. T.H.C., N.L.Q., G.W.C., L.P., A.E., B.Y. and P.H. performed and analyzed the experimental data. T.H.C. and T.A.R. wrote the manuscript.
Competing financial interests
The authors declare that they have no competing financial interests.
References
- 1.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 327:542–545. doi: 10.1126/science.1180794. 327/5965/542 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. S0092-8674(09)00523-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–229. doi: 10.1038/nature06642. nature06642 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiscornia G, Izpisua Belmonte JC. MicroRNAs in embryonic stem cell function and fate. Genes Dev. 24:2732–2741. doi: 10.1101/gad.1982910. 24/24/2732 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishijo K, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009;23:2681–2690. doi: 10.1096/fj.08-128116. fj.08-128116 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. 0504834102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. S1357272503000426 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. gr.082701.108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukada S, et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. 2007-0019 [pii] [DOI] [PubMed] [Google Scholar]
- 11.van Rooij E, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. 1139089 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. S0012-1606(04)00552-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka KK, et al. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. S1934-5909(09)00019-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. jhc.6R6995.2006 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Krutzfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. nature04303 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Grimson A, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. S1097-2765(07)00407-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khodadoust MS, et al. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res. 2009;69:6405–6413. doi: 10.1158/0008-5472.CAN-09-1063. 69/16/6405 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soares LM, Zanier K, Mackereth C, Sattler M, Valcarcel J. Intron removal requires proofreading of U2AF/3′ splice site recognition by DEK. Science. 2006;312:1961–1965. doi: 10.1126/science.1128659. 312/5782/1961 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Zammit PS, et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. jcb.200312007 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zammit PS, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. jcs.02908 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. 06-PLBI-RA-2054R2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. ncb1425 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim. 1995;31:773–779. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- 24.Bertoni C, et al. Enhancement of plasmid-mediated gene therapy for muscular dystrophy by directed plasmid integration. Proc Natl Acad Sci U S A. 2006;103:419–424. doi: 10.1073/pnas.0504505102. 0504505102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.