Abstract
• Background and Aims Hard-seeded (physical) dormancy is common among plants, yet mechanisms for dormancy release are poorly understood, especially in the tropics. The following questions are asked: (a) whether dormancy release in seed banks of the tropical shrub Parkinsonia aculeata (Caesalpiniaceae) is determined by wet heat (incubation under wet, warm to hot, conditions); and (b) whether its effect is modified by microclimate.
• Methods A seed burial trial was conducted in the wet–dry tropics (northern Australia) to compare dormancy release across different habitats (open, artificial cover, ground cover and canopy cover), burial depths (0, 3 and 20 cm) and burial durations (2, 6 and 14 weeks). Results were compared with predictions using a laboratory-derived relationship between wet heat and dormancy release, and microclimate data collected during the trial.
• Key Results Wet heat (defined as the soil temperature above which seeds were exposed to field capacity or higher for a cumulative total of 24 h) was 43·6 °C in the 0 cm open treatment, and decreased with increasing shade and depth to 29·5 °C at 20 cm under canopy cover. The dormancy release model showed that wet heat was a good predictor of the proportion of seeds remaining dormant. Furthermore, dormancy release was particularly sensitive to wet heat across the temperature range encountered across treatments. This resulted in a 16-fold difference in dormancy levels between open (<5 % of seeds still dormant) and covered (82 %) microhabitats.
• Conclusions These results demonstrate that wet heat is the principal dormancy release mechanism for P. aculeata when conditions are hot and wet. They also highlight the potential importance of microclimate in driving the population dynamics of such species.
Keywords: Caesalpiniaceae, dormancy release, hardseededness, legume, microclimate, models, Parkinsonia aculeata, physical dormancy, seed bank, temperature, tropics, wet heat
INTRODUCTION
Hardseededness, or physical dormancy, is caused by a densely packed layer of palisade cells impregnated with water-repellent substances which inhibits imbibition (Baskin and Baskin, 1998). It occurs across approx. 15 plant families including legumes (Morrison et al., 1998; Baskin et al., 2000) and is common among shrubs and trees in arid to tropical regions (Kigel, 1995; Baskin and Baskin, 1998). Physical dormancy, and the conditions under which it is released under natural conditions, remains poorly understood, especially in tropical regions (Baskin and Baskin, 1998; Morrison et al., 1998; Foley, 2001). This compares with seeds that have physiological dormancy, especially in Mediterranean and temperate regions where mechanisms underlying seed dormancy are relatively well known, and have led to the development of useful predictive models that assist in weed management (Forcella et al., 2000; Bradford, 2005), and helped explain plant distributions and predict the impact of climate change (Handley and Davy, 2005).
Until recently, most studies on physical dormancy have concentrated on artificial procedures for dormancy release (Morrison et al., 1998). Nonetheless, a wide range of factors that may potentially disrupt testa-imposed dormancy under natural conditions have been identified, with differing implications for seed bank dynamics and seedling emergence patterns. The best studied is high and/or diurnally fluctuating dry heat, through exposure either to extreme natural temperatures (McKeon and Nott, 1982; Lonsdale, 1993; Norman et al., 2002) or to dry-season fires (‘heat shock’: Auld and O'Connell, 1991; Teketay, 1996; Morrison et al., 1998). In fact, dry heat from fires is considered to be the principle dormancy release mechanism for many species in Mediterranean-type ecosystems (Morrison et al., 1998). Other possible mechanisms have been noted for a wide range of species with physical dormancy. These include temperature fluctuations at cold temperatures (Martin, 1945, in van Assche et al., 2003), alternate wetting and drying (Baskin and Baskin, 1984), wet heat (van Klinken and Flack, 2005), microbial activity (Lonsdale, 1993) and natural, physical scarification (Bower, 2004). However, the actual importance of most of these under natural conditions has not been tested.
Tropical regions are categorized by hot, wet summers and warm, dry winters, in contrast to winter rainfall Mediterranean climes where summers are hot and dry. It was therefore hypothesized that wet heat (incubation under wet, warm to hot, conditions) is likely to have an important role in dormancy release under those conditions. A strong positive relationship between water temperature and dormancy release has been noted for a wide range of species (Baskin and Baskin, 1998), but as far as can be determined has only been quantitatively described for the tropical shrub, Parkinsonia aculeata (Caesalpiniaceae) (van Klinken and Flack, 2005). Parkinsonia aculeata is typical of many tropical species with hard-seeded dormancy as seeds lack embryo dormancy and secondary dormancy mechanisms, and there is no evidence that light plays a role in germination (Everitt, 1983). Most seeds that will be released from dormancy (and subsequently imbibe) at a particular temperature when immersed in water will do so within 24 h. The proportion of seeds that are released from dormancy following 24 h of immersion is limited below approx. 25 °C, increases non-linearly up to a threshold temperature of 33·6 °C, and increases linearly above that threshold. Almost all seeds are released from dormancy at 40–45 °C. The most temperature-sensitive part of this relationship between wet heat and dormancy release coincides with temperatures typically encountered in tropical regions (Nix, 1981). Wet heat could therefore provide a strong environmental signal for dormancy release.
In this report it was tested whether exposure to wet heat alone can explain dormancy loss under field conditions. This was done by comparing the actual proportion of seeds that remain dormant in a seed burial trial with predictions based on a knowledge of the soil microclimate those seeds were exposed to, and the empirical relationship between dormancy release and wet heat (van Klinken and Flack, 2005). Microclimate is greatly influenced by both vegetation cover and soil depth, with increasing cover and depth both acting to dampen diurnal temperature oscillations (Lonsdale, 1993). The seed burial trial was therefore conducted across the range of shading conditions and soil depths that P. aculeata will typically encounter under natural conditions in upland habitats of the wet–dry tropics in order to best test the explanatory power of wet heat as an environmental cue for dormancy release. The ecological significance of the results is discussed.
MATERIALS AND METHODS
Seeds
Parkinsonia aculeata L. seeds are relatively large (approx. 9 mm long and 4 mm wide) and contained within indehiscent pods that hold up to ten seeds. Mature P. aculeata pods were harvested from trees in the Victoria River District (Auvergne Station, Northern Territory; 15°26′S 130°20′E) in November 2002, air-dried immediately at ambient conditions, and stored under laboratory conditions (approx. 20–25 °C). Any seed-feeders were removed on emergence to prevent reinfestation. Seeds were subsequently removed manually from pods to prevent damage to the seed coat. Only intact, fully developed seeds were used in the trial. Seed burial packets (12 × 12 cm), each with 50 intact, fully developed seeds, were made from plastic shade cloth (1·5 × 1·5 mm holes), folded over and stapled shut.
Site and replication
A trial was conducted to determine the effect of shading, burial depth and burial duration on the proportion of seeds that remained dormant. It was set up at the Tropical Ecosystems Research Centre (12°25′S 130°53′E) near Darwin in the Northern Territory. The site was flat and soil was loamy sand, about 1 m deep, derived from a lateritized sedimentary substrate (Myers et al., 1998). The site is located within the wet–dry tropics. It is hot all year, with mean monthly minima ranging from 20·0 (July) to 25·9 °C (November) and maxima from 29·8 to 33·3 °C. Mean annual rainfall is 1813 mm and is strongly seasonal, with most (89 %) falling between November and March.
Four types of shading treatments were compared: open (no ground or canopy cover), artificial cover (shade cloth only), ground cover (grass cover only) and canopy cover (forest cover only). The former three treatments were placed in a randomized block design (four replicates) on an open, grassed paddock, and within 8 m of the forest edge. Blocks were placed at 15 m intervals, and perpendicular to the forest, with plots (1·5 × 1·5 m) spaced at 3 m intervals. Canopy cover (forest) replicates (four) were placed within 8–22 m of their associated block, and 6–45 m from each other. The forest was Eucalyptus tetrodona F. Muell., E. miniata A. Cunn. Ex Schauer and Corymbia bleeseri (Blakely) KD Hill & LAS Johnson woodland (Wilson et al., 1990) with an understorey of leaf litter (2–5 cm deep).
Glyphosate (360 g L−1) was applied to bare ground and artificial cover treatments on 28 December 2002 (2 × 2 m plots) to remove ground cover. These plots remained bare for the duration of the trial. The artificial cover treatment (plastic shade cloth erected at 1 m, 70 % shade cover) was intended as an intermediate treatment between forest canopy and open treatments. The ground cover treatment was achieved by transplanting gamba grass (Andropogon gayanus Kunth.) on 6 December 2002 in 1·5 × 1·5 m blocks and watering it for the first week to allow establishment. Plots were subsequently kept at a height of 40 cm through regular pruning, and provided 100 % cover throughout the trial. Canopy cover plots were placed within the forest, and positioned to receive full canopy cover, but no herbaceous cover, although leaf litter was typically a few centimetres thick.
Three depths were compared for each shade treatment: 0 cm, 3 cm (which is the optimal depth for germination) and 20 cm (to represent conditions encountered by deep seed store). Seeds at 0 cm were covered with a thin film of soil to prevent direct contact with the air.
Seed packets were buried on 16 January 2003 (one per treatment-replicate-retrieval date), approximately coinciding with when most seeds in the wet–dry tropics would be expected to be incorporated into the seed bank (R. D. van Klinken, unpubl. res.), and retrieved after 2 (30 January 2003), 6 (27 February 2003) and 14 weeks (24 April 2003). Seed packets were buried by cutting a 25 cm deep hole (one retrieval per hole), inserting packets laterally into the walls of the holes, refilling the hole with the same soil, and carefully replacing any litter layer.
The effect of treatment on microclimate
The temperature and moisture conditions to which each treatment was exposed throughout the trial were estimated using weather data collected at the site. A weather station (Monitor Sensors®) was erected in the centre of the site to record rainfall (in 0·2 mm lots), air temperature and soil temperature. Soil temperature probes (surface-mounted transistor, ±0·2 °C) were placed at 3 cm in each shading replicate, and at one randomly selected replicate at 0 and 20 cm. They were buried together with seed packets by pushing the sensor horizontally into the wall of the hole. All data were collected at half-hourly time steps.
Lightening strikes and waterlogging resulted in data gaps of between a few hours and up to 9 d. Air temperature and rainfall data were therefore used from the Darwin Meteorological Station (23°49·2′S 133°54′E) 5 km away, which were in agreement with the measurements made (correlation of 0·966). Gaps in soil temperature data were obtained by interpolation assisted by predictions obtained by fitting generalized additive models to the probe temperatures (Hastie and Tibshirani, 1990). The modelled temperatures were functions of date, time of day and air temperature. The residuals were calculated for all times with temperature data. For times when temperature data were missing, linear interpolations in the residuals were calculated. Missing temperature readings were interpolated by the sum of the modelled temperature and the interpolated residual. Correlations between modelled and measured temperatures were mostly >0·93 (range: 0·83–0·99). Temperature data were available for 16 of the replicates for the first two retrieval periods, covering all combinations of shade regime and depth, and 11 of the replicates for the third retrieval period, covering all except the open treatment.
A soil moisture model was used to estimate when conditions at the trial were at or above field capacity. Soil moisture was estimated using a daily tipping bucket soil moisture model for the top 200 mm of soil. Field capacity was estimated to be at 75 % volume and dry soil at 55 % (McKenzie et al., 2004). Runoff was assumed above saturation, 25 mm d−1 drainage between saturation and field capacity, and 3·2 mm evapo-transpiration between field capacity and dry soil (Huxley et al., 2000). The resulting equation was:
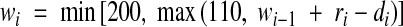 |
(1) |
where wi is the soil water (mm) on day i, ri is the rainfall (mm) on day i, and di is the drainage or evapo-transpiration (mm) on day i. If wi–1 >150 then di = 25, otherwise di = 3·2.
Determining seed dormancy and viability
The dormancy and viability of remaining, intact, seeds were determined under controlled conditions following seed retrieval. Seeds that were no longer intact at the time of retrieval had lost dormancy and subsequently either germinated or decayed in the seed burial bag (although these two possibilities could not be discriminated between based on seed remains).
Retrieved seeds were air-dried on retrieval and processed, together with two seed packets kept as controls under laboratory conditions (20–25 °C). Intact seeds from each treatment replicate were placed together in water at a constant 20 °C, which is below the point at which dormancy release becomes highly sensitive to temperature but not cool enough to affect germinability, for a maximum of 4 d, by which time imbibition rates approach zero (van Klinken and Flack, 2005). Imbibed seeds were removed daily and allowed to germinate on moist filter paper in Petri dishes at 25 °C, which is within the optimal temperature range for germination (van Klinken and Flack, 2005). Seeds were considered to have germinated once the radicle had emerged. Seeds that had not imbibed within 4 d were scarified with sandpaper and allowed to imbibe in water at 20 °C for a further 24 h prior to testing for germinability. Seeds were subsequently classified as ‘non-dormant and viable’ (imbibed within 4 d and subsequently germinated), ‘dormant’ (did not imbibe within 4 d) and ‘dormant and viable’ (did not imbibe within 4 d, but germinated following scarification and imbibition).
Analysis
All data were analysed in S plus (S plus 6·2 for windows, November 2003, Insightful Corp., Seattle, WA, USA).
Soil temperature data (half-hourly time steps) were ordered from hottest to coolest in order to determine the temperature above which seeds were exposed to a cumulative total of 24 h (48 time steps) both in total and when soil moisture was at or above field capacity (150 mm).
The seed counts, for dormant seeds and for non-dormant viable seeds, were analysed using a generalized linear model with a logistic link and an overdispersed binomial count distribution (McCullagh and Nelder, 1989). All main effects and interactions were fitted. The model fitted was
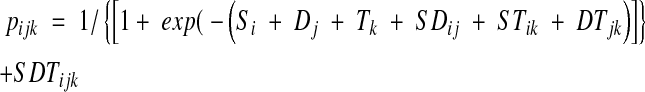 |
(2) |
where pijk = proportion of seeds released from dormancy at shade regime i, depth j and retrieval time k; Si = effect of shade regime i (1–4); Dj = effect of depth j (1–3); Tk = effect of seed retrieval time k (1–3); SDij = interaction of shade regime i and depth j; STik = interaction of shade regime i and retrieval time k; DTjk = interaction of depth j and retrieval time k; and SDTijk = interaction of shade regime i, depth j and retrieval time k.
Predictions for the proportion of seeds that remained dormant were made using all main effects and the two-way interactions of shade with depth and shade with retrieval time. For non-dormant viable seeds, all main effects and two-way interactions were used in making predictions. The effects of the treatment layout rows and columns were examined but were found not to be significant at the 5 % level and were excluded from the analysis.
Most seeds that are released from dormancy at temperatures above 30 °C do so within the first 24 h (van Klinken and Flack, 2005). The percentage of seeds that would remain dormant after each retrieval date was predicted by combining the empirical relationship between dormancy release and wet temperatures parameterized for seeds from the Victoria River District (van Klinken and Flack, 2005) with estimates of the temperature at which seeds were exposed to wet conditions (at or above field capacity) for a cumulative total of 24 h.
The proportion of seeds which had been released from dormancy after 24 h at a particular temperature was estimated by the equation
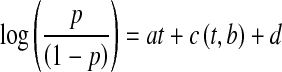 |
(3) |
where
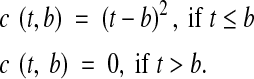 |
(4) |
p = proportion of seeds which have broken dormancy, t = temperature (°C), d = intercept term for seeds (=−8·47, s.e. = 0·32; van Klinken and Flack, 2005), b = 33·6 °C, and a = the slope of the curve above b (=0·257, s.e = 0·0091; van Klinken and Flack, 2005)
A confidence interval for individual observations was calculated for eqn (3) using the sum of an adjusted residual mean square error and the variance of the estimate of the expected value. The residual mean square error was adjusted for sample size (estimated with n = 20, van Klinken and Flack, 2005; confidence interval for n = 50 in this trial) assuming a binomial distribution.
RESULTS
The effect of treatment on microclimate
Air temperatures remained relatively constant through the trial, and were similar to those leading into the trial, averaging around 28 °C with a daily range of approx. 6 °C (Table 1). The primary differences between periods were in rainfall, with high rainfall leading into the trial, and relatively high and continuous rainfall through the first two trial periods. The final period (weeks 6–14) was relatively dry, marking the transition between wet and dry seasons. Over 1000 mm of rain fell during the trial. Rainfall patterns were reflected in modelled soil moisture patterns, with the soil rarely falling under field capacity (150 mm) in the first two trial periods, and being relatively dry in the final period (Table 1).
Table 1.
Temperatures (mean, maximum and minimum) and rainfall for the 2 weeks prior to the trial, and for each of the three periods during the trial
| Prior to trial | Period 1 | Period 2 | Period 3 | |
|---|---|---|---|---|
| No. of weeks | 2 | 2 | 4 | 8 |
| Air temperature (daily) | ||||
| Mean (range) | 27·9 (25·2–30·6) | 28·0 (26·9–29·8) | 27·3 (25·6–29·5) | 28·2 (25·2–30·0) |
| Minimum (range) | 25·1 (23·2–28·9) | 25·1 (22·6–27·5) | 24·7 (22·9–26·7) | 25·1 (21·2–27·7) |
| Maximum (range) | 31·2 (27·5–32·7) | 31·1 (29·3–32·5) | 30·0 (27·0–33·0) | 31·9 (27·6–35·7) |
| Rainfall | ||||
| Total (daily mean) | 300 (20·0) | 164 (10·9) | 722 (25·8) | 132 (2·4) |
| Percentage of days | 86 % | 86 % | 93 % | 34 % |
| Soil moisture | ||||
| Mean (min, max) | 166 (150–200) | 184 (143–200) | 182 (151–200) | 133 (110–176) |
| % time ≥field capacity | 85·7 % | 75·0 % | 100·0 % | 27·1 % |
Soil temperature replicates were within 2 °C for 99·95 % of recordings for ground cover (two replicates), 99·95 % for canopy cover (two replicates) and between 90·8 and 98·5 % for artificial cover (three replicates). Mean temperature values were used in the analyses.
The temperatures to which treatments were exposed, at or above field capacity, were higher than air temperatures in open and artificial cover treatments, and lower than air temperatures in ground cover and canopy cover treatments (Fig. 1). They varied with shade and depth treatments, and with how long seeds were buried (Fig. 1A). Maximum temperatures to which seeds were exposed under wet conditions for a cumulative total of 24 h increased between retrieval period 1 and 2, but there was little increase during period 3, in part because of the drier conditions during that period (Fig. 1A). Temperatures consistently decreased with depth, although it did interact with shade treatment. For example, differences were limited in canopy cover (1·1 °C) and ground cover treatments (1·4 °C), but were substantial in the open treatment, especially between 20 and 0 cm (8·9 °C) and 20 and 3 cm (6·6 °C) treatments. At any one depth, temperature was dependent on shade treatment, increasing from canopy cover, to ground cover, to artificial cover to open (Fig. 1A). For example, temperatures in the open treatments were 4·6 to 14·1 °C hotter than under canopy cover, depending on the depth.
Fig. 1.
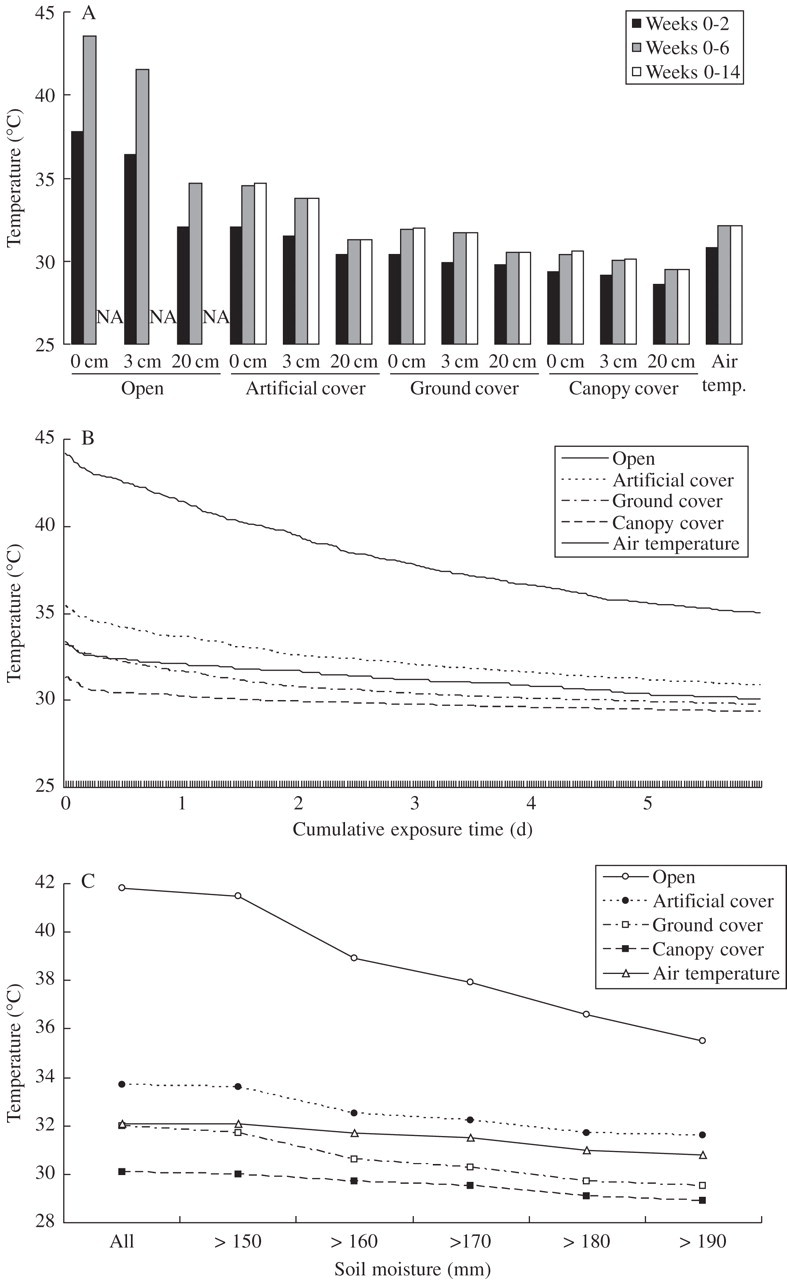
Maximum temperatures above which seeds were exposed (A) for a total of 48 half-hourly time steps (= cumulative 24 h period) at field capacity or above; (B) for the hottest 6 d worth of time steps (288) at field capacity or above; and (C) for a total of 48 time steps across a range of soil moisture thresholds. (A) Data for all treatments and retrieval dates. (B, C) Results for seeds buried at 3 cm (weeks 0–6 only). Note, temperatures after a cumulative 24 h in (B) and (C) (for soil moisture >150 mm) are the same as those shown in (A) for the respective treatments.
The wet temperatures to which treatments were exposed were relatively insensitive to cumulative exposure time (1–6 d) (Fig. 1B) and soil moisture thresholds (Fig. 1C), at least for the more buffered treatments. However, in open treatments, they varied by approx. 6 °C depending on whether it was 1 or 6 d of cumulative exposure time, and whether the soil moisture threshold for ‘wet heat’ was 150 mm or >190 mm.
The effect of treatment on seed longevity
Germination conditions within the laboratory seed tests were optimal. According to the present definitions of dormancy and viability, most seeds that were still dormant upon retrieval (n = 4731) were viable (99·6 %; confidence interval: 99·5–99·8 %), including all dormant seeds in 127 of the 144 treatment replicates. This compared with 100 % viability of dormant seeds within the controls.
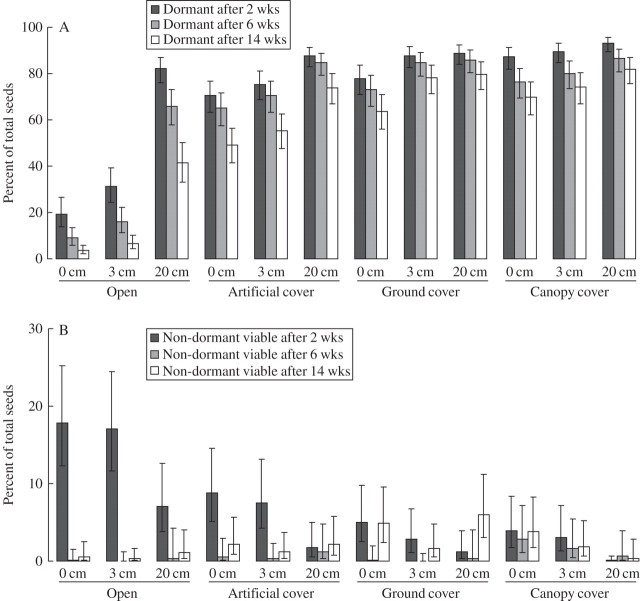
Overall, the proportion of seeds that were still dormant varied from 4 to 82 % after 14 weeks (Fig. 2A), compared with 86 % (s.e. 1·5 %) in the controls throughout this trial. There were considerable differences between shade treatments, depths and retrieval times in the proportion of total seeds that remained dormant (Table 2). There was also a strong interaction between shade and depth, and a less important, but statistically significant, interaction between shade and retrieval time (Table 2). The proportion of seeds that remained dormant decreased with decreasing shade cover, from canopy cover to ground cover (not statistically different at the 5 % level), artificial cover and open (Fig. 2A). It decreased with decreasing depth, although the effect was most evident in the less shaded sites (Fig. 2A). It also decreased with time, especially in the open and artificial cover treatments (Fig. 2A).
Fig. 2.
The proportion of total seeds that were still dormant (A) and that were non-dormant and viable (B) after each of the three retrieval dates (and 95 % confidence intervals). Note the different y-axis scales.
Table 2.
Analysis of deviance of seed burial trials for the proportion of total seeds that (i) remained dormant and (ii) were non-dormant and viable
| Dormant |
Non-dormant and viable |
||||
|---|---|---|---|---|---|
| Term | DF | Deviance | Probability | Deviance | Probability |
| Shade | 3 | 1352 | <0·001 | 29 | <0·001 |
| Depth | 2 | 419 | <0·001 | 24 | <0·001 |
| Retrieval time | 2 | 226 | <0·001 | 143 | <0·001 |
| Shade × depth | 6 | 168 | <0·001 | 14 | NS (P = 0·05) |
| Shade × retrieval time | 6 | 38 | <0·01 | 82 | <0·001 |
| Depth × retrieval time | 4 | 15 | NS (P = 0·1) | 22 | <0·001 |
| Shade × depth × retrieval time | 12 | 24 | NS (P = 0·1) | 19 | NS (P = 0·1) |
| Residual | 108 | 206 | 125 | ||
Relatively few of the original buried seeds were non-dormant and viable at the time of retrieval (Fig. 2B). This compares with the controls, where 11·3 % of total seeds were non-dormant and viable. The main exception was the first retrieval time in the 0 and 3 cm open treatments where up to 19 % of seeds were non-dormant and viable (Fig. 2B). There was a strong interaction between retrieval time and shade and depth (Table 2).
The buried seeds that were not either dormant (Fig. 2A), or non-dormant and viable (Fig. 2B), had either died or germinated. By the end of the trial (week 14), approx. 95 % of seeds in the open treatment (0 and 3 cm) had died or germinated, as compared with 54 % in the open 20 cm treatment, 46 % in the artificial shade treatment (0 and 3 cm), between 16 and 30 % in the other treatments, and 2·7 % of the control seeds.
Do seed physiology and microclimate predict seed longevity?
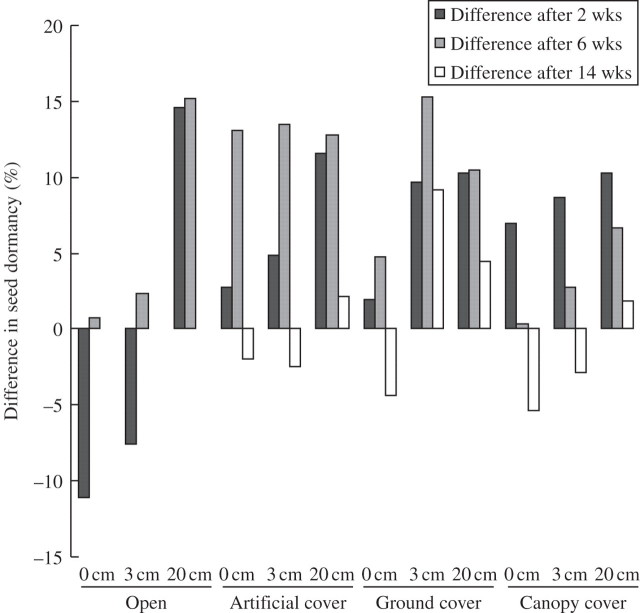
The proportion of seeds that remained dormant after 4 d in water at 20 °C was very similar for seeds used in this trial (89 %) and those sourced from the same site 12 months previously and used to develop the relationship between wet heat and dormancy release (eqn 3) (87 %) (van Klinken and Flack, 2005). The proportion of seeds that would remain dormant in each treatment (Fig. 2A) could therefore be predicted using eqn (3) and the microclimate data (Fig. 1A). Predictions were within 15 % of the observed levels of dormancy (Fig. 3). They were mostly overestimates, especially in the first and/or second periods, but fewer seeds were released from dormancy than expected at 0 and 3 cm depths in the open treatment. By the end of the trial estimates were within 2 and 9 %, and 1 and 15 % for open treatments after period 2 (Fig. 3).
Fig. 3.
Difference between mean actual proportion of seeds still dormant and the proportion predicted given the wet temperatures (Fig. 1A) to which they were exposed.
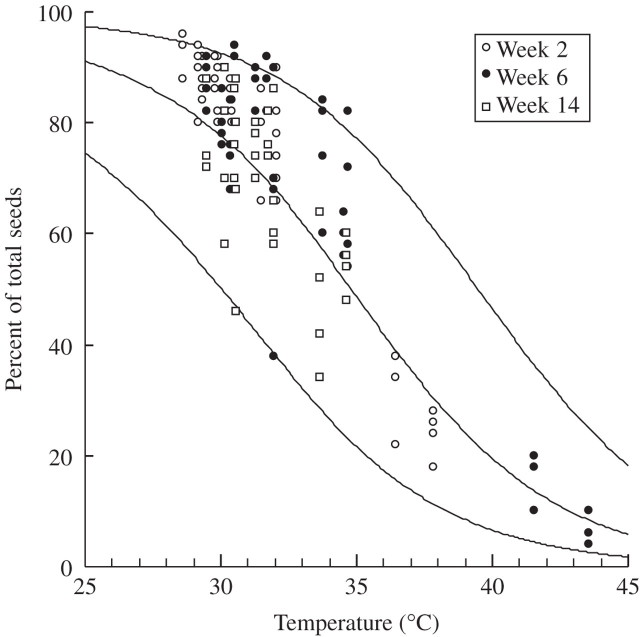
An alternative way to view the same data is to compare the actual temperatures to which seeds were exposed for a cumulative total of 24 h at or above field capacity (Fig. 1A), with those that the model would predict (Fig. 4). Actual temperatures were generally higher than required to achieve the observed proportion of seeds that were still dormant, especially in the first retrieval period. However, discrepancies mostly occurred at a temperature range at which dormancy release was relatively insensitive (i.e. below approx. 32 °C), and therefore resulted in relatively minor differences between actual and estimated proportions of seeds still dormant (Fig. 4). There were only a few data points (open treatment and artificial cover treatments) that coincided with the steepest part of the relationship between temperature and dormancy release, and they agreed well.
Fig. 4.
The observed proportion of seeds still dormant after each of the three retrieval dates and the temperature at which they were wet for at least 24 h. Each point is an individual observation. The solid line is the empirical relationship between wet heat and dormancy used to calculate predictions (Fig. 3; eqn 2), together with 95 % confidence intervals for individual observations.
DISCUSSION
The results demonstrate that wet season dormancy release of P. aculeata seeds could be entirely explained by the effect of wet heat, and that dormancy release within the seed bank could therefore be predicted by a relatively simple mechanistic model. Furthermore, the physical (linear) relationship between temperature and dormancy release, especially above the threshold temperature (van Klinken and Flack, 2005), and the fact that other species are also sensitive to wet heat (Baskin and Baskin, 1998), suggests that wet heat may be of more general importance, especially when seeds are exposed to hot, wet summers. This contrasts with Mediterranean climates where dry heat has been identified as the predominant dormancy release mechanism (Morrison et al., 1998). This does, however, need to be tested by describing the relationship between wet heat and dormancy release in other species, and by determining how wet heat actually results in the loss of dormancy in seeds. Different mechanisms for wet heat-related dormancy release can be expected across species, as is the case for dry heat (Morrison et al., 1998).
Seed dormancy provides an important mechanism for plant species to calibrate germination with environmental conditions in a way that will maximize the probability of recruitment (Baskin and Baskin, 1998; Caceres and Tessier, 2003). This is especially true for species such as P. aculeata whose seeds, once released from dormancy, will quickly germinate or decay when wet conditions are encountered. Parkinsonia aculeata seeds showed a simple yet highly effective gap detection mechanism under the climatic conditions in which the trial was conducted, with up to a 16-fold difference in the proportion of seeds that remained dormant between adjacent open and covered microhabitats. In fact, the buffering effect of vegetation was so strong that there was virtually no change in the proportion of seeds remaining dormant following burial under a closed canopy for almost a full wet season. There was also a lesser signal for depth, with fewer seeds released from dormancy at 20 cm. Germination would almost certainly not be successful at those depths (Barker et al., 1996), and remaining dormant might therefore allow seeds the opportunity to return to germinable depths.
Strong interactions between microclimate and dormancy release have important implications for the population dynamics of tropical plants at a range of scales. In tropical and arid regions, bare patches are common at the plant size scale, such as between grass tussocks and under trees following canopy death (Ludwig et al., 2003). At this scale, altered microclimate is a likely explanation for the frequently observed mass germination events following the death of parent trees of P. aculeata (R. D. van Klinken, unpubl. res.), and provides an alternative hypothesis for germination suppression such as allelopathy (Hierro and Callaway, 2003), low light (Denslow et al., 1990) and fluctuating high temperatures (McKeon and Nott, 1982). Significant microclimate effects are also likely to be seen at a landscape scale. For example, it would be expected that seed bank longevity, resulting from low rates of dormancy release, would be greatest in heavily vegetated habitats, and in wetlands where seeds may be essentially buffered by water for long periods. There are, however, insufficient data to speculate as to whether dormancy release through wet heat is expressly calibrated to respond to environmental signals, as is the case for some other dormancy release mechanisms (Baskin and Baskin, 1998).
One question not addressed in this trial is what the fate of seeds that remained dormant at the end of the trial would be in subsequent years. When predicting the proportion of seeds that remained dormant, it was assumed that the parameters for the relationship between wet heat and dormancy release remained constant through the trial, and also that there was no viability decline over time. These assumptions were upheld. However, if it remained unaltered between years, then most of the remaining fraction of seeds would be expected to remain dormant indefinitely, which is unlikely. The relationship between wet heat and dormancy release can differ between P. aculeata populations, with phase shifts in the driving variable (wet heat) of at least 6·6 °C (van Klinken and Flack, 2005). A phase shift that results in dormancy release occurring at cooler temperatures is one mechanism that could result in a between-year reduction in the proportion of seeds that remained dormant. Population-wide phase shifts could potentially result from changes in seed moisture content (Murdoch and Ellis, 1981) or weakening of seed coats, such as through repeated heating and cooling (McKeon and Nott, 1982; Lonsdale, 1993; Norman et al., 2002) or physical scarification. The importance of fluctuating dry heat has already been demonstrated experimentally for another tropical shrub with a similar seed type, Mimosa pigra (Mimosaceae) (Lonsdale, 1993).
Wet heat is also likely to interplay with episodic dormancy release events that can result from fire. The direct effects of fire on dormancy release (through heat shock) and seed mortality have already been documented for a wide range of species with similar seeds (Auld and O'Connell, 1991; Tieu et al., 2001). However, fire will also result in the creation of open ground. The resulting microclimatic changes would therefore be expected to result in mass dormancy release of remaining dormant seed, especially if fires coincided with hot and wet conditions.
The population-level consequences of the interplay between dormancy release and wet heat are yet to be properly explored. Results presented herein essentially span the sensitive range of the relationship between wet heat and dormancy release, with buffered treatments being at the lower end, and open, shallow treatments being at the higher end. This meant that dormancy release predictions were relatively insensitive to a number of assumptions, including that the effect of cumulative wet heat was the same as equivalent exposure to constant temperatures, soil moisture dynamics was independent of treatment, and soil moisture at or above field capacity has the same effect on dormancy release as inundation. Population models that have recently been developed for shrubs with seeds that are similar to P. aculeata (Kriticos, 2003; Buckley et al., 2004) could therefore be easily modified to accommodate the effects of wet heat simply by differentiating ‘open’ from ‘buffered’ microhabitats. However, landscape heterogeneity would ideally need to be considered at a relatively fine temporal (seasonal) and spatial (plant size) scale.
Acknowledgments
We thank Natural Heritage Trust and the Department of Agriculture (Western Australia) for financial support, Bert Lukitsch (NT DIPE) for evaluating seed dormancy and viability, Garry Cook (CSE) for assistance in soil moisture modelling, and Yvonne Buckley, Roger Lawes and Mark Ooi for comments on the draft manuscript.
LITERATURE CITED
- van Assche JA, Debucquoy KLA, Rommens WAF. 2003. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae). New Phytologist 158: 315–323. [Google Scholar]
- Auld TD, O'Connell MA. 1991. Predicting patterns of post-fire germination in eastern Australian Fabaceae. Australian Journal of Ecology 16: 53–70. [Google Scholar]
- Barker MJ, Dorney WJ, Lindsay AM, Vitelli J. 1996. Environmental factors influencing the establishment and growth of Acacia nilotica and Prosopis pallida. In: Shepherd RCH, ed. Eleventh Australian weeds conference. Melbourne: Weed Society of Victoria Inc., 223–227.
- Baskin CC, Baskin JM. 1998. Seeds: ecology, biogeography and evolution of dormancy and germination. New York: Academic Press.
- Baskin CC, Baskin JM, Li X. 2000. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology 15: 139–152. [Google Scholar]
- Baskin JM, Baskin CC. 1984. Environmental conditions required for germination of prickly acacia (Sida spinosa). Weed Science 32: 786–791. [Google Scholar]
- Bower JE. 2004. Diversified germination behaviour of Parkinsonia microphylla (foothill paloverde, Fabaceae). Madrono 51: 287–292. [Google Scholar]
- Bradford KJ. 2005. Threshold models applied to seed germination ecology. New Phytologist 165: 338–341. [DOI] [PubMed] [Google Scholar]
- Buckley YM, Rees M, Paynter Q, Lonsdale M. 2004. Modelling integrated weed management of an invasive shrub in tropical Australia. Journal of Applied Ecology 41: 547–560. [Google Scholar]
- Caceres CE, Tessier AJ. 2003. How long to rest: the ecology of optimal dormancy and environmental constraints. Ecology 84: 1189–1198. [Google Scholar]
- Denslow JS, Schultz JC, Vitousek PM, Strain BR. 1990. Growth response of tropical shrubs to treefall gap environments. Ecology 71: 165–179. [Google Scholar]
- Everitt JH. 1983. Seed germination characteristics of two woody legumes (Retama and Twisted Acacia) from south Texas. Journal of Range Management 36: 411–414. [Google Scholar]
- Foley ME. 2001. Seed dormancy: an update on terminology, physiological genetics, and quantitative trait loci regulating germinability. Weed Science 49: 305–317. [Google Scholar]
- Forcella F, Arnold RLB, Sanchez R. 2000. Modelling seedling emergence. Field Crops Research 67: 123–139. [Google Scholar]
- Handley RJ, Davy AJ. 2005. Temperature effects on seed maturity and dormancy cycles in an aquatic plant, Najas marina, at the edge of its range. Journal of Ecology 93: 1185–1193. [Google Scholar]
- Hastie TJ, Tibshirani RJ. 1990. Generalized additive models. London: Chapman and Hall, London.
- Hierro JL, Callaway RM. 2003. Allelopathy and exotic plant invasion. Plant and Soil 256: 29–39. [Google Scholar]
- Huxley LB, O'Grady AP, Eamus D. 2000. Evapotranspiration from eucalypt open-forest savanna of northern Australia. Functional Ecology 14: 183–194. [Google Scholar]
- Kigel J. 1995. Seed germination in arid and semiarid regions. In: Kigel J, Galili G, eds. Seed development and germination. New York: Marcel Dekker Inc., 645–699.
- van Klinken RD, Flack L. 2005. The relationship between wet heat and hard-seeded dormancy and germination. Weed Science 53: 663–669. [Google Scholar]
- Kriticos DJ. 2003. SPAnDX: a process-based population dynamics model to explore management and climate change impacts on an invasive alive plant, Acacia nilotica. Ecological Modelling 163: 187–208. [Google Scholar]
- Lonsdale WM. 1993. Losses from the seed bank of Mimosa pigra: soil micro-organisms vs. temperature fluctuations. Journal of Applied Ecology 30: 654–660. [Google Scholar]
- Ludwig J, Tongway D, Freudenberger D, Noble J, Hodgkinson K. 2003. Landscape ecology, function and management: principles from Australia's rangelands. Melbourne: CSIRO Publishing.
- McCullagh P, Nelder JA. 1989. Generalized linear models, 2nd edn. London: Chapman and Hall.
- McKenzie N, Jacquier D, Isbell R, Brown K. 2004. Australian soils and landscapes: an illustrated compendium. Melbourne: CSIRO Publishing.
- McKeon GM, Nott JJ. 1982. The effect of temperature on the field softening of hard seed of Stylonanthes humilis and S. hamata in a dry monsoonal climate. Australian Journal of Experimental Agriculture 33: 75–85. [Google Scholar]
- Morrison DA, McClay K, Porter C, Rish S. 1998. The role of the lens in controlling heat-induced breakdown of testa-imposed dormancy in native Australian legumes. Annals of Botany 82: 35–40. [Google Scholar]
- Murdoch AJ, Ellis RH. 1992. Longevity, viability and dormancy. In: Fenner M, ed. The ecology of regeneration in plant communities. Wallingford: CAB International, 193–229.
- Myers BA, Williams RJ, Fordyce I, Duff GA, Eamus D. 1998. Does irrigation affect leaf phenology in deciduous and evergreen trees of the savannas of northern Australia? Australian Journal of Ecology 23: 329–339. [Google Scholar]
- Nix HA. 1981. The environment of Terra Australis. In: Keast A, ed. Ecological biogeography of Australia. The Hague: Junk, 103–133.
- Norman HC, Cocks PS, Galwey NW. 2002. Hardseedness in annual clovers: variation between populations from wet and dry environments. Australian Journal of Agricultural Research 53: 821–829. [Google Scholar]
- Teketay D. 1996. Germination ecology of twelve indigenous and eight exotic multipurpose leguminous species from Ethiopia. Forest Ecology Management 80: 209–223. [Google Scholar]
- Tieu A, Dixon KW, Meney KA, Sivasitijamparam K. 2001. The interaction of heat and smoke in the release of seed dormancy in seven species from southwestern Western Australia. Annals of Botany 88: 259–265. [Google Scholar]
- Wilson BA, Brocklehurst PS, Clark MJ, Dickinson KJM. 1990. Vegetation survey of the Northern Territory, Australia. Technical Report no. 49, Land Conservation Unit, Conservation Commission of the Northern Territory, Palmerston, Australia.