Abstract
Despite many previous studies, controversy remains concerning the effects of dietary cholesterol on plasma cholesterol concentrations. In addition, the focus of previous studies has been fasting lipid and lipoprotein concentrations; there are no published studies with postprandial measurements. We studied the effects of four levels of dietary cholesterol intake on fasting lipid, lipoprotein, and apoprotein levels, as well as postprandial lipid levels, in a group of young, healthy men who were otherwise eating a low-fat, American Heart Association step 1 diet. Twenty young, healthy men completed a randomized, four-way crossover design study to test the effects of an American Heart Association step 1 diet containing 0, 1, 2, or 4 eggs per day. Dietary cholesterol ranged from 128 to 858 mg cholesterol per day. Each diet was eaten for 8 weeks, with a break between diets. Three fasting blood samples were obtained at the end of each diet period. In addition, blood samples were obtained just before and 2, 4, and 6 hours after ingestion of a standard lunch containing the various amounts of egg cholesterol. We also obtained blood 4 and 8 hours after the subjects ingested a standard, high-fat formula. Fasting plasma total cholesterol concentrations increased by 1.47 mg/dL (0.038 mmol/L) for every 100 mg dietary cholesterol added to the diet (P <.001). Low-density lipoprotein (LDL) cholesterol increased in parallel. Responsiveness varied but appeared to be normally distributed. Fasting plasma apoprotein B concentrations increased approximately 10% between the 0- and 4-egg diets and were correlated with changes in total and LDL cholesterol concentrations. Although there was a trend toward a greater response in men with an apoprotein E4 allele, this was not statistically significant. Fasting plasma cholesteryl ester transfer protein levels were higher only on the 4-egg diet, and changes in cholesteryl ester transfer protein levels between the 0- and 4-egg diets correlated with changes in total and LDL cholesterol. There were no differences in the postlunch or post-fat-formula responses of plasma lipids across the diets. Incubation of the 4-hour postlunch serum with J774 macrophages did not affect cell cholesteryl ester content at any level of dietary cholesterol. Cellular free cholesterol levels were slightly higher on each of the egg-containing diets versus the 0-egg diet. In summary, increases in dietary cholesterol resulted in linear increases in fasting total and LDL cholesterol in young, healthy men. The increases were less than expected based on previous studies, and this may have been due to the low saturated fat content of the background diet and/or the young age of the study group. Dietary cholesterol had no effect on postprandial plasma lipids either in response to the varying doses of cholesterol or after a standard high-fat meal. Increasing dietary cholesterol did not appear to result in an increased atherogenic potential of postprandial serum, as assessed by effects on cultured macrophages.
Keywords: diet, cholesterol, plasma, LDL, apoprotein B, apoprotein E, cholesteryl ester transfer protein, postprandial, macrophages
Despite many carefully controlled metabolic studies1–5 and detailed reviews of the available data,6–10 much controversy still exists regarding the effect of dietary cholesterol on plasma lipids and lipoproteins. The uncertainty regarding the potential for dietary cholesterol to raise plasma cholesterol concentrations derives in part from the lack of adequate dietary control in some studies, the extreme levels of dietary cholesterol used in other studies, and the variability in other diet components. Differences in the protocols used to study the effects of dietary cholesterol are compounded by both heterogeneity of responsiveness to dietary cholesterol11–13 and possible interactions between dietary cholesterol and fat.14,15
To gain a better perspective on the role of dietary cholesterol in the regulation of plasma cholesterol levels, we have initiated a series of studies in healthy young adults. We chose to study this group first because uncertainty concerning dietary recommendations is greatest in this population. In an attempt to isolate the effects of increasing dietary cholesterol and to give our results relevance to public health issues, we used a constant background diet consistent with the recommendations of the American Heart Association (AHA) and the National Cholesterol Education Program (NCEP).16 We also attempted to minimize effects of heterogeneity in responsiveness on overall outcome by studying every individual at four levels of dietary cholesterol. Finally, to maximize dietary control, we used a cafeteria setting and provided participants with all of their cholesterol-containing meals.
Methods
Subjects
Twenty-four subjects were recruited from a population of 150 healthy male medical and dental students, aged 22 to 31 years, who had their blood cholesterol tested at the Health Sciences campus of Columbia University in New York City. Cholesterol screening was carried out with an LDX lipid analyzer (Cholestech Corp) to obtain nonfasting, finger-stick blood cholesterol levels. Students with cholesterol concentrations between the 25th and 80th percentiles for age and sex were recruited. Individuals who expressed interest in participating in the study were invited to return after a 12-hour fast for the determination of plasma total cholesterol, triglyceride, and high-density lipoprotein (HDL) cholesterol levels from a venous blood sample.
Before the men were enrolled into the study, the coordinating dietitian interviewed them regarding dairy dietary intake, ethanol consumption, and smoking habits. Volunteers with extreme dietary habits or significant food intolerances were excluded. Individuals not willing to comply with limitations on alcohol intake to no more than two liquor drinks or five beers per week were excluded from participation in the study. Smoking was also a reason for disqualification. We also excluded volunteers with vigorous exercise regimens. None of the men had serious medical problems or were taking any medications that might affect plasma lipid levels.
The experimental protocol was reviewed and approved by the Institutional Review Board at Columbia University. Informed consent was obtained from all participants before screening and again before enrolling them in the study. Students did not receive monetary compensation for their participation in the study.
Protocol
This study was designed to determine the dose response to dietary cholesterol in healthy young men eating the step 1 diet recommended by the AHA and NCEP. We used a four-way crossover design in which the subjects ate each diet for 8 weeks. Breaks between diets were 3 days, 4 weeks, and 10 days. Each subject was randomly assigned to a different diet sequence; all possible diet sequences were used. Blood samples for determination of lipids and lipoproteins, apoprotein B (apoB), cholesteryl ester transfer protein (CETP), and apoprotein E (apoE) genotypes were obtained on 3 separate days at the end of each diet period. All fasting samples were obtained between 8 and 9 am after a 12-hour overnight fast. On 1 of the 3 days, blood samples were also obtained just before and at 2, 4, and 6 hours after the students consumed a standard AHA step 1 lunch that contained the quantity of cholesterol they had been eating daily during the previous 8 weeks. Thus, every subject had a postlunch response test at each level of dietary cholesterol, allowing us to observe the acute plasma response to differing cholesterol intakes. On another of the 3 days, blood samples were obtained at 4 and 8 hours after the students ingested a standard high-fat formula that contained 53 g fat and 60 000 U vitamin A per square meter of body surface. The fat meal provided 300 mg cholesterol as well. The students did not eat any other foods during the 8-hour period. All 20 subjects had the fat-formula test at the end of each diet period, allowing us to compare the long-term effects of different levels of dietary cholesterol on chylomicron metabolism.
The subjects ate lunches and dinners, Monday through Friday, under supervision, in the student dining facility. Monday breakfast was prepared and served by the principal investigator and the coordinating dietitian. Evening snacks and all other breakfasts were packaged and distributed at the preceding dinner. Weekend breakfasts and lunches were packaged and distributed on Friday nights. Dietary guidelines that stressed low cholesterol and fat intake (AHA step 1) were given for two self-selected weekend dinners. The subjects were instructed to keep a record of their weekend food consumption, and these records were reviewed by the dietitian every Monday at breakfast. Diet compliance was assessed and monitored daily using a self-administered form at each meal, by direct supervision in the dining hall, and by review of coded meal trays. Staff meetings with subjects were held on a regular basis as well. The study was conducted in a double-blind fashion, and only the dietary staff and the statisticians had knowledge of the subjects’ diet assignments.
Diets
The study diets consisted of foods prepared with fresh ingredients in accordance with computer-analyzed recipes and menu plans. Nutrient analysis of the composition of the research diets was done using the NUTRITION DATA SYSTEM program from the University of Minnesota (version 2.3). The caloric content of the AHA step 1 diet was 55% carbohydrate, 15% protein, and 30% fat, with 9% saturated fatty acids, 14% monounsaturated fatty acids, and 7% polyunsaturated fatty acids. The baseline content of cholesterol in all of the diets was 125 mg/d. The diets differed in their total cholesterol content, provided as 0, 1, 2, and 4 eggs per day. All diets were similar in appearance. Daily caloric requirements were estimated using data on each subject’s height, weight, diet history, and physical activity pattern. The subjects were assigned to one of three caloric groups (1900, 2200, or 2500 kcal/d). Participants were weighed every Monday before breakfast. Caloric adjustments were made either by shifting them to another caloric level or by provision of snacks designed to have the composition of the AHA step 1 diet except that they were virtually cholesterol free. The use of snacks allowed for flexibility in dairy caloric intake.
We used a 2-week menu cycle and served a variety of foods consisting of beef, pork, poultry, fish, dairy products, fruits, vegetables, grains and grain products, legumes, and desserts. Grade A large eggs were used, which, according to US Department of Agriculture (USDA) Handbook 8, contain 215 mg cholesterol per egg. The subjects were not aware of the number of eggs they were consuming. This was achieved by maintaining the egg bulk by using egg substitutes (Eggbeaters, Fleischmann Inc). The diets were formulated so that the fatty acid content remained the same despite increasing egg consumption. All of the eggs (and egg substitute) were served at lunch each day (including Saturday and Sunday). The basal level of cholesterol was distributed between the lunch and dinner meals.
Food samples were prepared for compositional analyses at the end of each diet period using USDA guidelines. Homogenates were prepared from 1 week’s meals from each of four research diets. Eight composite samples (two 1-week composites for each study diet) were sent to Hazelton Laboratories America (Madison, Wis) for analyses of protein, carbohydrate, total fat, individual fatty acids, and cholesterol.
Laboratory
Sampling
Blood samples were drawn into tubes containing EDTA (1.0 mg/mL) for plasma or into empty tubes for serum. The samples were placed immediately on ice and centrifuged at 2000 rpm for 20 minutes at 4°C within 1 hour of sampling. Plasma samples were stored at 4°C after the addition of aprotinin (Trasylol, FBA Pharmaceuticals) and azide. Plasma and serum samples were also stored in multiple aliquots at −70°C.
Plasma Lipids
Cholesterol and triglyceride concentrations were measured by enzymatic methods using a Hitachi 705 automated spectrophotometer. These measurements were performed with fresh plasma after each sampling. HDL cholesterol was measured by the same enzymatic method after precipitation of apoB-containing lipoproteins with magnesium arid dextran, using reagents supplied by Sigma.17 Low-density lipoprotein (LDL) cholesterol was estimated using the Lipid Research Clinics method.18 Our laboratory participates in the quality control program for lipid determinations administered by the Centers for Disease Control and Prevention (CDC), Atlanta, Ga. The interassay coefficients of variation were less than 3% for both cholesterol and triglyceride determinations.
Isolation of Lipoproteins
Very-low-density lipoproteins (VLDLs), intermediate-density lipoproteins (IDLs), LDL, and HDL were isolated from fasting plasma by sequential ultracentrifugation using a 50.3 Ti rotor in an L8-80 Beckman ultracentrifuge.19 Cholesterol and triglycerides were measured in each fraction as described above.
Apoprotein Assays
Serum apoB levels were measured by specific fluid-phase radioimmunoassay.20 Our laboratory participated in the Apolipoprotein Standardization Program administered by the CDC.21 All samples were assayed using a single radioiodinated LDL tracer. All samples from an individual man were analyzed in the same assay. The interassay and intra-assay coefficients of variation were 11% and 6%, respectively. Plasma CETP levels were determined by a specific radioimmunoassay as previously described.22 All samples from an individual man were analyzed in the same assay. The interassay and intra-assay coefficients of variation were 8.2% and 8.3%, respectively.
ApoE Genotyping
ApoE genotyping was performed by polymerase chain reaction (PCR) using the HaAI restriction enzyme.23 Briefly, leukocyte DNA was amplified by PCR using specifically synthesized oligonucleotide primers and Taq polymerase. The amplified apoE products were then digested with 5 U of HaAI enzyme at 37°C for 4 hours and the digest electrophoresed on a 12% nondenaturing polyacrylamide gel for 3 hours at a constant current of 10 mA. The gels were treated with ethidium bromide for 10 to 15 minutes and the DNA fragments visualized by UV illumination. DNA fragments of known size were used as markers.
Retinyl Ester Determination
Plasma retinol and retinyl palmitate levels were measured by reverse-phase high-performance liquid chromatography (HPLC) using a procedure similar to that described by Bieri et al.24 This method uses an internal-standard technique for the calculation of retinol and retinyl ester levels. The within-assay and between-assay coefficients of variation for retinol and retinyl ester determinations are less than 7%. For retinoid determinations, 100 µL plasma was denatured by addition of 100 µL ethanol containing internal-standard retinyl acetate, and the retinoids were extracted into hexane. The hexane extract was backwashed with water, evaporated to dryness under a gentle stream of nitrogen, and redissolved in benzene for injection onto the HPLC column. Chromatography was carried out on a 4.6 × 25 -mm Beckman 5 -µm Ultrasphere ODS column using 70% acetonitrile/15% methanol as solvent. Flow rate was 2.0 mL/min. Retinol and retinyl esters were detected by absorbance at 325 nm, and levels were determined from a standard curve relating integrated peak area ratios of the retinoid of interest and the internal-standard retinyl acetate to mass ratios of the two compounds. Standard curves were constructed using authentic retinol and retinyl palmitate.
Serum Incubation Studies With J774 Macrophages
Monolayer cultures of J774 murine macrophage-like cells were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics. For each experiment, the cells were plated in 35-mm dishes at a density of 106 cells per dish. Cell viability was determined by 0.04% trypan blue exclusion and was always greater than 90%. The average protein per dish was 1.0 mg. Cells were incubated for 18 hours in DMEM supplemented with 1% bovine serum albumin (control medium) or in control medium supplemented with 10% heat-inactivated serum obtained from the subjects after an overnight fast and 4 hours after the cholesterol-containing lunch. After the incubations, cells were scraped into the medium, and medium and cells were separated by low-speed centrifugation. Cells were then washed with phosphate-buffered saline three times, and aliquots were removed for determination of cell protein and measurement of cholesterol and cholesteryl ester by gas-liquid chromatography.25
Statistical Analysis
Each response variable was first analyzed for seasonal effects and carryover effects by a repeated-measures ANOVA, with period as the repeated-measure factor and diet and preceding diet as separate effects. No carryover effects were found. Seasonal effects were observed in all responses except total cholesterol. They were removed by adjusting the data from each period by that period’s effect relative to the overall mean. All subsequent statistical analyses were done with the adjusted values. The absolute values that we present are unadjusted; the lipid responses (slopes) are from the adjusted data.
Dose response to dietary cholesterol was analyzed by linear regression. For each subject, the response variable (eg, total cholesterol) at the four levels of dietary cholesterol was fitted by a straight-line function of dietary cholesterol and a slope determined. The adequacy of a linear model was determined by testing the significance of a quadratic term. After demonstrating that the linear model was adequate, the mean of all the subjects’ slopes was determined and tested to see if it was significantly different from zero (one-sample t test). With no missing data, this is equivalent to fitting a single slope to all subjects while allowing each subject to have a different y intercept. Keys et al4 suggested that the response to dietary cholesterol is linear with the square root of dietary cholesterol, rather than the absolute values. We also tested this model.
ApoB levels were determined only for the two extreme diets. Analysis was by a paired t test. Repeated-measures ANOVA was used to analyze CETP and the results of serum incubations with macrophages. The influence of apoE genotype on any response was analyzed by a simple one-way ANOVA using the absolute level of that response on a particular diet, or the change from 0 to 4 eggs, as the datum for each subject. Where appropriate, Spearman correlation coefficients were obtained between two measured responses.
The postlunch and post-fat-formula responses were calculated as the mean of the values determined after the meal, corrected for the baseline value; the latter was the level just before lunch in the case of the postlunch data and the fasting level in the case of the fat-formula test. The responses during the different diets were analyzed by repeated-measures ANOVA.
Results
Table 1 depicts the mean characteristics and baseline lipids of the 20 subjects who completed the study. The men were young and nonobese and had normal plasma lipid levels on a free-living diet. Body weight was constant throughout the study. Two of the original 24 men withdrew from the study during the first diet period, and two left the study after the second period.
TABLE 1.
Subject Characteristics
| Mean ± SD | Range | |
|---|---|---|
| Age, y | 24.4 ± 2.7 | 22–31 |
| Weight, kg | 77.9 ± 9.0 | 64–104 |
| BMI, kg/m2 | 24.2 ± 2.3 | 20.1–29.1 |
| TC, mg/dL | 168.8 ± 17.8 | 131–209 |
| TG, mg/dL | 90.3 ± 42.8 | 45–221 |
| LDL-C, mg/dL | 100.5 ± 21.5 | 45–148 |
| HDL-C, mg/dL | 50.3 ± 9.2 | 30–68 |
BMI indicates body mass index; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; and HDL-C, high-density lipoprotein cholesterol. Plasma lipid values were determined from a single blood sample obtained at the time of recruitment for the study, before initiation of diets. To convert milligrams per deciliter cholesterol to millimoles per liter, multiply by 0.02586. To convert milligrams per deciliter triglycerides to millimoles per liter, multiply by 0.01129.
The calculated and analyzed compositions of the four diets are presented in Table 2. The background nutrient composition, consisting of fats, carbohydrates, and protein, was essentially the same on all four diets. The quantities of each and the proportions of fat as saturated, monounsaturated, and polyunsaturated fatty acids met the guidelines of the step 1 diet. In fact, the total fat content of each diet was slightly lower than our goal, and this was associated with slightly lower than expected saturated and monounsaturated fatty acid content. Dietary cholesterol varied according to the number of eggs present in the diet. Each of the egg diets contained approximately 15% less cholesterol, by analysis, than was expected from calculations based on the USDA Food Composition Tables. On average, the addition of each egg to the diet increased the analyzed cholesterol content by approximately 180 mg. We do not know if this is the result of a systematic error in the USDA tables or in our method of compositing samples for analysis. We would point out, however, that our calculated responses to dietary cholesterol would be even smaller if we used the accepted value for egg cholesterol content (215 mg per egg yolk) rather than our measured values.
TABLE 2.
Composition of the Diets
| No. of Eggs | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 4 | |
| Cholesterol, mg/d | 125 (128) | 340 (283) | 555 (468) | 985 (858) |
| Fat | ||||
| Total | 30 (28.4) | 30 (27.1) | 30 (27.2) | 30 (28.2) |
| Saturated | 9 (8.4) | 9 (8.2) | 9 (8.1) | 9 (8.6) |
| Monounsaturated | 14 (12.2) | 14 (11.6) | 14 (11.6) | 14 (12.1) |
| Polyunsaturated | 7 (7.8) | 7 (7.3) | 7 (7.5) | 7 (7.5) |
| Carbohydrate | 55 (56.5) | 55 (55.9) | 55 (57.3) | 55 (54.2) |
| Protein | 15 (14.9) | 15 (14.9) | 15 (14.2) | 15 (14.9) |
Values are expressed as percent total caloric intake, except for cholesterol. Food samples were prepared and analyzed as described in “Methods.” The calculated values are followed, in parentheses, by the analyzed values. The calculated amounts of dietary cholesterol in the 1-, 2-, and 4-egg diets were based on an assumption that each egg contained 215 mg cholesterol.
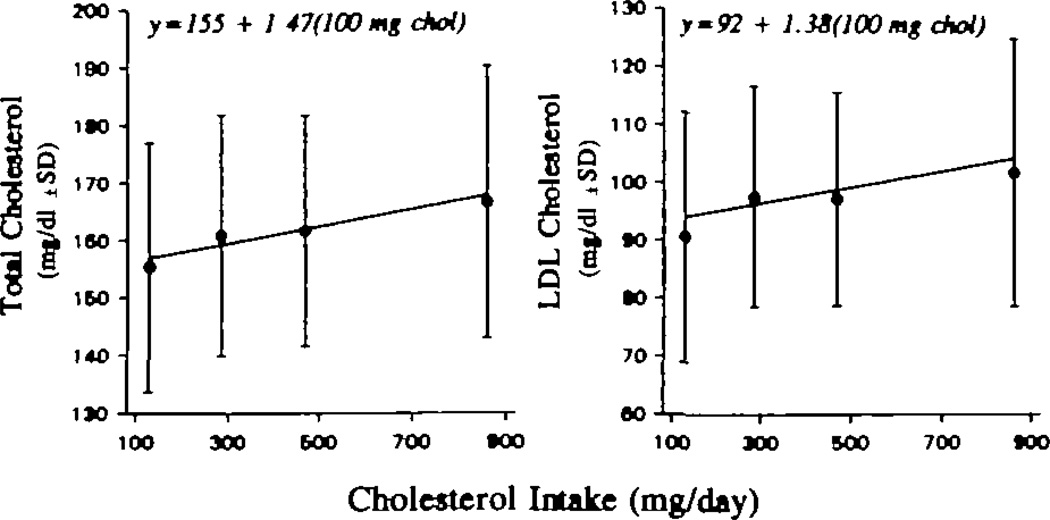
The dose responses of fasting plasma total and LDL cholesterol concentrations to increasing dietary cholesterol are depicted in Fig 1. The actual plasma cholesterol concentrations are plotted against the analyzed cholesterol content of the diets. There were statistically significant, linear increases in both plasma total and LDL cholesterol with increasing dietary cholesterol intake. Plasma total cholesterol increased 1.47 mg/dL (0.038 mmol/L) for each additional 100 mg dietary cholesterol per day (P <.001). The 95% confidence interval (CI) for the response was 0.73 to 2.2 mg/dL per 100 mg dietary cholesterol per day. Essentially all of the rise in total plasma cholesterol was accounted for by the rise in LDL cholesterol (1.38 mg/dL [0.036 mmol/L] per 100 mg dietary cholesterol per day) (P <.001), with a 95% CI of 0.81 to 1.95 mg/dL. When we attempted to fit the total and LDL cholesterol responses with a square root function, they were equally significant but with no improvement in the fit of the data. The estimated slopes for response were 0.60 mg/dL per square root milligrams dietary cholesterol per day for total cholesterol (95% CI, 0.29 to 0.91) and 0.57 mg/dL per square root milligrams dietary cholesterol per day for LDL cholesterol (95% CI, 0.32 to 0.81). The root mean squared error for total cholesterol (a measure of the variability in the data after the linear model was fitted) was 9.93 mg/dL with absolute levels and 9.96 mg/dL with the square roots of dietary cholesterol; for LDL cholesterol it was 8.4 mg/dL with both models. Thus, both models fit our data equally well. The mean concentrations of total and LDL cholesterol for the group at each level of dietary cholesterol are presented in Table 3.
Fig 1.
Graphs show responses of plasma total (left) and low-density lipoprotein (LDL) cholesterol (right) concentrations to increasing levels of dietary cholesterol intake. The individual points are the mean ± SD for the 20 men on each diet. The regression line is the mean of the individual regression lines for each of the 20 subjects. Plasma total cholesterol increased 1.47 mg/dL for each additional 100 mg/d of dietary cholesterol (P < .001). LDL cholesterol increased 1.38 mg/dL for each additional 100 mg/d of dietary cholesterol (P < .001). To convert milligrams per deciliter cholesterol to millimoles per liter, multiply by 0.02586.
TABLE 3.
Mean Plasma Lipids at Each Level of Dietary Cholesterol in 20 Subjects
| Mean (SD) Plasma Lipids, mg/dL | ||||
|---|---|---|---|---|
| Dietary Cholesterol,* mg/d |
TC | LDL-C | TG | HDL-C |
| 128 | 155.3 (21.6) |
90.6 (21.6) |
104.9 (31.3) |
43.7 (8.4) |
| 283 | 160.8 (21.0) |
97.6 (19.1) |
98.2 (23.4) |
43.5 (7.9) |
| 468 | 161.6 (20.1) |
97.2 (18.5) |
105.7 (45.8) |
43.2 (7.7) |
| 858 | 166.4 (23.6) |
101.8 (23.2) |
98.7 (25.2) |
44.9 (8.3) |
TC indicates total cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; and HDL-C, high-density lipoprotein cholesterol.
Measured amounts on each of the four diets.
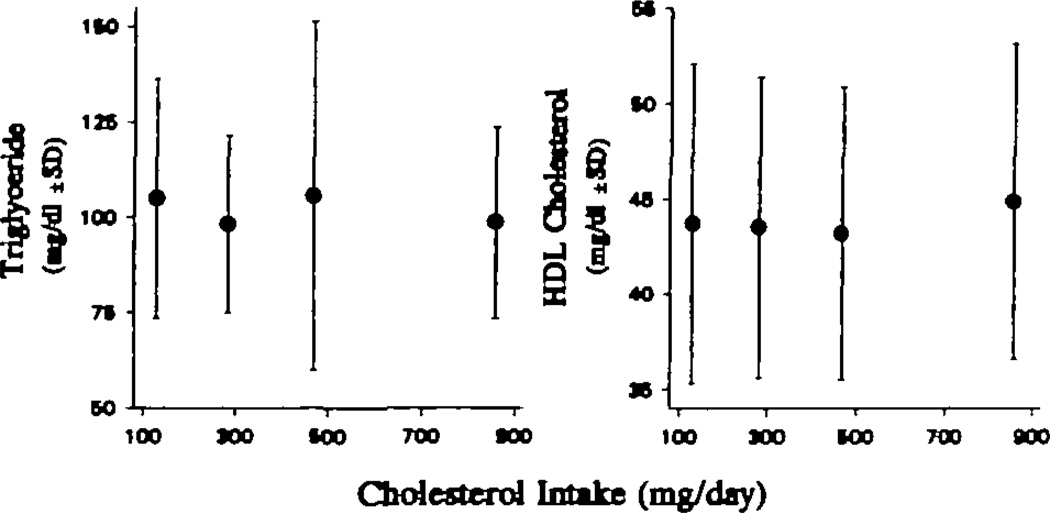
The dose responses of fasting plasma triglyceride and HDL cholesterol concentrations are presented in Fig 2. No significant response could be demonstrated by linear regression analysis. The mean levels of triglycerides and HDL cholesterol for the group at each level of dietary cholesterol are presented in Table 3. Increasing dietary cholesterol had no significant effect on plasma triglyceride levels or HDL cholesterol concentrations determined by precipitation.
Fig 2.
Graphs show responses of plasma triglycerides (left) and high-density lipoprotein (HDL) cholesterol (right) to increasing levels of dietary cholesterol intake. The individual points are the mean ± SD for the 20 men on each diet. There were no significant dose responses for either triglycerides or HDL cholesterol. To convert milligrams per deciliter cholesterol to millimoles per liter, multiply by 0.02586. To convert milligrams per deciliter triglycerides to millimoles per liter, multiply by 0.01129.
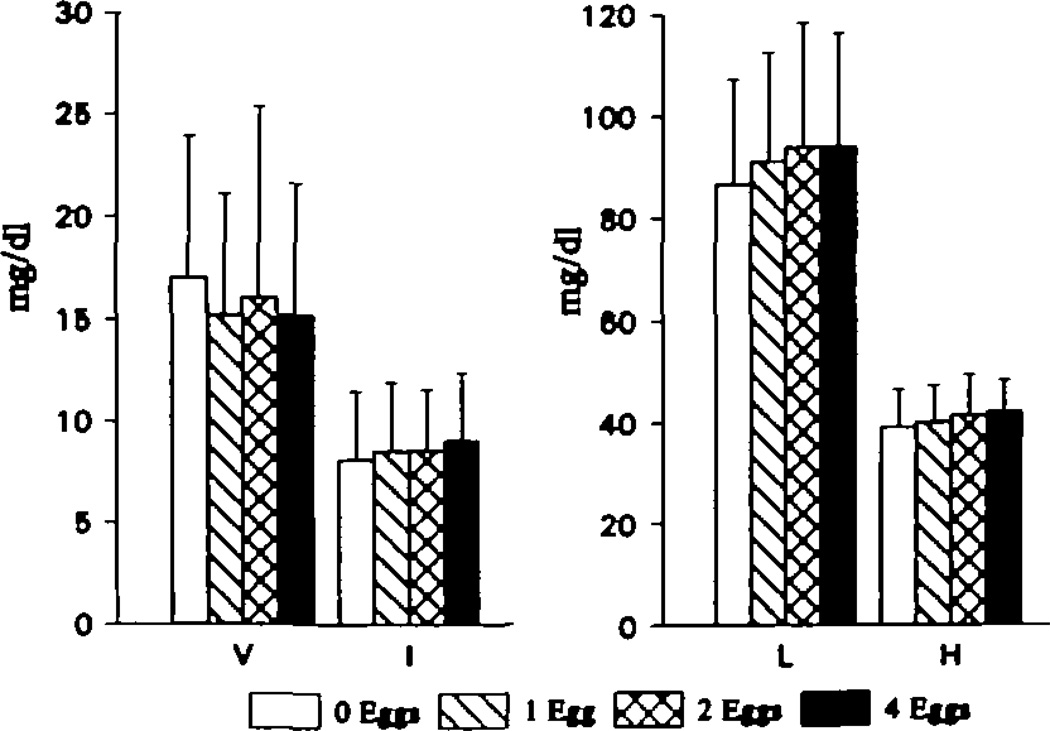
The cholesterol and triglyceride concentrations of ultracentrifugally isolated fasting VLDL, IDL, LDL, and HDL generally supported the data from whole plasma (Fig 3). There was no effect of increasing dietary cholesterol on VLDL or IDL cholesterol levels. In contrast, LDL cholesterol concentrations rose in a linear fashion (P <.05). Ultracentrifugal isolation indicated that there was also a significant rise in isolated HDL cholesterol levels in response to increasing dietary cholesterol consumption. The slope of the response was 0.29 ± 0.59 mg/dL per 100 mg dietary cholesterol per day (P <.05). The levels of HDL cholesterol isolated by ultracentrifugation were slightly lower at each level of dietary cholesterol than the levels determined by precipitation of plasma with magnesium and dextran. The lower HDL levels in the ultracentrifugally isolated fractions probably reflect less complete recovery compared with the precipitation method. There was no effect of dietary cholesterol on the triglyceride content of any lipoprotein fraction (data not shown).
Fig 3.
Bar graphs show cholesterol concentrations in individual lipoproteins isolated by ultracentrifugation. Very-low-density lipoprotein (V) and intermediate-density lipoprotein (I) levels are depicted in the left panel and low-density lipoprotein (L) and high-density lipoprotein (H) levels in the right panel. Low- and high-density lipoprotein cholesterol concentrations increased with increasing dietary cholesterol intake (P < .05). To convert milligrams per deciliter cholesterol to millimoles per liter, multiply by 0.02586.
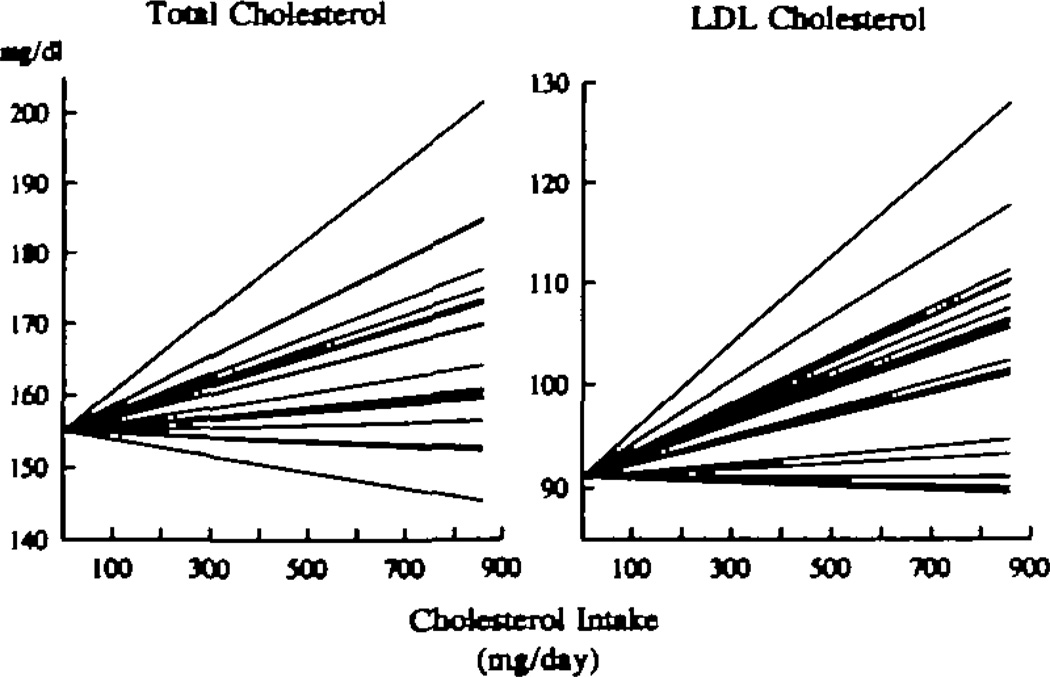
There was a wide distribution of individual dose responses for both total and LDL cholesterol (Fig 4). To facilitate visual inspection of the data, the total and LDL cholesterol responses of all of the subjects were adjusted to initial levels of 155.3 mg/dL and 90.6 mg/dL, respectively. These values correspond to the mean concentrations for all 20 subjects on the 0-egg diet. Three subjects actually had negative cholesterol responses to increasing eggs, while several responded at more than twice the mean. The distributions of the dose responses of total and LDL cholesterol of the group were consistent, within the limits of our small study sample size, with a normal distribution (Fig 5). A search for baseline and study variables that might predict an individual’s response indicated that the total cholesterol levels determined at the time of recruitment, when the subjects were eating their usual, free-living diets, were correlated with the response to dietary cholesterol during the study (r=.45 for total cholesterol; P <.05). There were no significant relations between response and other baseline lipids or characteristics, nor were there any significant relations between response and lipid concentrations on the 0-egg diet.
Fig 4.
Graphs show individual dose-response data for total (left) and low-density lipoprotein (LDL) (right) cholesterol levels with increasing dietary cholesterol. Each line is derived from regression analysis of an individual subject’s cholesterol levels on the four diets. To depict the range of responses more clearly, all individual responses have been adjusted to an initial level of 155.3 mg/dL for total cholesterol and 90.6 mg/dL for LDL cholesterol. These values represent the mean levels of each variable for the entire group on the 0-egg diet. The results indicate that there was a wide range of mostly positive responses to dietary cholesterol.
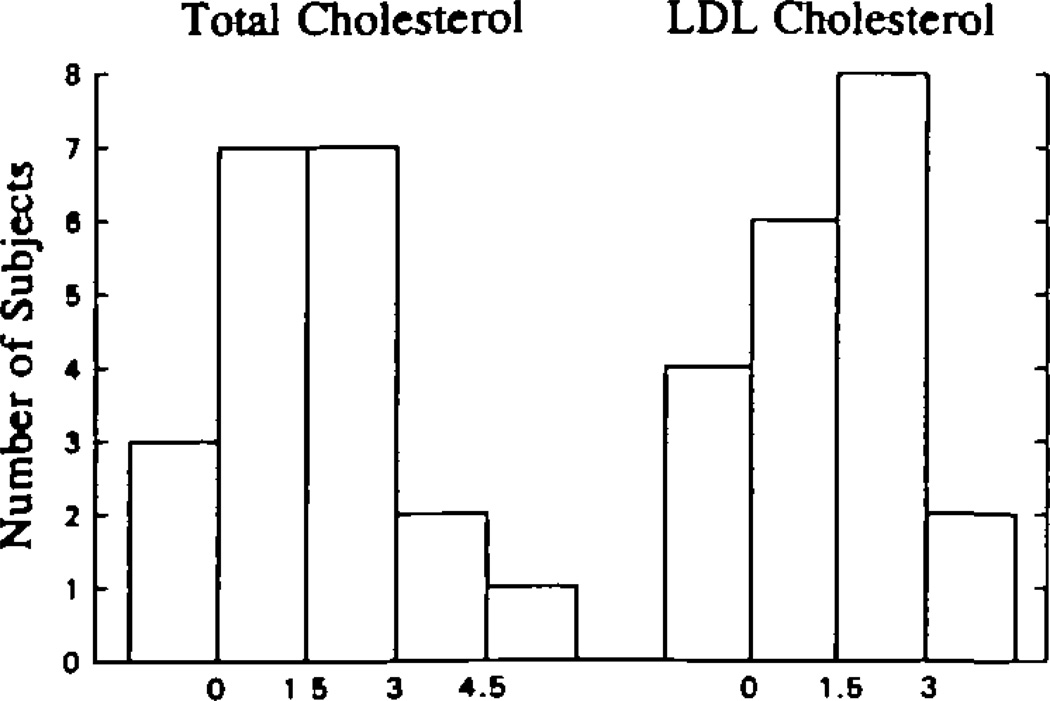
Fig 5.
Bar graphs show the distribution of plasma total (left) and low-density lipoprotein (LDL) cholesterol (right) responses to increasing dietary cholesterol. Each bar represents an interval of 1.5 mg/dL per 100 mg dietary cholesterol per day in the response to dietary cholesterol. The height of the bar indicates the number of subjects within any response interval. Responsiveness to dietary cholesterol appeared to be normally distributed. To convert milligrams per deciliter cholesterol to millimoles per liter, multiply by 0.02586.
ApoE genotyping indicated that the majority (12) of the subjects were E3/3. One of the subjects was E2/2, 2 subjects were E3/2, and 5 were E4/3. There were nonsignificant trends toward greater dose responses in total (2.13 ± 2.18 versus 1.24 ± 1.33 mg/dL per 100 mg dietary cholesterol per day; P=.28) and LDL (2.16 ± 1.57 versus 1.12 ± 1.02 mg/dL per 100 mg dietary cholesterol per day; P=.10) cholesterol in the 5 subjects with an E4 allele compared with the 15 subjects without that allele.
Fasting serum apoB levels were determined from samples obtained at the end of the 0- and the 4-egg diet periods. Consumption of the high-cholesterol diet was associated with a 10% increase in serum apoB levels (79.8 ± 22.4 versus 88.5 ± 28.4 mg/dL; Δ=8.7 ± 11.9 mg/dL; P <.01). There were no significant differences in the changes in apoB levels between 0 and 4 eggs among the different apoE isoform groups. There was a correlation (r=.76; P <.05) between individual LDL cholesterol responses to dietary cholesterol and changes in serum apoB levels.
Table 4 depicts the mean fasting CETP levels on each diet. We did not find a significant dose response in CETP to increasing dietary cholesterol intake. However, when the data for the 0-, 1-, and 2-egg diets were combined and compared with the mean CETP level on the 4-egg diet, the difference (0.15 ± 0.3 µ/mL) was statistically significant (P <.03). The CETP level was 6% higher on the 4-egg diet than on the other three diets combined. There was a significant, positive correlation between changes in CETP concentrations and changes in total (r=.65; P <.0l) and LDL (r=.67; P <.01) cholesterol levels between the 0- and 4-egg periods. We did not observe any relation between either CETP levels or changes in CETP levels and apoE genotypes. In addition, there was no relation between changes in CETP levels and changes in HDL cholesterol concentrations determined by either precipitation or ultracentrifugation.
TABLE 4.
Plasma Cholesteryl Ester Transfer Protein Levels at Each Level of Dietary Cholesterol in 20 Subjects
| Dietary Cholesterol,* mg/d |
CETP, µg/mL |
|---|---|
| 128 | 2.28 ± 0.56 |
| 283 | 2.15 ± 0.55 |
| 468 | 2.30 ± 0.49 |
| 858 | 2.40 ± 0.51 |
CETP indicates cholesteryl ester transfer protein. Values are mean ± SD.
Measured amounts on each of the four diets.
There was no effect of increasing dietary cholesterol consumption on the postlunch responses (calculated as areas above the prelunch levels) of total, LDL, and HDL cholesterol or triglycerides in the men (Fig 6, Table 5). Total cholesterol concentrations were essentially unchanged after lunch; LDL and HDL cholesterol levels decreased slightly at 2 and 4 hours before returning to baseline. Plasma triglycerides increased, as expected after ingestion of a fat-containing meal, but returned almost to baseline by 6 hours. The effect of increasing dietary cholesterol on fasting levels of total and LDL cholesterol did have an impact on the absolute values of these two parameters after lunch. Thus, total and LDL cholesterol levels were lowest on the 0-egg diet and highest on the 4-egg diet throughout the postlunch period.
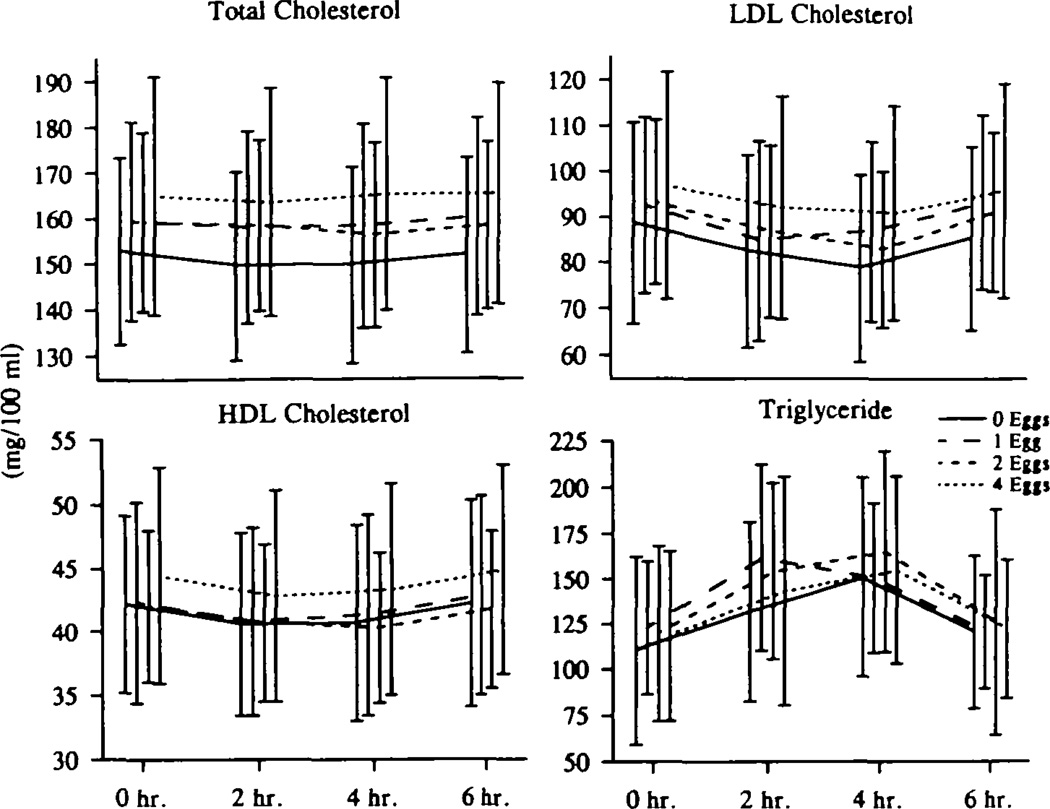
Fig 6.
Graphs show postlunch plasma lipid responses to increasing dietary cholesterol. Total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol and plasma triglyceride levels were measured just before and 2, 4, and 6 hours after a standard lunch containing each level of cholesterol. All 20 subjects had these measurements on each of the four diets. There were no differences between the responses (area) on different cholesterol-containing diets, after correcting for baseline (zero sample) differences. The absolute levels of total and LDL cholesterol were higher throughout the postlunch period on the 4-egg diet compared with the 0-egg diet.
TABLE 5.
Postprandial Cholesterol and Triglyceride Responses at Each Level of Dietary Cholesterol
| Postlunch Response | Post–Fat-Formula Response | |||||||
|---|---|---|---|---|---|---|---|---|
| Dietary Cholesterol,* mg/d |
TC | LDL-C | TG | HDL-C | TC | LDL-C | TG | HDL-C |
| 128 | −2.7 (4.7) |
−6.1 (4.3) |
23.8 (24.9) |
−0.9 (2.3) |
−0.5 (5.5) |
−14.8 (10.1) |
85.0 (57.3) |
−2.7 (3.5) |
| 283 | −0.5 (4.6) |
−3.9 (4.9) |
20.3 (23.4) |
−0.7 (1.4) |
−0.0 (6.5) |
−18.8 (11.6) |
111.4 (59.8) |
−3.5 (2.3) |
| 468 | −1.5 (4.9) |
−5.7 (5.2) |
27.6 (21.8) |
−1.0 (1.0) |
1.0 (6.8) |
−13.6 (14.7) |
88.0 (68.8) |
−3.0 (2.8) |
| 858 | −1.1 (5.7) |
−3.8 (5.5) |
21.2 (27.5) |
−0.7 (1.5) |
0.3 (5.9) |
−18.7 (13.2) |
112.8 (76.8) |
−3.5 (2.7) |
TC indicates total cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; and HDL-C, high-density lipoprotein cholesterol. Values are expressed in milligrams per deciliter. Postlunch (2, 4, and 6 hours after lunch) and post–fat-formula (4 and 8 hours after the fat load) responses were calculated as the mean (SD) of the values obtained during each test corrected for baseline (premeal) value. All 20 subjects had a postlunch test and a post–fat-fonmula test on each of the tour diets. Negative values indicate a fall in a level during the test. None of the responses on any diet was significantly different from any other response.
Measured amounts on each of the four diets.
Similarly, when the subjects consumed a standard, high-fat formula, the areas above baseline (which was the fasting plasma level that day) for total, LDL, and HDL cholesterol and for triglycerides did not differ on the different diets (Fig 7, Table 5). The effects of increasing dietary cholesterol on fasting levels of total and LDL cholesterol were evident, however, after ingestion of the high-fat formula, as the absolute levels of total and LDL cholesterol were consistently higher on the 4-egg versus the 0-egg diets. The high-fat formula also contained 60 000 U of vitamin A per square meter, which allowed us to monitor chylomicron metabolism. Long-term intake of diets high in cholesterol did not affect chylomicron metabolism in these men (Fig 8).
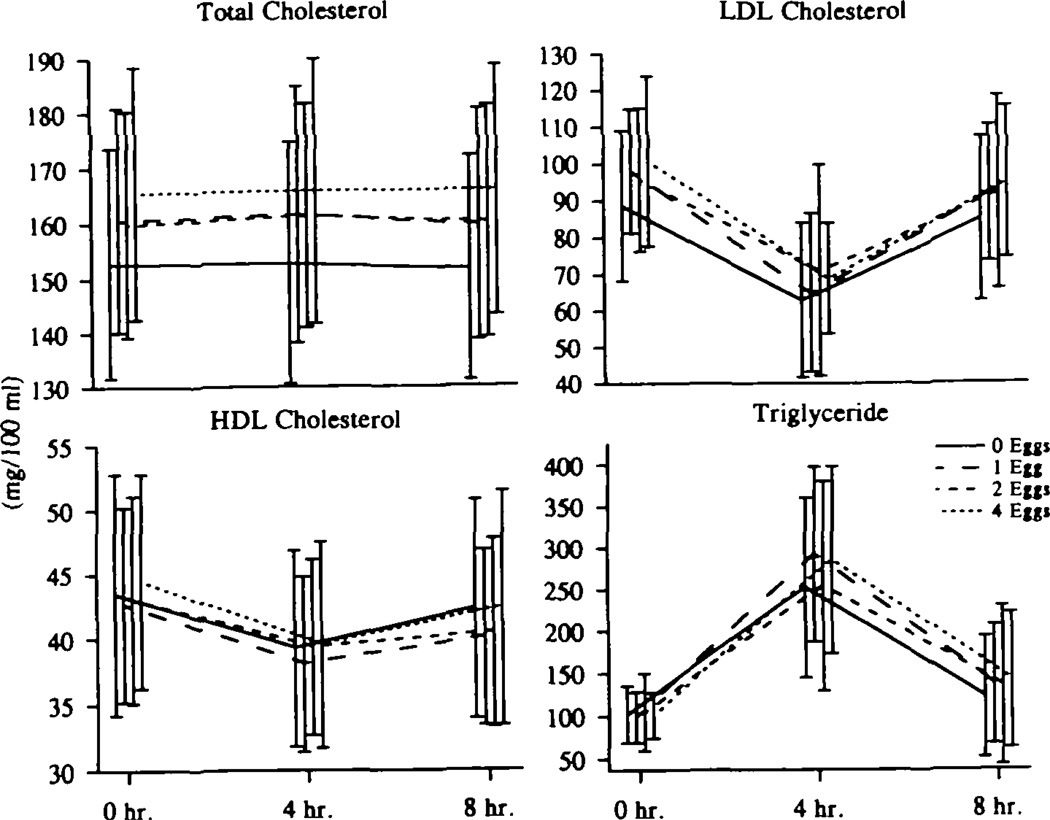
Fig 7.
Graphs show post–fat-formula plasma lipid responses to increasing dietary cholesterol. Total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol and plasma triglyceride levels were measured just before and 4 and 8 hours after a standard, high-fat formula providing 53 g fat per square meter, 60 000 U vitamin A per square meter, and 300 mg cholesterol. All 20 subjects had this test on each of the four diets. There was no effect of increasing dietary cholesterol intake on the response (area) to this high-fat meal, after correcting for fasting (zero) levels. Absolute levels of total and LDL cholesterol were higher throughout the post–fat-formula period on the 4-egg diet compared with the 0-egg diet.
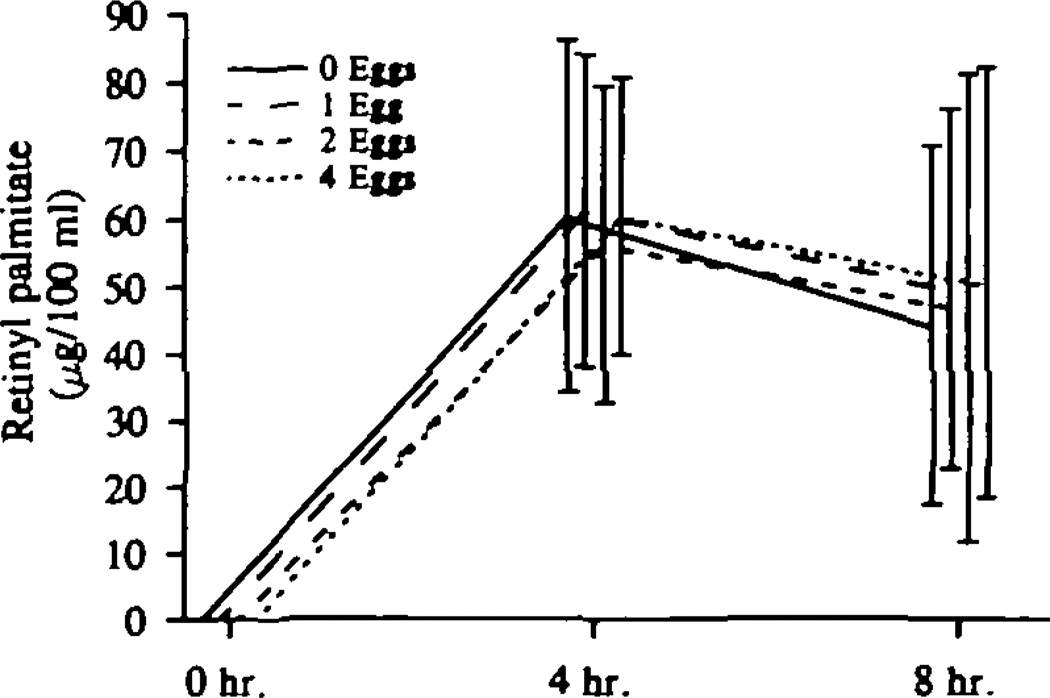
Fig 8.
Graph shows post–fat-formula chylomicron responses to increasing dietary cholesterol. Plasma retinyl palmitate concentrations (which represent >75% of retinyl esters in plasma) were measured at 4 and 8 hours after a standard, high-fat formula providing 53 g fat per square meter, 60 000 U vitamin A per square meter, and 300 mg cholesterol. All 20 subjects had this test on each of the four diets. There was no measurable retinyl palmitate in fasting plasma (zero sample). There was no effect of increasing dietary cholesterol on the retinyl palmitate responses to a standard, high-fat formula.
In an attempt to determine if either long- or short-term consumption of cholesterol increased the atherogenicity of postprandial serum, we incubated samples obtained 4 hours after the standard lunch meals with J774 cultured macrophages for 18 hours and compared total, free, and esterified cholesterol content of the cells. There were no differences in any of these measurements between any two diets (Table 6). There was, however, a significantly higher mean free cholesterol content on the 1-, 2-, and 4-egg diets together versus the 0-egg diet. The difference was 0.63 ± 0.11 µg/mg cell protein (P <.05).
TABLE 6.
Effects of Postlunch Serum on J774 Cells
| Dietary Cholesterol,* mg/d |
TC | FC | CE |
|---|---|---|---|
| 128 | 13.5 (2.9) |
7.5 (1.9) |
6.0 (2.0) |
| 283 | 14.0 (3.8) |
8.2 (2.5) |
5.7 (2.3) |
| 468 | 14.4 (4.1) |
8.1 (2.1) |
6.2 (2.8) |
| 858 | 14.4 (3.4) |
8.3 (2.2) |
6.1 (2.0) |
TC indicates total cholesterol; FC, free cholesterol; and CE, cholesteryl ester. Values are mean (SD), expressed in micrograms per milligram cell protein. There was no dose response of any of the variables to increasing dietary cholesterol. When the data from the 1-, 2-, and 4-egg diets were combined, FC was significantly increased vs the 0-egg diet (P <.05).
Measured amounts on each of the four diets.
Discussion
In this study of healthy young men eating an AHA step 1 diet, we have demonstrated a linear increase in plasma total and LDL cholesterol concentrations as dietary cholesterol consumption increased from very low (128 mg/d) to very high (858 mg/d) intakes. Our observed increases were, however, much less than generally accepted predictions. When we used the model proposed by Keys et al4,7 with the square root function of change in dietary cholesterol, our observed regression coefficient of .60 mg/dL was less than the value of 1.0 mg/dL predicted from the Keys’ equation, for a caloric intake of 2250 kcal/d, which was the average intake of our participants. (Keys et al4,7 estimated a regression coefficient of 1.5 mg/dL when dietary cholesterol is expressed as 100 mg/1000 kcal; this is equivalent to a regression coefficient of 1.0 mg/dL when dietary cholesterol is expressed as 100 mg/2250 kcal, as we have done.) In fact, the response predicted by Keys’ equation was greater than the upper 95% CI (0.91 mg/dL) for our group of subjects. Only four of our 20 men had a response greater than 1.0 mg/dL per square root milligrams cholesterol per day. The regression analyses by Hegsted et al3,26 would have predicted an even greater increase, of approximately 4 mg/dL for every 100 mg cholesterol added to the dairy diets eaten by our men, which is much greater than either the mean response of 1.47 mg/dL or the upper 95% CI of 2.2 mg/dL. Only one of our 20 subjects had a value greater than 3.5 mg/dL per 100 mg dietary cholesterol per day, as shown in Fig 5.
Why do our results differ? A review of the literature indicates that the fatty acids present in the background diet may have a critical role in determining response to dietary cholesterol. In most of the studies conducted by Hegsted et al3 and Keys et al,4 high total or saturated fat diets were fed with varying amounts of cholesterol. Mattson et al5 used a 40% fat diet that was high in saturated fat as their background diet. It seems clear from the Faribault Study,14 however, that the quantities of total and saturated fat in the diet have a very significant impact on responsiveness to dietary cholesterol. In that large study, the change in plasma cholesterol associated with an increase of approximately 500 mg of dietary cholesterol per day was close to that predicted by others3–5 when the background diet was high in total and saturated fat but was very similar to our prediction when the background diet was much lower in total or saturated fat. Schonfeld et al15 also demonstrated marked differences in responsiveness to dietary cholesterol as they changed the ratio of polyunsaturated to saturated fatty acids (P-S ratio) in the diet.
The importance of the background diet is also clear from a comparison of metabolic studies done by Packard et al27 and by our group.28 The former investigators, using a diet with an extremely low P-S ratio of 0.2, found clear increases in LDL production on a high-cholesterol diet. We were unable, using a diet with a P-S ratio of 0.8, to demonstrate an effect of increasing dietary cholesterol on LDL metabolism.28 It must be noted, however, that neither McNamara et al29 nor Kestin et al30 observed effects of changing P-S ratios on the response to dietary cholesterol. The mean change in plasma cholesterol in those studies was very low, approximately 1 mg/dL per 100 mg dietary cholesterol per day, and was unaffected by the P-S ratio. In both of those investigations, the diets were higher in total fat than in our study. In addition, a review of more than 100 studies in the literature by McNamara8 led him to conclude that plasma cholesterol increases approximately 2 mg/dL for every 100 mg dietary cholesterol intake per day, regardless of the background diet. We believe, however, that the interaction between dietary saturated fat and cholesterol intake must be investigated in more detail, particularly in view of the data from Clifton et al31 and Katan et al32 suggesting that there is a correlation between responsiveness to these two nutrients.
We also found that the increase in total cholesterol was due almost entirely to an increase in LDL cholesterol, which rose 1.38 mg/dL (0.036 mmol/L) per 100 mg dietary cholesterol. The increases in LDL cholesterol that we observed were correlated closely with increases in serum apoB concentrations, indicating that LDL composition was unchanged and that there were more LDL particles in the plasma on the 4-egg diet. It is not clear whether this increase resulted from greater production of LDL particles27 or reduced clearance of LDL via receptor-mediated uptake into cells.33
We did not find any change in HDL cholesterol concentrations determined by the standard precipitation method but did see increases in HDL cholesterol levels with increasing dietary cholesterol intake when we isolated HDL by ultracentrifugation. This discrepancy may result from an increase in apoE-enriched HDL on the highest cholesterol diet.34,35 This form of HDL, which usually accounts for a very small proportion of HDL cholesterol, may not be measured accurately by the precipitation method but is isolated in the HDL fraction by ultracentrifugation. Thus, even though ultracentrifugation was associated with less complete isolation of HDL at all levels of dietary cholesterol (due to losses common to this methodology), it did demonstrate small but significant increases in HDL cholesterol with higher cholesterol intakes. Overall, we think that our results indicate that, as suggested by others,34,35 increasing dietary cholesterol was associated with increases in apoE-enriched HDL. The role of apoE-enriched HDL in human physiology is not fully defined, although it may return cholesteryl esters directly to the liver.36
We observed a very wide range of responsiveness to dietary cholesterol in these healthy, young men. In fact, several individuals had negative or zero responses to increasing dietary cholesterol, while a few had responses that were more than twice the group mean. Individual variability in responsiveness to dietary cholesterol has been studied closely by several groups. Beynen et al12 and Katan and Beynen13 have concluded that there are “true” hyperresponders and hyporesponders to dietary cholesterol, but that individual variability is high even within these groups, making it difficult to characterize subjects on the basis of a single study. The data from McNamara et al29 indicate that responsiveness is determined by the ability to suppress endogenous cholesterol synthesis in the face of increased dietary cholesterol intake. They concluded that approximately two thirds of normal individuals can compensate extremely well for increased dietary cholesterol consumption. Quintao et al37 and Nestel and Poyser38 also concluded that inhibition of endogenous cholesterol synthesis was central to an individual’s ability to compensate for increased dietary intake. Mistry et al11 observed a wide range of responsiveness to dietary cholesterol that appeared to correlate with LDL receptor binding to freshly isolated monocytes; those subjects with greater binding capacity at baseline were less responsive to increased dietary cholesterol intake. In our small study, individual responses of both total and LDL cholesterol to dietary cholesterol appeared to be normally distributed. We did find that the “free-living” cholesterol concentrations in our men (obtained at the time of recruitment) correlated positively with the changes in plasma cholesterol between the 0- and 4-egg diets. We are presently conducting a study of repeated challenges with high-cholesterol diets in a similar group of men.
ApoE genotype appears, in some39–41 but not all42–44 studies, to modify responsiveness to dietary cholesterol consumption, possibly by regulating intestinal absorption of this nutrient.45 In this relatively small group of subjects we did not find a significant association between the presence of the E4 allele and an increased response to dietary cholesterol. Recent studies with another group of men and with women have also failed to associate apoE genotype with responsiveness to dietary cholesterol (unpublished data). Whether apoE genotyping can be used to identify hyperresponders to dietary cholesterol remains to be determined in larger, prospectively designed studies. Well-controlled studies will also be needed to investigate the relation between other genetic markers, such as the polymorphisms in apoB46,47 and in apoA-IV,48 and responsiveness to dietary cholesterol.
Plasma CETP appears to play a key role in the reverse transport of cholesterol from tissues to the liver.49 Recent studies have demonstrated increases in plasma CETP concentrations and hepatic CETP mRNA levels in primates fed very-high-cholesterol diets.50,51 Although we did not observe a dose-response relation between dietary cholesterol and plasma CETP levels, our findings of increased CETP levels on the 4-egg diet and of a correlation between increases in CETP and cholesterol concentrations support the previous studies in animals. Martin et al44 recently reported a similar correlation between changes in CETP levels and changes in LDL cholesterol with high-cholesterol feeding. The physiological basis of a link between plasma cholesterol and CETP responses to a high-cholesterol diet remains to be determined. One possible explanation is that increased plasma levels of LDL cholesterol may somehow stimulate CETP gene expression. The fact that Martin et al44 observed greater increases in both plasma LDL cholesterol and CETP concentrations when their subjects consumed high-cholesterol diets supports such a link. On the other hand, increased CETP levels may, by mediating the generation of cholesteryl ester–enriched VLDL remnants, lead to downregulation of hepatic LDL receptors and further increases in LDL concentrations. Martin et al44 also noted a relation between changes in CETP levels and changes in HDL2 cholesterol concentrations. We did not see a correlation between changes in CETP and changes in total HDL cholesterol levels.
The association between dietary cholesterol and coronary heart disease is complex and may not be derived simply from the effects of dietary cholesterol on fasting plasma lipid and lipoprotein concentrations.9 For example, cholesterol-enriched remnant lipoproteins isolated from cholesterol-fed animals can transform cultured macrophages into foam cells,52 and such remnantlike lipoproteins have been isolated from humans fed high-cholesterol diets.53 With this in mind, we investigated both the short- and long-term effects of increasing dietary cholesterol intake on postprandial lipid metabolism. We used a standard lunch containing each of the levels of dietary cholesterol to investigate the acute effects of varying cholesterol intake on the background of the same level of dietary cholesterol consumed for 8 weeks. We did not observe any differences in the plasma lipid response, calculated as the area above baseline, between the different cholesterol-containing lunches. Thus, there was no short-term effect of different dietary cholesterol loads. On the other hand, we did see the background effect of the highest cholesterol diet, in that all of the postlunch total and LDL cholesterol concentrations were higher on the 4-egg diet compared with the 0-egg diet.
We explored the effects of dietary cholesterol on postprandial lipid metabolism further by determining the response to a standard, high-fat meal. This test, which provided the same amount of dietary cholesterol (300 mg) during all four diet periods, isolated the long-term effects of increasing dietary cholesterol. We did not see any effects of long-term consumption of high-cholesterol diets on chylomicron (as measured by triglyceride levels) or chylomicron remnant (as measured by retinyl palmitate levels) metabolism. The latter results are of particular interest in view of the uncertainty concerning the pathway for chylomicron remnant removal from plasma. Our inability to observe an effect of high cholesterol intake on chylomicron remnant removal is compatible with studies by Weintraub et al,54 in which retinyl ester clearance was normal in subjects with heterozygous familial hypercholesterolemia, and by Eriksson et al,55 in which groups with a wide range of LDL receptor activity had similar rates of clearance of a cholesterol-rich fat emulsion. Our present data suggest that either LDL receptor–mediated catabolism was not affected by increased cholesterol intake, despite increases in fasting LDL cholesterol and apoB levels, or that another receptor, such as the LDL receptor–related protein, is responsible for remnant removal. In any event, we found no evidence for accumulation of postprandial lipoproteins during the higher-cholesterol diet periods. The absence of increased postprandial lipoproteins during consumption of high-cholesterol diets was mirrored by the absence of any striking effects of postprandial serum on cholesterol metabolism in cultured macrophages.
In summary, we have demonstrated that increases in dietary cholesterol intake are associated with modest, linear increases in total and LDL cholesterol levels in young, healthy men consuming a low-fat diet. The wide range of individual responsiveness we observed was consistent with a normal distribution, but given our sample size, our results are not inconsistent with previous studies indicating that hyporesponders and hyperresponders exist in the population; we neither determined sterol balance11,29,37,38 nor conducted repeated studies.12,13 We did find a correlation between the plasma cholesterol concentration on a free-living, “average American diet” and responsiveness to dietary cholesterol in our study. This suggests that students most responsive to an average American diet, higher in saturated fat and cholesterol, were most responsive to simply increasing dietary cholesterol. Plasma CETP levels increased only on the highest-cholesterol diets, but this response was not related to changes in HDL cholesterol. Finally, we did not find any significant effects of increased dietary cholesterol on postprandial lipid metabolism. Future studies must focus on identifying predictors for hyperresponsiveness to dietary cholesterol so that dietary counseling can be used in the most efficacious manner.
Acknowledgments
This study was supported by grants HL-36000, HL-21006, and RR-645 from the National Institutes of Health and a grant from the American Egg Board. Support was also provided by Fleischmann Inc. The authors wish to thank Colleen Ngai, Nelson Fontanez, Jeffrey Jones, Minnie Myers, Anne Gleeson, and Inge Hansen for their excellent technical assistance; Colleen Johnson, Roberta Holeman, and the diet staff for their outstanding work in preparing and serving meals; and Ludmilla Karameros for assistance with the manuscript.
References
- 1.Conner WE, Hodges RE, Bleiler RE. Effect of dietary cholesterol upon serum lipids in man. J Lab Clin Med. 1961;57:331–342. [PubMed] [Google Scholar]
- 2.Connor WE, Hodges RE, Bleiler RE. The serum lipids in men receiving high cholesterol and cholesterol-free diets. J Clin Invest. 1961;40:894–901. doi: 10.1172/JCI104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. Am J Gin Nutr. 1965;17:281–295. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- 4.Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet, II: the effect of cholesterol in the diet. Metabolism. 1965;14:759–765. doi: 10.1016/0026-0495(65)90002-8. [DOI] [PubMed] [Google Scholar]
- 5.Mattson FH, Erickson BA, Kligman AM. Effects of dietary cholesterol on serum cholesterol in man. Am J Clin Nutr. 1972;25:589–594. doi: 10.1093/ajcn/25.6.589. [DOI] [PubMed] [Google Scholar]
- 6.McGill HC. The relationship of dietary cholesterol to serum cholesterol concentration and to atherosclerosis in man. Am J Clin Nutr. 1979;2:2664–2702. doi: 10.1093/ajcn/32.12.2664. [DOI] [PubMed] [Google Scholar]
- 7.Keys A. Serum cholesterol response to dietary cholesterol. Am J Clin Nutr. 1984;40:351–359. doi: 10.1093/ajcn/40.2.351. [DOI] [PubMed] [Google Scholar]
- 8.McNamara DJ. Relationship between blood and dietary cholesterol. Adv Meat Res. 1990;6:63–87. [Google Scholar]
- 9.Stamler J, Shekelle R. Dietary cholesterol and human coronary heart disease. Arch Pathol Lab Med. 1988;112:1032–1040. [PubMed] [Google Scholar]
- 10.Stehbens WE. The controversial role of dietary cholesterol and hypercholesterolemia in coronary heart disease and atherogenesis. Pathology. 1989;21:213–221. doi: 10.3109/00313028909061061. [DOI] [PubMed] [Google Scholar]
- 11.Mistry P, Miller NE, Laker M, Hazzard WR, Lewis B. Individual variation in the effects of dietary cholesterol on plasma lipoproteins and cellular cholesterol homeostasis in man. J Clin Invest. 1981;67:493–502. doi: 10.1172/JCI110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beynen AC, Katan MB, Van Zutphen LFM. Hypo- and hyperresponders: individual differences in the response of serum cholesterol concentration to changes in diet. Adv Lipid Res. 1987;22:115–171. doi: 10.1016/b978-0-12-024922-0.50008-4. [DOI] [PubMed] [Google Scholar]
- 13.Katan MB, Beynen AC. Characteristics of human hypo and hyper-responders to dietary cholesterol. Am J Epidemiol. 1987;125:387–399. doi: 10.1093/oxfordjournals.aje.a114545. [DOI] [PubMed] [Google Scholar]
- 14.National Diet-Heart Study Research Group. Faribault second study: National Diet–Heart Study final report. Circulation. 1968;37 suppl 1:I-260–I-274. [Google Scholar]
- 15.Schonfeld G, Patsch W, Rudel LL, Nelson C, Epstein M, Olson RE. Effects of dietary cholesterol and fatty acids on plasma lipoproteins. J Gin Invest. 1982;69:1072–1079. doi: 10.1172/JCI110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summary of the second report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. JAMA. 1993;269:3015–3023. [PubMed] [Google Scholar]
- 17.Warnick GR, Nguyen T, Albers AA. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin Chem. 1985;31:217–222. [PubMed] [Google Scholar]
- 18.Manual of Laboratory Operations: Vol I, Lipid and Lipoprotein Analysis. Washington, DC: National Institutes of Health; 1974. Lipid Research Clinic Program; pp. 75–628. Dept of Health, Education, and Welfare publication 9NIH0. [Google Scholar]
- 19.Havel RA, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;4:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson JC, Rubinstein A, Bukberg PR, Brown WV. Apolipoprotein E enriched lipoprotein subclasses in normolipidemic subjects. J Lipid Res. 1983;24:886–898. [PubMed] [Google Scholar]
- 21.Smith SJ, Cooper GR, Henderson LO, Hannon WH the Apolipoprotein Standardization Collaborating Group. An international collaborative study on standardization of apolipoproteins A–I and B. Clin Chem. 1987;33:2240–2249. [PubMed] [Google Scholar]
- 22.Marcel YL, McPherson R, Hogue M, Czarnecka H, Zawadzki Z, Weech PK, Whitlock ME, Tall AR, Milne RW. Distribution and concentration of cholesteryl ester transfer protein in plasma of normolipidemic subjects. J Clin Invest. 1990;85:10–17. doi: 10.1172/JCI114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hixson J, Vernier D. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhAI. J Lipid Res. 1991;31:545–548. [PubMed] [Google Scholar]
- 24.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of α-tocopherol and retinol in plasma or red cells by high-pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa TT, MacGee J, Morrison JA, Glueck CJ. Quantitative analysis of cholesterol in 5 to 20 µl of plasma. J Lipid Res. 1974;15:286–291. [PubMed] [Google Scholar]
- 26.Hegsted DM. Serum-cholesterol response to dietary cholesterol: a re-evaluation. Am J Clin Nutr. 1986;44:299–305. doi: 10.1093/ajcn/44.2.299. [DOI] [PubMed] [Google Scholar]
- 27.Packard CJ, McKinney L, Carr K, Shepherd J. Cholesterol feeding increases low density lipoprotein synthesis. J Clin Invest. 1983;72:45–51. doi: 10.1172/JCI110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginsberg H, Le N-A, Mays C, Gibson J, Brown WV. Lipoprotein metabolism in non-responders to increased dietary cholesterol. Arteriosclerosis. 1981;1:463–470. doi: 10.1161/01.atv.1.6.463. [DOI] [PubMed] [Google Scholar]
- 29.McNamara DJ, Kolb R, Parker TS, Batwin H, Samuel P, Brown CD, Ahrens EH., Jr Heterogeneity of cholesterol homeostasis in man. J Clin Invest. 1987;79:1729–1739. doi: 10.1172/JCI113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kestin M, Clifton PM, Rouse IL, Nestel PJ. Effect of dietary cholesterol in normolipidemic subjects is not modified by nature and amount of dietary fat. Am J Clin Nutr. 1989;50:528–532. doi: 10.1093/ajcn/50.3.528. [DOI] [PubMed] [Google Scholar]
- 31.Clifton PM, Kestin M, Abbey M, Drysdale M, Nestel PJ. Relationship between sensitivity to dietary fat and dietary cholesterol. Arteriosclerosis. 1990;10:394–401. doi: 10.1161/01.atv.10.3.394. [DOI] [PubMed] [Google Scholar]
- 32.Katan MB, Berns MAM, Glatz JFC, Knuiman JT, Nobels A, deVries JHM. Congruence of individual responsiveness to dietary cholesterol and to saturated fat in humans. Lipid Res. 1988;29:883–892. [PubMed] [Google Scholar]
- 33.Yokode M, Hammer RE, Ishibashi S, Brown MZS, Goldstein JL. Diet-induced hypercholesterolemia in mice: prevention by overexpression of LDL receptors. Science. 1990;250:1273–1275. doi: 10.1126/science.2244210. [DOI] [PubMed] [Google Scholar]
- 34.Mahley RT, Innerarity L, Bersont TP, Lipson A, Margolis S. Alterations in human high-density lipoproteins, with or without increased plasma-cholesterol, induced by diets high in cholesterol. Lancet. 1978;2:807–8O9. doi: 10.1016/s0140-6736(78)92588-6. [DOI] [PubMed] [Google Scholar]
- 35.Cole TG, Patsch W, Kuisk I, Gonen B, Schonfeld G. Increases in dietary cholesterol and fat raise levels of apoprotein E-containing lipoproteins in the plasma of man. J Clin Endocrinol Metab. 1983;56:1108–1115. doi: 10.1210/jcem-56-6-1108. [DOI] [PubMed] [Google Scholar]
- 36.Rinninger F, Pittman RC. Regulation of the selective uptake of high density lipoprotein-associated cholesteryl esters by human fibroblasts and HepG2 hepatoma cells. J Lipid Res. 1988;29:1179–1194. [PubMed] [Google Scholar]
- 37.Quintao E, Grundy SM, Ahrens EH., Jr Effect of dietary cholesterol on the regulation of total body cholesterol in man. Lipid Res. 1971;12:233–247. [PubMed] [Google Scholar]
- 38.Nestel PJ, Poyser A. Changes in cholesterol synthesis and excretion when cholesterol intake is increased. Metab Clin Exp. 1976;25:1591–1599. doi: 10.1016/0026-0495(76)90112-8. [DOI] [PubMed] [Google Scholar]
- 39.Manttari M, Koskinen P, Ehnholm C, Huttunen JK, Manninen V. Apolipoprotein E polymorphism influences the serum cholesterol response to dietary intervention. Metabolism. 1991;40:217–221. doi: 10.1016/0026-0495(91)90179-z. [DOI] [PubMed] [Google Scholar]
- 40.Lehtimaki T, Moilanen T, Solakivi T, Laippala P, Ehnholm C. Cholesterol-rich diet induced changes in plasma lipids in relation to apolipoprotein E phenotype in healthy students. Ann Med. 1992;24:61–66. doi: 10.3109/07853899209164146. [DOI] [PubMed] [Google Scholar]
- 41.Tikkanen MJ, Huttunen JK, Ehnholm C, Pietinen P. Apolipoprotein E4 homozygosity predisposes to serum cholesterol elevation during high fat diet. Arteriosclerosis. 1990;10:285–288. doi: 10.1161/01.atv.10.2.285. [DOI] [PubMed] [Google Scholar]
- 42.Boerwinkle E, Brown SA, Rohrbach K, Gotto AM, Jr, Patsch W. Role of apolipoprotein E and B gene variation in determining response of lipid, lipoprotein, and apolipoprotein levels to increased dietary cholesterol. Am J Hum Genet. 1991;49:1145–1154. [PMC free article] [PubMed] [Google Scholar]
- 43.Savolainen MJ, Rantala M, Kervinen K, Jarvi L, Suvanto K, Rantala T, Kesaniemi YA. Magnitude of dietary effects on plasma cholesterol concentration: role of sex and apolipoprotein E phenotype. Atherosclerosis. 1991;86:145–152. doi: 10.1016/0021-9150(91)90210-t. [DOI] [PubMed] [Google Scholar]
- 44.Martin LJ, Connelly PW, Nancoo D, Wood N, Zhang ZJ, Maguire G, Quinet E, Tall AR, Marcel YL, McPherson R. Cholesterol ester transfer protein and high density lipoprotein responses to cholesterol feeding in men: relationship to apolipoprotein E genotype. J Lipid Res. 1993;34:437–446. [PubMed] [Google Scholar]
- 45.Kesaniemi YA, Ehnholm C, Miettinen TA. Intestinal cholesterol absorption efficiency in man is related to apoprotein E phenotype. J Clin Invest. 1987;80:578–581. doi: 10.1172/JCI113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boerwinkle E, Brown S, Rohrbach K, Gotto A, Patsch W. Role of apolipoprotein E and B gene variation in determining response of lipid, lipoprotein, and apolipoprotein levels to increased dietary cholesterol. Am J Hum Genet. 1991;49:1145–1154. [PMC free article] [PubMed] [Google Scholar]
- 47.Talmud PJ, Boerwinkle E, Xu CF, Tikkanen MJ, Pietinen P, Huttunen JK, Humphries S. Dietary intake and gene variation influence the response of plasma lipids to dietary intervention. Genet Epidemiol. 1992;9:249–260. doi: 10.1002/gepi.1370090404. [DOI] [PubMed] [Google Scholar]
- 48.McCombs RJ, Marcadis DE, Weinber RB. Attenuated hypercholesterolemic response of apolipoprotein A-IV-1/2 heterozygotes to a high cholesterol diet. Clin Res. 1993;41:191A. doi: 10.1056/NEJM199409153311104. Abstract. [DOI] [PubMed] [Google Scholar]
- 49.Tall AR. Plasma lipid transfer proteins. J Lipid Res. 1986;27:361–367. [PubMed] [Google Scholar]
- 50.Quinet EA, Tall A, Ramakrishnan R, Rudel L. Plasma lipid transfer protein as a determinant of the atherogenicity of monkey plasma lipoproteins. J Clin Invest. 1991;87:1559–1566. doi: 10.1172/JCI115169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinet EM, Agellon LB, Kroon PA, Marcel YL, Lee Y-C, Whitlock ME, Tall AR. Atherogenic diet increases cholesterol ester transfer protein (CETP) mRNA levels in rabbit liver. J Clin Invest. 1990;85:357–363. doi: 10.1172/JCI114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldstein JL, Ho YK, Brown MS. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine β-very low density lipoproteins. J Biol Chem. 1978;255:1839–1848. [PubMed] [Google Scholar]
- 53.Nestel P, Tada N, Billington T, Huff M, Fidge N. Changes in very low density lipoproteins with cholesterol loading in man. Metabolism. 1982;32:398–405. doi: 10.1016/0026-0495(82)90117-2. [DOI] [PubMed] [Google Scholar]
- 54.Weintraub MS, Eisenberg S, Breslow JL. Different patterns of postprandial lipoprotein metabolism in normal, type IIa, type III, type IV hyperlipoproteinemic individuals: effects of treatment with cholestyramine and gemfibrozil. J Clin Invest. 1987;79:1110–1119. doi: 10.1172/JCI112926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eriksson M, Angelin B, Henriksson P, Ericsson S, Vitols S, Berglund L. Metabolism of lipoprotein remnants in humans. Arterioscler Thromb. 1991;11:827–837. doi: 10.1161/01.atv.11.4.827. [DOI] [PubMed] [Google Scholar]