Abstract
• Background and Aims Intervascular pit membranes were examined within Ericales to determine the distribution and structure of torus-like thickenings.
• Methods Forty-nine species representing 12 families of the order Ericales were investigated using light, scanning and transmission electron microscopy. They were compared with four species of Oleaceae to determine the true nature of the thickenings.
• Key Results Pit membranes with torus-like thickenings were observed in seven species of Ericaceae and were found to be amorphous, plasmodesmata-associated structures with an irregular distribution. These pseudo-tori show major differences compared with true tori with respect to their distribution and ultrastructure. Genuine tori, which are strongly correlated with round pit apertures in narrow tracheary elements, were found in two species of Osmanthus (Oleaceae).
• Conclusions The pseudo-tori found in some Ericaceae are considered to be similar to pit membrane thickenings previously recorded in Rosaceae. While true tori appear to be functionally significant in terms of efficiency and safety of water transport, the possible function of pseudo-tori could be associated with the role of plasmodesmata during differentiation of tracheids, fibre-tracheids or narrow vessels.
Keywords: Ericaceae, Ericales, Oleaceae, pit membrane, plasmodesmata, pseudo-torus, torus, tracheary elements
INTRODUCTION
Pits represent small openings or depressions in the secondary cell wall of wood cells and are among the most conspicuous wood anatomical structures in plants. Their structure and biological significance in the xylem have fascinated plant anatomists for centuries (Schacht, 1859). Although pits in tracheary elements are characteristically bordered (i.e. the pit membrane is overarched by the secondary cell wall), the pit structure in vascular plants shows wide structural variation with respect to pit size, shape, pit membrane characteristics, border configuration, pit-field arrangement, and presence or absence of vestures (Schmid, 1965; Bauch et al., 1972; Jansen et al., 2001). Due to this structural variation, pits are very useful for wood identification, forensic research, or palaeobotany (IAWA Committee, 2004). The structure and distribution of bordered pits may also be used to define cell types (Baas, 1986; Carlquist, 1986a, b). Moreover, since water-conducting xylem elements are of limited length, pits also play a key role in lateral water transport in sapwood of living plants, linking water uptake in roots with transpiration in leaves. Minute pores in the pit membranes allow the flow of water between adjacent conductive elements and, at the same time, limit the passage of air bubbles and pathogens (Tyree and Zimmermann, 2002). Recent advances in the field of xylem physiology have stimulated a renewed interest in the micromorphology and ultrastructure of bordered pits in vessel elements and tracheids as it is now apparent that certain pit characteristics may affect flow resistance and vulnerability to air entry (Tyree and Sperry, 1989; Cochard et al., 1992; Becker et al., 2003; Choat et al., 2004; Pitterman et al., 2005). Because pit membranes also affect the penetration of liquids, preservatives and gases in timber, research on pit membranes provides interesting applications in the field of wood technology, including the paper and pulp industry (Bailey, 1913; Flynn, 1995; Watanabe et al., 1998; Singh et al., 1999). Nevertheless, our current knowledge of pit membranes is based on a relatively small number of plant species and hampered by technological difficulties in dissecting the exact nature of pit membranes without creating artefacts (Wheeler, 1983; Sano and Fukazawa, 1994; Pesacreta et al., 2005).
The frequently cited statement that angiosperms show a homogeneous pit membrane and that gymnosperms are characterized by a torus–margo pit membrane holds true for the majority of angiosperms and gymnosperms, although some observations have been reported to which this generalization does not apply. Ohtani and Ishida (1978) are assumed to be the first anatomists to record a torus–margo configuration in some Daphne and Osmanthus species. Since then, tori have been found in five angiosperm families (i.e. Cannabaceae, Oleaceae, Rosaceae, Thymelaeaceae, Ulmaceae) (Wheeler, 1983; Dute and Rushing, 1987, 1988, 1990; Dute et al., 1992, 2004; Jansen et al., 2004; S. Jansen, unpubl. res.). It is unlikely that the torus structure in these families is homologous, because two different types of tori have been described in angiosperms based on differences in their ontogeny and chemical composition (Coleman et al., 2004). These observations raise interesting questions about the systematic distribution and functional significance of torus-bearing pit membranes in angiosperms and it is suggested that tori evolved more than once within that group (Morrow and Dute, 1999). Besides these observations, torus-like pit membranes have also been reported in several species of Rosaceae and in Ribes sanguineum (Grossulariaceae). However, it is now evident that these features do not represent true tori as they are primary wall thickenings associated with plasmodesmata in narrow tracheary elements (Parameswaran and Liese, 1973, 1981; Barnett, 1987a, b; Lachaud and Maurousset, 1996). The function of these plasmodesmata associated thickenings is still unclear and their systematic distribution in angiosperms is currently restricted to a few Rosaceae and one Grossulariaceae species.
Obviously, the distribution of pit membrane thickenings is an interesting character in seed plant phylogeny. Variation in the structure of pit membranes may also play a functional role in water transport or could reflect various processes in xylem differentiation (Hacke et al., 2004; Sperry and Hacke, 2004). Since it has been claimed that tori and other types of pit membrane thickenings have been overlooked in at least some angiosperm groups, the enlarged order Ericales sensu APG was screened in order to explore the occurrence of these characters (Anderberg et al., 2002; Bremer et al., 2002; APG II, 2003). Based on light and electron microscopy, this paper aims to determine the distribution and true nature of pit membrane thickenings in some of the Ericales. In addition, observations in the Ericales are compared with representatives of the Oleaceae in which true tori have previously been reported (Ohtani and Ishida, 1978; Dute and Rushing, 1987, 1988).
MATERIALS AND METHODS
Materials
For light microscopy (LM) and scanning electron microscopy (SEM) dried wood samples were used from several wood collections and botanical gardens. Acronyms used in the material list below correspond with the wood collections listed in the index xylariorum (Stern, 1988). For transmission electron microscopy (TEM), young stem segments were collected from the Royal Botanic Gardens (RBG), Kew. The nomenclature of all species followed the currently accepted species delimitation according to the International Plant Names Index (http://www.ipni.org). The following 53 species representing 13 families were investigated with reference to their origin and collectors if known:
Actinidiaceae
Actinidia polygama Franch. and Sav.: USA, M. Nee 43547 (MADw 47991); Saurauia villosa Buscal.: Guatemala (Izabal), Withford and Stadtmiller s.n. (Tw 38694).
Clethraceae
Clethra lanata Mart. and Galeotti: Mexico, L. Lebacq s.n. (Tw 24571).
Cyrillaceae
Cliftonia nitida Gaertn.f.: USA, H.H. Smith (MADw 28029); Cyrilla racemiflora L.: Cuba (Santiago de Cuba), R. Dechamps 12377 (Tw 49847); Purdiaea nutans Planch.: Venezuela (Amazonas), B. Maguire et al. 42133 (Tw 36535).
Ebenaceae
Diospyros virginiana L.: USA (Virginia), F. Scruggs s.n. (Tw 19293).
Ericaceae
Andromeda ferruginea Walter.: USA, A. Curtis 60 (Kw 11721); Arbutus unedo L.: UK, D. Rabaey s.n. (RBG, Kew; 1969–10463); Arctostaphylos alpinus Spreng.: Norway (Hordaland), R. Dechamps 6054c (Tw 38561); A. arbutoides Hemsl.: Costa Rica (Prov. Cartago, Cerro de la Muerte, 3300 m), M. Wiemann and W. Jimenez 7 (Uw 30891); A. uva-ursi Spreng.: UK, D. Rabaey s.n. (RBG, Kew; 1985–2627); Azalea sp.: Belgium (Leuven, Kasteelpark Arenberg), D. Rabaey s.n.; Calluna vulgaris L.: Belgium (Leuven, Institute of Botany and Microbiology), D. Rabaey s.n.; Cassiope tetragona (L.) D.Don: Greenland (Angmagsalik), Daniels, de Molenaar, Feruerda (Uw 16028); Chamaedaphne calyculata (L.) Moench: Germany (Botanic Garden, Bochum), E. Smets s.n.; Comarostaphylis discolor (Hook.) Diggs: Mexico (Chiapis: Municipio Motozintlu de Mendoza, 3000 m), Breedlove (Dw 40352); Dimorphanthera dekockii J.J.Sm. var. publiflora Sleumer: New Guinea, Vink 17307 (Uw 18316); Dracophyllum strictum Hook.f.: New Zealand, H. J. Dentzman 2085 (MADw 5075); D. urvilleanum A.Rich.: New Zealand (Campbell's Island), J.D. Hooker (Kw 11742); D. verticillatum Labill.: New Caledonia (SJRw 14126); Empetrum hermaphroditum Hagerup: Greenland (Angmagsalik), Daniels, de Molenaar, Feruerda (Uw 15996); Enkianthus campanulatus Nichols: Japan, 4360 (BR); Erica arborea L.: Belgium (Leuven, Institute of Botany and Microbiology), D. Rabaey s.n.; E. mannii (Hook.f.) Beentje subsp. usambarensis (Alm and T.C.E.Fr.) Beentje: Kenya (Samburu district, 2500m), B. Bytebier et al. 187 (BR); E. rosacea (L.Guthrie) E.G.H.Oliv.: South Africa (top of Snyberg Mountain), E.G.H. Oliver 11997 (NBG); E. rugegensis Engl.: Rwanda (2300 m), G. Bouxin 1311 (Tw 26015); E. strigosa Wendl.: Tanzania (Kilimanjaro, 2800 m), G. Volkers (Kw 11650); Ledum palustre L.: Poland (Bialystok), F. Schweingruber (Tw 40584); Leiophyllum buxifolium Ell.: origin unknown, (SJRw 52884); Loiseleuria procumbens (L.) Desv.: Switzerland (Arose, 2550 m), J. van der Burgh (Uw 9188); Lyonia neziki Nakai and Hara: Japan (Kumamoto), For. Exp. Stat. 2244 (Tw 17276); Macleania pentaptera Hoerold.: Colombia (Valle), J. L. Luteyn and M. Lebrón-Luteyn 6957 (NY); Oxydendrum arboreum (L.) DC.: USA (Ohio), A.W. Green 245 (Tw 19787); Richea continentis B.L.Burtt: Australia (Mount Sterling, Victoria), collector unknown (Kw 38484); Rhododendron ponticum L.: Belgium (Leuven, Kasteelpark Arenberg), D. Rabaey s.n.; Vaccinium corymbosum L.: Canada (Quebec), R. Dechamps 5003 (Tw 33895); V. corymbosum L.: UK, D. Rabaey s.n. (RBG, Kew; 1999–3980); V. stanleyi Schweinf.: Democratic Republic Congo (Prov. Kivu, Mount Kahun, 2500 m), P. Deuse 55 (BR); V. uliginosum L.: Norway (Hordaland), R. Dechamps 6033 (Tw 38581).
Fouquieriaceae
Fouquieria splendens Engelm.: USA, E.W. Karls s.n. (MADw 30415).
Marcgraviaceae
Marcgravia pedunculosa Triana and Planch.: Suriname, Land- en bosbouwbeheer 15302 (Uw 21928); Norantea guianensis Aubl.: Colombia, Fuchs and Zonella (USw 38181).
Oleaceae
Nestegis apetala (Vahl) L.A.S.Johnson: UK, D. Rabaey s.n. (RBG, Kew; 1966–67112); Osmanthus serratulus Rehder: UK, D. Rabaey s.n. (RBG, Kew; 1973–14933); O. suavis King ex C.B.Clarke: UK, D. Rabaey s.n. (RBG, Kew; 1992–326); Phillyrea angustifolia: UK, D. Rabaey s.n. (RBG, Kew; 1974–1825).
Pentaphylacaceae
Pentaphylax euryoides Gardner and Champ.: China, Inst. Wood Acad. 53110 (Tw 50516).
Saraceniaceae
Heliamphora tatei Gleason: Venezuela, M. Nee 31174 (MADw 44264).
Sapotaceae
Argania spinosa Skeels: Morocco (Rabat Prov.), J. Lewalle 6909 (Tw 26747); Synsepalum attenuatum Hutch. et Dalziel.: Democratic Republic of Congo, J. Louis 7181 (Tw 38460).
Styracaceae
Pterostyrax corymbosum Siebold and Zucc.: China (Guangdong), Forest Research Institute 1074 (Tw 42092).
Theaceae
Camellia furfuracea (Merr.) Cohen-Stuart: China, Inst. Wood Acad. 35 (Tw 50431); Eurya inaequilatera Melch.: Bolivia, B.A. Krukoff 11044 (MADw 35884).
Methods
All wood samples were investigated using light microscopy. Thin sections (10–15 μm) were prepared using a sliding microtome (Reichert, Vienna, Austria). A mixture of safranin and alcian blue (35 : 65, v/v) was used as the staining solution. After staining, sections were washed with distilled water, dehydrated with ethanol and cleared with Parasolve (Prosan, Merelbeke, Belgium). The sections were embedded in Euparal (Agar Scientific Ltd, Essex, UK). Observations were carried out with a Dialux 20 (Leitz, Wetzlar, Germany) using a ×100 oil immersion objective. Pictures were taken with a DP50-CU digital camera (Olympus, Hamburg, Germany). Terminology follows the IAWA Committee (1989). Quantitative data were based on counts of ten measurements using longitudinal sections. Measurements were conducted on LM images using the programme AnalySIS 3.2 (Soft Imaging Systems GmbH, Munster, Germany) as on SEM and TEM pictures using Carnoy 2·0 (Schols et al., 2002).
Based on LM observations the following seven species with pit membrane thickenings were selected for SEM: Arctostaphylos alpina, Empetrum hermaphroditum, Lyonia neziki, Vaccinium corymbosum, V. uliginosum, Osmanthus serrulatus and O. suavis. Small blocks (±3 mm thick) were cut from dry wood samples and attached to stubs. The samples were coated either with gold using a sputter coater (Spi-Supplies, West Chester, PA, USA) or with gold/palladium using an EMITECH K550 sputter coater (Emitech Ltd, Ashford, UK). Observations were carried out at the Laboratory of Plant Systematics (K.U.Leuven) with a Jeol JSM 6360 SEM (Jeol Ltd, Tokyo, Japan) and with a Hitachi S-4700 field-emission SEM (Hitachi High Technologies Corp., Tokyo, Japan) at the Jodrell Laboratory (RBG, Kew).
Five species (Arbutus unedo, Arctostaphylos uva-ursi, Vaccinium corymbosum, Osmanthus suavis and O. serrulatus) were prepared for TEM observations to determine the ultrastructure of pit membrane thickenings. Fresh wood samples from 1- to 2-year-old branches were collected at the RBG, Kew. Small segments from thin branches were cut into 2-mm3 pieces and fixed overnight in Karnovsky's fixative at room temperature (Karnovsky, 1965). After washing in 0·05 m phosphate buffer, the specimens were postfixed in 1 % buffered osmium tetroxide for 4 h at room temperature, washed and dehydrated through a graded ethanol series. The ethanol was gradually replaced with LR White resin (London Resin Co., Reading, UK) over several days. The resin was polymerized at 60 °C and 400 mmHg for 18–24 h. Embedded samples were trimmed and sectioned with an ultramicrotome (Ultracut, Reichert-Jung, Austria). Semi-thin sections cut with a dry glass knife were heat-fixed to glass slides, stained with 0·5 % toluidine blue in 0·1 m phosphate buffer and mounted in DPX (Agar Scientific, Stansted, UK). Ultra-thin sections (90–150 nm) were cut using a diamond knife. The sections were attached to formvar grids and stained with uranyl acetate and lead citrate using a LKB 2168 ultrostainer (LKB-Produkter AB, Bromma, Sweden). Observations were carried out using a Jeol JEM-1210 TEM at 80 kV accelerating voltage and digital images were taken using a MegaView III camera (Soft Imaging System, Münster, Germany).
RESULTS
Intervascular pits showed an even thickness of the pit membrane in the majority of the species studied based on LM observations (Fig. 1A, B). Torus-like thickenings, however, were found in the following seven Ericaceae species: Arbutus unedo (Fig. 1C), Arctostaphylos alpina, A. uva-ursi (Fig. 1D), Empetrum hermaphroditum (Fig. 1E), Lyonia neziki, Vaccinium corymbosum and V. uliginosum. Similar features were observed in Osmanthus serrulatus (Fig. 1F) and O. suavis. Although the thickenings associated with membranes of bordered pits were usually difficult to detect and almost beyond the resolution of the LM, major differences could be distinguished between the thickenings observed in some Ericaceae and the thickenings found in two species of Osmanthus.
Fig. 1.
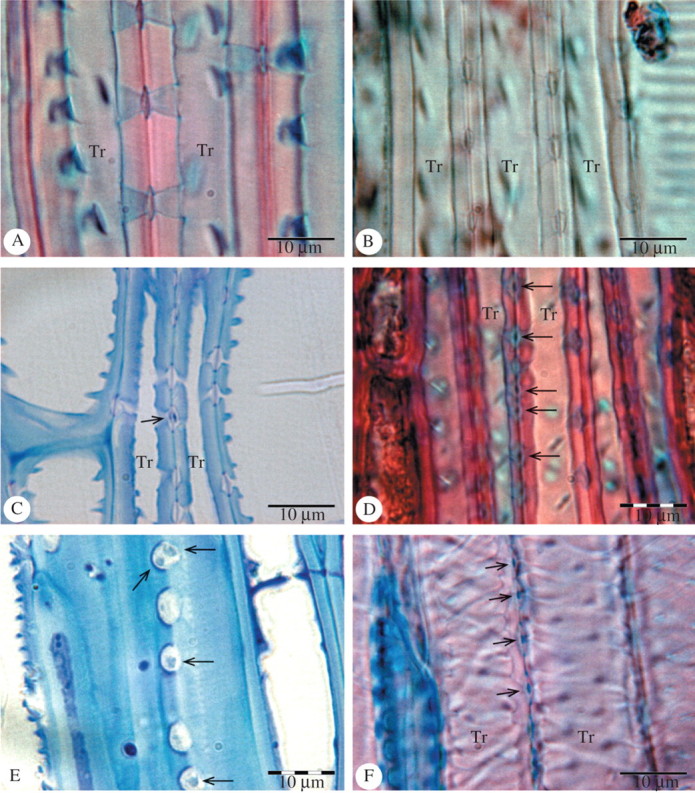
LM illustrations of pit membrane thickenings in Ericaceae and Oleaceae. Sections (C) and (E) are semi-thin sections stained with toluidine blue; sections (A), (B), (D) and (F) are 10–15 μm thick and stained with safranin and alcian blue. (A) Radial section of Andromeda ferruginea showing even pit membranes in narrow tracheary elements (Tr). (B) Radial section of Dracophyllum strictum showing narrow tracheary elements (Tr) with bordered pits without pit membrane thickenings. (C) Longitudinal section of Arbutus unedo (Ericaceae) showing a single pseudo-torus (arrow) and even pit membranes in narrow tracheary elements (Tr) with helical thickenings. (D) Radial section of Arctostaphylos uva-ursi (Ericaceae) with various pseudo-tori (arrows) in narrow tracheary elements (Tr). (E) Radial section of Empetrum hermaphroditum (Ericaceae). Surface view of bordered pits with thickenings (arrows) on pit membranes. Note two thickenings associated with the upper pit membrane. (F) Tangential section of Osmanthus serrulatus (Oleaceae) showing narrow tracheary elements (Tr) with tori (arrows).
The thickenings found in Ericaceae were not consistently present in all pit membranes within a single cell (Fig. 1C) and seemed to occur in different cell types, namely vasicentric tracheids, vascular tracheids and vessel elements. After staining the sections with safranin and alcian blue, the pit membrane thickenings were visible as brownish, glassy and spherical structures with an axial diameter between 1·7 and 2·1 μm and a horizontal diameter between 1·2 and 1·4 μm. Helical thickenings were clearly visible in tracheary elements with pit membrane thickenings in Arbutus unedo (Fig. 1C) and Arctostaphylos uva-ursi. The pits of all Ericaceae species with membrane thickenings were more or less round to elliptical and did not differ in shape and size from other Ericaceae species studied. The mean axial diameter of the pit border was 4·8 μm, varying from 4·2 to 5·3 μm, and the mean horizontal diameter was 4·0 μm, varying from 3·7 to 4·5 μm. Pit apertures in all specimens studied were elongate to slit-like, except for Osmanthus. Pit membranes of Osmanthus serrulatus and O. suavis bore characteristic torus-like thickenings and were visible as blue lens-shaped structures (Fig. 1F) with a mean axial and horizontal diameter of 1·1 and 0·9 μm, respectively. They were found on membranes of bordered pits in narrow tracheids and narrow vessel elements. Helical thickenings were usually associated with the cell walls of these elements. The mean axial and horizontal diameter of pit borders in Osmanthus serrulatus and O. suavis was 2·8 and 2·4 μm, respectively.
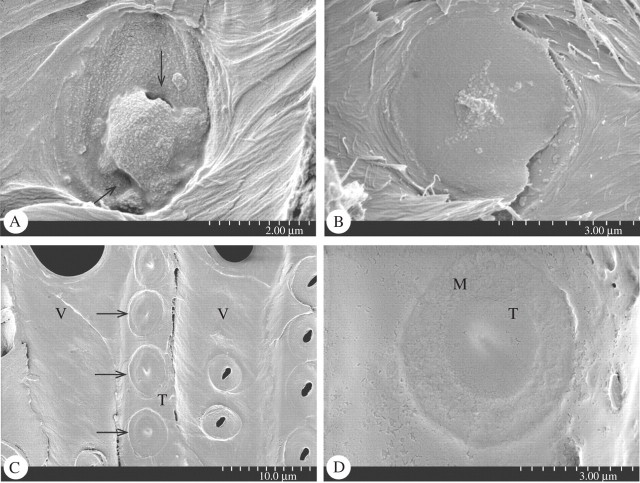
Interesting differences between pit membrane thickenings in Ericaceae and Oleaceae were noticed based on SEM observations. The pit membrane thickening in some Ericaceae was amorphous, irregular in shape and varying in size from 1·4 μm to 2·6 μm (Fig. 2A, B). Vaccinium corymbosum had relatively large thickenings (mean axial diameter, 2·0 μm; mean horizontal diameter, 1·4 μm) and sometimes two or even more thickenings were found to be associated with a single pit membrane (Fig. 1E). On some pit membranes, openings were noticed near the edge of the thickenings, giving these a hollow appearance (Fig. 2A). The structures found in other Ericaceae species were smaller than in V. corymbosum, but were similar in their irregular distribution, variable shape and asymmetrical location on the pit membrane. They were found to take a central as well as more eccentric position on the pit membrane. Torus-like thickenings were clearly visible and consistently associated with bordered pits in tracheids and narrow vessel elements of Osmanthus serrulatus and O. suavis (Fig. 2C, D). The mean axial and horizontal diameter of these pit membrane thickenings was 1·7 μm and 1·6 μm in O. serrulatus, and 2·8 μm and 2·5 μm in O. suavis, respectively. They appeared as round, massive pads surrounded by more even of the pit membrane.
Fig. 2.
SEM illustrations of pit membrane thickenings in Ericaceae and Oleaceae. (A) Pit membrane of Vaccinium corymbosum (Ericaceae) bearing a large pseudo-torus with an eccentric position and two openings (arrows). (B) Pit membrane of Arctostaphylos alpina (Ericaceae) bearing an amorphous, relatively small pseudo-torus. (C) Overview of torus-bearing pit membranes (arrows) on a tracheid (Tr) of Osmanthus suavis (Oleaceae) with vessel elements (V) on the left and right. (D) Detail of a pit membrane of Osmanthus suavis (Oleaceae) with a torus (T) surrounded by a margo (M).
The three Ericaceae species studied with TEM showed pit membrane thickenings composed of two different parts: primary wall thickenings and cap-like structures on the outer side of the pit membrane. Plasmodesmata or plasmodesmata remnants were usually associated with the thickenings (Fig. 3A–C). They initiated at the surface of the thickenings and point towards the centre of the membrane (Fig. 3B, C). Depending on the ontogenetic stage of the cell, the cap-like structures were not always distinctly developed (Fig. 3A–D). The cap-like structures were much more electron dense than the primary wall thickenings. After cytoplasmic autolysis, which leads to cell death, only the secondary wall-like cap and plasmodesmata remnants appeared unaltered, giving the entire structure a hollow appearance (Fig. 3B, C). TEM observations confirmed the irregular shape and inconsistent distribution of these structures within tracheary elements. The shape of the thickenings varied from a massive round structure to an electron dense opaque cap. The mean thickness of the cap-like structure was 0·3 μm, and the entire structure was on average 1·0 μm thick in Arbutus unedo. The diameter of even membranes did not seem to differ between the three Ericaceae species examined and was on average 0·2 μm.
Fig. 3.
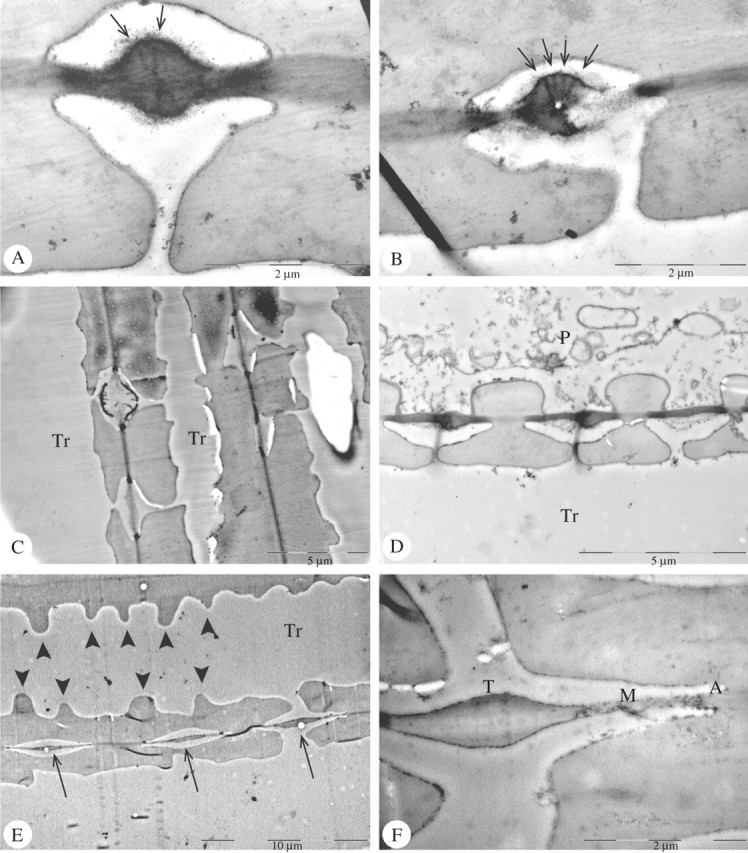
TEM illustrations of pit membrane thickenings in Ericaceae and Oleaceae. (A) Pit membrane with pseudo-torus of Arbutus unedo (Ericaceae) and plasmodesmata (arrows) pointing to the middle of the thickening. (B) Pit membrane of Arbutus unedo (Ericaceae) with a partially hydrolysed pseudo-torus including plasmodesmata (arrows). (C) Narrow tracheary elements (Tr) of Arctostaphylos uva-ursi (Ericaceae) with non-thickened pit membranes and one pseudo-torus with a cap-like structure and remnants of plasmodesmata. (D) Longitudinal section showing a pit between a tracheary element (Tr) and a parenchyma cell (P). The thickenings are most distinct on the tracheid side of the pit pair. (E) Narrow tracheary elements (Tr) of Osmanthus suavis (Oleaceae) with helical thickenings (arrowheads) and tori (arrows) on each pit membrane. (F) Detail of a torus-bearing pit membrane between two narrow tracheary elements in Osmanthus suavis (Oleaceae). An even margo (M) surrounds a torus (T), which is located in the middle of the pit membrane and is composed of various layers. At the periphery of the pit membrane is an annulus (A).
Torus-like thickenings were found to be consistently associated with pit membranes in Osmanthus serrulatus and O. suavis (Fig. 3E, F). These thickenings could be seen with TEM as composed of two different layers (Fig. 3F). Pad-like structures could be distinguished on the pit membrane external surface as a more electron opaque layer than the compound middle lamella between the pads. The entire pit membrane thickenings in the two Osmanthus species were on average 0·6 μm thick. Moreover, an even area was clearly visible as well as an electron dense annulus at the periphery of the membrane (Fig. 3F). Helical thickenings on the secondary cell walls of tracheary elements with pit membrane thickenings were observed in both species (Fig. 3E).
DISCUSSION
The majority of the species studied showed homogeneous intervascular pit membranes with an even thickness. This type of pit membrane has been well-illustrated in the literature (e.g. Côté, 1958; Schmid, 1965; Pesacreta et al., 2005). The results presented in this study clearly illustrate that torus-like pit membrane thickenings occur in several Ericaceae and that these thickenings cannot be interpreted as genuine tori. It is suggested that the thickenings observed in a small number of Ericaceae should be termed ‘pseudo-tori’, because these encrusting materials associated with the pit membrane may easily be mistaken for tori based on LM observations only. In this way, the artificial nature of pseudo-tori corresponds to pseudo-vestures, which are encrusting materials in pit cavities giving the false impression of projections from the secondary cell wall (IAWA Committee, 1989; Jansen et al., 1998).
The present observations of four Oleaceae species, however, confirm the occurrence of true tori in the two species of Osmanthus studied. Both SEM and TEM observations indicate that these pit membranes have a typical torus–margo configuration. Interestingly, the presence of a torus-bearing pit membrane is often correlated with the occurrence of ring-porosity as well as with the following pit characteristics: (a) round to oval pit apertures that are relatively small; (b) secondary wall thickenings with helical thickenings; and (c) an indistinct pit canal that is either very short or entirely lacking (Dute et al., 2004; Jansen et al., 2004). These character combinations are clearly visible in Osmanthus serrulatus and O. suavis and may explain the absence of tori in Nestegis apetala and Phillyrea angustifolia. Indeed, round to oval pit apertures occur in narrow tracheary elements in Osmanthus, but not in Nestegis and Phillyrea. Other species of Oleaceae, in which tori have been observed, include Osmanthus americanus, O. fragrans, O. heterophyllus, O. fortunei, O. rigidus, O. insularis and O. aurantiacus (Ohtani and Ishida, 1978; Dute and Rushing, 1987, 1988). According to recent phylogenetic insights, Osmanthus is included in subtribe Oleinae (Wallander and Albert, 2000). It might be that additional records of tori can be found in Oleaceae, but that their detection has simply been overlooked in previous studies. It is also possible that torus-bearing pit membranes have evolved more than once within the Oleaceae family. A close relationship between Osmanthus, Picconia, Phillyrea, Nestegis and Notelaea was suggested by molecular data and morphological support, including two wood anatomical synapomorphies, viz. dendritic vessel distribution and vascular tracheids (Baas et al., 1988). Although the latter two features are characteristic of at least a few torus-bearing angiosperms, observations made by Jansen et al. (2004) do not reveal tori in Nestegis apetala and Phillyrea angustifolia. More species have to be examined to determine the exact taxonomic distribution of tori in this group. Moreover, observations by Parameswaran and Gomes (1981) suggest that pseudo-tori occur in Ligustrum lucidum, which is included in the tribe Oleeae, because the irregular, pad-like thickenings on the pit membranes of vessels are very similar to pseudo-tori previously described in Pyrus and Prunus.
Although observations of thin sections (±10 μm) and the use of safranin in combination with alcian blue may help to detect tori (Jansen et al., 2004), careful observations of the shape of the pit aperture as well as the distribution of pit membrane thickenings may be most useful to identify their nature. Indeed, the above-mentioned characters correlated with true tori are not found in the Ericaceae species with pseudo-tori. True tori are strongly correlated with circular to oval pit apertures and are not found in association with linear, slit-like pit apertures (Wright, 1928; Beck et al., 1982; Dute et al., 1996, 2001; Jansen et al., 2004). Moreover, true tori are consistently present in all pits within a single cell and show a diameter that is characteristically greater in size than the pit aperture.
The present SEM and TEM observations show that the pseudo-tori observed in a few members of the Ericaceae are very similar to the thickenings associated with plasmodesmata as previously reported in Pyrus, Prunus, Ribes and Sorbus (Parameswaran and Liese, 1973, 1981; Barnett, 1987a, b; Lachaud and Maurousset, 1996). This paper presents the first record of this feature in Ericaceae. Within this family, the seven species with pseudo-tori belong to three different tribes, which are not closely related (Kron et al., 2002). Moreover, not all species within a single genus bear pit membranes with pseudo-tori. For instance, Vaccinium corymbosum and V. uliginosum show pseudo-tori, while Vaccinium stanleyi has pit membranes with an even, homogeneous thickness. Although the authors' observations suggest that pseudo-tori show a wider systematic distribution than previously believed, it is unclear why pseudo-tori are only found in some Ericaceae and not in any of the other Ericales families examined. Ongoing research on pseudo-tori within Vaccinium suggests that most species with this character are from cold to temperate climates, which is also reflected in a more narrow vessel diameter, than species that show no pseudo-torus (D. Rabaey, unpubl. res.).
There are major ultrastructural differences between tori and pseudo-tori. A torus consists of a round to oval thickening located in the centre of the membrane and results either from a thickening of the primary walls of the pit membrane (e.g. Ulmus, Celtis), or from pads of secondary wall material deposited upon the pit membrane (e.g. Daphne, Osmanthus) (Dute and Rushing, 1988, 1990; Dute et al., 1990). Also, plasmodesmata are not encountered in tori at any time during their ontogeny. In general, plasmodesmata are not associated with pit membranes of intervessel pits and pits between tracheids, but they are common in pit pairs between fibres and parenchyma cells (Barnett, 1982; Yang, 1986). Recent work illustrates the occurrence of pseudo-tori associated with fibre-tracheids in many genera within the Rosaceae, and in few representatives of the Elaeagnaceae and Rhamnaceae (S. Jansen, unpubl. res.). Primary wall thickenings associated with plasmodesmata are not consistently located in the centre of the pit membrane. As illustrated by observations of V. corymbosum, sometimes two thickenings are found on a single pit membrane. Depending on the ontogenetic stage of the cell, pseudo-tori can be seen as a primary thickening of the pit membrane containing branched plasmodesmata or as a secondary wall-like cap. They seem to differ in their resistance to hydrolytic enzyme released at cell death. At the end of the cell maturation, these cap-like structures remain largely unaltered after cytoplasmic autolysis, while the central part of the pit membrane thickening is hydrolysed, resulting in the hollow appearance of the cap (Barnett, 1987a, b; Lachaud and Maurousset, 1996).
It seems obvious that pit membrane modifications such as tori and pseudo-tori may affect the efficiency and/or safety of lateral water transport in tracheary elements. Indeed, the porous margo considerably facilitates efficient water transport through relatively large pores in the pit membrane, while the torus is functional when sealing off the pit aperture to avoid air seeding (Sperry, 2003; Pitterman et al., 2005). This explains the similar shape of the pit aperture and torus, and the slightly greater diameter of the functional torus compared with the aperture. The function of pseudo-tori is unknown, but it is highly unlikely that they have a similar function as tori because of their irregular occurrence as well as their amorphous shape, which frequently does not correspond in size and shape to pit apertures. Thus, the eccentric position of the pseudo-torus on a pit membrane seems to obviate a role in pit aspiration for this structure. Lachaud and Maurousset (1996) suggested that the function of plasmodesmata may change with time. Plasmodesmata may be functional for transferring molecules which co-ordinate cellular processes during cell differentiation, for instance phytohormones such as gibberellins (Kwiatkowska, 1991; Lucas et al., 1993). It was also suggested that plasmodesmata play a role in releasing hydrolytic enzymes into cells that are programmed for death (Juniper, 1977). Although this could be possible, absence of plasmodesmata from the pit membranes of vessel elements and tracheids indicates that pits develop without the need for plasmodesmatal connections to the symplast (Barnett and Harris, 1975; Barnett, 1981, 1982). This suggests that plasmodesmata are not always a prerequisite for pit formation and do not necessarily disrupt deposition of cellulose near the pit (Carr, 1976; Juniper, 1977).
The diameter of plasmodesmata varies between 20 and 200 nm and is on average 50 nm. Minor differences in diameter are a result of the arrangement of proteins, which are able to alter their conformation to increase or decrease their size exclusion limit. In this way, plasmodesmata may inhibit particular molecules to pass from one cell to another. Another way to regulate the transport through plasmodesmata is to plug the ends of the intercellular channels. One way to block plasmodesmata is by callose deposition, which has been related to rapid control mechanisms like wounding or pathogenesis. Callose plugs have been observed for instance in pit fields of cells that were infected by potato virus X (Allison and Shalla, 1974; Roberts and Oparka, 2003). Formation of an amorphous resistant cap could be another way to block off plasmodesmata. Barnett (1987a) suggested that the secondary wall-like caps prevent passage of autolytic enzymes from a dying cell to its living neighbouring cells via plasmodesmata. Lachaud and Maurousset (1996), however, noticed that some plasmodesmata in Sorbus torminalis appeared to perforate the cap-like structure and that the cap does not seem to seal the plasmodesmata. Alternatively, Barnett (1987a) suggested that the primary thickenings of the pit membrane may act to support plasmodesmatal protrusions, which may have a minimum length to be structurally and functionally complete.
Acknowledgments
We thank Dr R. R. Dute (Auburn University, USA) for valuable advice and useful suggestions and Dr Suzy Huysmans (K.U.Leuven) for reading over an earlier version of this paper. We thank the directors of the National Botanic Garden of Belgium (BR), New York Botanical Garden (NY), RBG, Kew and the directors of the xylaria in Madison (MADw, SJRw), Kew (Kw), Tervuren (Tw), Utrecht (Uw) and Delft (Dw). We thank the Bentham-Moxon Trust (RBG, Kew) and the Systematics Research Fund for financial support. Research at the Laboratory of Plant Systematics is supported by research grants from of the K.U.Leuven (OT/05/35) and the Fund for Scientific Research—Flanders (Belgium) (G.0268.04 and G.0250.05). F.L. is a post-doctoral fellow of the Fund for Scientific Research—Flanders (Belgium).
LITERATURE CITED
- Allison AV and Shalla TA. (1974) The ultrastructure of local lesions induced by potato virus X: a sequence of cytological events in the course of infection. Phytopathology 64784–793. [Google Scholar]
- Anderberg AA, Rydin C, Källersjö M. (2002) Phylogenetic relationships in the order Ericales s.l: analysis of molecular data from five genes from plastid and mitochondrial genomes. American Journal of Botany 89667–687. [DOI] [PubMed] [Google Scholar]
- APG. (2003) An update of the angiosperm phylogeny group classification for the orders and families of flowering plants. APG II. Botanical Journal of the Linnean Society 141399–436. [Google Scholar]
- Baas P. (1986) Terminology of imperforate tracheary elements—in defense of libriform fibres with minutely bordered pits. International Association of Wood Anatomists Bulletin, New Series 782–86. [Google Scholar]
- Baas P, Esser PM, Van der Westen MET, Zandee M. (1988) Wood anatomy of the Oleaceae. International Association of Wood Anatomists Bulletin, New Series 9103–182. [Google Scholar]
- Bailey IW. (1913) The preservation treatment of wood. II. The structure of the pit membranes in tracheids of conifers and their relation to the penetration of gases, liquids, and finely divided solids into green and seasoned wood. Forestry Quarterly 1112–20. [Google Scholar]
- Barnett JR. (1981) Secondary xylem cell development. In Barnett JR (Ed.). Xylem cell development(Castle House Publications, Tunbridge Wells) pp. 47–95.
- Barnett JR. (1982) Plasmodesmata and pit development in secondary xylem elements. Planta 155251–260. [DOI] [PubMed] [Google Scholar]
- Barnett JR. (1987a) The development of fibre-tracheid pit membranes in Pyrus communis L. International Association of Wood Anatomists Bulletin, New Series 8134–142. [Google Scholar]
- Barnett JR. (1987b) Changes in the distribution of plasmodesmata in developing fibre-tracheid pit membranes of Sorbus aucuparia L. Annals of Botany 59269–279. [Google Scholar]
- Barnett JR and Harris JM. (1975) Early stages of bordered pit formation in radiate pine. Wood and Science Technology 9233–241. [Google Scholar]
- Bauch J, Liese W, Schultze R. (1972) The morphological variability of the bordered pit membranes in gymnosperms. Wood Science and Technology 6165–184. [Google Scholar]
- Beck CB, Coy K, Schmid R. (1982) Observations on the fine structure of Callixylon wood. American Journal of Botany 6954–76. [Google Scholar]
- Becker P, Gribben RJ, Schulte PJ. (2003) Incorporation of transfer resistance between tracheary elements into hydraulic resistance models for tapered conduits. Tree Physiology 231009–1019. [DOI] [PubMed] [Google Scholar]
- Bremer B, Bremer K, Heidari N, Erixon P, Olmstead RG, Anderberg AA, et al. (2002) Phylogenetics of asterids based on 3 coding and 3 non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. Molecular Phylogenetics and Evolution 24274–301. [DOI] [PubMed] [Google Scholar]
- Carlquist S. (1986a) Terminology of imperforate tracheary elements. International Association of Wood Anatomists Bulletin, New Series 775–81. [Google Scholar]
- Carlquist S. (1986b) Terminology of imperforate tracheary elements—a reply. International Association of Wood Anatomists Bulletin, New Series 7168–170. [Google Scholar]
- Carr DJ. (1976) Plasmodesmata in growth and development. In Gunning BES and Robards AW (Eds.). Intercellular communication in plants: studies on plasmodesmata(Springer Verlag, Berlin) pp. 243–289.
- Choat B, Jansen S, Zwieniecki MS, Smets E, Holbrook NM. (2004) Changes in pit membrane porosity due to deflection and stretching: the role of vestured pits. Journal of Experimental Botany 551569–1575. [DOI] [PubMed] [Google Scholar]
- Cochard H, Cruiziat P, Tyree MT. (1992) Use of positive pressures to establish vulnerability curves—further support for the air-seeding hypothesis and implications for pressure-volume analysis. Plant Physiology 100205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CM, Prather BL, Valente MJ, Dute RR, Miller ME. (2004) Torus lignification in hardwoods. International Association of Wood Anatomists Bulletin, New Series 25435–448. [Google Scholar]
- Côté WA. (1958) Electron microscope studies of pit membrane structure. Forest Products Journal 8296–301. [Google Scholar]
- Dute RR and Rushing AE. (1987) Pit pairs in the wood of Osmanthus americanus (Oleaceae). International Association of Wood Anatomists Bulletin, New Series 8237–244. [Google Scholar]
- Dute RR and Rushing AE. (1988) Notes on torus development in wood of Osmanthus americanus (L.). International Association of Wood Anatomists Bulletin, New Series 941–51. [Google Scholar]
- Dute RR and Rushing AE. (1990) Torus structure and development in the woods of Ulmus alata Michx, Celtis laevigata Willd, and Celtis occidentalis L. International Association of Wood Anatomists Bulletin, New Series 1171–83. [Google Scholar]
- Dute RR, Rushing AE, Perry JW. (1990) Torus structure and development in species of Daphne. International Association of Wood Anatomists Bulletin, New Series 11401–412. [Google Scholar]
- Dute RR, Rushing AE, Freeman JD. (1992) Survey of intervessel pit membrane structure in Daphne species. International Association of Wood Anatomists Bulletin, New Series 13113–123. [Google Scholar]
- Dute RR, Freeman JD, Henning F, Barnard LD. (1996) Intervascular pit membrane structure in Daphne and Wikstroemia—systematic implications. International Association of Wood Anatomists Journal 17161–181. [Google Scholar]
- Dute RR, Miller ME, Carollo RR. (2001) Intervascular pit structure in selected species of Thymelaeaceae. Journal of Alabama Academy of Science 7214–26. [Google Scholar]
- Dute RR, Martin AL, Jansen S. (2004) Intervascular pit membranes with tori in wood of Planera aquatica J.F.Gmel. Journal of the Alabama Academy of Science 757–21. [Google Scholar]
- Flynn KA. (1995) A review of the permeability, fluid flow, and anatomy of spruce (Picea spp.). Wood and Fiber Science 27278–284. [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. (2004) Analysis of circular bordered pit function. II. Gymnosperm tracheids with torus-margo pit membranes. American Journal of Botany 91386–400. [DOI] [PubMed] [Google Scholar]
- IAWA Committee. (1989) IAWA list of microscopic features for hardwood identification. International Association of Wood Anatomists Bulletin, New Series 10219–332. [Google Scholar]
- IAWA Committee. (2004) IAWA List of microscopic features for softwood identification. International Association of Wood Anatomists Journal 251–70. [Google Scholar]
- Jansen S, Smets E, Baas P. (1998) Vestures in woody plants: a review. International Association of Wood Anatomists Journal 19347–382. [Google Scholar]
- Jansen S, Baas P, Smets E. (2001) Vestured pits: their occurrence and systematic importance in eudicots. Taxon 251–70. [Google Scholar]
- Jansen S, Choat B, Vinckier S, Lens F, Schols P, Smets E. (2004) Intervascular pit membranes with a torus in the wood of Ulmus (Ulmaceae) and related genera. New Phytologist 16351–59. [DOI] [PubMed] [Google Scholar]
- Juniper BE. (1977) Some speculations on the possible role of the plasmodesmata in the control of differentiation. Journal of Theoretical Biology 66583–592. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. Journal of Cell Biology 27137A–138A. [Google Scholar]
- Kron KA, Judd WS, Stevens PF, Crayn DM, Anderberg AA, Gadek PA, et al. (2002) A phylogenetic classification of Ericaceae: molecular and morphological evidence. Botanical Review 68335–423. [Google Scholar]
- Kwiatkowska M. (1991) Autoradiographic studies on the role of plasmodesmata in the transport of gibberellin. Planta 183294–299. [DOI] [PubMed] [Google Scholar]
- Lachaud S and Maurousset L. (1996) Occurrence of plasmodesmata between differentiating vessels and other xylem cells in Sorbus torminalis L. Crantz and their fate during xylem maturation. Protoplasma 191220–226. [Google Scholar]
- Lucas WJ, Ding B, Van der Schoot C. (1993) Tansley Review No. 58. Plamsodesmata and the supracellular nature of plants. New Phytologist 125435–476. [DOI] [PubMed] [Google Scholar]
- Morrow AC and Dute RR. (1999) Electron microscopic investigation of the coating found on torus-bearing pit membranes of Botrychium dissectum, the common grape fern. International Association of Wood Anatomists Journal 20359–373. [Google Scholar]
- Ohtani J and Ishida S. (1978) Pit membrane with torus in dicotyledonous wood. Mokuzai Gakkaishi 24673–675. [Google Scholar]
- Parameswaran N and Gomes AV. (1981) Fine structural aspects of helical thickenings and pits in vessels of Ligustrum lucidum Ait. (Oleaceae). International Association of Wood Anatomists Bulletin, New Series 2179–185. [Google Scholar]
- Parameswaran N and Liese W. (1973) Anomalous structures in the bordered pits of fibre-tracheids of Ribes sanguineum. Wood and Fiber 576–79. [Google Scholar]
- Parameswaran N and Liese W. (1981) Torus-like structures in interfibre pits of Prunus and Pyrus. International Association of Wood Anatomists Bulletin, New Series 289–93. [Google Scholar]
- Pesacreta TC, Groom LH, Rials TG. (2005) Atomic force microscopy of the intervessel pit membrane in the stem of Sapium sebiferum (Euphorbiaceae). International Association of Wood Anatomists Journal 26397–426. [Google Scholar]
- Pitterman J, Sperry JS, Hacke UG, Wheeler JK, Sikkema EH. (2005) Torus-margo pits help conifers compete with angiosperms. Science 3101924. [DOI] [PubMed] [Google Scholar]
- Roberts AG and Oparka KJ. (2003) Plasmodesmata and the control of symplastic transport. Plant, Cell and Environment 26103–124. [Google Scholar]
- Sano Y and Fukazawa K. (1994) Structural variations and secondary changes in pit membranes in Fraxinus mandshurica var. japonica. International Association of Wood Anatomists Journal 15283–291. [Google Scholar]
- Schacht H. (1859) Ueber die Tüpfel der Gefäss- und Holzzellen. Botanische Zeitung 17238–239. [Google Scholar]
- Schmid R. (1965) Fine structure ot pits in hardwoods. In Côté WA (Ed.). Cellular ultrastructure of woody plant(Syracuse University Press, New York, NY) pp. 291–304.
- Schols P, Dessein S, D'Hondt C, Huysmans S, Smets E. (2002) Carnoy: a new digital measurement tool for palynology. Grana 41124–126. [Google Scholar]
- Singh A, Dawson B, Franich R, Cowan F, Warnes J. (1999) The relationship between pit membrane ultrastructure and chemical impregnability of wood. Holzforschung 53341–346. [Google Scholar]
- Sperry JS. (2003) Evolution of water transport and xylem structure. International Journal of Plant Science 164S115–S127. [Google Scholar]
- Sperry JS and Hacke UG. (2004) Analysis of circular bordered pit function. I. Angiosperm vessels with homogenous pit membranes. American Journal of Botany 91369–385. [DOI] [PubMed] [Google Scholar]
- Stern WL. (1988) Index xylariorum—institutional wood collections of the world 3. International Association of Wood Anatomists Bulletin, New Series 9203–252. [Google Scholar]
- Tyree MT and Sperry JS. (1989) Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Plant Molecular Biology 4019–38. [Google Scholar]
- Tyree MT and Zimmermann MH. (2002) Xylem structure and the ascent of sap 2nd edn (Springer Verlag, Berlin).
- Wallander E and Albert VA. (2000) Phylogeny and classification of Oleaceae based on rps16 and trnL-F sequence data. American Journal of Botany 871827–1841. [PubMed] [Google Scholar]
- Watanabe U, Imamura Y, Iida I. (1998) Liquid penetration of precompressed wood. VI. Anatomical characterization of pit fractures. Journal of Wood Science 44158–162. [Google Scholar]
- Wheeler EA. (1983) Intervascular pit membranes in Ulmus and Celtis native to the United States. International Association of Wood Anatomists Bulletin, New Series 479–88. [Google Scholar]
- Wright JG. (1928) The pit-closing membrane in the wood of the lower gymnosperms. Proceedings and Transactions of the Royal Society of Canada, Series 3 2263–94. [Google Scholar]
- Yang KC. (1986) The ultrastructure of pits in Paulownia tomentosa. Wood and Fiber Science 18118–126. [Google Scholar]