Abstract
• Background The reconstruction of biological processes and human activities during the last glacial cycle relies mainly on data from biological remains. Highly abundant tissues, such as wood, are candidates for a genetic analysis of past populations. While well-authenticated DNA has now been recovered from various fossil remains, the final ‘proof’ is still missing for wood, despite some promising studies.
• Scope The goal of this study was to determine if ancient wood can be analysed routinely in studies of archaeology and palaeogenetics. An experiment was designed which included blind testing, independent replicates, extensive contamination controls and rigorous statistical tests. Ten samples of ancient wood from major European forest tree genera were analysed with plastid DNA markers.
• Conclusions Authentic DNA was retrieved from wood samples up to 1000 years of age. A new tool for real-time vegetation history and archaeology is ready to use.
Keywords: Ancient DNA, fossil wood, trnL intron, probability of authenticity, Abies, Pinus, Fagus, Quercus
INTRODUCTION
Ancient DNA studies have been a matter of fascination as well as of irritation (e.g. Nickle et al., 2002). After a period of relative dilettantism, a number of criteria of authenticity have been introduced (Cooper and Poinar, 2000) and applied to an increasing number of studies of ancient DNA (aDNA) retrieval from fossil animal and human remains. At the time being, there is only a small number of studies on DNA from ancient plant remains (Gugerli et al., 2005). In most of them detailed information on control experiments and on measures to prevent contamination are missing. Even more critical is the lack of independent replications in different laboratories, a requirement which obviously could not be fulfilled by studies using up the entire sample in a single experiment, e.g. pollen grains (Suyama et al., 1996; Parducci et al., 2005) or Prunus fruit stones (Pollmann et al., 2005). Hence, there is an urgent demand for proving the authenticity of DNA obtained from ancient plant remains.
Ancient wood is found in high abundance and samples are usually large enough to be divided and sent to different laboratories. From this point of view, wood is an ideal target for ancient plant DNA studies applying the criteria of authenticity. Do we have a chance of routinely analysing ancient wood remains in studies of archaeology or phylogeography?
In this study we tackle this question, since forest trees are important elements in ecosystems that have been subjected to large range shifts during the climate cycles of the Quaternary. Furthermore, the DNA of ancient wood constructions may tell us more about historic human migrations. A systematic experiment with a blind testing design was set up in two aDNA laboratories and the results were subjected to rigorous statistical tests. It was possible to isolate and analyse authentic DNA from ancient wood of major European forest tree genera.
MATERIALS AND METHODS
Experimental design
Ten ancient wood samples, 300–11 500 years old, were chosen to be identified to the genus level by the analysis of plastid DNA. These remains had been determined previously by paleobotanists on the basis of wood anatomy (Table 1). For the blind test, specimens were divided and then shipped to the aDNA laboratories. These fragments were labelled only with a code, lacking information about genus or age. The codes were different across laboratories so that no exchange of information about individual samples was possible.
Table 1.
Details of the ancient wood samples
| Sample | Genus | Age (years) | Conservation | Location | Contact or reference |
|---|---|---|---|---|---|
| MRS 48 | Abies | 400* | Ice cave | Devoluy, France | J. L. de Beaulieu (current author) |
| CHA 1 | Pinus | 8300† | Glacis terrace | Charanc, France | Miramont et al. (2000) |
| G51C | Pinus | 11 500† | Clay sediment | Zurich, Switzerland | M. Schaub, K. F. Kaiser (Swiss Federal Institute for Forest, Snow and Landscape Research, WSL, Switzerland) |
| MRS46 | Pinus | 3800† | Glacis terrace | Bachasette, France | J. L. de Beaulieu (as above) |
| A555 | Fagus | 1000‡ | Clay sediment | Charavines colletière, France | C. Bourquin-Mignot (Laboratory of Chrono-Ecologie, UMR CNRS 6565, Besançon, France) |
| MT2 | Quercus | 300* | Waterlogged | Montbeliard, France | O. Girardclos (Centre d'Études Dendrochronologiques et de Recherches en Environnement, Besançon, France) |
| URLS F10 | Fagus | 1300† | Waterlogged | Lago Scuro, Italy | Giraudi (2005) |
| URLS F5 | Fagus | 3300–3600‡ | Waterlogged | Lago di Bolsena, Italy | L. Sadori (Dipartimento di Biologia Vegetale, Università ‘La Sapienza’, Rome, Italia) |
| L1 | Quercus | 800* | Waterlogged | Limé ‘Le gros Buisson’, France | C. Bourquin-Mignot (as above) |
| M40 | Abies | 300* | Waterlogged | Lons-le-Saunier, France | O. Girardclos (as above) |
* Dendrochronological dating; † 14C dating; ‡ archaeological dating.
Two primer pairs were designed to differentiate among forest tree genera. Each primer pair amplified a small fragment of the chloroplast trnL intron smaller than 100 bp long in the most common tree species of Europe. Primer pair I was characterized by the following primers: forward primer I 5′-GGG TAA TCC TGA GCC AAA T-3′ and reverse primer 5′-CAT TGA GTC TCT GCA CCT ATC-3′. Primer pair II amplified a shorter fragment and shared its reverse primer with primer pair I. The sequence of forward primer II was 5′-CAA ATA AGG GTT GAG AAG AAA GC-3′. Primer pair I was chosen, because it mainly differentiates among gymnosperms, while primer II is suited for the identification of angiosperm tree genera.
The aDNA laboratories of the participating institutions were physically isolated from the laboratories working with fresh samples. To prevent contamination, they were equipped with filter systems for incoming air, overpressure systems, decontamination by UV-light and special protective gear for the experimenters. All samples which gave signals after amplification were sequenced as a matter of principle.
All wood samples were shipped in air-tight plastic bags and were directly transferred to the aDNA laboratories after shipping. They were then dried (if necessary) and stored on silica gel to be extracted in a dry condition. For DNA extraction a layer of wood was removed from the wood fragments with a sterile scalpel and discarded. With a second sterile scalpel a few flakes of wood were scraped off the cleaned area and transferred directly into a sterile 2-mL reaction tube together with a UV-sterilized stainless-steel ball. The wood flakes were pulverized in a shaking mill (Retsch) at room temperature. Extraction of the pulverized wood remains was performed with the Plant DNA Mini Kit (Qiagen, Germany) according to the manufacturer's instructions, apart from the following deviations from the protocol: the extraction buffer was injected through the lid of the reaction tubes by means of disposable sterile syringes and needles to avoid spilling of the dry wood powder.
Blank extractions were performed simultaneously, starting out with an empty reaction tube containing just a sterile stainless-steel ball, and were treated exactly the same for the rest of the analysis.
PCR reactions were prepared under a separate PCR or laminar flow hood in the clean-air laboratories using a dedicated set of pipettes. Extraction and PCR set-up were performed on different days. Blank PCR reactions were performed by adding the appropriate amount of sterile ultra-pure water to the reaction. Reactions were carried out in a volume of 25 μL (Lab A) containing 1 U Fast Start Taq DNA polymerase (Roche Diagnostics, Switzerland), 2 μL extract, 2 mm MgCl2, 0·1 mm dNTPs and 0·2 μm of each primer. Lab B prepared the PCR reactions in a volume of 50 μL containing 2 U AmpliTaq Gold (Applied Biosystems, USA), 1 μL extract and identical concentrations of the remaining ingredients. After preparation of the PCR reactions in the clean-air laboratories the closed reaction tubes were transferred to the regular laboratories and subjected to thermocycling. A denaturation step of 4 min (Lab A) or 9 min (Lab B) at 95 °C was followed by 50 cycles of 1 min 95 °C, 1 min 55 °C and 1 min 72 °C. The procedure ended with a final elongation step of 10 min at 72 °C.
Subsequent analyses, such as electrophoresis, cloning and sequencing, were carried out in regular laboratories. PCR products were first checked on agarose gels and detected fragments were then either cloned and sequenced (Lab A) or directly sequenced using the PCR primers (Lab B). PCR products were cloned with the TOPO TA Cloning Kit (Invitrogen, The Netherlands) according to the instructions of the manufacturer. Sequencing reactions were performed with the BigDye Terminator Sequencing Kit (Applied Biosystems, USA) from plasmid DNA (Lab A) or PCR products purified with the Qiagen MinElute Kit (Lab B). Electrophoresis was carried out in ABI Prism 310 automatic sequencers (Applied Biosystems).
Statistical analysis
For statistical analysis, ‘authentic’ was defined as DNA present in the sample before the beginning of the experiment, and it was assumed that it is not possible to decide whether a result is authentic by examining the sequence. The lack of woody plant sequences from controls suggests that this is a conservative assumption. It was also assumed that authentic and contaminant DNA occur and are amplified independently. The probabilities γ and γ2 and their 95 % profile confidence intervals were estimated as described in Spencer and Howe (2004). Because replicate extractions from a given sample are being analysed, γ and γ2 tell us whether an extract from this sample is likely to contain authentic DNA, but do not tell us how likely it is that extracts from other samples will contain authentic DNA.
RESULTS
Three of ten samples (MRS48, A555, M40; Fig. 1) were correctly identified to the genus level by both laboratories from multiple extracts each. Three samples (CHA1, G51C, MT2) were identified correctly by one of the two laboratories only, and therefore did not fulfil the basic criterion of independent replication. Sequences of herbaceous plant species were retrieved from samples MRS46 (Senecio) and URLS F10 (Poa) and a mixture of woody plant sequences was found in sample CHA1 (Populus, Eucalyptus, Cupressus, Ficus). From two samples (URLS F5, L1) no DNA fragments could be amplified by either laboratory.
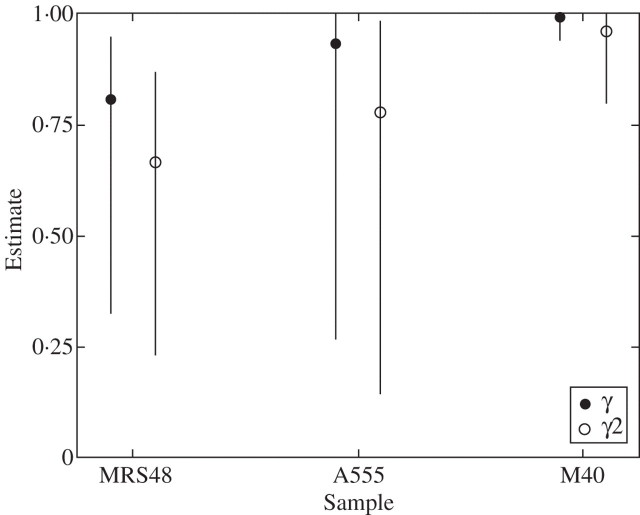
Fig. 1.
DNA sequences from ancient wood samples MRS48, M40 and A555 aligned with published sequences of (a) Abies and (b) Fagus from the respective cpDNA region. Example sequences from both laboratories (A and B) are displayed.
Figure 1 displays original sequences of the samples that could be replicated independently. Differences from the published sequences (GenBank accession numbers: AF327571, AF327582) are visible. Such differences are common for amplification products from aDNA, which has been modified chemically over time (Lindahl, 1993; Willerslev and Cooper, 2005). This is the most likely explanation for the differences from the published Abies (fir) sequence (Fig. 1A). In the case of the Fagus (beech) sequences (Fig. 1B), some of the differences might also have been caused by the repetitive nature of the sequence, which can even cause variation among clones of the same PCR product (Liepelt et al., 2001) or problems with direct sequencing, making it hard to determine the exact number of repeats.
Extraction and PCR controls yielded readable sequences in a few cases. Table 2 gives an overview of the number of extractions and controls for the three samples correctly identified by both laboratories. None of the sequences retrieved from controls could be attributed to woody plant species. Nevertheless, they were conservatively considered to represent laboratory contamination for the subsequent statistical analysis.
Table 2.
Total number of extractions and controls performed in laboratories A and B
| Sample | Extractions | Successful extractions | Extraction controls | Positive extraction controls | PCR controls | Positive PCR controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | Total | A | B | Total | A | B | Total | A | B | Total | A | B | Total | A | B | Total | |
| MRS48 | 4 | 15 | 19 | 3 | 7 | 10 | 2 | 15 | 17 | 0 | 2 | 2 | 2 | 15 | 17 | 0 | 4 | 4 |
| A555 | 4 | 4 | 8 | 2 | 4 | 6 | 2 | 4 | 6 | 0 | 1 | 1 | 2 | 4 | 6 | 0 | 0 | 0 |
| M40 | 4 | 13 | 17 | 4 | 10 | 14 | 2 | 13 | 15 | 0 | 0 | 0 | 2 | 13 | 15 | 0 | 1 | 1 |
For the three samples correctly identified by both laboratories, the maximum likelihood method was used to estimate the probability γ that a positive result was at least partly from authentic DNA, and the probability γ2 that a positive result was entirely from authentic DNA. All three samples exhibited high probabilities of authenticity (γ > 0·80, Fig. 2). For γ2 the probabilities were lower (>0·66) except for sample M40, which still exhibited a very high probability of 0·96 (Fig. 2). Therefore, based only on the numbers of positive results observed, there is strong evidence that there was woody plant DNA present in all three samples before the experiments began, and that it was possible to amplify this DNA. The overlapping confidence intervals in Fig. 2 also show that the probabilities of such DNA being present were not significantly different between the three samples. The confidence intervals were much wider for A555 than the other two samples because fewer experiments were carried out on this sample.
Fig. 2.
Estimated probabilities of authenticity for DNA sequences from three samples of ancient wood. γ, probability that amplified DNA is at least partially from the ancient wood sample; γ2, probability that amplified DNA is purely from the ancient wood sample. Error bars indicate the 95 % confidence interval.
DISCUSSION
The results fulfil the following criteria of authenticity suggested by Cooper and Poinar (2000): (a) the work was carried out in suitable, dedicated and properly equipped laboratories; (b) a large number of extraction and PCR controls was carried out, of which few were contaminated by non-woody species; (c) results were reproducible in each laboratory; (d) results made sense biologically, since the wood had been identified using anatomical criteria as well; (e) some sequences were degraded in ways typical of ancient DNA; (f) independent replications of the results in different laboratories were successful under the conditions of a blind-testing experiment. Independent replicates are considered as one of the strongest criteria of authenticity by several authors (Austin et al., 1997; Cooper and Poinar, 2000; Hofreiter et al., 2001; Willerslev and Cooper, 2005). The idea of blind testing to authenticate aDNA sequences was suggested by Yang et al. (1997). The blind-testing design on wood from different genera allowed an additional independent control of the results by anatomical criteria in combination with the use of universal primers differentiating among genera.
Recently some of the criteria of authenticity have been critically reviewed (Gilbert et al., 2005) and it was suggested to build a strong case of evidence instead of adhering to these criteria as to a checklist. The greatest challenge in aDNA studies is the differentiation between authentic DNA and contaminant DNA. In the case of ancient wood, the risk of contamination during handling and analysis can be considered to be lower than with human or microbial DNA which is essentially omnipresent (e.g. Gilbert et al., 2005; Willerslev and Cooper, 2005). Thus, although it was not possible to test for all of the nine criteria of authenticity, strong evidence for the authenticity of the results has been provided.
Previous studies relying on relatively fresh wood (Fladung et al., 2004; Asif and Cannon, 2005; Deguilloux et al., 2006) and reports of DNA from ancient Quercus (Dumolin-Lapègue et al., 1999; Deguilloux et al., 2002) and Cryptomeria wood (Tani et al., 2003) suggested the possibility of DNA survival in ancient wood remains, which was confirmed by the present study. As far as is known, this is the first time that DNA sequences from ancient wood samples have been authenticated in a thorough experimental set-up including two clean-air laboratories, extensive controls and statistical evaluation of the results. Here, it was possible to analyse authentic DNA from wood as old as 1000 years. Depending on the mode of conservation and the climate at the excavation site, it appears plausible that even older samples could be analysed successfully (Deguilloux et al., 2006). For future studies, in addition to strict measures avoiding contamination, it is proposed to use independent replication of results and a carefully designed system of controls as standard procedures in the field of plant aDNA studies.
At the moment aDNA studies on human and animal remains make up the majority of publications in this field, while plant studies are still quite rare (Gugerli et al., 2005). It is hoped that these results will promote additional aDNA studies on trees. A large number of potential applications exists. The study of ancient tree DNA could provide powerful tools for ‘real-time’ historical biogeography and archaeology and may increase our knowledge about the postglacial history of forest ecosystems and the impact of human activities during the Holocene.
Supplementary Material
Acknowledgments
We are grateful to C. Miramont, L. Sadori, C. Begeot, M. Schaub, K. F. Kaiser, C. Bourquin-Mignot and O. Girardclos for providing ancient wood remains and to L. Bertel for assistance in the laboratory. This work was financially supported by the European Union Project FOSSILVA (CT-1999-00036) and the Swiss State Secretariat for Education and Research SER (CT-99.0689-2).
REFERENCES
- Asif MJ and Cannon CH. (2005) DNA extraction from processed wood: a case study for the identification of an endangered timber species (Gonstylus bancanus). Plant Molecular Biology Reporter 231–8. [Google Scholar]
- Austin JJ, Smith AB, Thomas RH. (1997) Palaeontology in a molecular world: the search for authentic ancient DNA. Trends in Ecology Evolution 12303–306. [DOI] [PubMed] [Google Scholar]
- Cooper A and Poinar HN. (2000) Ancient DNA: do it right or not at all. Science 2891139. [DOI] [PubMed] [Google Scholar]
- Deguilloux M-F, Pemonge M-H, Petit RJ. (2002) Novel perspectives in wood certification and forensics: dry wood as a source of DNA. Proceedings of the Royal Society of London Series B: Biological Sciences 269 pp. 1039–1046. [DOI] [PMC free article] [PubMed]
- Deguilloux M-F, Bertel L, Celant A, Pemonge M-H, Sadori L, Magri D, et al. (2006) Genetic analysis of archaeological wood remains: first results and prospects. Journal of Archaeological Science 331216–1227. [Google Scholar]
- Dumolin-Lapègue S, Pemonge M-H, Gielly L, Taberlet P, Petit RJ. (1999) Amplification of oak DNA from ancient and modern wood. Molecular Ecology 82137–2140. [DOI] [PubMed] [Google Scholar]
- Fladung M, Nowitzki O, Ziegenhagen B, Markussen T. (2004) Identification of transgenes from wood of genetically transformed poplar trees. Wood Science and Technology 38207–215. [Google Scholar]
- Gilbert MTP, Bandelt H-J, Hofreiter M, Barnes I. (2005) Assessing ancient DNA studies. Trends in Ecology and Evolution 20541–544. [DOI] [PubMed] [Google Scholar]
- Giraudi C. (2005) Middle to Late Holocene glacial variations, periglacial processes and alluvial sedimentation on the higher Apennine massifs (Italy). Quaternary Research 64176–184. [Google Scholar]
- Gugerli F, Parducci L, Petit RJ. (2005) Ancient plant DNA: review and prospects. New Phytologist 166409–418. [DOI] [PubMed] [Google Scholar]
- Hofreiter M, Serre D, Poinar HN, Kuch M, Pääbo S. (2001) Ancient DNA. Nature Reviews Genetics 2353–359. [DOI] [PubMed] [Google Scholar]
- Liepelt S, Kuhlenkamp V, Anzidei M, Vendramin GG, Ziegenhagen B. (2001) Pitfalls in determining size homoplasy of microsatellite loci. Molecular Ecology Notes 1332–335. [Google Scholar]
- Lindahl T. (1993) Instability and decay of the primary structure of DNA. Nature 362709–715. [DOI] [PubMed] [Google Scholar]
- Miramont C, Sivan O, Rosique T, Edouard JL, Jorda M. (2000) Subfossil tree deposits in the Middle Durance (Southern Alps, France): environmental changes from Allerod to Atlantic. Radiocarbon 42423–435. [Google Scholar]
- Nickle DC, Learn GH, Rain MW, Mullins JI, Mittler JE. (2002) Curiously modern DNA for a ‘250 million-year-old’ bacterium. Journal of Molecular Evolution 54134–137. [DOI] [PubMed] [Google Scholar]
- Parducci L, Suyama M, Lascoux M, Bennett KD. (2005) Ancient DNA from pollen: a genetic record of population history in Scots pine. Molecular Ecology 142873–2882. [DOI] [PubMed] [Google Scholar]
- Pollmann B, Jacomet S, Schlumbaum A. (2005) Morphological and genetic studies of waterlogged Prunus species from the Roman vicus Tasgetium (Eschenz, Switzerland). Journal of Archaeological Science 321471–1480. [Google Scholar]
- Spencer M and Howe CJ. (2004) Authenticity of ancient-DNA results: a statistical approach. American Journal of Human Genetics 75240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama Y, Kawamuro K, Kinoshita I, Yoshimura K, Tsumura Y, Takahara H. (1996) DNA sequence from a fossil pollen of Abies spp. from Pleistocene peat. Genes & Genetic Systems 71145–149. [DOI] [PubMed] [Google Scholar]
- Tani N, Tsumura Y, Sato H. (2003) Nuclear gene sequences and DNA variation of Cryptomeria japonica samples from the postglacial period. Molecular Ecology 12859–868. [DOI] [PubMed] [Google Scholar]
- Willerslev E and Cooper A. (2005) Ancient DNA. Proceedings of the Royal Society Series B 272 pp. 3–16. [DOI] [PMC free article] [PubMed]
- Yang H, Golenberg EM, Shoshani J. (1997) A blind testing design for authenticating ancient DNA sequences. Molecular Phylogenetics and Evolution 7261–265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.