Abstract
• Background and Aims Anoxia leads to an energy crisis, tolerance of which varies from plant to plant. Although the apoplast represents an important storage and reaction space, and engages in the mediation of membrane transport, this extracellular compartment has not yet been granted a role during oxygen shortage. Here, an attempt is made to highlight the importance of the apoplast during oxygen stress and to test whether information about it is transferred systemically in Hordeum vulgare.
• Methods Non-invasive ion-selective microprobes were used which, after being inserted through open stomata, directly contact the apoplastic fluid and continuously measure the apoplastic pH and changes to it.
• Key Results (a) Barley leaves respond to oxygen stress with apoplastic alkalinization and membrane depolarization. These responses are persistent under anoxia (N2; O2 < 3%) but transient under hypoxia. (b) Being applied to the root, the information ‘anoxia’ is signalled to the leaf as an increase in pH, whereas ‘hypoxia’ is not: flooding of the roots within the first 2 h has no effect on the leaf apoplastic pH, whereas anoxia (N2) or chemical anoxia (NaCN/salicylic hydroxamic acid) rapidly increase the leaf apoplastic pH. (c) Under anoxia, the proton motive force suffers a decrease by over 70 %, which impairs H+-driven transport.
• Conclusions Although anoxia-induced apoplastic alkalinization is a general response to stress, its impact on the proton motive force (reduction) and thus on transport mediation of energy-rich compounds is evident. It is concluded that anoxia tolerance depends on how the plant is able to hold the proton motive force and H+ turnover at a level that guarantees sufficient energy is harvested to overcome the crisis.
Keywords: Anoxia, apoplastic pH, barley, hypoxia, pH, proton motive force, systemic signalling
INTRODUCTION
A fast and typical response to anoxia/hypoxia is the rapid acidification of the cytoplasm, which in many cases ranges around half a pH unit (e.g. Sanders and Slayman, 1982; Roberts et al., 1984a; Fan et al., 1988; Menegus et al., 1991; Fox et al., 1995; Felle, 1996; Gout et al., 2001; Schulte et al., 2006). After some minutes, cytoplasmic pH stabilizes at a new level and, depending on the degree of tolerance towards anoxic conditions, may drop further to reach values at which cells can become damaged (Roberts et al., 1984b; Gibbs and Greenway, 2003). Whereas the effect of anoxia on cytoplasmic pH and its impact on cellular metabolism have been the subject of major interest and discussion in the past (Felle, 2005), the role of the apoplast in it has drawn little attention so far. The apoplast of higher terrestrial plants represents an essential storage and reaction space that connects cellular tissues, engages in transport of matter, enzyme catalysis and defence reactions, and also in gas exchange and storage of oxygen (Sattelmacher, 2001). Therefore, flooding of tissues and waterlogging may have far-reaching consequences for the plant, in that the basic ionic milieu of the apoplast may be altered or, in rarer cases, in that gas may be forced out and possibly be replaced by some unfavourable fluid. Obviously, this will impair the capacity of a plant to tolerate oxygen shortage for an extended time, because transport of energy-rich compounds within the apoplast or from the apoplast into the symplast, as well as enzymatic activities, would be heavily impaired then. For instance, the carbohydrate reserves that are recycled to be broken down in glycolysis and fermentation have to pass the apoplast from the site of storage to the cytoplasm. In case the apoplastic ionic milieu (especially the pH) has changed, the scarce energy that could be gained from fermentation cannot be harvested accordingly, with the consequence that the time span of anoxia tolerance will decline rapidly. Regardless of whether the apoplast gets flooded or not, O2 depletion will deactivate (not necessarily inactivate) the plasma membrane H+ ATPase (H+ pump), which will result in immediate depolarization (Felle, 1996) from which an apoplastic pH shift might occur. Apoplastic pH is one of the most important parameters for transmembrane transport of organic matter. Organic compounds like sugars, amino acids, etc. are driven from the apoplast into the cytoplasm by H+ symport which critically depends on the so-called proton motive force (pmf). Since this is composed of membrane potential and transmembrane pH gradient, it is obvious that pump deactivation and apoplastic (and cytoplasmic) pH changes will have a strong impact on the pmf and hence on the ability to translocate energy-rich organic compounds. Since the time of survival of a plant under hypoxia/anoxia depends critically on the availability of usable energy (Greenway and Gibbs, 2003), the tolerance of plants to hypoxia/anoxia must therefore be directly related to the ability of the plant to recruit energy-rich compounds from reserves and to transport them accordingly to the site of catalysis, i.e. from the apoplast into the cytoplasm. To get an idea how the conditions in the apoplast change in the case of insufficient oxygen, ion activities (with special emphasis on pH) within the apoplast have to be monitored continuously, non-invasively and directly within the apoplast before and during the event of oxygen shortage. Until recently, it had been difficult or almost impossible to measure the response of the apoplast to anoxic situations in the apoplast of an intact plant without causing severe injury to the tissue.
The development of more suitable techniques like those of fluorescent dyes (e.g. Hoffmann and Kosegarten, 1995; Mühling et al., 1995; Taylor et al., 1996; Oja et al., 1999), green fluorescent protein (e.g. Gao et al., 2004; Zheng et al., 2004) or the adaptation of the ion-selective microelectrode technique to detect non-invasively ion activities in the leaf apoplast (Hanstein and Felle, 1999; Felle et al., 2004) has closed this gap. In the present study, to investigate the importance of the apoplast during oxygen stress, apoplastic pH was measured in barley leaves while conditions of anoxia or hypoxia were imposed either on the leaf itself or on the root system.
MATERIALS AND METHODS
Plant material and growth conditions
Barley (Hordeum vulgare L.) ‘Ingrid’ was grown at 20 °C, 50–70 % humidity, and with a 1- to 6-h photoperiod (60 μmol m−2 s−1 photon flux density). In the experiments, either intact three- to four-leaved plants or single detached leaves were used.
Cuvette and apoplastic measurements
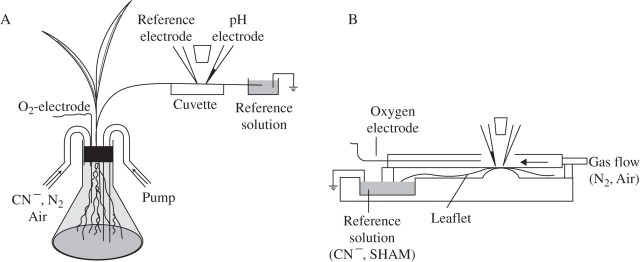
The basic measuring arrangement is shown in Fig. 1A. Roots of intact plants were put into a 100-mL Erlenmeyer flask. The leaf on which measurements were to be made was fixed with double-sided adhesive tape on the bottom of the (dry) cuvette. The tip of the leaf was cut off and put into the reference solution (5 mm KCl, 0·1 mm CaCl2, buffered with 2 mm Mes/Tris to pH 5·0) which, through an Ag/AgCl electrode, was connected to earth. Access to the apoplastic fluid was achieved by carefully (without damaging cells) inserting blunt voltage- and ion-selective microelectrodes into the sub-stomatal cavities of open stomata (Felle et al., 2000). Contact of the electrodes with the apoplastic fluid was signalled by the closing of the electrical circuit. In rare cases where displacement of the electrode tips did occur, the electrodes were either repositioned or the measurement was terminated. Since the electrical resistance from the sensitive tip through the apoplastic network to the bath reference was high (1–20 MΩ, depending on the distance from the veins), a blunt leaf voltage reference microelectrode was placed in a neighbouring stoma to continuously record the apoplastic voltage and changes thereof. Since the ion-selective electrode also picks up these voltage changes, the difference between the signals of these two electrodes was recorded as the net trace representing the ion activity of interest. This approach guaranteed that non-physiological voltage interferences picked up by both electrodes were filtered out (Hanstein and Felle, 1999; Felle et al., 2004). When detached leaves were used, the basipetal end of the leaf rested in the reference solution (Fig. 1B).
Fig. 1.
Principle of apoplastic pH measurements. (A) On a leaf of an intact plant. The roots were placed in an Erlenmeyer flask. The leaf was fixed with double-adhesive tape on a dry Plexiglass cuvette beneath the electrodes. The (cut) leaf tip rested in a chamber that held the exchangeable reference solution, but also harboured the earth electrode. Exchange of fluid (e.g. CN−) or gas flow (air, N2) was achieved through pipes while recording. An O2-minielectrode constantly controlled the O2. (B) On a detached leaf. The cuvette consists of two chambers: a wet (reference) chamber, which holds an exchangeable solution and the earth electrode. Added agents (e.g. FC or cyanide) were transported to the site of measurement through the transpiration stream. The leaf blade was fixed and the electrodes were placed in the dry chamber, as described in (A). An extra gas-flow cuvette with a small opening at the top to allow the approach of the electrodes could be placed on top of the leaflet. As in (A), an oxygen electrode constantly measured the O2.
Infiltration of the leaf apoplast
The infiltrate solution (5 mm KCl, 0·1 mm CaCl2, buffered with 2 mm Mes/Tris to pH 5·0) was taken up in a glass pipette (tip diameter 2–4 μm) using a ‘pico pump’ (WPI). After placing the pipette in an open stoma, exact amounts of the fluid were ejected, controlled by the pico-pump. The fluid was distributed within the apoplast at random and was gradually absorbed. The response to this treatment was monitored by a pH microelectrode placed in a neighbouring stoma (Hanstein and Felle, 2004).
Ion-selective microelectrodes
The electrical set-up for the fabrication of ion-selective microelectrodes and their application to apoplastic tests in leaves has been described in detail previously (e.g. Hanstein and Felle, 1999; Felle et al., 2000, 2004). The tips of the electrodes were 2–4 μm in diameter, blunt and heat-polished to prevent injuries to the cells in case of unintended contact. Pipettes were pulled on a two-stage patch-clamp puller (List Instruments, Darmstadt, Germany) and silanized internally, using a 0·2 % tributylsilane/chloroform solution. After heat-stabilization at 200 °C for 1 h, the cooled pipettes were back-filled with the respective sensor cocktail. To give the sensor enough firmness to stay in place, the cocktail (# 95297 H+; # 60398 K+; Fluka Chemical Corp. Milwaukee, WI, USA) was dissolved in a mixture of polyvinylchloride and tetahydrofuran (40 mg mL−1) at a ratio of 70 : 30 (v/v). After evaporation of the tetrahydrofuran, the remaining firm gel was topped up with the undiluted sensor cocktail, followed by the backfill-solution, which in the case of the pH-sensitive electrode contained 0·5 m KCl plus 0·1 m Mes/Tris adjusted to pH 6. After due equilibration, these electrodes gave stable responses for several weeks, when stored in a dry chamber. The electrodes were connected to a high-impedance amplifier (FD 223; WP-Instruments, Sarasota, FL, USA), which simultaneously measured and subtracted the signals coming from the ion-selective and the voltage reference electrodes, to obtain the net kinetics of the free ion concentration under investigation. The signals were recorded on a chart recorder (L 2200; Linseis, Selb, Germany). Intracellular pH measurements were carried out with double-barrelled microelectrodes, fabricated as described (e.g. Felle and Bertl, 1986; Felle, 1987). Briefly: ‘piggy-back’ double-barrelled capillaries, both with a solid filament but of different diameter (OD 1·5/0·75 mm; Hilgenberg, Malsfeld, Germany or WPI, USA) were pulled to about 0·5 μm tips. The barrel with the larger diameter was internally silanized and filled with the sensor cocktail and backfill solution as described above. The slimmer barrel was filled with 0·5 m KCl and represented the reference electrode. Since plant cells develop turgor pressures in the range 7–10 bar, the sensor cocktail had to be protected from being pushed away from the very tip. A home-built manual pressure controller kept the sensor in place during intracellular pH measurements. Small displacements of the sensor after impalement were corrected using a micrometer screw.
Oxygen measurements
Although O2 measurements accompanied most experiments by continuously monitoring O2 using an O2 mini-electrode (ISO2; WPI), the kinetics of which are not shown in the figures. Values are given as a percentage. The electrode had a tip diameter of 2 mm. After due polarization, the electrode was calibrated for measurements in the gas phase by zeroing the instrument with pure nitrogen in a closed vessel. By flushing with pure oxygen, 100 % was obtained. Air (depending on humidity and temperature) showed a value around 21 %. For aqueous calibration, water was bubbled for a few minutes with air, and dissolved oxygen was calculated according to pO2 = 21 % (1−pH2O/760), where pH2O denotes the partial pressure of water vapour.
RESULTS
In the series of tests presented here, apoplastic pH of the barley leaves rested at 4·97 ± 0·16 s.e. (n = 36). This value accounts for light, 22–24 °C room temperature and the presence of a reference solution, which comprised 5 mm KCl, 0·1 mm CaCl2 buffered with 2 mm Mes/Tris to pH 5·0. Alterations of either of these conditions will change the apoplastic pH to some extent (Hanstein and Felle, 1999; Felle et al., 2004, 2005). To understand the full impact anoxia/hypoxia may have on the apoplast and its ionic milieu (mainly pH), the following situations were investigated: (a) control experiments to find out what impact the changes in H+ pump activity have on the barley leaf apoplast; (b) flooding and infiltrating the leaf apoplast; (c) imposing anoxia/hypoxia to part of a leaf; (d) imposing anoxia/hypoxia to the roots while the shoot remained well aerated.
The apoplastic ion milieu depends critically on the H+ pump activity
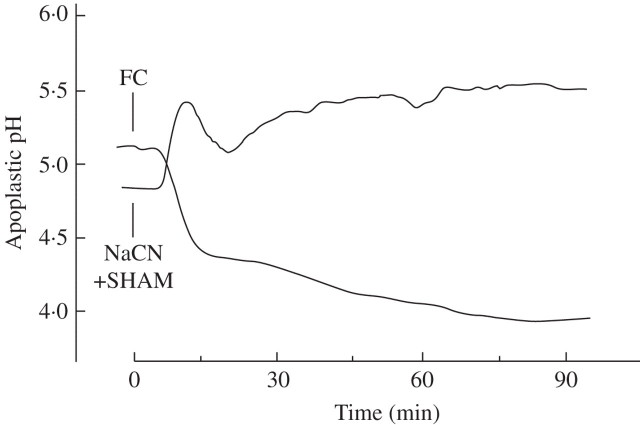
Since the plasma membrane H+ pump is the universal primary transporter which, by exporting H+, builds up an inwardly directed electrochemical proton gradient across the plasma membrane, not only the transmembrane H+gradient, but also the gradients of all other membrane permeable ions are affected as soon as the pump transport activity is altered. To have a control that indicates what happens to the apoplastic pH when the H+ pump activity is changed, the leaf apoplast was treated with the H+ pump-stimulating fungal toxin fusicoccin (FC) (Marrè, 1979) or with the H+ pump-deactivating cyanide (which inhibits cytochrome c: O2-oxidoreductase), supplemented with salicylic hydroxamic acid (SHAM) to knock out a cyanide-insensitive alternative oxidase; the combination of these compounds is equivalent to real anoxia. As shown in Fig. 2, 10 μm FC, added to the cut end of a barley leaf, caused a fast decrease in apoplastic pH from 5·1 to 4·4 and then, more slowly, to approx. 4·0. On the other hand, 5 mm cyanide (+ 0·2 mm SHAM) rapidly shifted the apoplastic pH from 4·9 to 5·6. These results show that, depending on H+ pump activity, the apoplastic pH may vary considerably.
Fig. 2.
Apoplastic pH of barley leaves responding to 10 μm FC or to 5 mm NaCN + 0·2 mm salicylic hydroxamic acid (SHAM). Agents were added to the leaf according to Fig. 1B and reached the measuring site after being carried through the xylem stream, as described in Felle et al., (2000). The curves are representative of three experiments (FC) or four experiments (NaCN/SHAM).
The response to infiltration of barley leaves
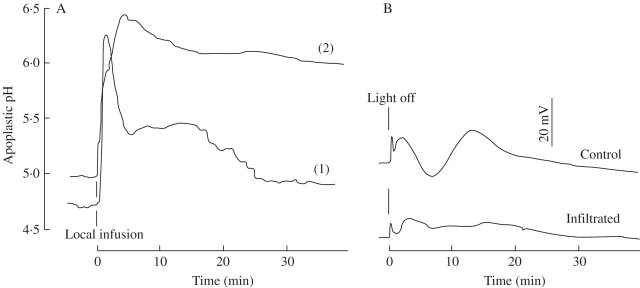
Infiltration of the barley leaf apoplast with the reference solution causes an immediate and rapid increase in apoplastic pH by 1·5–1·7 units. Figure 3A shows that this response is transient, provided the leaf was able to absorb the fluid, so that after about 30 min the pH returns close, albeit not entirely, to its starting value (curve 1). When absorption was prevented by larger amounts of fluid being injected, which then caused flooding of major areas around the measuring site, the apoplastic pH only partially recovered within the observation time and remained around 6·1 (curve 2). Figure 3B shows that during the state of infiltration (conditions curve 2), basic responses, e.g. the typical voltage response following the switch from light to dark, are heavily impeded.
Fig. 3.
Apoplastic pH of barley leaves responding to infiltration. (A) Local infiltration through nanolitre pressure infusion with a solution that comprised 5 mm KCl, 0·1 mm CaCl2. Curve 1: only the immediate area around the measuring site was infiltrated, as detected by microscopic observation. Curve 2: a larger area was infiltrated. (B) Voltage responses to a ‘light off’ test following leaf infiltration according to pattern 2, or without infiltration (control). Representative curves of three equivalent tests each. For experimental details, see Materials and Methods.
Direct effects of N2 on the leaf
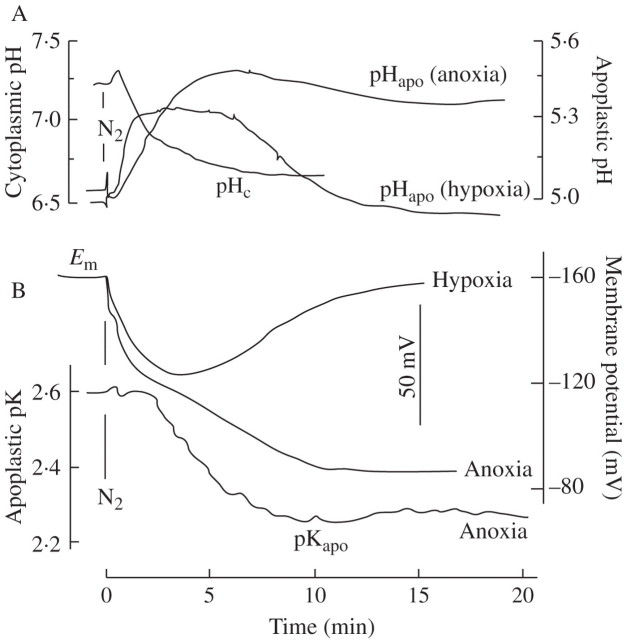
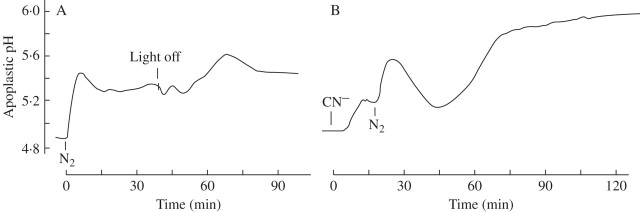
The leaf apoplast responds to a decrease in oxygen caused by N2 flushing with a rapid increase in pH by 0·3–0·6 units. As shown in Fig. 4A, the pH recovers spontaneously when O2 reduction is incomplete (hypoxia) but remains elevated when O2 is reduced to 3 % or less (as tested online with the O2 electrode; data not shown). Simultaneously, the cytoplasmic pH decreases by about 0.6 units, a response demonstrated for a variety of plants with different techniques in a number of laboratories (e.g. Roberts et al., 1984a; Fan et al., 1988; Menegus et al., 1991; Fox et al., 1995; Felle, 1996; Gout et al., 2001; Schulte et al., 2006). As shown in Fig. 4B, the response of the membrane potential is in line with the apoplastic pH, showing a persistent depolarization under anoxic conditions, but a transient depolarization with hypoxia. Apoplastic K+ (measured as pK), after a lag phase increases by a factor of about 2, from 2·6 mm to 5·5 mm, within the first 20 min, but increases further in the next 40 min to almost 10 mm (Table 1), indicating a constant K+ leak.
Fig. 4.
Short-term effects of anoxia or hypoxia on the barley leaf. After positioning the electrodes in the substomatal cavity (pHapo; pKapo) and in mesophyll cells (pHc; Em), N2 was flushed across the leaves, as shown in Fig. 1B, while the O2 concentration was constantly monitored (not given). (A) Apoplastic and cytoplasmic pH; (B) membrane potential and apoplastic K+ (measured as pK). Traces are each representative of at least five experiments (A), and three experiments (B).
Table 1.
Transport parameters that determine the proton motive force (pmf) for H+-mediated symport from the barley leaf apoplast into the cytoplasm of adjacent cells
| Em (mV) | pHcyt | pHapo | [K+]apo (mm) | pmf (mV) | |
|---|---|---|---|---|---|
| Before oxygen stress | −161 ± 2·73 | 7·32 ± 0·16 | 4·88 ± 0·15 | 2·6 ± 0·13 | −305 |
| Hypoxia (20 min) | −159 ± 4·33 | 6·73 ± 0·21 | 4·92 ± 0·13 | 2·8 ± 0·19 | −266 |
| Anoxia (20 min) | −81 ± 3·42 | 6·62 ± 0·15 | 5·34 ± 0·11 | 5·5 ± 0·22 | −156 |
| Anoxia (2 h) | −65 ± 3·85 | 6·42 ± 0·13 | 6·06 ± 0·11 | 9·9 ± 0·10 | −86 |
Em, membrane potential as measured in mesophyll cells; pHcyt, cytoplasmic pH as measured with a pH-sensitive double-barrelled microelectrode; pHapo, apoplastic pH as measured with a blunt pH-sensitive micro-electrode within the sub-stomatal cavity.
Values given denote average values ± s.e., calculated from five experiments each.
To calculate the pmf, pH gradients (pHcyt−pHapo) have been transformed into millivolts: (pHcyt−pHapo) × −59 mV.
Longer exposures to N2 and cyanide yield even stronger apoplastic pH responses. As shown in Fig. 5A, apoplastic pH responds to N2 flushing across the leaf with a typical rapid increase of about 0·6 units and a partial recovery around 15 min (see Fig. 4A), but then increases further following ‘light off’. Apoplastic pH responds likewise to cyanide, added to the cut end of the leaf and transported to the site of measurement through the xylem stream, with a rapid increase which is markedly enhanced by N2, flushed across the leaf when cyanide is present.
Fig. 5.
Apoplastic pH of barley leaves responding to (A) N2, flushed across a barley leaf followed by ‘light off’ (in the presence of N2 ) or (B) to 5 mm NaCN, followed by N2-flushing (in the presence of cyanide). The kinetics are representative of three tests each.
Anoxia imposed at the root is signalled to the leaf
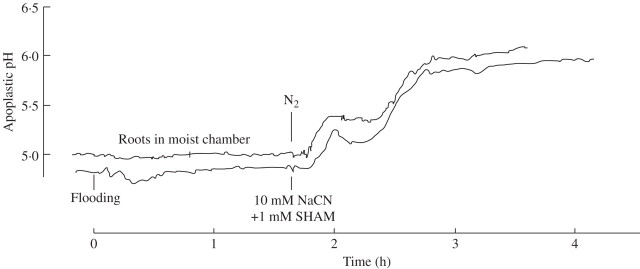
It was demonstrated recently that stress signals are transferred from root to shoot (leaf) via the xylem and that apoplastic alkalinization—picked up in the sub-stomatal cavity of leaves—is typical of the information obtained (Wilkinson, 1999; Felle et al., 2005). When flooding occurs, the roots are affected first. Thus it is of interest if and to what extent the information ‘oxygen shortage’ at the roots reaches the leaves as an apoplastic change in the ionic milieu. To avoid a hypoxic reaction of the root before the actual experiment, the entire root system was placed in a 100-mL Erlenmeyer flask containing a small quantity of water to keep the atmosphere around the roots moist. The roots were allowed to adapt to the conditions for 1–2 h, during which the apoplastic pH was continuously monitored. As soon as the pH baseline was straight or showed only minor fluctuations (<0·05 pH units within 10 min), N2 was flushed into the otherwise closed Erlenmeyer flask. About 8 min after flushing the Erlenmeyer flask with N2, the apoplastic pH within the leaf being measured (20–30 cm away) began to increase rapidly to reach a transitory level about 0·4 pH units above the control, but increased further to reach a pH that seemed to level off around 6 (Fig. 6, upper curve). Flooding of the roots with the reference solution (following adaptation in the moist atmosphere) had no effect on the leaf apoplast at all but, after exchanging the reference solution with a NaCN/SHAM solution, the apoplastic pH changed in a similar manner to that observed in the presence of N2, indicating the equivalence of both treatments.
Fig. 6.
Apoplastic pH of a barley leaf responding to treatment at the root, as indicated. Flooding (lower curve): roots of an intact plant (adapted to a moist atmosphere) were rapidly flooded with a solution consisting of 1 mm KCl, 0·1 mm CaCl2, weakly buffered with 1 mm Mes/Tris to pH 6. After almost 2 h the reference solution was removed by suction and replaced by 10 mm NaCN + 1 mM SHAM (dissolved in the reference solution and adjusted to pH 6). Upper curve: roots resting in a moist atmosphere, after due adjustment, were flushed with N2, while O2 was monitored constantly (not shown). Curves are representative examples of three equivalent experiments (N2) and four experiments (cyanide). See Materials and Methods.
DISCUSSION
The question has been addressed whether the apoplast plays a role in anoxia tolerance during oxygen stress. To raise this problem is relevant because oxygen shortage sooner or later leads to an energy shortage that has to be dealt with by the affected organ or by the plant as a whole. As recently reviewed by Gibbs and Greenway (2003), anoxia in plants reduces the rate of energy production by 65–97 % compared with the rate in air, with the consequence that adaption to this situation means coping with an energy crisis. Since the apoplast is that extracellular space within the plant through which energy-rich compounds have to pass before they are taken up into the cells through H+-symport, apoplastic pH was studied during anoxia or hypoxia using pH-sensitive micro-probes. Use of this method is advantageous for several reasons: (a) it measures changes in ion activities continuously from the first moment of the experiment; (b) it is entirely non-invasive, i.e. ion activities are measured directly without cutting, injuring or disturbing the tissue in any way, which guarantees genuine physiological responses; (c) it permits very long-lasting measurements and one can even collect data during experimentally unfavourable times (e.g. during the night); (d) the method also permits the detection of signals coming from distant organs that were affected or tested for a certain purpose, i.e. from root to shoot. The main questions to be answered were: (a) How does the apoplastic pH of a certain plant organ (leaf or root) respond to oxygen shortage? (b) Which way is the message ‘oxygen shortage’ transferred from root to shoot? (c) Is the apoplastic ion milieu altered by oxygen shortage to an extent that is critical enough to cause potential problems for transport of energy-rich compounds?
Oxygen shortage at the leaf
Regardless of whether the leaves were treated with low oxygen (N2) or chemical anoxia (cyanide), apoplastic pH always increased. This is in line with other stress responses (e.g. drought, salt or low temperature; Wilkinson, 1999; Felle et al., 2005) and indicates that the anoxia-induced response to pH is not specific. However, the data in Fig. 4 show clearly that hypoxia was experienced by the apoplast, and responded to, in different ways to anoxia. Whereas under hypoxia the increase in apoplastic pH was clearly transient, under anoxia (<3 % O2) the apoplast remained about 0·5 units above the control for as long as the experiments lasted (see also Fig. 5). The explanation for this is that the small amount of oxygen left, together with the energy gained in fermentation, is used by the cells to produce energy that can be used for membrane transport, etc. The observation that membrane depolarization, measured under the same conditions, was also transient whereas it was not under anoxia, supports this view. Obviously, the H+ pump still had sufficient energy to repolarize the plasma membrane, probably facilitated by the acidification of the cytoplasm which is equivalent to an increase in its transport substrate (H+). A similar response comes from the apoplastic K+. In plants, K+ is the ion whose distribution across the plasma membrane is closest to the NNernst condition. This means that membrane potential changes will have an immediate effect on apoplastic K+, as is in fact shown in Fig. 4B. Apoplastic [K+], measured under anoxic conditions, rapidly increased from 2·6 mm to approx. 5·5 mm within the first 10 min and increased further, but more slowly to reach 10 mm after 2 h. The reason for this K+ loss from the cells obviously results from the depolarization of the plasma membrane through which the normally inwardly directed driving force for K+ is inverted after passing the K+ equilibrium potential. This notion is supported by the finding that no K+ loss was measured during hypoxia where the membrane potential spontaneously re-polarized (Fig. 3B and Table 1).
At first sight, cytoplasmic acidification and apoplastic alkalinization, shown in Fig. 4A, seem to match each other, thus suggesting a causal relationship. Referring to Fig. 4A, the largest apoplastic pH increase occurred around 6 min after anoxia onset from 5·0 to 5·49 (= 0·49 units). This is accompanied by a cytoplasmic acidification from 7·25 to 6·70 (= 0·55 units). In an effort to bring these values into a quantitative relationship, buffer capacities and volumes of both spaces have to be considered. Cytoplasmic buffer capacity is 40–60 mm (Sanders and Slayman, 1982; Felle, 1987; Guern et al., 1991), that of the apoplast 5–8 mm per pH unit (Hanstein and Felle, 1999; Oja et al., 1999). In the work cited it was demonstrated that within the cytoplasmic pH range considered here, the cytoplasmic buffer capacity does not change considerably, i.e. the capacity ratio of cytoplasm versus apoplast would be between 5 and 12. If it is assumed that the volumes of apoplast and cytoplasm in the substomatal cavity (the measuring site) are about equal, then the protons lost from the apoplast would have acidified the cytoplasm to 6·2 (buffer ratio 12) or to 5·85 (buffer ratio 5). Alternatively, it would have to be assumed that the volume of the cytoplasm was at least five times that of the apoplast at the measuring site, which appears unlikely. Clearly, in judging the cytoplasmic acidification, intracellular stores like the vacuole also have to be considered. However, as reviewed recently (Felle, 2005), only a few of the vacuolar pH changes under anoxia reported would be in the range of cytoplasmic acidification. Thus, the fast apoplastic pH increase may not mirror the cytoplasmic acidification, but could rather be a secondary consequence of K+ leaking out (Fig. 4B) and could be due to a shift in the strong ion difference (Stewart, 1983; Good, 1988; Ullrich and Novacki, 1990; Gerendas and Schurr, 1999; Greenway and Gibbs, 2003). Additionally, organic acid anions, which may leave the cells following the activation of anion channels, may also contribute.
Root-to-shoot signalling
In the present work, situations were tested in which either the entire plant or only parts of it were exposed to real or chemical anoxia. Since the root is usually the first part of the plant to be flooded and in many cases remains so, it was of interest whether the information about oxygen shortage would be transferred to the shoot or leaf. It was interesting, but not quite unexpected, to observe that, at least within the first 2 h, mere flooding of the root system with a reference solution (in ion content similar to the apoplastic fluid) had no effect at all (Fig. 6). Clearly, the experience of reduced oxygen was not sent to the leaf as a pH message, thus not identifying this hypoxic condition to be a strong stress situation to the root, otherwise the leaf apoplast would have been alkalized as it is in a variety of stress situations (Felle et al., 2005). On the other hand, either flushing the roots with N2 or submersing them in NaCN/SHAM, after allowing time for transpiration-driven xylem transport to take place, caused a pH increase of at least 1 pH unit (Fig. 6). The similarity of the kinetics indicates a common mode of action.
Is infiltration of the apoplast a problem?
Although infiltration of the apoplast may be a relatively rare naturally occurring event during flooding or water logging, wherever it happens, it has fatal consequences. The apoplast is very sensitive to the slightest mechanical and chemical disturbances, as well as to alterations in the ion content and to oxygen shortage. Whereas the rapid and transient increase in pH by almost 2 units following local nano-infusion is probably caused by mechanical stress, i.e. by displacing the gas and subsequent wetting the apoplast surface, the longer-lasting increased pH (about pH 6) may in fact be due to oxygen shortage through which the H+ pump activity gets reduced, with the consequence that no or very few H+ ions are exported. This interpretation is supported by control tests (Fig. 2) in which the activity of the H+ pump was manipulated by FC (stimulation) or cyanide (deactivation). From the light/dark responses shown in Fig. 3B, it is also obvious that an infiltrated apoplast will not be fully functional any more, i.e. transport of any kind across the plasma membrane of the cells involved is heavily impeded through the change in the ionic milieu and increase in the aqueous phase. Clearly, not all flooding situations may be as drastic, but similar scenarios should be considered for plant organs with surfaces which are not water repellent. Mostly, however, the apoplast will not be infiltrated and the gas within the apoplast will not be displaced. When the entire plant gets flooded, it is very likely that the leaves will not permit external fluid into the apoplast (unless injuries exist). The roots, however, through their basic function as uptake organs will permit exchange of water and dissolved components therein into the cortex, at least up to the Casparian band. Therefore, whatever components the flooding water contains, some of them will get into the cortex. In fact, earlier experiments with intact plants have shown that, apart from the major ions (K+, Na+, Ca2+, Cl−), organic compounds like abscisic acid (and probably a variety of others) are also quickly taken up and transported into the shoot, providing they are able to jump the endodermis barrier (Felle et al., 2005). Flooding the roots had no measurable effect, as long as the concentrations of the major ions were not too different from the apoplastic ion content. However, a different ion concentration within the flood fluid will be experienced as stress and transferred immediately to the leaf as an apoplastic pH change (Felle et al., 2005). This means that not necessarily the hypoxic situation per se but rather the ion content of the fluid may be the cause of some of the apoplastic pH changes.
The transmembrane driving forces under anoxic conditions
Uptake of energy-rich compounds (e.g. sugars) that are unloaded from the phloem into the apoplast to be taken up into the adjacent cells, is mediated by proton-driven symporters and, therefore, depends critically not only on the electrochemical proton gradient across the plasma membrane, but also on the activity of the H+ pump which provides the driving transmembrane proton circulation. In barley leaves, the main relevant transport parameters that would be affected by oxygen shortage have been measured: membrane potential, apoplastic pH, cytoplasmic pH and apoplastic K+. As shown in Fig. 4 and Table 1, cytoplasmic pH drops while the apoplastic pH increases; at the same time the membrane potential massively depolarizes from −161 to −81 mV. Calculating the pmf yields, on average, −305 mV before anoxia and −156 mV during anoxia (20 min). Within the first 2 h of anoxia the situation gets worse. As shown in Fig. 6, the apoplastic pH increases to about 6·1, meaning that the transmembrane pH gradient is just 0·5 units (provided cytoplasmic pH did not drop further). Simultaneously, K+ keeps leaking out and reaches approx. 10 mm while the membrane potential drops to –65 mV, thus leaving a pmf of just –86 mV (Table 1). This is a loss of 72 % of the inwardly directed driving force. By thermodynamic standards this may still be enough to drive some symport but, since the H+ turnover with a largely deactivated H+ pump is rather low, it can be predicted that the energy situation will be aggravated. Interestingly, hypoxia does not cause such drastic effects. As shown in Fig. 4, both membrane depolarization and apoplastic pH changes are transient and apparently recover fully within minutes. This means that only very low oxygen partial pressures will get the barley tissue into trouble. One reason for this is certainly the fact that the H+ pump works more efficiently at acidic pH, because a drop from pH 7·32 to 6·73 meant that the free cytoplasmic H+ concentration increased by a factor of about 4, which enables the H+ pump to export more H+ with less energy into the apoplast. This not only repolarizes the plasma membrane but also brings back the apoplastic pH to almost normal values (Table 1). Under these conditions, the pmf is –266 mV, i.e. only slightly below the control value.
CONCLUSIONS
This study shows that in barley the apoplast may be crucial in dealing with a shortage of oxygen. In this, the changes in apoplastic pH observed during anoxia or hypoxia may be more than just a tolerated side effect. Under conditions of normoxia, the apoplast plays an active role as a storage compartment as well as in mediating and controlling transport of matter over a long distance and from cell to cell, functions that largely rely on the apoplastic ion milieu, especially on the pH. Since anoxia increases the apoplastic pH to 6 or over, functions of enzymes (e.g. invertase) and transporters with pH optima around 5 are impeded. Moreover, the transmembrane driving force (pmf) for metabolizable compounds drops drastically and thus aggravates the anoxic energy shortage. Therefore, it is concluded that anoxia tolerance depends a good deal on how the plant is able to hold pmf and H+ turnover at a level that guarantees sufficient energy harvest to overcome the crisis. The validity of this interpretation will have to be tested by measuring apoplastic pH during oxygen stress in species of varying tolerance to anoxia.
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft. I would like to thank Professors Hank Greenway, Michael Jackson and Tim Colmer for helpful discussions on this issue during the ISPA meeting in Perth (Australia).
LITERATURE CITED
- Fan TW-M, Higashi RM, Lane AN. (1988) An in vivo 1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Archives of Biochemistry and Biophysics 266592–606. [DOI] [PubMed] [Google Scholar]
- Felle H. (1987) Proton transport and pH control in Sinapis root hairs: a study carried out with double-barrelled pH micro-electrodes. Journal of Experimental Botany 38340–354. [Google Scholar]
- Felle HH. (1996) Control of cytoplasmic pH under anoxic conditions and its implication for plasma membrane proton transport in Medicago sativa root hairs. Journal of Experimental Botany 47967–973. [Google Scholar]
- Felle HH. (2005) pH regulation in anoxic plants. Annals of Botany 96519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H and Bertl A. (1986) The fabrication of H+-selective liquid-membrane micro-electrodes for use in plant cells. Journal of Experimental Botany 371416–1428. [Google Scholar]
- Felle HH, Hanstein S, Steinmeyer R, Hedrich R. (2000) Dynamics of ionic activities in the apoplast of the sub-stomatal cavity of intact Vicia faba leaves during stomatal closure evoked by ABA and darkness. The Plant Journal 24297–304. [DOI] [PubMed] [Google Scholar]
- Felle HH, Herrmann A, Hanstein S, Hueckelhoven R, Kogel K-H. (2004) Apoplastic pH signalling in barley leaves attacked by the powdery mildew fungus Blumeria graminis f.sp. hordei. Molecular Plant–Microbe Interactions 17118–123. [DOI] [PubMed] [Google Scholar]
- Felle HH, Herrmann A, Hückelhoven R, Kogel K-H. (2005) Root-to-shoot signalling: apoplastic alkalinization, a general stress signal and defence factor in barley. Protoplasma 22717–24. [DOI] [PubMed] [Google Scholar]
- Fox GG, McCallan GG, Ratcliffe RG. (1995) Manipulating cytoplasmic pH under anoxia: a critical test of the role of pH in the switch from aerobic to anaerobic metabolism. Planta 195324–330. [Google Scholar]
- Gao DJ, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C. (2004) Self-reporting arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiology 134898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerendas J and Schurr U. (1999) Physicochemical aspects of ion relations and pH regulation in plants—a quantitative approach. Journal of Experimental Botany 501101–1114. [Google Scholar]
- Gibbs J and Greenway H. (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 301–47. [DOI] [PubMed] [Google Scholar]
- Good NE. (1988) Active transport, ion movements and pH changes. I. The chemistry of pH changes. Photosynthesis Research 19225–236. [DOI] [PubMed] [Google Scholar]
- Gout E, Boisson A-M, Aubert S, Douce R, Bligny R. (2001) Origin of the cytoplasmic pH changes during anaerobic stress in higher plant cells: carbon-13 and phosphorous-31 nuclear magnetic resonance studies. Plant Physiology 125912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H and Gibbs J. (2003) Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology 30999–1036. [DOI] [PubMed] [Google Scholar]
- Guern J, Felle H, Mathieu Y, Kurkdjian A. (1991) Regulation of intracellular pH in plant cells. International Reviews of Plant Cytology 127111–173. [Google Scholar]
- Hanstein S and Felle HH. (1999) The influence of atmospheric pH on the apoplastic pH of green leaves: a non-invasive approach with pH-sensitive microelectrodes. New Phytologist 143333–338. [Google Scholar]
- Hanstein SM and Felle HH. (2004) Nano-infusion: an integrating tool to study elicitor perception and signal transduction in intact leaves. New Phytologist 161595–606. [DOI] [PubMed] [Google Scholar]
- Hoffmann B and Kosegarten H. (1995) FITC-Dextran for measuring apoplast pH and apoplastic pH gradients between various cell types in sunflower leaves. Physiologia Plantarum 95327–335. [Google Scholar]
- Marrè E. (1979) Fusicoccin: a tool in plant physiology. Annual Review of Plant Physiology 30273–288. [Google Scholar]
- Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E. (1991) Response to anoxia in rice and wheat seedlings. Plant Physiology 95760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühling KH, Plieth C, Hansen UP, Sattelmacher B. (1995) Apoplastic pH of intact leaves of Vicia faba as influenced by light. Journal of Experimental Botany 46377–382. [Google Scholar]
- Oja V, Savchenko G, Jakob B, Heber U. (1999) pH and buffer capacities of apoplastic cell compartments in leaves. Planta 209239–249. [DOI] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M. (1984a) Cytoplasmic acidosis as determinant of flooding intolerance in plants. Proceedings of the National Academy of Sciences of the USA 816029–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M. (1984b) Further evidence that cytoplasmic acidosis is a determinant of flooding intolerance in plants. Plant Physiology 77492–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D and Slayman CL. (1982) Control of intracellular pH. Predominant role of oxidative metabolism not proton transport in the eukaryotic microorganism Neurospora. Journal of General Physiology 80377–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelmacher B. (2001) The apoplast and its significance for plant mineral nutrition. New Phytologist 149167–192. [DOI] [PubMed] [Google Scholar]
- Schulte A, Lorenzen I, Boetcher M, Plieth C. (2006) A novel fluorescent pH probe for expression in plants. Plant Methods 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA. (1983) Modern quantitative acid-base chemistry. Canadian Journal of Physiology and Pharmacology 611444–1461. [DOI] [PubMed] [Google Scholar]
- Taylor DP, Slattery J, Leopold AC. (1996) Apoplastic pH in corn root gravitropism: a laser scanning microscopy measurement. Physiologia Plantarum 9735–38. [PubMed] [Google Scholar]
- Ullrich CI and Novacki AJ. (1990) Extra- and intracellular pH and membrane potential changes induced in K+, Cl−, H2PO4−, and NO3− uptake and fusicoccin in root hairs of. Limnobium stoloniferum. Plant Physiology 941561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. (1999) pH as a stress signal. Plant Growth Regulation 2987–99. [Google Scholar]
- Zheng HQ, Kunst L, Hawes C, Moore C. (2004) A GFP-based assay reveals a role for RDH3 in transport between the endoplasmic reticulum and golgi apparatus. The Plant Journal 37398–414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.