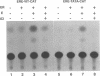
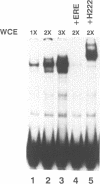
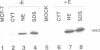Abstract
We have developed a transient transfection system using the Cytomegalovirus (CMV) promoter to express the human estrogen receptor (ER) at very high levels in COS-1 cells and have used it to study the interaction of agonist and antagonist receptor complexes with estrogen response element (ERE) DNA. ER can be expressed to levels of 20-40 pmol/mg or 0.2-0.3% of total soluble protein and all of the soluble receptor is capable of binding hormone. The ER binds estradiol with high affinity (Kd 0.2 nM), and is indistinguishable from native ER in that the receptor is capable of recognizing its cognate DNA response element with high affinity, and of transactivating a transgene in an estradiol-dependent manner. Gel mobility shift assays reveal interesting ligand-dependent differences in the binding of receptor complexes to ERE DNA. Receptors occupied by estradiol or the type I antiestrogen transhydroxytamoxifen bind to DNA response elements when exposed to the ligand in vitro or in vivo. Likewise, receptors exposed to the type II antiestrogen ICI 164,384 in vitro bind to ERE DNA. However, when receptor exposure to ICI 164,384 is carried out in vivo, the ER-ICI 164,384 complexes do not bind to ERE DNA, or do so only weakly. This effect is not reversed by subsequent incubation with estradiol in vitro, but is rapidly reversible by in vivo estradiol exposure of intact COS-1 cells. This suggests there may be some cellular process involved in the mechanism of antagonism by the pure antiestrogen ICI 164,384, which is not observed in cell-free extracts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Bellingham D. L., Cidlowski J. A. Stable overproduction of intact glucocorticoid receptors in mammalian cells using a selectable glucocorticoid responsive dihydrofolate reductase gene. Mol Endocrinol. 1989 Nov;3(11):1733–1747. doi: 10.1210/mend-3-11-1733. [DOI] [PubMed] [Google Scholar]
- Brown M., Sharp P. A. Human estrogen receptor forms multiple protein-DNA complexes. J Biol Chem. 1990 Jul 5;265(19):11238–11243. [PubMed] [Google Scholar]
- Burnside J., Darling D. S., Chin W. W. A nuclear factor that enhances binding of thyroid hormone receptors to thyroid hormone response elements. J Biol Chem. 1990 Feb 15;265(5):2500–2504. [PubMed] [Google Scholar]
- Chang T. C., Shapiro D. J. An NF1-related vitellogenin activator element mediates transcription from the estrogen-regulated Xenopus laevis vitellogenin promoter. J Biol Chem. 1990 May 15;265(14):8176–8182. [PubMed] [Google Scholar]
- Dahlman K., Strömstedt P. E., Rae C., Jörnvall H., Flock J. I., Carlstedt-Duke J., Gustafsson J. A. High level expression in Escherichia coli of the DNA-binding domain of the glucocorticoid receptor in a functional form utilizing domain-specific cleavage of a fusion protein. J Biol Chem. 1989 Jan 15;264(2):804–809. [PubMed] [Google Scholar]
- Edwards D. P., Kühnel B., Estes P. A., Nordeen S. K. Human progesterone receptor binding to mouse mammary tumor virus deoxyribonucleic acid: dependence on hormone and nonreceptor nuclear factor(s). Mol Endocrinol. 1989 Feb;3(2):381–391. doi: 10.1210/mend-3-2-381. [DOI] [PubMed] [Google Scholar]
- Eul J., Meyer M. E., Tora L., Bocquel M. T., Quirin-Stricker C., Chambon P., Gronemeyer H. Expression of active hormone and DNA-binding domains of the chicken progesterone receptor in E. coli. EMBO J. 1989 Jan;8(1):83–90. doi: 10.1002/j.1460-2075.1989.tb03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., White R., Hoare S., Sydenham M., Page M., Parker M. G. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. K., Nemmers L. A., Beckman W. C., Jr, Davis V. L., Curtis S. W., Korach K. S. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology. 1991 Oct;129(4):2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G. L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986 Mar 7;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Sobel N. B., King W. J., Jensen E. V. Immunochemical studies of estrogen receptors. J Steroid Biochem. 1984 Jan;20(1):51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- Hirst M. A., Northrop J. P., Danielsen M., Ringold G. M. High level expression of wild type and variant mouse glucocorticoid receptors in Chinese hamster ovary cells. Mol Endocrinol. 1990 Jan;4(1):162–170. doi: 10.1210/mend-4-1-162. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Murphy C. S. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev. 1990 Nov;11(4):578–610. doi: 10.1210/edrv-11-4-578. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Kendra K. L., Norman M. J., Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987 Aug 15;47(16):4355–4360. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Miller M. A., Mullick A., Sheen Y. Y. Antiestrogen action in breast cancer cells: modulation of proliferation and protein synthesis, and interaction with estrogen receptors and additional antiestrogen binding sites. Breast Cancer Res Treat. 1985;5(3):231–243. doi: 10.1007/BF01806018. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen J. A., Carlson K. E., Heiman D. F., Robertson D. W., Wei L. L., Katzenellenbogen B. S. Efficient and highly selective covalent labeling of the estrogen receptor with [3H]tamoxifen aziridine. J Biol Chem. 1983 Mar 25;258(6):3487–3495. [PubMed] [Google Scholar]
- Klein-Hitpass L., Cato A. C., Henderson D., Ryffel G. U. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 1991 Mar 25;19(6):1227–1234. doi: 10.1093/nar/19.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Kushner P. J., Hort E., Shine J., Baxter J. D., Greene G. L. Construction of cell lines that express high levels of the human estrogen receptor and are killed by estrogens. Mol Endocrinol. 1990 Oct;4(10):1465–1473. doi: 10.1210/mend-4-10-1465. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIndoe J. H., Woods G. R., Etre L. A. The specific binding of estradiol and estrone and the subsequent distribution of estrogen-receptor complexes within MCF-7 human breast cancer cells. Steroids. 1982 Mar;39(3):245–258. doi: 10.1016/0039-128x(82)90145-3. [DOI] [PubMed] [Google Scholar]
- Mak P., McDonnell D. P., Weigel N. L., Schrader W. T., O'Malley B. W. Expression of functional chicken oviduct progesterone receptors in yeast (Saccharomyces cerevisiae). J Biol Chem. 1989 Dec 25;264(36):21613–21618. [PubMed] [Google Scholar]
- Martinez E., Wahli W. Cooperative binding of estrogen receptor to imperfect estrogen-responsive DNA elements correlates with their synergistic hormone-dependent enhancer activity. EMBO J. 1989 Dec 1;8(12):3781–3791. doi: 10.1002/j.1460-2075.1989.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D., White J. H., Chambon P. The human oestrogen receptor functions in yeast. Nature. 1988 Jul 7;334(6177):31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Katzenellenbogen B. S., Miller M. A., Ziegler Y. S., Katzenellenbogen J. A. Characterization of the estrogen receptor and its dynamics in MCF-7 human breast cancer cells using a covalently attaching antiestrogen. Endocrinology. 1984 Jul;115(1):143–153. doi: 10.1210/endo-115-1-143. [DOI] [PubMed] [Google Scholar]
- Mukherjee R., Chambon P. A single-stranded DNA-binding protein promotes the binding of the purified oestrogen receptor to its responsive element. Nucleic Acids Res. 1990 Oct 11;18(19):5713–5716. doi: 10.1093/nar/18.19.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. B., Towle H. C. Identification of nuclear factors that enhance binding of the thyroid hormone receptor to a thyroid hormone response element. Mol Endocrinol. 1989 Sep;3(9):1434–1442. doi: 10.1210/mend-3-9-1434. [DOI] [PubMed] [Google Scholar]
- Pham T. A., Elliston J. F., Nawaz Z., McDonnell D. P., Tsai M. J., O'Malley B. W. Antiestrogen can establish nonproductive receptor complexes and alter chromatin structure at target enhancers. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3125–3129. doi: 10.1073/pnas.88.8.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Mutagenesis of cysteines in the hormone binding domain of the human estrogen receptor. Alterations in binding and transcriptional activation by covalently and reversibly attaching ligands. J Biol Chem. 1991 Jun 15;266(17):10880–10887. [PubMed] [Google Scholar]
- Schena M., Yamamoto K. R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science. 1988 Aug 19;241(4868):965–967. doi: 10.1126/science.3043665. [DOI] [PubMed] [Google Scholar]
- Toney T. W., Katzenellenbogen B. S. Antiestrogen action in the medial basal hypothalamus and pituitary of immature female rats: insights concerning relationships among estrogen, dopamine, and prolactin. Endocrinology. 1986 Dec;119(6):2661–2669. doi: 10.1210/endo-119-6-2661. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling A. E., Bowler J. Novel antioestrogens without partial agonist activity. J Steroid Biochem. 1988 Oct;31(4B):645–653. doi: 10.1016/0022-4731(88)90014-3. [DOI] [PubMed] [Google Scholar]
- Wrenn C. K., Katzenellenbogen B. S. Cross-linking of estrogen receptor to chromatin in intact MCF-7 human breast cancer cells: optimization and effect of ligand. Mol Endocrinol. 1990 Nov;4(11):1647–1654. doi: 10.1210/mend-4-11-1647. [DOI] [PubMed] [Google Scholar]
- Young C. Y., Qiu S. D., Prescott J. L., Tindall D. J. Overexpression of a partial human androgen receptor in E. coli: characterization of steroid binding, DNA binding, and immunological properties. Mol Endocrinol. 1990 Dec;4(12):1841–1849. doi: 10.1210/mend-4-12-1841. [DOI] [PubMed] [Google Scholar]