Abstract
Angle-resolved low-coherence interferometry (a/LCI) is an optical biopsy technique that measures scattered light from tissue to determine nuclear size with submicron-level accuracy. The a/LCI probe can be deployed through the accessory channel of a standard endoscope and provides feedback to physicians to guide physical biopsies. The technique has been validated in animal and ex vivo human studies, and has been used to detect dysplasia in Barrett’s esophagus patients in vivo. In a recent clinical study of 46 Barrett’s esophagus patients, a/LCI was able to detect dysplasia with 100% sensitivity and 84% specificity. This report reviews the technique and discusses its potential clinical utility.
Keywords: Barrett’s esophagus, dysplasia, early detection, endoscopy, light scattering, nuclear morphology, optical biopsy
Barrett’s esophagus (BE) is a metaplastic transformation of the epithelial lining in the lower esophagus that is associated with an increased risk of esophageal adenocarcinoma, the incidence of which has increased dramatically in the last half century [1,2]. The poor outlook and survival rates of esophageal adenocarcinoma patients – in addition to difficulties surrounding the accepted BE surveillance technique of systematic physical biopsy, such as interobserver disagreement, delay to diagnosis and low sampling area – point to the need for improved surveillance and detection methods [3,4].
Optical biopsy provides a compelling alternative method for assessing tissue health that has drawn increasing interest in recent years. This group of technologies analyze the spectral and spatial characteristics of scattered light to measure cellular and/or tissue morphology [5]. Examples include, but are not limited to, laser confocal microscopy [6], optical coherence tomography [7], elastic-scattering spectroscopy [8] and light-scattering spectroscopy [9]. Angle-resolved low-coherence interferometry (a/LCI) is a novel light-scattering technique that has recently demonstrated potential clinical utility [10–13].
a/LCI combines the depth-resolving ability of low-coherence techniques, such as optical coherence tomography, with the ability to measure subcellular morphologies of light scattering [14]. By isolating the angular scattering distribution from cellular nuclei at various tissue depths, a/LCI is able to provide biomarkers based on morphology that are highly correlated with the presence of dysplasia, of both low- and high-grade. The technique was first demonstrated in animal models, and subsequently used in ex vivo human studies, as well as recently in a pilot in vivo study of 46 BE patients undergoing routine surveillance endoscopy [13].
This article summarizes the technical basis behind the a/LCI technique, describes the validation studies that have been performed to demonstrate its utility in identifying dysplasia in BE patients and provides insight as to its potential clinical utility in the next 5 years.
a/LCI
A noninvasive light-scattering technique, a/LCI measures the angular intensity distribution of light scattered by a tissue sample in order to quantify subcellular morphology as a function of depth in the tissue [10]. For each depth layer, the collected angular scattering signal is processed to extract signatures from cell nuclei, which are then analyzed using a Mie theory-based light-scattering model to produce measurements of average nuclear diameter with submicron-level accuracy [11,15,16].
There are a number of potential biological and medical applications for a/LCI. Previous studies have ranged from characterizing in vitro cancer cells to detecting dysplasia in BE patients. In studies of dysplasia using animal models and ex vivo human tissues, a/LCI provided high sensitivity and specificity in differentiating nondysplastic and dysplastic tissues [17–20]. Notably, it has provided measurements that confirm that neoplastic tissue transformation is accompanied by an increase in the average cell nuclei size [12,13,17,21,22].
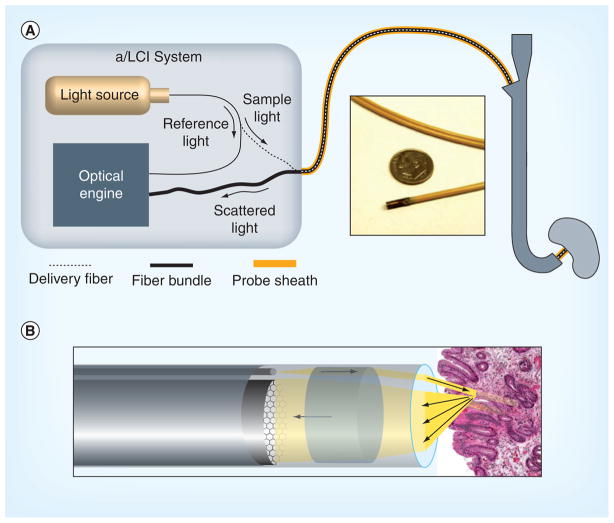
Recent engineering developments have led to a clinical a/LCI system for the in vivo examination of BE patients [13,14]. The endoscopic system incorporates a thin, flexible fiber probe to examine tissue sites that are difficult to access otherwise. As shown in Figure 1, this system uses a single fiber to deliver light from the source (Superluminescent Diode, Superlum Ltd, Russia: 832-nm center wavelength, 19-nm bandwidth) to the tissue, and an 18,000-element fiber bundle to collect scattered light. Both the delivery fiber and fiber bundle are sheathed and protected in a 2.5-mm diameter, 230-cm-long endoscopic probe, which can be passed through the accessory channel of a standard endoscope. No difficulty was reported in articulating the endoscope while the a/LCI probe was deployed. During measurement, the probe is placed in direct contact with the tissue and light scattering data are acquired in a fraction of a second, minimizing the opportunity for artifacts to arise owing to patient motion. Feedback from the instrument operator to the endoscopist was provided to ensure that sufficient contact was achieved in order to obtain a good signal. Individual fibers within the fiber bundle (~160 fibers across its diameter) collect light scattered as a function of the angle with a resolution of 0.035 rad (2°) over a range of 0.56 rad (32°), and transmit them back to the optical engine for optical processing and electronic readout.
Figure 1. Configuration of the clinical angle-resolved low-coherence interferometry system.
(A) High-level a/LCI system diagram. Light from the source is split into a reference arm, which provides the optical engine with a depth reference for sample signal, and a sample arm, which delivers most light power to illuminate the tissue. Scattered light is collected by the fiber bundle and sent back to the optical engine. Outside of the a/LCI instrument box, both the delivery fiber and the fiber bundle are protected by probe sheathing. Inset shows the probe tip compared with a US dime. (B) Detail of the a/LCI probe tip. Light is delivered as a collimated beam to the tissue. Scattered light is collected across the face of the fiber bundle for transport back to the a/LCI system.
a/LCI: Angle-resolved low-coherence interferometry.
Reproduced with permission from [14].
Since its first use, the a/LCI technique has seen significant developments to improve its speed, reduce its footprint and enable in vivo capabilities. The translation of this technique from laboratory to clinical operation and eventual deployment as a cancer screening tool will continue through further technical development and clinical study.
Validation of a/LCI for detecting dysplasia
Animal models
The potential of a/LCI for detecting dysplasia was first validated through two studies using the rat esophageal carcinogenesis model. In the first study, the mean nuclear size of cells in the basal layer of excised esophagi from 42 rats was retrospectively compared with histological classification, and a positive correlation was identified between increased nuclear size and neoplastic progression [11]. The average nuclear diameter was seen to increase from 9.55 ± 0.23 μm in normal tissues to 10.5 ± 0.56 μm in low-grade dysplasia (LGD), and to 14.4 ± 0.21 μm for high-grade dysplasia, with statistically significant differences (p < 0.001 between normal and LGD; p < 0.005 between normal and low- and high-grade dysplasia). To distinguish between normal and dysplastic tissues, a decision threshold was determined using logistic regression, yielding 80% sensitivity (eight out of ten) and 100% specificity (17 out of 17). The diagnostic capacity of the approach was verified in a second a/LCI rat study, where the decision threshold from the first study was used to prospectively grade tissue health [17]. Subsequent comparison between a/LCI nuclear morphology data with histological tissue classification provided 91% sensitivity (20 out of 22) and 97% specificity (58 out of 60) for detecting dysplasia, confirming the efficacy of nuclear size as a cancer biomarker.
Ex vivo human tissue study
Following the concept validation through animal experiments, technological advances in a/LCI instrumentation have resulted in prototype endoscopic systems with much improved data acquisition speed (~40 ms). These developments enabled an initial investigation of a/LCI for detecting esophageal dysplasia in ex vivo human tissues [12,16].
In one study, esophageal tissues from three BE patients who underwent esophagogastrectomies were scanned within 2 h of resection at multiple tissue locations using a/LCI in the laboratory [12]. Biopsies from these locations were then evaluated by a pathologist. For gastric (n = 5) and dysplastic (n = 13) tissues, the mean nuclear diameters were found to be 10.1 μm and 12.9 μm, respectively. A secondary metric, nuclear density, which is the relative refractive index of nuclei to cytoplasm, was also found to be a statistically significant discriminator. With both metrics combined, a complete discrimination was achieved between these two tissue populations.
Another study with ex vivo human tissue investigated the diagnostic power of a/LCI as a function of interrogation depth in the tissue [16]. When data from superficial depths were analyzed, excellent sensitivity (100%; six out of six) was obtained, but specificity (56%; five out of nine) was low. When the signal from a deeper tissue segment near to the basal layer was considered, specificity was improved (78%; seven out of nine) with an unchanged sensitivity. This result confirmed the findings from the animal studies that the deep basal layer could provide the greatest diagnostic potential for a/LCI.
While these initial human results were encouraging, the small number of patients and biopsies involved were not sufficient to define the diagnostic power of a/LCI. An in vivo study of a larger population was needed to assess the clinical utility of the approach.
In vivo pilot clinical study
The first in vivo human study of a/LCI was enabled by the clinical system described above, which features an endoscope-compatible fiber-optic probe [13,14]. In this study, 46 patients undergoing routine upper-endoscopic screening for BE were enrolled at one of two endoscopy centers. During each procedure, the physician brought the a/LCI probe into direct contact with the esophageal epithelium through the accessory channel of the endoscope and between three and six tissue sites were scanned. Following each of these optical biopsies, the a/LCI probe was retracted but a circular indentation was left on the tissue, as shown in Figure 2. A coregistered standard biopsy was subsequently taken at the marked site to enable later evaluation by a pathologist. The disease state of each tissue site was then correlated with the a/LCI measurements of nuclear morphology in order to assess the diagnostic ability of the method.
Figure 2. Clinical operation of the angle-resolved low-coherence interferometry probe.
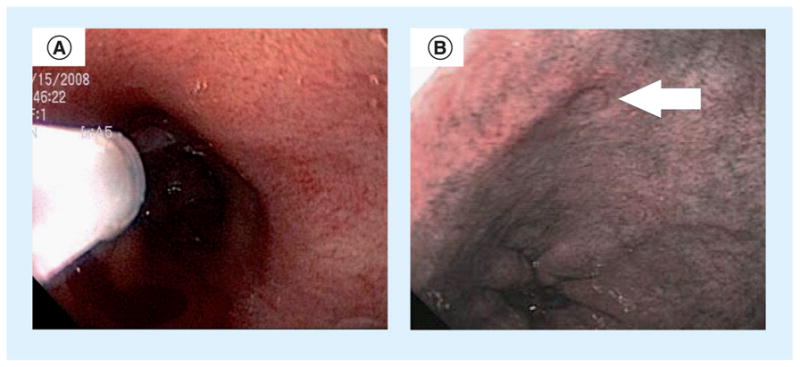
(A) Angle-resolved low-coherence interferometry probe tip deployed inside the esophagus of a patient through the accessory channel of the endoscope. (B) Arrow indicates a temporary mark left by the probe tip after measurement used to guide coregistration of physical biopsy.
Reproduced with permission from [9].
In total, 172 coregistered optical and physical biopsy pairs were taken in the study, and were pathologically dichotomized as dysplastic (n = 13) and nondysplastic (n = 159 [consisting of 70 normal and 89 Barrett’s mucosa samples]). A scatter plot of these data, shown in Figure 3, revealed that an enlarged nuclear diameter from the deep epithelial layer (200–300 μm in depth) showed a strong statistical correlation (p < 0.001) with the presence of dysplasia. This finding was consistent with the results from previous ex vivo human studies. To evaluate the diagnostic ability of this metric, a receiver operating characteristic was developed, demonstrating good overall performance in discrimination, with an area under the curve of 0.91. Furthermore, the receiver operating characteristic curve identified an optimal decision line at 11.84 μm for the classification of tissue health. In Figure 3, this decision line yielded a sensitivity of 100% (13 out of 13; 95% CI: 0.75–1.00), a specificity of 84% (134 out of 159; 95% CI: 0.78–0.90), an overall accuracy of 86% (147 out of 172; 95% CI: 0.80–0.91) and positive and negative predictive values of 34% (13 out of 38; 95% CI: 0.20–0.51) and 100% (134 out of 134; 95% CI: 0.97–1.00), respectively. Performance in discriminating dysplastic from nondysplastic Barrett’s mucosa was similar to that for the overall study (100% sensitivity; 95% CI: 0.75–1.00; and 85% specificity; 95% CI: 0.76–0.92).
Figure 3. Scatter plot with each biopsy plotted as a function of its nuclear size and density, and categorized by its pathological diagnosis.
Dashed black line indicates an optimized decision line between the dysplastic and nondysplastic populations, yielding 100% sensitivity and 84% specificity.
BE: Barrett’s esophagus; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; SQ: Squamocolumnar.
Reproduced with permission from [9].
This pilot in vivo study represented a significant step forward in the development of an a/LCI-based clinical diagnostic for dysplasia in the esophagus. However, limitations of this study must be considered when placing it into perspective. First, this was a pilot study and, as such, includes a limited number of patients and biopsies, such that future trials are still needed to more broadly validate the technology. Second, the output of dysplastic versus nondysplastic tissues was dichotomized, in that varying degrees of dysplasia were not differentiated. This was a pragmatic step as a limited number of dysplastic biopsies were observed, and this enabled the demonstration of proof-of-principle that the approach has the capacity to detect dysplasia. Finally, the gold standard in this study was histopathology, such that any errors in classification would impact performance characteristics. The impact of this limitation was mitigated by requiring confirmation assessments on dysplastic biopsies, as is commonly performed in clinical practice.
Expert commentary
The a/LCI technology described here was specifically developed to address the clinical need to assess the health of epithelial tissues. This arises from the limitations of the traditional approach of histopathological evaluation of biopsy samples, including limited tissue coverage, delay between procedure and diagnosis and, ultimately, an inherent variability due to tissue sampling and pathological classification. Instead, a/LCI provides a potential alternative method for evaluating tissue health through depth-resolved measurements of nuclear morphology, without requiring tissue removal or the use of exogenous contrast agents. As described here, a/LCI measurements of increased nuclear diameter correlate highly with the presence of dysplasia, especially for measurements at the base of the epithelial layer.
Application to the detection of dysplasia in BE is a good target for a/LCI. The increased risk of developing cancer in BE tissues results in a need for surveillance. The current regimen is time consuming but is the only accepted approach for early detection, leading to frustration among clinicians. In the preliminary study discussed here, the a/LCI technology was shown to provide good performance characteristics for this application, suggesting a potential clinical role. As a clinical tool, a/LCI could be used to direct the endoscopist to tissue regions that are more likely to harbor dysplasia. The extremely high negative predictive value of the technique would suggest that tissue sites without enlarged nuclei are most likely nondysplastic and a physical biopsy may be avoided. Thus, a/LCI has the potential to reduce the number of physical biopsies needed to adequately monitor the health of BE tissues, although further validation in a broader population is needed to justify adoption.
Finally, the completion of the first-in-human study is a significant advance in the development of the a/LCI technology. After previous applications in animal and ex vivo human tissues, the translation of this technology to use during endoscopy represents an engineering milestone.
Five-year view
In the next 5 years, future development of a/LCI will continue to advance this technology, while also expanding clinical use. A randomized controlled clinical study of efficacy and safety will be needed to justify its use in monitoring of BE tissues. Further technical developments will improve clinical utility and ergonomic ease of use as the technology evolves from prototype to clinical tool. Expansion of a/LCI to surveillance of other epithelial tissue sites also seems likely, owing to the common feature of enlarged cell nuclei in neoplastic transformation. Compelling targets for the a/LCI technology include the colon, where large numbers of biopsies are needed to monitor patients with inflammatory bowel disease, and the cervix, where cytology provides a suitable screening method, but additional tools are needed to locate potentially dysplastic regions.
Key issues.
Barrett’s esophagus is a metaplastic transformation of the esophagus that is associated with an increased risk for esophageal adenocarcinoma.
Improved methods for screening for dysplasia in Barrett’s esophagus patients are needed owing to limitations to the current standard of care.
Angle-resolved low-coherence interferometry (a/LCI) is a point-scanning optical biopsy technique that measures angular light-scattering distributions from depths beneath the tissue surface in order to measure nuclear morphology and identify the presence of dysplasia.
a/LCI has been validated in animal studies, ex vivo human studies and in a pilot in vivo clinical study of 46 patients.
Clinically, a/LCI has the potential to provide intraoperative feedback to guide the selection of biopsy sites by the endoscopist.
Further studies are needed to validate the technique and spur clinical adoption.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work was supported by grants from the National Cancer Institute (NCI R21-CA109907, R33-109907, R01-CA138594), the National Science Foundation (BES 03-48204) and a grant from the Coulter Translational Partnership. NG Terry has a consulting relationship with Oncoscope, Inc. A Wax has a financial interest in Oncoscope, Inc., the company that holds proprietary rights to the technology described in this article. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373(9666):850–861. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Mason AC, Desmond RA, El-Serag HB. Temporal trends (1973–1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98(7):1627–1633. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 4.Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost–utility analysis. Ann Intern Med. 2003;138(3):176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 5.Wilson BC. Detection and treatment of dysplasia in Barrett’s esophagus: a pivotal challenge in translating biophotonics from bench to bedside. J Biomed Opt. 2007;12(5):051401. doi: 10.1117/1.2795688. [DOI] [PubMed] [Google Scholar]
- 6.Meining A, Saur D, Bajbouj M, et al. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: an examiner blinded analysis. Clin Gastroenterol Hepatol. 2007;5(11):1261–1267. doi: 10.1016/j.cgh.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Vakoc BJ, Shishko M, Yun SH, et al. Comprehensive esophageal microscopy by using optical frequency-domain imaging (with video) Gastrointest Endosc. 2007;65(6):898–905. doi: 10.1016/j.gie.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovat LB, Johnson K, Mackenzie GD, et al. Elastic scattering spectroscopy accurately detects high grade dysplasia and cancer in Barrett’s oesophagus. Gut. 2006;55(8):1078–1083. doi: 10.1136/gut.2005.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backman V, Wallace MB, Perelman LT, et al. Detection of preinvasive cancer cells. Nature. 2000;406(6791):35–36. doi: 10.1038/35017638. [DOI] [PubMed] [Google Scholar]
- 10.Wax A, Yang C, Backman V, Kalashnikov M, Dasari RR, Feld MS. Determination of particle size by using the angular distribution of backscattered light as measured with low-coherence interferometry. J Opt Soc Am A Opt Image Sci Vis. 2002;19(4):737–744. doi: 10.1364/josaa.19.000737. [DOI] [PubMed] [Google Scholar]
- 11.Wax A, Yang CH, Backman V, et al. Cellular organization and substructure measured using angle-resolved low-coherence interferometry. Biophys J. 2002;82(4):2256–2264. doi: 10.1016/S0006-3495(02)75571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Pyhtila JW, Chalut KJ, Boyer JD, et al. In situ detection of nuclear atypia in Barrett’s esophagus by using angle-resolved low-coherence interferometry. Gastrointest Endosc. 2007;65(3):487–491. doi: 10.1016/j.gie.2006.10.016. Ex vivo human study with angle-resolved low-coherence interferometry (a/LCI) [DOI] [PubMed] [Google Scholar]
- 13•.Terry NG, Zhu Y, Rinehart MT, et al. Detection of dysplasia in Barrett’s esophagus with in vivo depth-resolved nuclear morphology measurements. Gastroenterology. 2011;140(1):42–50. doi: 10.1053/j.gastro.2010.09.008. Clinical pilot study of a/LCI in patients with Barrett’s esophagus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu YZ, Terry NG, Woosley JT, Shaheen NJ, Wax A. Design and validation of an angle-resolved low-coherence interferometry fiber probe for in vivo clinical measurements of depth-resolved nuclear morphology. J Biomed Opt. 2011;16(1) doi: 10.1117/1.3520130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pyhtila JW, Graf RN, Wax A. Determining nuclear morphology using an improved angle-resolved low coherence interferometry system. Opt Express. 2003;11(25):3473–3484. doi: 10.1364/oe.11.003473. [DOI] [PubMed] [Google Scholar]
- 16•.Brown WJ, Pyhtila JW, Terry NG, et al. Review and recent development of angle-resolved low-coherence interferometry for detection of precancerous cells in human esophageal epithelium. IEEE J Sel Top Quantum Electron. 2008;14(1):88–97. Reports on an ex vivo human study and a/LCI signal processing. [Google Scholar]
- 17.Wax A, Pyhtila JW, Graf RN, et al. Prospective grading of neoplastic change in rat esophagus epithelium using angle-resolved low-coherence interferometry. J Biomed Opt. 2005;10(5):1785–1791. doi: 10.1117/1.2102767. [DOI] [PubMed] [Google Scholar]
- 18.Chalut KJ, Ostrander JH, Giacomelli MG, Wax A. Light scattering measurements of subcellular structure provide noninvasive early detection of chemotherapy-induced apoptosis. Cancer Res. 2009;69(3):1199–1204. doi: 10.1158/0008-5472.CAN-08-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wax A, Pyhtila JW. In situ nuclear morphology measurements using light scattering as biomarkers of neoplastic change in animal models of carcinogenesis. Dis Markers. 2008;25(6):291–301. doi: 10.1155/2008/584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalut KJ, Kresty LA, Pyhtila JW, et al. In situ assessment of intraepithelial neoplasia in hamster trachea epithelium using angle-resolved low-coherence interferometry. Cancer Epidemiol Biomark Prev. 2007;16(2):223–227. doi: 10.1158/1055-9965.EPI-06-0418. [DOI] [PubMed] [Google Scholar]
- 21.Kelloff GJ, Sigman CC. Assessing intraepithelial neoplasia and drug safety in cancer-preventive drug development. Nat Rev Cancer. 2007;7(7):508–518. doi: 10.1038/nrc2154. [DOI] [PubMed] [Google Scholar]
- 22.Wax A, Yang C, Muller MG, et al. In situ detection of neoplastic transformation and chemopreventive effects in rat esophagus epithelium using angle-resolved low-coherence interferometry. Cancer Res. 2003;63:3556–3559. [PubMed] [Google Scholar]