Abstract
• Background and Aims Many Orchidaceous species are threatened globally by development and over-collection from their natural habitats for horticultural purposes. Artificial propagation from seeds is difficult in most terrestrial orchids native to temperate regions. Seed production is another limiting factor in the artificial propagation for these species because of the lessened probability of pollination and the destruction of fruit by insect larvae. Members of the genus Cephalanthera are distributed across Europe, Asia and North America. C. falcata is a temperate species of East Asia and an endangered species in Japan. As successful propagation from seeds of this species has never been reported, a reproducible method is described here for seed production in situ and propagation using immature seeds in asymbiotic culture in vitro.
• Methods Effects of hand-pollination and bagging treatment of ovaries were examined. Young capsules were collected every 10 d from 50 d after pollination until 120 d after pollination. Immature seeds obtained from these capsules were cultured asymbiotically on modified Kano medium and ND medium. Seed viability was examined within TTC (2,3,5-triphenyl tetrazolium chloride) test solution and histological observations were made on viable seeds by paraffin embedding at each collection stage.
• Key Results and Conclusions Hand-pollination followed by bagging treatment of ovaries with aluminium foil was effective for insect control during fruit development, and successfully yielded capsules. Of the capsules, 74·5 % survived to full maturity. The highest frequency (39·8 %) of seed germination was obtained with seeds harvested 70 d after pollination. The frequency declined with progress of seed maturity on the mother plant. Minimal germination was observed with seeds harvested 100 d or later after pollination. Histological observation suggests that accumulation of such substances as lignin in the inner integument surrounding the embryo during seed maturation plays an important role in induction of dormancy.
Keywords: Orchidaceae, Cephalanthera falcata, seed dormancy, seed germination, seed production, immature seed, inner integument
INTRODUCTION
Cephalanthera falcata is a terrestrial orchid species that is distributed in China, the Korean Peninsula and Japan. It grows at the edges and on floors of broadleaved forests. The plant is 30–70 cm high and its bright lemon-yellow flowers bloom in raceme during April and May. This species is threatened through deforestation and over-collection for horticultural purposes. C. falcata is categorized as vulnerable (VU; heightened and increasing danger of extinction) in the Red Data Book of the Environment Agency of Japan (Environment Agency of Japan, 2000). Only fragmentary information is available for the cultivation of this species. Plants collected from natural habitats usually become quiescent and eventually disappear within 3–4 years.
Generally, seed germination of terrestrial orchid species from temperate regions is difficult (Arditti et al., 1982b; Rasmussen, 1995; Miyoshi and Mii, 1998). Information relating to the factors that complicate seed germination of these species remains fragmentary and obscure. For many orchid species, higher frequencies of germination have been achieved by culturing immature seeds than by culturing mature seeds (Withner, 1955; Linden, 1980; Arditti et al., 1982a, b; Ballard, 1987; Mitchell, 1989; DePauw and Remphrey, 1993; Rasmussen, 1995; Light and MacConaill, 1998). It has been postulated that dormancy is induced by some undefined changes during seed development and maturation, accumulation of some inhibitory substances such as phenolics in Cymbidium goeringii (Kako, 1976) and abscisic acid in Dactylorhyza maculata and Epipactis helleborine (van der Kinderen, 1987), induction of a physiologically dormant state in embryos (Arditti et al., 1982a), or by increasing impermeability of the embryos during seed maturation (Miyoshi and Mii, 1988). It appears that no studies have elucidated the reasons for increased germination frequencies of immature seeds of the Orchidaceae based on a series of histological observations of developing seeds on the mother plant.
Low fruit set is inferred to be characteristic of this family (Ackerman and Zimmerman, 1994) and low fruit productivity has been reported among non-autogamous species as a result of high levels of fruiting failure (Neiland and Wilcock, 1998). In fact, in the natural habitat of C. falcata, capsules full of fertile seeds are rarely observed; a 3-year preliminary observation revealed fewer opportunities for pollination as well as damage of pollinated ovaries during development by insects. When ovaries were bagged after hand-pollination, however, successful production of capsules was achieved in some orchid species (Hasegawa et al., 1987; Yamazaki et al., 2001). In orchidaceous plants that are difficult to cultivate, hand-pollination followed by bagging treatment was performed in situ to harvest enough seeds for propagation in vitro (Ballard, 1987; DePauw and Remphrey, 1993).
In the present study, as a first approach for seed propagation of C. falcata, hand-pollination followed by bagging treatment was examined as a means of reliable seed production. Developing fruits were collected at 10-d intervals from 50 to 120 d after pollination and the seeds were sown asymbiotically in vitro to determine the optimum time for seed collection to achieve high frequencies of germination. TTC (2,3,5-triphenyl tetrazolium chloride) testing was conducted to determine the viability of non-germinated seeds as used previously for other orchid seeds (Vujanovic et al., 2000).
Although Kano medium, which contains the commercial fertilizer Hyponex® (Hyponex Japan Corp., Osaka, Japan), has been used successfully as the instant medium for seed germination of orchids (Shimasaki and Umemoto, 1990), details of the constituent components of Hyponex have not been released. In the present study, comparisons were made regarding the frequencies of seed germination and protocorm formation of C. falcata between Kano medium and completely defined ND medium (Tokuhara and Mii, 1993). Furthermore, some developing fruits collected at the same time as seed sowing were also fixed with formalin-aceto-alcohol (FAA) and used for observations specifically addressing the developing embryos and inner seed integument by using a paraffin-sectioning method.
Based on these results, the mechanisms of changes in the germination frequency during seed development and maturation are discussed with respect to morphological changes in seed tissues.
MATERIALS AND METHODS
Hand-pollination and bagging treatment
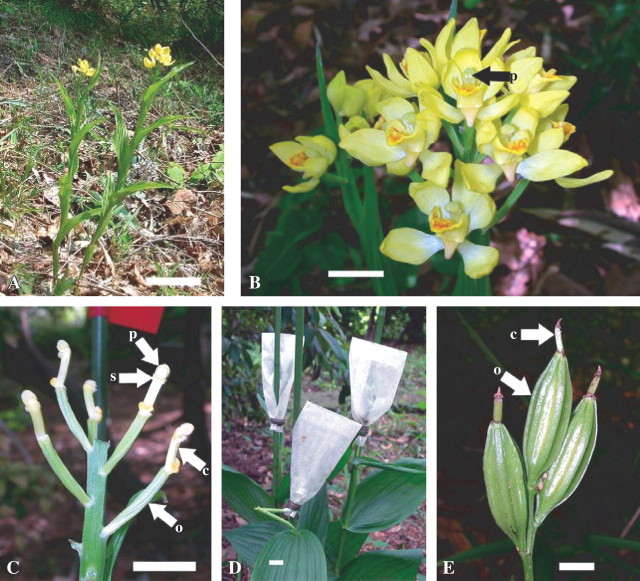
Fifty-five Cephalanthera falcata Blume stems, growing naturally in Quercus acutissima Carruthers and Q. serrata Thunb. ex. Murray forest (Fig. 1A), Tamagawa University campus (Tokyo, Japan), were used. To increase capsule productivity, hand-pollination and bagging treatment were conducted at the full bloom period of C. falcata from 17 to 24 April, 2002 (Fig. 1B). On 38 stems, sepals and petals of a total of 80 flowers were removed with forceps to avoid further contamination of expected fruits with micro-organisms. The pollinia were transferred onto the column of the same flower to achieve self-pollination (Fig. 1C). The number of hand-pollinated flowers was limited to three for each plant to avoid the inhibition of capsule development caused by excess set of capsules. After pollination, remaining unpollinated flowers or buds on the same flower stem were eliminated. Only flowers with two intact pollinia were used for pollination because they were considered to have not been previously visited by insect pollinators (DePauw and Rumphery, 1993). The effect of bag material, namely aluminium foil and parchment paper, on the production of capsules was evaluated. Bagging treatment was conducted just after pollination by using aluminium foil bags of approx. 5 × 10 cm made by folding aluminium foil of 12 × 12 cm, bags of parchment paper of approx. 5 cm wide × 10–13 cm long, or no bags (control). Stems were staked with steel rods to prevent lodging (Fig. 1D). On the remaining 17 stems, 106 flowers that were confirmed to be unpollinated were used as controls (flowers without hand-pollination or bagging treatment).
Fig. 1.
Hand-pollination and bagging treatment in Cephalanthera falcata in situ. (A) Plants in their natural habitat. (B) The inflorescence at the time used for hand-pollination and bagging treatment. (C) Tepals were removed before hand-pollination. (D) Bagging treatment with parchment paper covering a few ovaries. The stem in the front was pulled by a crow and bent. (E) Fully grown intact ovaries which were protected from insects by parchment paper. Scale bars: 10 cm in A, 1 cm in B–E. c, column; o, ovary; p, pollinia; s, stigma.
Two weeks after the pollination treatments, the number of capsules was counted to clarify the effect of hand-pollination on fruit set. Surviving capsules were counted 5 months after initiation of each treatment to elucidate the effects of bagging treatments on the control of insects and pests. Parchment paper bags were occasionally changed with new ones when they were damaged by heavy rain or attacked by snails or crows (Fig. 1D).
In vitro germination and TTC staining of seeds during seed formation
A total of 776 flowers were hand-pollinated from 83 stems of C. falcata and covered with the parchment paper bags from 27 April to 4 May, 2003. Overall, 331 fruits were set and used for the experiment. A random harvest of three capsules was first conducted 50 d after hand-pollination and repeated at 10-d intervals until 120 d. Three capsules for each occasion were surface sterilized with 70 % ethanol for 2 min in a screw-capped sample tube (30 mL). For each capsule, approx. 100–200 seeds were sown in each well of a six-well plate (Falcon Multiwell, Becton Dickinson Labware, Franklin Lakes, NJ, USA) (Miyoshi and Mii, 1998). Sets of six replicate wells were prepared for each capsule. Each well contained 7 mL of 2 g L−1 Gelrite-solidified modified Kano medium (MK medium; Table 1A). After sowing seeds, a six-well plate was sealed with two layers of Parafilm (Pechiney Plastic Packaging, Menasha, WI, USA) and incubated at 24 °C in darkness. Approximately one-third of the seeds that remained within these capsules were used for histological observations.
Table 1.
Components of culture media used in the present study
| Elements | g L−1 |
|---|---|
| (A) MK medium (modified from Kano, 1965)* | |
| Hyponex (N-P-K = 6·5–6–19) | 3 |
| Peptone | 2 |
| Sucrose | 30 |
| pH = 5·2 | |
| Elements | mg L−1 |
|---|---|
| (B) ND medium (Tokuhara and Mii, 1993) | |
| NH4NO3 | 480 |
| KNO3 | 200 |
| Ca(NO3)2.4H2O | 470 |
| KCl | 150 |
| MgSO4.7H2O | 250 |
| KH2PO3 | 550 |
| MnSO4.4H2O | 3 |
| ZnSO4.7H2O | 0·5 |
| H3BO3 | 0·5 |
| CuSO4.5H2O | 0·025 |
| Na2MoO4.2H2O | 0·025 |
| CoCl2.6H2O | 0·025 |
| Concentrated H2SO4 | 0·5 μL L−1 |
| myo-Inositol | 100 |
| Nicotinic acid | 1 |
| l-cysteine | 1 |
| Thiamine hydrochloride | 1 |
| Pyridoxine hydrochloride | 1 |
| Adenine | 1 |
| Calcium pantothenate | 1 |
| (+)-Biotin | 0·1 |
| Fe-EDTA | 21 |
| Sucrose | 20 g L−1 |
| pH = 5·4 | |
These culture media were solidified with 2 g L−1 Gerlite®.
* The original Kano medium (Kano, 1965) contained 35 g L−1 sucrose and was solidified with 15 g L−1 agar.
Each well was examined 300 d after seed sowing under a stereoscopic microscope (magnification 60×, SZH-ILLK, Olympus Optical Co. Ltd, Tokyo, Japan). The process of seed germination was divided into the following six categories according to developmental stages of embryos, which were modifications of those given by Miyoshi and Mii (1995):
Stage 0: ‘No germination’ stage. No growth of embryo occurs.
Stage 1: ‘Pre-germination’ stage. Embryo swells to fill the seed coat.
Stage 2: ‘Germination’ stage. Embryo emerges from the seed coat.
Stage 3: ‘Protocorm’ stage. Embryo is completely discharged from the seed coat.
Stage 4: ‘Rhizoid’ stage. Rhizoids are formed on the protocorm surface.
Stage 5: ‘Shoot’ stage. Shoot is differentiated from the protocorm.
For the viability test, 300–500 seeds from each capsule were put in a sample tube and stained with 1 % TTC solution for 40–48 h at 30 °C in the dark (Miyoshi and Mii, 1981; Van Waes and Debergh, 1986; Lauzer et al., 1994). The seeds stained red were evaluated as viable under a stereoscopic microscope.
Evaluation of the effects of basal media and illumination on seed germination and protocorm development
A mixture of seeds that were collected aseptically from five capsules 80 d after hand-pollination in 2003 was sown on ND medium (see Table 1B; Tokuhara and Mii, 1993) and MK medium (Table 1A; modified from Kano, 1965), which were contained in each well of a six-well plate (Falcon Multiwell). A set of six replicate wells was prepared for each medium. They were incubated in continuous darkness to evaluate the effect of basal medium on the germination of seed and development of protocorm.
To examine the effects of illumination on seed germination and protocorm development, seeds collected from one capsule 80 d after pollination were sown on MK medium in 2002. They were cultured under a 12-h photoperiod illumination of approx. 40 μmol m−2 s−1 provided by white fluorescent lamps, or in continuous darkness. Three sets of 50–100 seeds each were sown in Petri dishes, which contained 25 mL of culture medium.
Observation of embryo development and morphological changes of the inner integument
Two capsules were serially collected for histological observation on the same days when the seeds were sown to evaluate germination frequencies. These two capsules together with what remained of the three capsules used for the germination test, as described previously, were cut into approx. 5-mm-thick cross-sections with a razor blade. The pieces were then fixed in sample tubes containing FAA with aspiration for 10 min. They were dehydrated through an ethyl alcohol series and embedded in paraffin (melting point 54–56 °C) with a graded series of tertiary butyl alcohol. The paraffin blocks were sectioned serially at 10 μm thickness using a microtome. Sections were stained with haematoxylin and safranine combinations. Each section was examined under a light microscope (100×, CX-41, Olympus Optical Co. Ltd).
RESULTS
Effects of hand-pollination and bagging treatment
Table 2 shows the effects of hand-pollination and bagging treatment on formation and survival of capsules. Hand-pollination greatly enhanced capsule formation with or without bagging treatment. Frequencies of capsule formation were 100 % 2 weeks after hand-pollination, but only 7·5 % without hand-pollination. Five months after the initiation of each treatment, all capsules without bagging treatment were eaten by larvae of one fly species (unidentified). By contrast, 74·5 and 35·3 % of capsules that had been bagged with aluminium foil and parchment paper survived, respectively (Fig. 1E). Approximately 20 % of capsules were decayed in the aluminium bag because of contamination with micro-organisms until the final examination 5 months after hand-pollination. The surviving capsules fully matured in autumn and contained 12–49 mg (average 31 mg estimated on 20 capsules) seeds per capsule; approx. 90 % of such seeds contained fully developed embryos (as confirmed by stereomicroscopy; mean of eight capsules).
Table 2.
Effects of hand-pollination and bagging of ovaries on production of Cephalanthera falcata capsules
| Treatment | Capsules setting (%) | ||||
|---|---|---|---|---|---|
| Hand pollination | Bagging treatment | No. of stems used | No. of flowers pollinated | 2 weeks after hand-pollination | 5 months after hand-pollination |
| 17 | (106) ‡ | 7·5 | 0 | ||
| + | 8 | 19§ | 100 | 0 | |
| + | +* | 14 | 27§ | 100 | 74·5 |
| + | +† | 16 | 34§ | 100 | 35·3 |
* With aluminium foil.
† With parchment paper.
‡ Without hand-pollination treatment.
§ Number of flowers pollinated per stem was less than three.
Changes in germination ability in vitro and stainability of seeds with TTC during seed formation
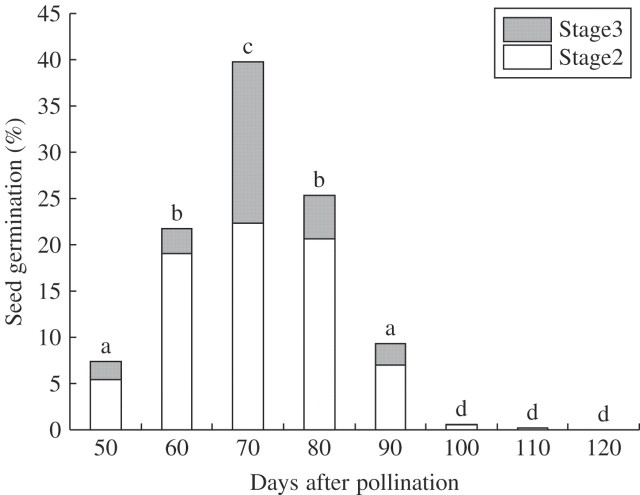
Germination of the seeds collected 50–90 d after pollination was initiated 20 d after sowing and continued to occur even at 180–220 d after sowing. Incubation for longer than 300 d showed no further seed germination. The time of capsule harvest markedly affected germination frequency 300 d after sowing in C. falcate (Fig. 2). A roughly consistent triphasic pattern was observed with respect to the germination of immature and mature seeds during development and maturation of C. falcata seeds. The highest frequency of germination (Stages 2 and 3) of 39·8 % was obtained with seeds harvested 70 d after hand-pollination. A decline in germination frequency was noted with seeds harvested 80 d after pollination. Only minimal germination was observed with seeds harvested 100–120 d after pollination. Seeds harvested 70 d after pollination also showed the highest frequencies of protocorm formation (17·5 %, Stage 3). In this experimental series, browning of the medium was observed in the culture of seeds harvested 60–80 d after pollination, which gave higher germination frequencies than other cultures. By contrast, seeds harvested 50 and 90–120 d after pollination showed no or little browning of the culture medium.
Fig. 2.
Effect of harvesting time of seeds on seed germination in Cephalanthera falcata 300 d after seed sowing. Germination percentages marked by different letters above bars are significantly different at the 1 % level (t-test).
Table 3 shows the results of stainability of seeds by TTC. Most seeds harvested 50–60 d after pollination were not stained. For seeds harvested 70–100 d after pollination, 84·3–98·6 % were stained. For seeds harvested 110 d after pollination, identification of stained embryos was difficult because of the dense dark-brown coloration within the inner integument surrounding the embryo.
Table 3.
Effects of time of harvest during seed formation on stainability of embryos of Cephalanthera falcata by use of 1 % triphenyl tetrazolium chloride
| Days after pollination (d) | ||||||
|---|---|---|---|---|---|---|
| 50 | 60 | 70 | 80 | 90 | 100 | |
| Stainability (%)* | 0a | 2·6a | 95·3b | 84·3b | 90·7b | 98·6b |
*The same letters indicate not significantly different at P = 0·01 (t-test).
Effect of basal media and illumination on seed germination and protocorm development
Frequencies of seeds in Stage 0 (no germination stage; Fig. 3A) were higher in ND medium cultures than in MK medium cultures (38·4 and 16·8 %, respectively). By contrast, frequencies of seeds at Stage 1 (pre-germination stage) were higher for seeds cultured on MK medium than those on ND medium (57·8 and 35·9 %, respectively; Fig. 3B). Frequencies of seeds that developed further than Stage 2 300 d after sowing were almost identical between the MK medium and ND medium cultures (25·3 and 25·7 %, respectively). However, significant differences were indicated in the frequencies in each of the further stages between the two media (Table 4 and Fig. 3). Frequency of protocorm formation (Stage 3; Fig. 3D) on ND medium (10·9 %) was over twice as high as that on MK medium (4·7 %). Frequencies of the seeds attained to rhizoid (Stage 4; Fig. 3E) and shoot (Stage 5; Fig. 3F) stages were observed only on ND medium.
Fig. 3.
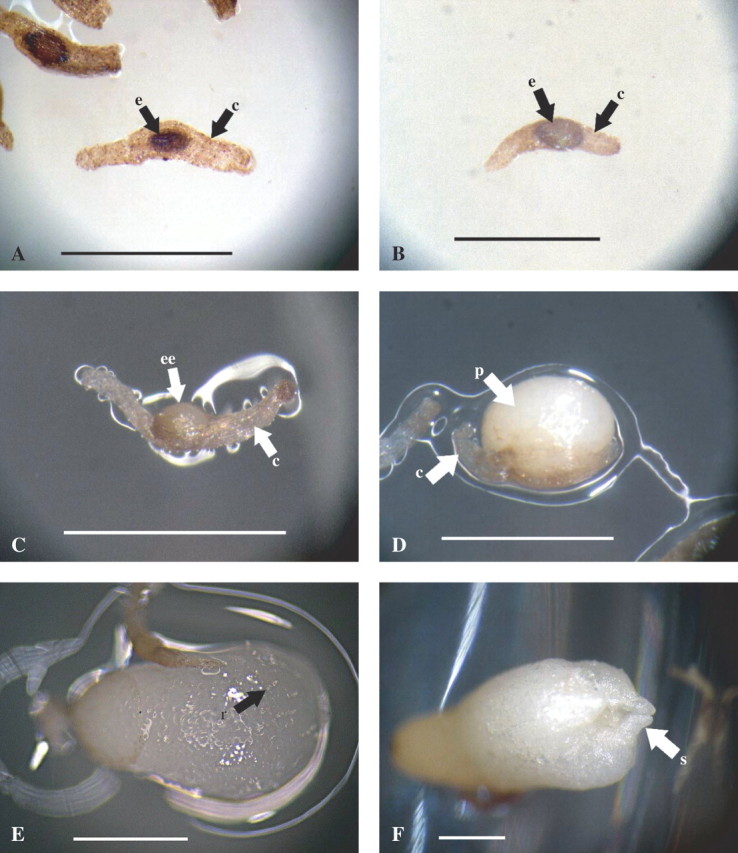
The successive developmental stages of Cephalanthera falcata from seed germination to protocorm formation in asymbiotic culture in vitro. (A) Stage0: ‘No germination stage’, no growth of embryo occurs. (B) Stage 1: ‘Pre-germination stage’, embryo swells in the width of seed coat. (C) Stage 2: ‘Germination stage’, embryo emerges from the seed coat. (D) Stage3: ‘Protocorm stage’, embryo is discharged from the seed coat. (E) Stage 4: ‘Rhizoid stage’, rhizoids are formed on the surface of the protocorm (arrow). (F) Stage 5: ‘Shoot stage’, shoot is differentiated in protocorm. Scale bars = 1 mm. e, embryo; c, seed coat; ee, emerging embryo; p, protocorm; r, rhizoid; s, shoot.
Table 4.
Effect of basal medium on seed germination and protocorm formation in Cephalanthera falcata
| Developmental stages in germination and protocorm formation (%) | ||||||
|---|---|---|---|---|---|---|
| Medium | 0 | 1 | 2 | 3 | 4 | 5 |
| MK† | 16·8 | 57·8 | 20·6 | 4·7 | 0 | 0 |
| ND‡ | 38·4** | 35·9** | 13·1** | 10·9** | 0·9* | 0·8** |
The values in each column were significantly different at P = 0·05 (*) and 0·01 (**) (t-test). Seeds were cultured for 300 d after sowing.
† Modified from Kano (1965).
In continuous darkness, the proportion of germinated seeds of those harvested 80 d after pollination was 42 % (Stages 2 and 3), whereas only 17 % of seeds had germinated under 12-h illumination (Table 5).
Table 5.
The effect of illumination on seed germination and protocorm formation of Cephalanthera falcata
| Developmental stages in germination and formation of protocorm (%) | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Light | 45 | 38 | 0 | 17 | 0 | 0 |
| Dark | 14** | 44 | 14* | 28** | 0 | 0 |
Modified MK medium was used. Seeds were cultured for 100 d after sowing. The values in each column were significantly different at P = 0·05 (*), and 0·01 (**) (t-test).
Embryo development and morphological changes of the inner integument
Successive development of the embryo and inner integument in seeds of C. falcata was revealed by the paraffin sectioning method (Fig. 4). Embryogenesis was initiated 50 d after pollination. The inner integument, which consisted of 2–3 cell layers, was clearly identified (Fig. 4A). The layer inside the inner integument was stained slightly with safranine 60 d after pollination; staining was also recognized clearly in some areas surrounded by the outer cell layer of the inner integument (Fig. 4B). The layer inside the inner integument stained red by safranine 70 d after pollination (Fig. 4C), and this staining was more dense 80 d after pollination (Fig. 4D). Embryo formation was almost completed 90 d after pollination; the layer inside the inner integument became thicker and stained more densely (Fig. 4E, I). At 100 d after pollination, the layer inside the inner integument became less intensely stained and that outside the inner integument was compressed into a thin, densely stained layer (Fig. 4F). These two layers were more clearly distinguishable 110 and 120 d after pollination. The layer inside the inner integument was almost colourless at this point. The gap between the inner integument and the embryo was visible even at mature stage (Fig. 4G, H, J).
Fig. 4.
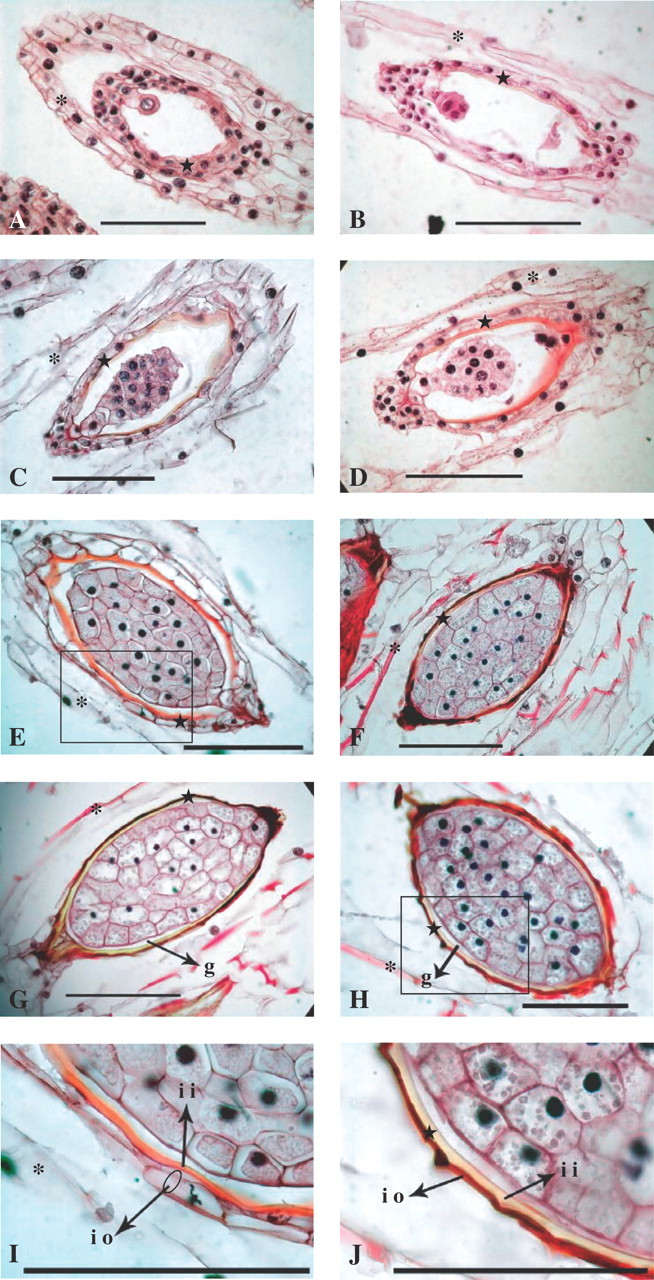
The successive developmental stages of embryo and inner integument (inner seed coat) during seed formation of Cephalanthera falcata: (A) 50 d after pollination, (B) 60 d after pollination, (C) 70 d after pollination, (D) 80 d after pollination, (E) 90 d after pollination, (F) 100 d after pollination, (G) 110 d after pollination, (H) 120 d after pollination. (I) Detail of E (inner integument 90 d after pollination). (J) Detail of H (inner integument 120 d after pollination). Scale bars = 100 μm. Star, inner integument; asterisks, outer integument. io, inner integument (a layer outside); ii, inner integument (a layer inside); g, the gap between embryo and inner integument.
DISCUSSION
Cephalanthera falcata does not seem to have any tendency for auto-pollination, which is found in 5–20 % of Orchidaceae species (Catling, 1990), given that flowers covered with bags without hand-pollination formed no capsules (data not shown). Only 7·5 % of flowers formed capsules without hand-pollination 2 weeks after initiation of the experiment (Table 2). Study of pollination has been intensively conducted in other Europe members of the genus Cephalanthera (van der Cingel, 1995). However, no previous reports have investigated the pollinator(s) in C. falcata.
In the family Orchidaceae, except for auto-pollinating species, successful pollination by insects is generally low as capsule formation is pollinator-limited. The pollinator of C. falcata was not identified, although it was noted that one-third of the flowers in inflorescence of a plant of this species growing near a beehive produced capsules without hand-pollination. Further observation will be required to clarify the role of honeybees as pollinators for the effective production of capsules in this species.
In the present study, hand-pollination resulted in capsule formation 2 weeks after pollination: the frequency was 100 % irrespective of bagging treatment. However, some young capsules were observed to drop 2–4 weeks after hand-pollination (data not shown). Given that Nagashima (1979) reported that fertilization occurs at around 38–40 d after pollination in C. falcata, the drop of these young capsules might result from a pre-fertilization event, such as inhibition of growth of the pollen tubes in the stigma. In addition, consumption of young capsules by insects during maturation reduces capsule production. Hasegawa et al. (1987) revealed in his preliminary study that 68 % of ovaries formed mature capsules by hand-pollination in combination with in situ bagging treatment of Cypripedium japonicum. However, Hasegawa et al. did not give the frequencies of capsule formation in early stages of post-pollination. Furthermore, the contribution of hand-pollination and bagging treatments on the production of capsule was not investigated.
In the present study, flies laid eggs in some capsules, which resulted in loss of the capsules. With bagging with aluminium foil, however, no gaps were observed and the frequency of surviving capsules was approx. 40 % higher than that bagging with parchment paper bags. The difference in survival frequencies between the two bagging treatments might be attributable to the difference in the degree of protection of capsules from infestation by egg-laying insects. The aluminium foil was more suitable as a material for bagging treatment than the parchment paper, although capsule decay was observed in approx. 20 % of the pollinated flowers using aluminium foil bags (data not shown). Increased seed productivity should be achieved by the use of durable transparent materials, which will enable ease of fruit set, that have good ventilation properties for in situ bagging of capsule to inhibit their decay but to prevent invasion of insects.
Cephalanthera falcata grows in the forest floor where sunlight is limited by the leaf canopy. The dispersed seeds may fall into the litter and impregnate soil surface layers. Table 5 shows that illumination during culture inhibited seed germination of this species. Inhibition of seed germination by illumination in C. falcata might indicate the mobility of seeds into the space below the soil surface in its natural habitat prior to germination.
Bidartondo et al. (2004) reported that European members of the genus Cephalanthera have a dual and simultaneous symbiotic relationship with endomycorrhiza and ectomycorrhiza. Symbiosis with ectomycorrhiza might serve an important role in the growth of Cephalanthera plants. The difficulties encountered with cultivation of C. falcata might be attributable to the lack of establishment of a symbiotic relationship between the orchid and an ectomycorrhizal fungus, which share a symbiotic relationship with trees. It was noted here that protocorms remained white after 300 d of culture, even under illumination. This would suggest a strong heterotrophic relationship between this species and mycorrhizal fungi in the juvenile phase. An efficient method for raising large numbers of protocorms asymbiotically has been developed here. These protocorms will be further utilized to examine changes in the mode of the symbiotic relationship among orchids, endomycorrhizal and ectomycorrhizal fungi, and trees in their life cycles.
Fully matured seeds of terrestrial orchid species from northern temperate regions are much more difficult to germinate in vitro than immature seeds (Arditti et al., 1982a; Ballard, 1987; Mitchell, 1989; Light and MacConaill, 1998). Although the reasons underlying the difficulty in seed germination of these terrestrial species have not been elucidated, two salient hypotheses regarding germination at seed maturity have been advanced: induction of dormancy through accumulation of inhibitory substances and through increasing embryo impermeability (Stoutamire, 1974; Kako, 1976; Linden, 1980; van der Kinderen, 1987; Miyoshi and Mii, 1988, 1995; DePauw and Remphrey, 1993; Rasmussen, 1995).
A triphasic pattern of germination was observed in this study. In phase I, germination frequency of seeds of C. falcata increased to the peak value of 39·8 % 70 d after pollination. In phase II, a decline in germination frequency was observed with seeds harvested 80–90 d after pollination. Little or no germination occurred in phase III, the period of 100–120 d after pollination. Pale red coloration of the inner integument (Fig. 4C) by staining with safranine of seeds harvested 70 d after pollination suggested that initiation of lignification occurred in this layer. Almost all seeds harvested 80–100 d after pollination were viable given that the staining frequency by TTC was 84–99 %. Therefore, seeds at this stage of development had already reached a dormant state.
In observations of seed development of orchidaceous plants, the inner integument has often been referred to as ‘carapace’ (Rasmussen, 1995; syn. carapax, Lucke, 1981; van der Kinderen, 1995; Yeung et al., 1996). It has a putative role in the control of dormancy, but information on this subject remains fragmented and obscure. In the present study, the ‘carapace’ was observed to comprise two layers. Increased coloration of the carapace by safranine suggests the accumulation of lignin. The layer inside the inner integument, which was stained by safranine 90 d after pollination, became less stained 100 d after pollination (Fig. 4E, F). Such discoloration of a layer inside the inner integument at this stage could be explained by cutinization. The layer outside the inner integument was compressed, and was considered to be fully lignified 100 d after pollination (Figs 4 F and J). Thus, the ‘carapace’ of seeds 100 d after pollination or later consists of two layers, one lignified and one cutinized, giving these seeds different characteristics from those 90 d after pollination or earlier. This ‘carapace’ may have an important role physically and/or chemically in the regulation of seed germination of this species. Cutinization and lignification could be postulated to strengthen the inner integument, and the ‘carapace’ could be postulated to inhibit embryo growth by mechanical restriction (Miyoshi and Sato, 1997) or via chemical reactions. Further experimentations will be needed to evaluate these possibilities.
Intact embryos of mature seeds, such as those harvested 140 d after pollination, were not stained by TTC solution. However, embryos from those seeds in which the ‘carapace’ was peeled by forceps and soaked in TTC showed an intense red coloration. A possible explanation is that the TTC solution cannot be absorbed through the ‘carapace’ of the embryos 100 d after pollination or later. In seeds younger than 100 d, the TTC solution may have impregnated to the embryos through minute openings, such as holes in the suspensors. When seeds are fully mature, those small openings could be plugged by accumulation of a secondary metabolites(s), possibly lignin or cutin.
In the present experiments, higher frequencies of germination were observed with browning of the medium. Mii (1976) reported that in anther cultures of tobacco, plantlet emergence paralleled anther browning, suggesting that browning might induce the development of plants from pollen. Further experiments are necessary to evaluate whether browning of the medium has a stimulatory effect on germination of C. falcata.
This study examined differences in the frequencies of seed germination and subsequent development on two basal media. Frequency of seeds at stage 0 (‘no germination stage’) was twice as high in ND medium (38·4 %) than in MK medium (16·8 %) (Table 4). However, seeds at Stages 4 and 5 were only observed in ND medium (1·7 % in total). This suggests that MK medium is more suitable for seed germination initiation and that ND medium might favour formation of protocorms with rhizoids (Stage 4) and shoots (Stage 5) from germinated seeds. Further examination should elucidate the components of the ND medium that control germination and formation of rhizoids and shoots in vitro.
Acknowledgments
We thank Prof. Katsumi Kataoka, Dr Hiroyuki Kohara and Dr Yushi Hida for their help with these experiments. We also thank Dr Tokiko Nagashima for suggestions regarding the paraffin sectioning method, and Tokushi Momiyama, Masakazu Onoduka and Susumu Tomono for retrieving plant materials and technical help. Finally, we thank the Moritani Foundation for financial support. This work was supported partly by a Grant-in-Aid for Scientific Research (B) (16310159) to K. Miyoshi from Japan Society for the Promotion of Science.
LITERATURE CITED
- Ackerman JD and Zimmerman J. (1994) Bottlenecks in the life histories of orchids: resources, pollination, population structure, and seedling establishment. In Pridgeon AM (Ed.). Proceedings of the 14th World Orchid Conference(HMSO, Edinburgh) pp. 125–129.
- Arditti J, Michaud JD, Oliva AP. (1982a) Practical germination of North American and related orchids: Epipactis atrorubens, E. gigantea and E. helleborine. American Orchid Society Bulletin 51162–171. [Google Scholar]
- Arditti J, Clements MA, Fast G, Hadley G, Nishimura G, Ernst R. (1982b) Orchid seed germination and seedling culture. A manual. In Arditti J (Ed.). Orchid biology. Reviews and perspectives, II(Cornell University Press, Ithaca, NY) pp. 243–370.
- Ballard WW. (1987) Sterile propagation of Cypripedium reginae from seeds. American Orchid Society Bulletin 56935–946. [Google Scholar]
- Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ. (2004) Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proceedings of the Royal Society of London, Series B 2711799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catling PM. (1990) Auto-pollination in the Orchidaceae. In Arditti J (Ed.). Orchid biology: reviews and perspectives(Timber Press, V. Portland, OR) pp. 121–158.
- van der Cingel NA. (1995) Systematic account. Genus Cephalanthera. An atlas of orchid pollination. European orchids(A. A. Balkema, Rotterdam) pp. 74–77.
- DePauw MA and Remphrey WR. (1993) In vitro germination of three Cypripedium species in relation to time of seed collection, media, and cold treatment. Canadian Journal of Botany 71879–885. [Google Scholar]
- Environment Agency of Japan. (2000) Vascular plants. Threatened wildlife of Japan—Red Data Book 2nd edn (Japan Wildlife Research Center, Tokyo) (in Japanese).
- Hasegawa A, Nakasugi M, Goi M. (1987) A seed harvesting method of Cypripedium japonicum Thunberg. Technical Bulletin of the Faculty of Agriculture Kagawa University 3863–70. [Google Scholar]
- Kako S. (1976) Study on the germination of seeds of Cymbidium goeringii. In Torigata H (Ed.). Seed formation and sterile culture of orchidsTokyo Seibundoshinkosha pp. 174–237 (in Japanese).
- Kano K. (1965) Studies on the media for orchid seed germination. Memoirs of the Faculty of Agriculture Kagawa University 201–79. [Google Scholar]
- van der Kinderen G. (1987) Absisic acid in terrestrial orchid seeds: a possible impact on their germination. Lindleyana 284–87. [Google Scholar]
- van der Kinderen G. (1995) Observation on in situ germination of Epipactis helleborine (L. ) Crantz. Lindleyana 10223–231. [Google Scholar]
- Lauzer D, St-Arnaud M, Barabé D. (1994) Tetrazolium staining and in vitro germination of mature seeds of Cypripedium acaule (Orchidaceae). Lindleyana 9197–204. [Google Scholar]
- Light MHS and MacConaill M. (1998) Factors affecting germinable seed yield in Cypripedium calceolus var. pubescens (Wild.) Correll and Epipactis helleborine (L.) Crantz (Orchidaceae). Botanical Journal of the Linnean Society 1263–26. [Google Scholar]
- Linden B. (1980) Aseptic germination of seeds of Northern Terrestrial orchids. Annales Botanici Fennici 17174–182. [Google Scholar]
- Lucke E. (1981) Samenstruktur und Samenkeimung europäischr Orchideen nach Veylet sowie weitere Untersuchungen (Teil 1). Die Orchidee 32182–188. [Google Scholar]
- Mii M. (1976) Relationships between anther browning and plantlet formation in anther culture of Nicotiana tabacum L. Zeitschrift fur Pflanzenphysiologie 80206–214. [Google Scholar]
- Mitchell RB. (1989) Growing hardy orchids from seeds at Kew. The Plantsman 11152–169. [Google Scholar]
- Miyoshi K and Mii M. (1981) Enhancement of seed germination of Calanthe izu-insularis. Journal of the Japanese Society for Horticultural Science 50(Suppl. 1), 332–333 (in Japanese). [Google Scholar]
- Miyoshi K and Mii M. (1988) Ultrasonic treatment for enhancing seed germination of terrestrial orchid, Calanthe discolor, in asymbiotic culture. Scientia Horticulturae 35127–130. [Google Scholar]
- Miyoshi K and Mii M. (1995) Enhancement of seed germination and protocorm formation in Calanthe discolor (Orchidaceae) by NaOCl and polyphenol absorbent treatments. Plant Tissue Culture Letters 12267–272. [Google Scholar]
- Miyoshi K and Mii M. (1998) Stimulatory effects of sodium and calcium hypochlorite, pre-chilling and cytokinins on the germination of Cypripedium macranthos seed in vitro. Physiologia Plantarum 102481–486. [Google Scholar]
- Miyoshi K and Sato T. (1997) Removal of the pericarp and testa of seeds of Japonica and Indica rice (Oryza sativa) at various oxygen concentrations has opposite effects on germination. Physiologia Plantarum 991–6. [Google Scholar]
- Nagashima T. (1979) Studies on the seed germination and embryogenesis in a few species of Orchidaceae. Bulletin of Keisen College of Horticulture 1277–111 (in Japanese). [Google Scholar]
- Neiland MRM and Wilcock CC. (1998) Fruit set, nectar reward, and rarity in the Orchidaceae. American Journal of Botany 851657–1671. [PubMed] [Google Scholar]
- Rasmussen HN. (1995) Terrestrial orchids from seed to mycotrophic plant(Cambridge University Press, New York).
- Shimasaki K and Umemoto S. (1990) Micropropagation of a terrestrial Cymbidium species using rhizomes developed from seeds and pseudobulbs. Plant Cell, Tissue Organ Culture 22237–244. [Google Scholar]
- Stoutamire W. (1974) Terrestrial orchid seedlings. In Withner CL (Ed.). The orchids. Scientific studies(Wiley and Sons, New York) pp. 101–128.
- Tokuhara K and Mii M. (1993) Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Reports 137–11. [DOI] [PubMed] [Google Scholar]
- Van Waes JM and Debergh PC. (1986) Adaptation of the tetrazolium method for testing the seed viability, and scanning electron microscopy study of some Western European orchids. Physiologia Plantarum 66435–442. [Google Scholar]
- Vujanovic V, St-Arnaud M, Barabe D, Thibeault G. (2000) Viability testing of orchid seed and the promotion of colouration and germination. Annals of Botany 8679–86. [Google Scholar]
- Withner CL. (1955) Ovule culture and growth of Vanilla seedlings. American Orchid Society Bulletin 51380–392. [Google Scholar]
- Yamazaki J, Momiyama T, Fukuda H. (2001) Seed harvesting method of Cephalanthera falcata Blume and Cremastra appendiculata Makino. Journal of the Japanese Society for Horticultural Science 70(Suppl. 2), 176 (in Japanese). [Google Scholar]
- Yeung E, Zee SY, Ye XL. (1996) Embryology of Cymbidium sinense: embryo development. Annals of Botany 78105–110. [Google Scholar]