Abstract
We report for the first time single wall carbon nanotubes (SWNTs)-based chemiresistive affinity sensors for highly sensitive and selective detection of small and/or weak-/un-charged molecules using displacement format. The detection of glucose, a small weakly charged molecule, by displacement of plant lectin, Concavalin A, bound to polysaccharide, dextran, immobilized on SWNTs, with picomolar sensitivity and selectivity over other sugars and human serum proteins is demonstrated as a proof-of-concept.
In recent years, there has been an increasing interest on use of one-dimensional (1-D) nanostructures, such as nanowires, nanobelts and nanotubes, as transducer elements in affinity (bio)sensors. Use of nanomaterials provide high sensitivity with low limit of detection and in conjunction with molecular recognition element, high selectivity, for label-free, rapid and low-cost, multiplexed and point-of-care/field detection of various analytes. Single-walled carbon nanotubes (SWNTs) are one such class of nanomaterials that have been used extensively as sensing elements due to their excellent electrical properties, ultra-high surface area to volume ratio and surface atoms which are extremely sensitive to any surface adsorption/reaction events. SWNTs modified with biorecognition molecules such as antibodies, aptamers or DNA have been successfully used to detect various targets ranging from proteins,1 viruses,2 bacteria, 3 yeast,4 DNA/RNA5 and even mammalian cancer cells.6 Majority of these SWNT-based biosensors are affinity sensors wherein the binding of the analyte, generally a large charged antigen, to the bioreceptor immobilized on the surface of SWNTs leads to change in conductance of SWNTs channel.
Small, charged or uncharged, molecules constitute a large group of analytes of interest in the fields of environmental monitoring and health care. The detection of these analytes using SWNTs-based chemiresistive/field-effect transistor sensors using the traditional modes of affinity-based sensing might be ineffective as their binding to the recognition molecule might not generate measurable change in conductance/resistance. Nanobiosensors that can detect and quantify such small molecules with high sensitivity and selectivity are therefore in urgent need. In an effort to achieve these objectives, we have for the first time employed the displacement immuno-assay/sensor format7 on a SWNTs-based chemiresistive platform and demonstrated its effectiveness. In the displacement mode of operation, the SWNTs are initially functionalized with an analog of the target analyte having lower binding affinity to the biological recognition molecule than the actual analyte followed by binding to the biological recognition element such as antibody. Upon addition of the sample analyte to the device it competes with the analog for the bioreceptor and displaces it off the SWNTs channel leading to a change in the sensor conductance.
Figure 1 shows the schematic of the displacement-based chemiresistive affinity (bio)sensor. The principle behind the displacement mode based sensors is similar to the competitive mode of immunoassay. The well-characterized glucose-Concavalin A-dextran system8 was evaluated as a model system to demonstrate displacement based chemiresistive mode of sensing. Concanvalin A (ConA), a plant lectin that binds non-covalently to some carbohydrates, is a metalloprotein with four carbohydrate binding pockets and can exist as a dimeric form at low pH or tetrameric form at neutral pH9. Polysaccharide dextran and monosaccharide glucose are among the carbohydrates that reversibly bind to ConA, with glucose displaying higher binding affinity which means that upon introduction of glucose to ConA-dextran complex, glucose displaces dextran from ConA (Figure 1). The binding of the ConA to carbohydrates results in changes to its conformation and alters its isoelectric point to far from neutral pH leading to accumulation of positive charge.10 On the other hand, both glucose and dextran are electrically neutral over a wide pH range in free form as well as in the bound state to ConA. Thus, using this system in the chemiresistive configuration it's the binding and removal of ConA from the SWNTs that will result in conductance change due to its positive charge.
Figure 1.
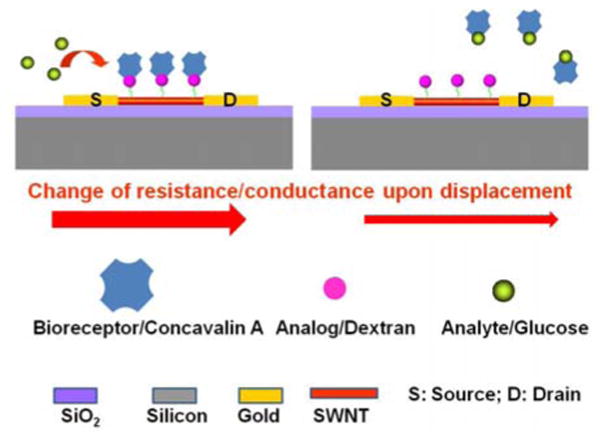
Schematic of displacement-based chemiresistive biosensor.
The process starts with preparation of AC dielectrophoretically aligned SWNT across a pair of 3 μm spaced microfabricated gold electrodes. In brief, the procedure involved addition of 0.1 μl drop of SWNTs suspended in dimethyl formamide and applying AC voltage at a frequency of 4 MHz (amplitude 0.3 V peak to peak) across the electrodes. The aligned SWNTs were then annealed in place by heating at 300°C for an hour under inert environment maintained by continuously flowing nitrogen gas containing 5% hydrogen. This was followed by modification with dextran by overnight incubation at room temperature with 1 wt % phenoxy dextran (DexP) in water, incubation with 0.1% Tween20 to block any naked/bare sites on SWNTs to prevent any non-specific adsorption and finally with 14 μM ConA solution prepared in 10 mM phosphate buffer supplemented with 0.5 mM CaCl2 and 0.1 mM MnCl2 (PB) for 2 h at room temperature. ConA being a metaloprotein requires transition metal ions, manganese and calcium, for its binding 11. Because dextran cannot bind to the SWNTs by itself, hydrophobic dextran derivative, phenoxy dextran (DexP), was synthesized (Please see Supplementary Information) to noncovalently modify the SWNTs 12. The fabrication and sensing processes were monitored by recording the current-voltage (I-V) characteristics between +1V to -1V of the device after each step using a semiconductor parameter analyzer. As shown in Figure 2, the current at the same corresponding voltage of the SWNT device decreased upon incubation with both dextran and ConA. The first decrease is a consequence of the modification of SWNTs with the DexP molecules by means of π-π stacking interactions between SWNTs and phenoxy groups and the later is attributed to accumulation of the positive charge on the ConA molecules and/or a scattering potential effect resulting from its binding to dextran.13
Figure 2.
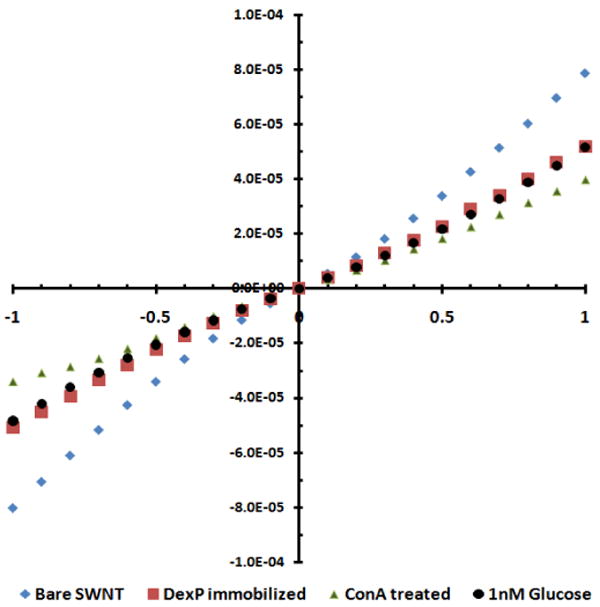
I-V characteristics of the biosensor at various stages of fabrication and upon addition of glucose.
To examine the displacement principle of detection and functionality of the biosensor for glucose, the biosensor was incubated with 1 nM glucose in PB for 1 min, washed three times with PB followed by once with deionized water and the I-V was recorded. As shown in Figure 2, the conductance of the device reverted to the original value of dextran-modified SWNTs, confirming the displacement sensing modality for the weakly charged and small size glucose. Specificity of the biosensor was also evaluated by measuring the response of DexP and Tween20 blocked SWNTs without conjugated ConA for glucose, Mg2+, and Ca2+. As shown in Figure 3, the sensors conductance remained unaffected when incubated with 1 nM of glucose, 0.1 mM MnCl2 and 0.5 mM CaCl2 in 10 mM phosphate buffer, confirming the sensor response a result of highly specific competition between dextran and glucose for ConA binding sites. Figure 4A shows normalized response [(R – R0)/R0, where R is the resistance after exposure to glucose and R0 is resistance after exposure to buffer determined from the inverse of the slope of the I-V curve from +0.1 V to -0.1 V] of the biosensor as a function of analyte concentration in buffer. The response was linear in the range of 1 pM to 1 nM and 0.039 per pM glucose (slope of the calibration plot) sensitivity. This lower detection limit is superior than the 50 nM and 3.7 mM reported for ConA-cyclodextrin/dextran solution based assays using fluorescence resonance energy transfer between CdTe quantum dot and gold nanoparticle14 and infra red absorbance of carbon nanotubes deaggregation12, respectively, and is attributed to the high sensitivity of chemiresistive/FET mode of transduction.
Figure 3.
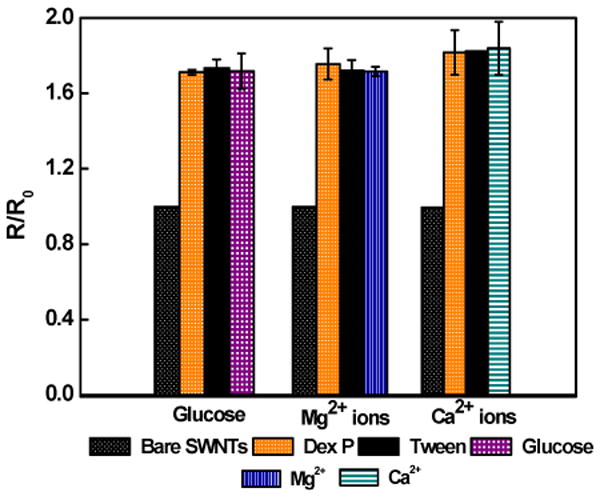
Response of SWNTs modified with DexP and Tween20 minus the ConA to 1 nM glucose, 0.1 mM MnCl2 and 0.5 mM CaCl2 prepared in 10 mM phosphate buffer.
Figure 4.
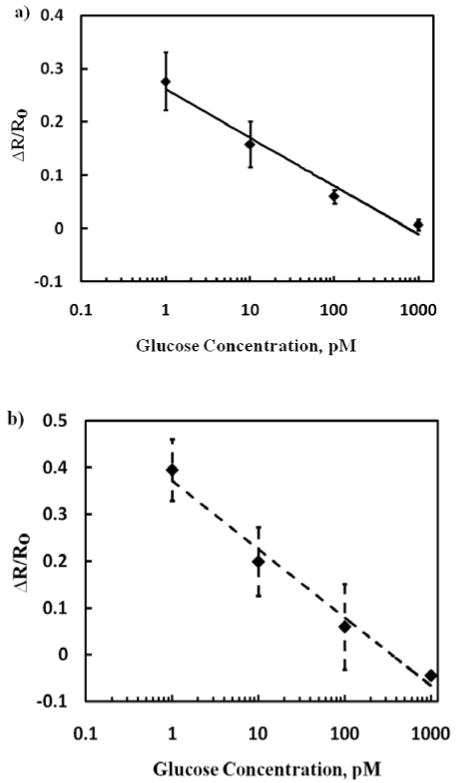
Biosensor calibration for glucose in 10mM phosphate buffer (a) and in human plasma (b). Data points are average of four independent sensors (for each investigation) prepared at different times and error bars represent ±1 standard deviation. Regression coefficient for the buffer sample is 0.97 and for spiked plasma sample is 0.98.
The biosensor selectivity was evaluated against sugars, sucrose and galactose. As illustrated in Figure 5, a two-fold higher concentration (2 nM) of sucrose, a disaccharide of glucose and fructose, was required to return the device resistance to the original value. On the other hand, there was no decrease in the device resistance when incubated with 1 nM monosaccharide galactose. However, when the same sensor was treated with 1 nM glucose, ConA was completely displaced from the sensor surface, confirming the sensor functionality. These selectivity results are in agreement with the literature 8 that ConA has lower affinity to sucrose in comparison with glucose and no affinity for galactose. Mechanism of operation of sensor although well proven, these sugar specificity tests further augment this fact. Furthermore, a nearly identical sensitivity when analyzing glucose spiked in human plasma (Figure 4B) demonstrated no interference from plasma components, i.e. no matrix effect, and potential application of the sensor for blood glucose measurement.
Figure 5.
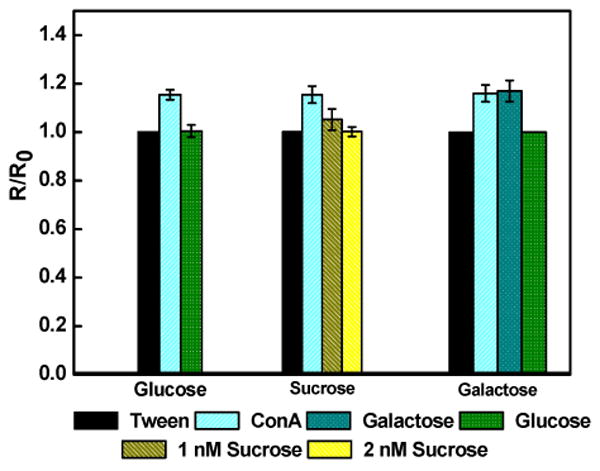
Biosensor selectivity for various sugars (1 nM in 10mM phosphate buffer). Data points are average of 3 independent sensors prepared at different times and error bars represent ±1 standard deviation
To conclude, we have built a SWNTs-based sensor displaying novelty in two aspects: first, the adaptation of the displacement mode of biosensing with chemiresistive sensor for detection of small molecules which otherwise are difficult to detect by chemiresistive/FET transduction principle and second, an enzyme-free chemiresistive glucose sensor with sensitivity in the picomolar range and exquisite selectivity. The ability to detect such low glucose concentration for the reported sensor would find potential applications in monitoring glucose in unconventional body fluids such as interstitial fluid extracted by iontophoresis, tears, saliva and urine and intracellular concentration at single cell level in metabolomic studies.15 While the displacement detection principle was demonstrated for glucose, it can also be applied to other weakly or uncharged molecules. Furthermore, the biosensor sensitivity can be amplified by augmenting the charge of the displacing moiety.
Supplementary Material
Acknowledgments
We acknowledge the support of grants GR-83237501 from the U.S. Environmental Protection Agency, CBET-0617240 from National Science Foundation and U01ES016026 from National Institutes of Health.
Footnotes
Supporting Information Available: Phenoxy dextran synthesis, SWNT suspension preparation and instrumentation details. This material is available free of charge via Internet at http://pubs.acs.org.
References
- 1.(a) Wang CW, Pan CY, Wu HC, Shih PY, Tsai CC, Liao KT, Lu LL, Hsieh WH, Chen CD, Chen YT. Small. 2007;3:1350–1355. doi: 10.1002/smll.200600723. [DOI] [PubMed] [Google Scholar]; (b) Chen RJ, Bangsaruntip S, Drouvalakis KA, Kam NWS, Shim M, Li YM, Kim W, Utz PJ, Dai HJ. Proc Natl Acad Sci U S A. 2003;100:4984–4989. doi: 10.1073/pnas.0837064100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) So HM, Won K, Kim YH, Kim BK, Ryu BH, Na PS, Kim H, Lee JO. J Am Chem Soc. 2005;127:11906–11907. doi: 10.1021/ja053094r. [DOI] [PubMed] [Google Scholar]; (d) Chen RJ, Choi HC, Bangsaruntip S, Yenilmez E, Tang XW, Wang Q, Chang YL, Dai HJ. J Am Chem Soc. 2004;126:1563–1568. doi: 10.1021/ja038702m. [DOI] [PubMed] [Google Scholar]; (e) Park DW, Kim YH, Kim BS, So HM, Won K, Lee JO, Kong KJ, Chang H. J Nanosci Nanotechnol. 2006;6:3499–3502. [PubMed] [Google Scholar]; (f) Cella LN, Sanchez P, Zhong W, Myung NV, Chen W, Mulchandani A. Anal Chem. 2010;82:2042–2047. doi: 10.1021/ac902791q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh J, Yoo S, Chang YW, Lim K, Yoo KH. Curr Appl Phys. 2009;9:E229–E331. [Google Scholar]
- 3.(a) So HM, Park DW, Jeon EK, Kim YH, Kim BS, Lee CK, Choi SY, Kim SC, Chang H, Lee JO. Small. 2008;4:197–201. doi: 10.1002/smll.200700664. [DOI] [PubMed] [Google Scholar]; (b) Villamizar RA, Maroto A, Rius FX, Inza I, Figueras MJ. Biosens Bioelectron. 2008;24:279–283. doi: 10.1016/j.bios.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Villamizar RA, Maroto A, Rius FX. Sens Actuators, B. 2009;136:451–457. [Google Scholar]
- 5.(a) Dastagir T, Forzani ES, Zhang R, AMlani I, Nagahara LA, Tsui R, Tao NJ. Analyst. 2007;132:738–740. doi: 10.1039/b707025j. [DOI] [PubMed] [Google Scholar]; (b) Tang X, Bansaruntip S, Nakayama N, Yenilmez E, Chang YI, Wang Q. Nano Lett. 2006;6:1632–1636. doi: 10.1021/nl060613v. [DOI] [PubMed] [Google Scholar]; (c) Dong X, Lau CM, Lohani A, Mhaisalkar SG, Kasim J, Shen Z, Ho X, Rogers JA, Li LJ. Adv Mater. 2008;20:2389–2393. [Google Scholar]
- 6.Shao N, Wickstrom E, Panchapakesan B. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/46/465101. Art. 465101. [DOI] [PubMed] [Google Scholar]
- 7.(a) Sheikh SH, Mulchandani A. Biosens Bioelectron. 2001;16:647–652. doi: 10.1016/s0956-5663(01)00193-2. [DOI] [PubMed] [Google Scholar]; (b) Narang U, Gauger PR, Ligler FS. Anal Chem. 1997;69:2779–2785. [Google Scholar]; (c) Vianello F, Signor L, Pizzariello A, Di Paolo ML, Scarpa M, Hock B, Giersch T, Rigo A. Biosens Bioelectron. 1998;13:45–53. doi: 10.1016/s0956-5663(97)00079-1. [DOI] [PubMed] [Google Scholar]; (d) Kronkvist K, Lövgren U, Svenson J, Edholm LE, Johansson G. J Immunol Methods. 1997;200:145–153. doi: 10.1016/s0022-1759(96)00199-8. [DOI] [PubMed] [Google Scholar]; (e) Kaptein WA, Zwaagstra JJ, Venema K, Ruiters MHJ, Korf J. Sens Actuators, B. 1997;45:63–69. [Google Scholar]; (f) Wemhoff GA, Rabbany SY, Kusterbeck AW, Ogert RA, Bredehorst R, Ligler FSJ. Immunol Methods. 1992;156:223–230. doi: 10.1016/0022-1759(92)90029-s. [DOI] [PubMed] [Google Scholar]
- 8.(a) Schultz JS. U.S. Patent 4 344 438 (A) 1982; (b) Mansouri S, Schultz JS. Bio-Technol. 1984;2:885–890. [Google Scholar]; (c) Russell RJ, Pishko MV, Gefrides CC, McShane MJ, Cote GL. Anal Chem. 1999;71:3126–3132. doi: 10.1021/ac990060r. [DOI] [PubMed] [Google Scholar]; (d) Ballerstadt R, Evans C, McNichols R, Gowda A. Biosens Bioelectron. 2006;22:275–284. doi: 10.1016/j.bios.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 9.So LL, Goldstein IJ. Biochim Biophys Acta. 1968;165:398–404. doi: 10.1016/0304-4165(68)90218-3. [DOI] [PubMed] [Google Scholar]
- 10.Gordon SH, Irwin JG. Eur J Biochem. 1970;16:549–556. [Google Scholar]
- 11.Summer JB, Howell SF. J Biol Chem. 1936;115:582. [Google Scholar]
- 12.Barone PW, Strano MS. Angew Chem, Int Ed. 2006;45:8138–8141. doi: 10.1002/anie.200603138. [DOI] [PubMed] [Google Scholar]
- 13.(a) Gruner G. Anal Bioanal Chem. 2006;384:322–335. doi: 10.1007/s00216-005-3400-4. [DOI] [PubMed] [Google Scholar]; (b) Star A, Gabriel JCP, Bradley K, Gruner G. Nano Lett. 2003;3:459–463. [Google Scholar]; (c) Heller I, Janssens AM, Mannik J, Minnot ED, Lemay SG, Dekker C. Nano Lett. 2008;8:591–595. doi: 10.1021/nl072996i. [DOI] [PubMed] [Google Scholar]
- 14.Tang B, Cao L, Xu K, Zhuo L, Ge J, Li Q, Yu L. Chem Eur J. 2008;14:3637–3644. doi: 10.1002/chem.200701871. [DOI] [PubMed] [Google Scholar]
- 15.(a) Lee MC, Kabilan S, Hussain A, Yang X, Blyth J, Lowe CR. Anal Chem. 2004;76:5748–5755. doi: 10.1021/ac049334n. [DOI] [PubMed] [Google Scholar]; (b) Ge XD, Tolosa L, Rao G. Anal Chem. 2004;76:1403–1410. doi: 10.1021/ac035063p. [DOI] [PubMed] [Google Scholar]; (c) Yamaguchi M, Mitsumori M, Kano Y. IEEE Eng Med Biol Mag. 1998;17:59–63. doi: 10.1109/51.677170. [DOI] [PubMed] [Google Scholar]; (d) Miyashita M, Ito N, Ikeda S, Murayama T, Oguma K, Kimura J. Biosens Bioelectron. 2009;24:1336–1340. doi: 10.1016/j.bios.2008.07.072. [DOI] [PubMed] [Google Scholar]; (e) O'Connell TM, Ardeshirpour F, Asher SA, Winnike JH, Yin X, George J, Guttridge DC, He W, Wysong A, Willis MS, Couch ME. Metabolomics. 2008;4:216–225. [Google Scholar]; (f) Fehr M, Takanaga H, Ehrhardt DW, Frommer WB. Mol Cell Biol. 2005;25:11102–11112. doi: 10.1128/MCB.25.24.11102-11112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


