Abstract
This review discusses the potential of ultraviolet C (UVC) irradiation as an alternative approach to current methods used to treat localized infections. It has been reported that multidrug-resistant microorganisms are equally sensitive to UVC irradiation as their wild-type counterparts. With appropriate doses, UVC may selectively inactivate microorganisms while preserving viability of mammalian cells and, moreover, is reported to promote wound healing. UVC is also found in animal studies to be less damaging to tissue than UVB. Even though UVC may produce DNA damage in mammalian cells, it can be rapidly repaired by DNA repair enzymes. If UVC irradiation is repeated excessively, resistance of microorganisms to UVC inactivation may develop. In summary, UVC should be investigated as an alternative approach to current methods used to treat localized infections, especially those caused by multidrug-resistant microorganisms. UVC should be used in a manner such that the side effects would be minimized and resistance of microorganisms to UVC would be avoided.
Keywords: antibiotic resistance, bacteria, DNA repair, fungi, localized infections, mammalian cells, resistance to UVC, ultraviolet C irradiation, wound healing, wound infection
The prevalence of infectious diseases caused by multidrug-resistant microorganisms is relentlessly increasing worldwide owing to the excessive use of antibiotics [1]. Recently, a dangerous new mutation (named NDM-1) [2] that makes some bacteria resistant to almost all antibiotics has been found in the USA in patients with urinary tract infections [3]. Antibiotic resistance has led to a major research effort to find alternative antimicrobial approaches to which, it is hypothesized, microorganisms will not be easily able to develop resistance.
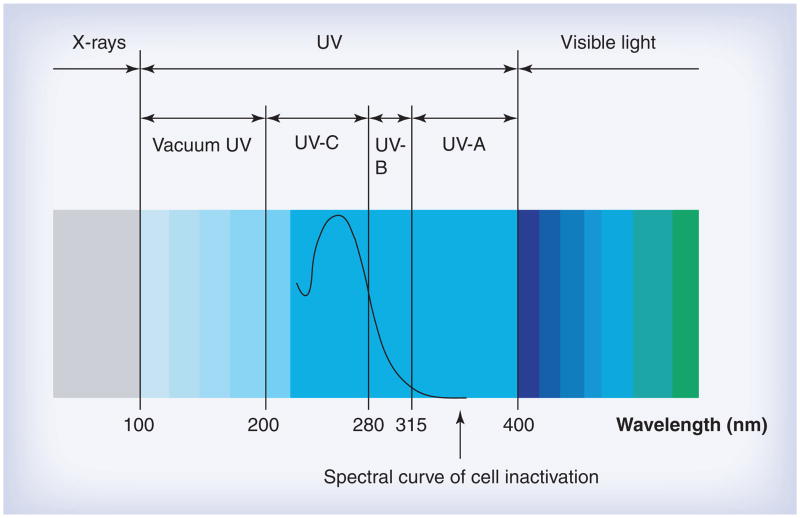
Ultraviolet (UV) irradiation is electromagnetic irradiation with a wavelength (100–400 nm) shorter than that of visible light (400–700 nm), but longer than x-rays (<100 nm) (Figure 1). UV irradiation is divided into four distinct spectral areas including vacuum UV (100–200 nm), UVC (200–280 nm), UVB (280–315 nm) and UVA (315–400 nm) (Figure 1) [4]. The mechanism of UVC inactivation of microorganisms is to damage the genetic material in the nucleus of the cell or nucleic acids in the virus [5]. The UVC spectrum, especially the range of 250–270 nm, is strongly absorbed by the nucleic acids of a microorganism and, therefore, is the most lethal range of wavelengths for microorganisms. This range, with 262 nm being the peak germicidal wavelength, is known as the germicidal spectrum [6]. The light-induced damage to the DNA and RNA of a microorganism often results from the dimerization of pyrimidine molecules. In particular, thymine (which is only found only in DNA) produces cyclobutane dimers. When thymine molecules are dimerized, it becomes very difficult for the nucleic acids to replicate and if replication does occur it often produces a defect that prevents the microorganism from being viable.
Figure 1. Spectrum of ultraviolet light.
UV: Ultraviolet.
Although it has been known for the last 100 years that UVC irradiation is highly germicidal, the use of UVC irradiation for prevention and treatment of localized infections is still in the very early stages of development. Most of the studies are confined to in vitro and ex vivo levels, while in vivo animal studies and clinical studies are much rarer. Studies that have examined UVC inactivation of antibiotic-resistant bacteria have found them to be as equally susceptible as their naive counterparts [7]. Within the UVC range, 254 nm is easily produced from a mercury low-pressure vapor lamp and has been shown to be close to the optimal wavelength for germicidal action. Because the delivery of UVC irradiation to living tissue is a localized process, UVC for infectious diseases is likely to be applied exclusively to localized infections.
In this review, we will discuss the potential of UVC irradiation as an alternative approach to localized infections. The topics include the efficacy of UVC for localized infections, effects of UVC on wound healing, effects of UVC on mammalian tissue and cells, and the possibility of the resistance development of microorganisms to UVC. To the best of our knowledge, this is the first comprehensive review on UVC for localized infections.
UVC irradiation for infections
The mechanism of UVC inactivation of microorganisms is to cause cellular damage by inducing changes in the chemical structure of DNA chains [5]. The consequence is the production of cyclobutane pyrimidine dimers (CPDs) causing distortion of the DNA molecule, which might cause malfunctions in cell replication and lead to cell death.
In vitro/ex vivo studies
An ex vivo study was carried out by Taylor et al. to investigate the use of UVC irradiation (254 nm) for the prophylaxis of surgical site infections [8]. The authors modeled a ‘clean’ surgical wound lightly contaminated with airborne bacteria by using agar, ovine muscle and ovine adipose tissue, respectively. It was found that airborne bacteria were inhibited more rapidly and more completely on agar than on muscle. A coating of blood over the microorganisms on muscle substantially reduced the effectiveness of UVC. At an irradiance of 1.2 mW/cm2 calculated at the lamp aperture, 1 min UVC irradiation time reduced bacterial colony forming units (CFUs) by 99.1% on agar, 97.1% on muscle (p = 0.046) and 53.5% on muscle coated with blood (p < 0.001). The combination of pulsed jet lavage and UVC was tested with the intention to remove the blood coated over the bacteria prior to UVC irradiation. The bacterial CFUs were reduced by 97.7% with the combination of pulsed jet lavage and UVC.
Conner-Kerr et al. examined the effectiveness of UVC irradiation at 254 nm in inactivating antibiotic-resistant strains of Staphylococcus aureus and Enterococcus faecalis in vitro [7]. Bacterial suspensions at 108 CFU/ml were prepared and plated on agar medium and then exposed to UVC irradiation. The calculated irradiance at the device aperture was 15.54 mW/cm2 and distance between UVC lamp and agar medium was 25.4 mm. For the methicillin-resistant strain of S. aureus (MRSA), inactivation rates were 99.9% at 5 s and 100% at 90 s. For vancomycin-resistant E. faecalis (VRE), inactivation rates were 99.9% at 5 s and 100% at 45 s. These findings suggest that UVC at 254 nm is bactericidal for antibiotic-resistant strains of S. aureus and E. faecalis at times as short as 5 s.
In a similar study, Rao et al. reported a complete (100%) eradication of the microorganisms on agar at the UVC doses ranging from a minimum of 5 s irradiation (methicillin-resistant, coagulase-negative Staphylococcus and Streptococcus pyogenes) to a maximum of 15 s irradiation (methicillin-susceptible S. aureus and Enterococci species) [9]. The irradiance used was 5 mW/cm2 calculated at the lamp aperture and lamp-agar distance was 10 cm.
By using a prototype solid-state UVC light-emitting diode device at 265 nm, Dean et al. evaluated the efficacy of UVC for treating corneal bacterial infections in vitro [10]. Agar plate lawns of S. aureus, Escherichia coli, Pseudomonas aeruginosa and S. pyogenes were exposed to UVC irradiation at an irradiance of 1.93 mW/cm2 (calculated at the target surface) for varying length of time. The study demonstrated that a 1-s exposure to UVC (1.93 mJ/cm2) was sufficient to induce 100% inhibition of growth for all the bacterial species tested. In this study, human corneal epithelial cells cultured on glass cover slips were also exposed to corresponding doses of UVC from the same device.
An idea of using UVC irradiation for disinfection of catheter biofilms was reported by Bak et al. [11]. In this study, the investigators determined the dose requirement for UVC disinfection of catheter biofilms. Contaminated urinary catheters from patients (n = 67) were used as test samples. The microorganisms identified from the catheter biofilms included E. coli (n = 32), coagulase-negative Staphylococcus (n = 22), E. faecalis (n = 13), Streptococcus (n = 13), P. aeruginosa (n = 12), Coryneforms (n = 7) and so on. Mean killing rates of the bacteria in catheter biofilms were 89.6 (11.8 mJ/cm2), 98 (47 mJ/cm2) and 99% (1400 mJ/cm2). The UVC exposures were calculated at the target surface.
Mohr et al. described the use of UVC for pathogen reduction of platelet concentrates [12]. The application of strong agitation of loosely fixed platelet concentrate bags resolved one of the problems related to the use of UVC for pathogen inactivation: namely, the quenching of the irradiation in protein-containing and turbid solutions or cell suspensions. Agitation allowed the penetration of UVC irradiation for inactivation of six bacterial species, including Gram-positive Bacillus cereus, S. aureus and Staphylococcus epidermidis, and Gram-negative E. coli, Klebsiella pneumoniae, P. aeruginosa. All bacteria species tested were reduced by more than 4 log10 at 400 mJ/cm2 (calculated at the surface of quartz plate where platelet concentrate samples were placed). The study also proved that platelet damage by UVC irradiation was limited under the conditions used. The in vitro functions and the other variables measured were only moderately influenced, and the storage stability of the of platelet concentrates was not impaired. Glucose consumption was slightly enhanced and lactate accumulation was slightly increased in comparison to the untreated control samples. By contrast, irradiation of platelet concentrates with UVC irradiation leads to more enhanced platelet metabolism, as evidenced by lactate accumulation and a stronger decrease in pH during storage.
Sullivan and Conner-Kerr compared the inactivation efficacies of UVC on pathogenic bacteria and fungi, in both single suspensions and mixed suspensions in vitro [13]. The calculated irradiance at the device aperture was 15.54 mW/cm2, with the distance between the UVC lamp and suspension surface 25.4 mm. Upon exposure to UVC, a 99.9% inactivation rate was obtained at 3–5 s for the bacteria (P. aeruginosa and Mycobacterium abscessus) tested. By contrast, 15–30 s of UVC treatment was required to obtain 99.9% inactivation of the fungi (Candida albicans, Aspergillus fumigatus) tested.
Dai et al. tested the ability of UVC to inactivate dermatophyte suspensions in vitro and to sterilize an ex vivo model of nail infection [14]. Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum and Microsporum canis suspensions were irradiated with UVC (254 nm) at a dose of 120 mJ/cm2 (calculated at the suspension surface) and surviving fungal cells quantified. T. rubrum infecting porcine hoof slices and human toenail clippings was irradiated with UVC at the doses of 36–864 J/cm2. In vitro studies showed that 3–5 log10 of cell inactivation in dermatophyte suspensions were produced with 120 mJ/cm2 UVC irradiation. Depending on factors such as the thickness and infectious burden of the ex vivo cultures, the radiant exposure of UVC needed for complete sterilization was usually in the order of tens to hundreds of J/cm2.
Animal studies
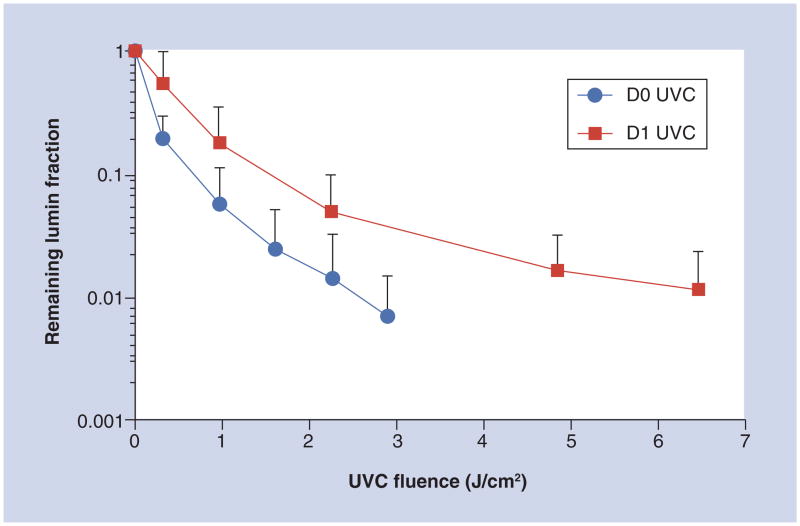
There has been, rather surprisingly, only one reported animal study on the use of UVC irradiation to treating infections. Dai et al. investigated the use of UVC irradiation (254 nm) for treatment of C. albicans infection in mouse third-degree burns [15]. The C. albicans strain was stably transformed with a version of the Gaussia princeps luciferase gene that allowed real-time bioluminescence imaging of the progression of C. albicans infection. UVC treatment with a single exposure carried out on day 0 (30 min postinfection) gave an average 2.16-log10 (99.2%) loss of fungal luminescence when 2.92 J/cm2 UVC had been delivered, while UVC at 24 h postinfection gave a 1.94-log10 (95.8%) reduction of fungal luminescence after 6.48 J/cm2 (Figure 2). The UVC exposures were calculated at the surfaces of mouse burns. Statistical analysis demonstrated that UVC treatment carried out on both day 0 and day 1 significantly reduced the fungal burden of infected burns by 99.2% (p = 0.003) and 97.7% (p = 0.004), respectively. UVC was found to be superior to a topical antifungal drug, nystatin cream (p = 0.028).
Figure 2. Dose–responses of mean fungal luminescence of the mouse burns infected with Candida albicans and treated by use of a single ultraviolet C exposure on day 0 (30 min; n = 11) and day 1 (24 h; n = 12) postinfection, respectively.
The data are displayed as mean plus SD.
UVC: Ultraviolet; D0: Day 0; D1: Day 1.
Reprinted with permission from [15].
Clinical studies
The first clinical study was reported by Taylor et al. who used UVC irradiation (254 nm) for the disinfection of surgical wounds during total joint arthroplasty procedures [16]. UVC irradiation commenced 10 min after the operation, allowing the wound to be exposed to a conventional open-air environment. Two different UVC irradiances, 0.1 and 0.3 mW/cm2 (calculated at the lamp aperture), were used. Bacteria in wounds were measured by imprinting with 47-mm diameter 5-μm mixed cellulose acetate and nitrate membrane filters. After 10 min of UVC irradiation, the average bacterial CFU in wounds was reduced by 87% with 0.1 mW/cm2 (n = 18; p < 0.001) and 92% with 0.3 mW/cm2 (n = 13; p < 0.001), compared with that with conventional environment (n = 13).
Shimomura et al. examined the efficacies of UVC irradiation (254 nm) on the prevention of catheter exit-site infections [17]. First, bacterial cultures of swabbed fluid from the catheter exit site were obtained from 68 continuous ambulatory peritoneal dialysis outpatients six times during the 24-month observation period. Second, the bactericidal effects of UVC irradiation on the catheter exit-site were examined. The authors found that:
In spite of disinfection of the catheter exit site by the strict application of povidone-iodine once or twice a day, 23–45% of the cases were found to be microorganism positive;
In the nasal cavity, S. aureus was detected in 20–25% of patients. There was a high incidence of exit-site infection among the S. aureus nasal carriers;
UVC irradiation was performed (twice per day, 30–60 s each time) in 18 cases that constantly revealed bacteria on culture at the catheter exit site. Ten cases (55%) became culture negative, three cases showed a microbial decrease and five cases remained unchanged. These results suggest that UVC can eliminate bacteria and can be of prophylactic use for exit-site infections.
Thai et al. investigated the use of UVC for the treatment of cutaneous ulcer infections [18]. In this study, three patients with chronic ulcers infected with MRSA were treated with UVC at 254 nm. UVC irradiation was applied to each wound for 180 s, with the irradiance of 15.54 mW/cm2 (calculated at the UVC device aperture) and wound-lamp distance of 25.4 mm. In all three patients, UVC treatment reduced the relative amount of bacteria in wounds and facilitated wound healing. Two patients had complete wound closure following 1 week of UVC treatment. In a later study performed by the same group, 22 patients with chronic ulcers exhibiting at least two signs of infection and critically colonized with bacteria received a single 180-s treatment of UVC. Semi-quantitative swabs taken immediately before and after UVC treatment were used to assess changes in the bacterial bioburden present within the wound bed [19]. A statistically significant (p < 0.0001) reduction in the relative amount of bacteria following a single treatment of UVC was observed. The greatest reduction in semi-quantitative swab scores following UVC treatment were observed for wounds colonized with P. aeruginosa and wounds colonized with only one species of bacteria. Significant (p < 0.05) reductions in the relative amount of bacteria were also observed in 12 ulcers in which MRSA was present.
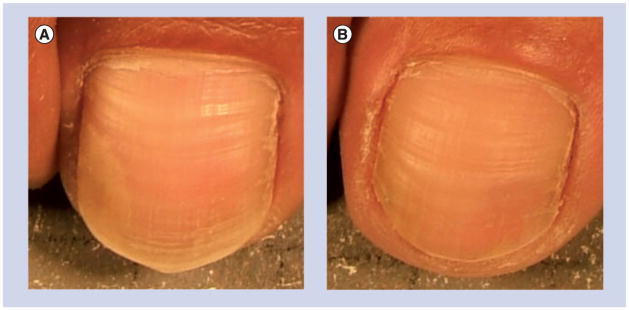
A study using UVC to treat toenail onychomycosis was reported by Boker et al.[20]. Thirty patients with mild-to-moderate onychomycosis involving no more than 35% of the great toenail were equally randomized to receive 4 weekly UVC treatments with either a low-pressure mercury lamp delivering a total UVC dose of 22 J/cm2 at the surfaces of the treated toenails or via a xenon pulsed-light device delivering a total UVC dose of 2–4 J/cm2 at the surface of the treated toenails. The investigator’s global assessment (IGA) scale was used to assess treatment efficacy. In total, 60% of patients treated with the xenon pulsed-light device showed an improvement of at least 1 point on their week-16 IGA scale compared with baseline (p < 0.01). An image depicting the result of a patient in the xenon-pulsed light group is presented in Figure 3. Of patients treated with the low-pressure mercury lamp, 26% had at least a 1-point improvement in their week-16 IGA score (p < 0.01). The treatments with both devices were well tolerated. Minor and uncommon side effects included temporary mild erythema of the irradiated toe.
Figure 3. Ultraviolet C treatment of toenail onychomycosis.
(A) Before ultraviolet C treatment. (B) 28 weeks after ultraviolet C treatment.
In summary, in vitro studies have reported that multidrug-resistant pathogenic microorganisms are highly sensitive to UVC inactivation. Generally, bacterial cells are found to be more sensitive to UVC than fungal cells.
The UVC doses required to inactivate a therapeutically sufficient fraction of microorganisms in vivo (e.g., 180 s irradiation time in study of Thai et al. [19]) may be orders of magnitude higher than those for in vitro (e.g., 5 s irradiation time in the study of Conner-Kerr et al. [7]). This is because the energy of UVC irradiation attenuates exponentially when penetrating into tissue.
One advantage of using UVC over antibiotics is that UVC can eradicate microorganisms in a much faster manner (2–3-log10 eradication of microorganism population in vivo could be achieved in less than 1 h), while antibiotics usually take several days to take effect, especially in burns and chronic wounds that frequently have impaired blood perfusion. UVC may also be much more cost effective than the commonly used antibiotics.
Effects of UVC irradiation on wound healing
In addition to the eradication of microorganisms that can impede wound healing, it is hypothesized that judicious UV exposure might be beneficial for wound healing and restoration of skin homeostasis. The effects of UVC on wound healing include hyperplasia and enhanced re-epithelialization or de-squamation of the leading edge of periulcer epidermal cells, granulation tissue formation and sloughing of necrotic tissue [21]. In addition, UV exposure of wounds might stimulate and restore normal melanocyte number and distribution in re-epithelialized wounds while preventing hypopigmentation [22]. Furthermore, exposure of re-epithelialized wounds to UV irradiation might exert a photo protective effect in the skin by the production of melanin by melanocytes [22]. It is therefore proposed that moderate UV exposure should be commenced early in the healing process of cutaneous wounds [21,22]. Physical therapists have used UVC irradiation as a therapeutic modality for wound healing for many years; however, the physician community has been slow to adopt this technology [23].
In vitro studies
An in vitro study was reported by Morykwas and Mark on the effects of UVC irradiation at 254 nm on dermal fibroblasts [24]. Fifteen newborn foreskin fibroblast cultures were treated with UVC light. The investigators observed that, in comparison to nonirradiated fibroblast cultures, those fibroblasts irradiated with UVC had a decreased amount of fibronectin bound to cell surfaces (mean: 14%) and an increased amount of fibronectin released into the medium (mean: 42%). In addition, collagen lattices constructed with irradiated fibroblasts contracted significantly faster at 7 days. The authors suggested that fibronectin release led to increased healing via wound contraction.
Animal studies
Basford et al. compared the efficacy of several approaches for wound healing by using a pig model, including HeNe laser (632.8 nm), UVC irradiation (254 nm), occlusion and air exposure [25]. UVC-treated wounds were given two minimal erythemal dose treatments, twice daily, 6 days a week. Although UVC treatment showed a tendency towards healing faster than air-exposed wounds, the tendency did not reach a clinical significance. The authors concluded that there was no advantage in using UVC treatment.
Suo et al. investigated the effect of UVC on the expression of TGF-β in rat wounds [26]. Three full-thickness wounds were made on the dorsal surface of each rat (n = 30) and then treated with UVC respectively at 0 mJ/cm2 (no UVC), 15 mJ/cm2 and 60 mJ/cm2 daily for 3 successive days. The UVC exposures were calculated at the wound surfaces. The expression of TGF-β was measured at both the mRNA level and protein level by in situ hybridization and immunohistochemistry. The authors observed that, on day 7 after wounding, the expression of TGF-β in the wounds treated with 15 mJ/cm2 UVC was higher than that in the wounds treated with 60 mJ/cm2 and the control wounds without UVC (p < 0.05). While on day 21, the expression of TGF-β in the wounds treated with 60 mJ/cm2 was higher than that in the wounds treated with 15 mJ/cm2 as well as the control wounds without UVC treatment. The authors concluded that at the early stage of wound healing, 15 mJ/cm2 UVC treatment promotes the expression of TGF-β and might be beneficial for accelerating wound healing. The level of TGF-β expression was upregulated at the later stage at the UVC dose of 60 mJ/cm2.
In a later study using the same rat wound model [27], the authors from the same group investigated the effects of UVC irradiation at different doses on the expression of basic fibroblast growth factor (bFGF) in rat wounds. Full-thickness wounds made on the dorsal surfaces of rats (n = 30) were respectively treated with UVC at 0, 15 and 60 mJ/cm2 on a daily basis for 3 days. On day 7 after wounding, the expression of bFGF in the wounds treated with both 15 mJ/cm2 or 60 mJ/cm2 UVC was higher than that in the control wounds without UVC (p < 0.01), and the bFGF expression in the wound treated with 60 mJ/cm2 UVC was higher than that in the wounds treated with 15 mJ/cm2 UVC. On day 14 after wounding, the bFGF expression in the wounds treated with 60 mJ/cm2 significantly decreased, and was lower than the wounds treated with 15 mJ/cm2 and the wounds without UVC (p < 0.01). The authors concluded that in the early stage of wound healing, UVC promotes the expression of bFGF in granulation tissues. The effect of UVC with 60 mJ/cm2 was acute, and the effect of UVC with 15 mJ/cm2 was chronic.
Clinical studies
A very early clinical study on the effect of UVC on wound healing can be found in the year 1965. In this study, Freytes et al. tested the use of UVC irradiation at 254 nm emitted from a mercury-vapor lamp for the treatment of indolent ulcers in three patients [28]. The ulcerated area was exposed to UVC irradiation for 150 s (minimum erythema dose). Treatments were repeated once a week. The first patient had a deeply ulcerated area 25.4 mm (1 inch) in diameter. The patient received four treatments, at the end of which the ulcer was approximately 6.35 mm (0.25 inches) in size, clean and with good granulation tissue. The second patient had an ulcer of approximately 63.5 mm (2.5 inches) in diameter. The patient received four treatments and complete healing was achieved. The third patient had a multiple sclerosis and decubitus ulcer resistant to conventional treatment. The ulcer was 2 inches in diameter and 0.25 inches in depth. The patient received five treatments, at the end of which the ulcer was approximately 0.5 inches in diameter, clean and with good granulation tissue.
Nussbaum et al. compared the use of UVC in combination with ultrasound (UVC/US) for wound healing of pressure ulcers with the use of low-level laser and standard nursing care alone. UVC was emitted from a cold-quartz lamp at 250 nm [29]. Treatment parameters for UVC were based on wound appearances using erythema dosages. Ultrasound treatment was delivered at a frequency of 3 MHz and at a spatial average temporal average intensity of 0.2 W/cm2 (1:4 pulse ratio). Twenty patients were randomly assigned to the three groups. Results showed that UVC/US treatment had a greater effect on wound healing than nurse care, either alone or combined with laser. The average weekly rates of healing were 53.5, 32.4 and 23.7% for UVC/US group, standard nurse care alone group and laser group, respectively.
In summary, variable results have been reported on the effects of UVC irradiation on wound healing. The discrepancies of the results might be due to the various parameters of UVC used in different studies. For all the reported animal and clinical studies on the effects of UVC on wound healing, UVC irradiation was applied to noninfected wounds. While most of the studies reported positive results of enhanced wound healing by UVC light, no study reported any negative results that UVC delayed wound healing. While pathogenic microorganisms impede the healing of infected wounds, one can expect that the eradication of microorganisms by UVC would enhance wound healing in infected wounds.
Effects of UVC irradiation on mammalian tissue & cells
It is well known that prolonged UV irradiation is damaging to human tissue and particularly to skin. UVB irradiation of skin has been particularly well studied, and is accepted as the main cause of skin cancer [30]. Exposure of skin to prolonged UVB irradiation is recommended to be avoided by appropriate application of sunscreen and choice of lifestyle [26,31]. However, under appropriate risk–benefit analysis, UVB treatments are still delivered to various areas of the bodies of millions of patients in the USA every year for the treatment of a larger number of cutaneous disorders [32]. It has been documented that UVB therapy is an effective therapeutic option with an excellent safety profile and well-documented side effects [33,34].
UVC irradiation of human skin has been much less studied, but is also known to cause the same kind of damage [35]. In proposing to employ UVC irradiation to treat localized infections, it is clearly important to test the effect of UVC with effective antimicrobial doses on normal mammalian cells and tissue to ensure that unacceptable damage is not inflicted.
Selectivity of UVC inactivation of microorganisms over that of mammalian cells
The safety issue of the UVC treatment requires that the pathogenic microorganisms be selectively inactivated while the cells in normal tissue cells are spared. Sosnin et al. compared the in vitro susceptibilities to UVC irradiation between living mammalian cells and bacteria [36]. The light source was a narrow-band UVC lamp with the emission peak at 206 nm. Chinese hamster ovary (CHO-K1) cells (fibroblasts) and E. coli (which is considered to be one of the most resistant species to UV irradiation within the enterobacteria group) were used in the study. The fibroblasts were in confluent monolayer cultures and the E. coli cultures were colonies on agar plates. The authors found that the UVC dose that led to necrosis in fibroblasts was more than ten-times higher than that needed for inactivation of E. coli. A 2-log10 inactivation of E. coli was achieved at approximately 25 mJ/cm2, and this dose did not cause any adverse effects on the fibroblasts. The authors concluded that UVC irradiation may become a method of selective bacterial decontamination of wounds without killing the host cells that strive to repair the wound. The authors also pointed out that the possible DNA damage in mammalian cells, which survived the UVC treatment, should be investigated in a long-term study.
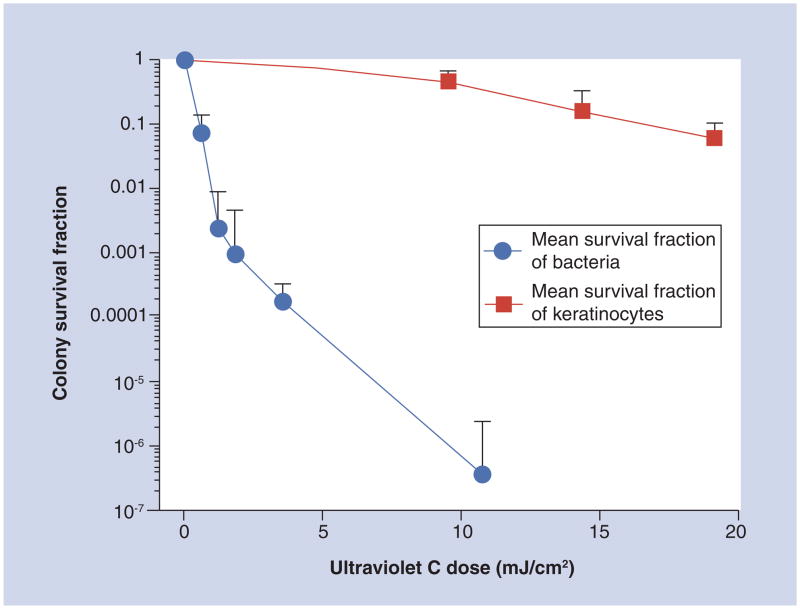
Another in vitro study was conducted by Dai et al. on the selectivity of UVC inactivation of bacteria in suspensions over keratinocytes in confluent monolayer cultures [37]. On average, when 11 mJ/cm2 UVC irradiation had been delivered, as shown in Figure 4, the viability loss of the keratinocytes was only approximately 0.24 log10 (~57%), while over 6-log10 inactivation of bacteria (average value of P. aeruginosa and S. aureus) was achieved under similar conditions, resulting in a more than 5-log10 selective inactivation of bacteria over keratinocytes. If we consider a 2-log10 (99%) inactivation of bacteria as a sufficiently therapeutic fraction, the viability loss of the keratinocytes would be only approximately 6% at the same UVC dose.
Figure 4. Comparison of averaged fluence-dependent survival fractions in response to ultraviolet C irradiation of bacteria with that of keratinocytes using identical irradiation conditions and colony-forming assays.
The error bars represent the SD.
Reprinted with permission from [37].
In another study carried out by the same group, the authors compared the in vitro susceptibilities to UVC irradiation between keratinocytes and C. albicans [15]. It was found that UVC selectively inactivated C. albicans compared with keratinocytes in a UVC dose-dependent manner. On average, when 19.2 mJ/cm2 UVC irradiation had been delivered, the viability loss of keratinocytes was approximately 1.22 log10, while a 3.02-log10 inactivation of C. albicans was achieved at the same irradiation dose (p < 0.001), resulting in an early 2-log10 selective inactivation of C. albicans over keratinocytes. If we consider a 2-log10 (99%) inactivation of C. albicans as the therapeutically effective fraction, the viability loss of keratinocytes is approximately 0.77 log10 (18.9%) at the comparable UVC dose.
To assess the safety of using UVC irradiation to treat corneal bacterial infections, Dean et al. compared the in vitro sensitivity to UVC (265 nm) of human primary corneal epithelial cells with that of corneal pathogenic bacteria [10]. The authors found that an exposure of confluent monolayer human corneal epithelial cells to 57.95 mJ/cm2 UVC (calculated at the culture surface) gave no statistically significant decrease (p = 0.877) in the ratio of live to dead cells when compared with the nonirradiated control cultures, while an exposure to only 1.93 mJ/cm2 UVC was sufficient to induce 100% inhibition of growth of all the bacterial species tested on agar plates. The authors suggested UVC at appropriate doses could potentially be beneficial in treating corneal surface infections, without causing significant adverse effects.
Effects of UVC on host tissue
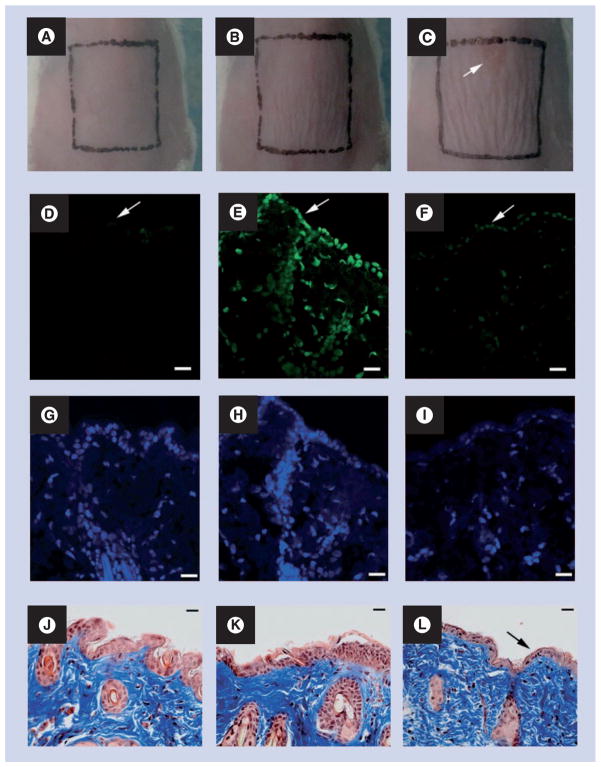
In an animal study using BALB/c mice, Dai et al. investigated whether mouse skin could tolerate UVC irradiation at the antifungal dose [15]. Figure 5A–C show representative morphologies of BALB/c mouse skin before, immediately after and 24 h after being exposed to UVC at a radiant exposure of 6.48 J/cm2, which is the effective antifungal dose for treating infections on day 1 postinfection. It can be observed that there is mild wrinkling of the skin evident immediately after UVC and this is slightly more pronounced at 24 h after UVC exposure, but the wrinkling disappeared in succeeding days. A small lesion (top of square in Figure 5C) was also observed, which occurred at 24 h after UVC exposure, but the lesion eventually healed without problem. CPD-positive nuclei were observed in the immunofluorescence micrograph of the biopsy taken immediately after UVC exposure (Figure 5E). However, the damage was extensively repaired at 24 h after UVC exposure where only traces of fluorescence remained (Figure 5F). Mild epidermal shrinkage was seen at 24 h after UVC exposure (Figure 5L).
Figure 5. Effect of ultraviolet C at the effective antifungal dose on mouse skin.
(A–C) Morphologies of a representative mouse skin before, immediately after, and 24 h after being exposed to ultraviolet C at a dose of 6.48 J/cm2, respectively.
(D–F) Representative immunofluorescence micrographs of cyclobutane pyrimidine dimers in skin cell nuclei. (G–I) Micrographs of diamidino-2-phenylindole counterstaining of cell nuclei. (J–L) Micrographs of Masson’s trichrome-stained sections. Biopsies were taken before (D, G, J), immediately after (E, H, K) and 24 h after (F, I, L) being exposed to UVC at a dose of 6.48 J/cm2, respectively, from the same mouse. Arrow in (C): UVC-induced lesion on mouse skin. Arrows in (D–F): mouse skin surface. Arrow in (L) shows epidermal shrinkage. Scale bars shown in panels (D–L) are 20 μm.
Reprinted with permission from [15].
In another animal study using hairless mice, Sterenborg et al. compared the carcinogenic effect of UVC (254 nm) in mice with that of UVB (313 nm) [38]. UVC or UVB irradiation was applied daily at a dose of 57.5–460 mJ/cm2 (calculated at the lamp aperture) per day. In all dose groups, most of the animals developed large numbers of tumors at some stage of the experiment. The large majority were classified as squamous cell carcinomas. For both UVC and UVB, the tumor induction time was proportional to a power function of the daily dose (the power was −0.2 and −0.58 for UVC and UVB, respectively). Throughout the whole range of daily doses used in the experiment, UVC was less carcinogenic than UVB. An intriguing difference between the two types of radiation was that the tumors induced by UVC appeared much more scattered over the irradiated parts of the animals than the UVB-induced tumors.
In summary, microorganisms are found to be more sensitive to UVC than mammalian cells. As a result, with appropriate doses, pathogenic microorganisms may be selectively inactivated by UVC with minimum nonspecific damage to mammalian cells. This is crucial in the application of UVC irradiation for localized infections. Studies did find that UVC at the effective antimicrobial doses can cause DNA damage to mammalian cells to some extent. However, at that same time, it has been found that the UVC-induced DNA damages can be rapidly repaired by the DNA repairing enzymes of the host. To further minimize the UVC-induced DNA damage, one can combine the use of protective agents (e.g., green tea) [39] and DNA repair agents (e.g., DNA repair liposomes) [40] with UVC irradiation. Green tea could be applied to the UVC irradiated area during the UVC treatment and liposomes could be applied after the UVC treatment. In addition, the intact skin surrounding the area to be treated could be screened from UVC irradiation [15,19].
Will microorganisms develop resistance to UVC?
The rapid rate of replication of microorganisms allows them to adapt to environmental stresses with some facility. Favorable mutations in DNA can arise and if these mutations lead to a competitive advantage they will spread to the whole microbial population. This consideration is in fact largely responsible for the emergence of drug resistance in clinical therapy. Since one of the primary functions of UVC is to damage DNA of microorganisms, is it possible to generate mutants with increased resistance to UVC?
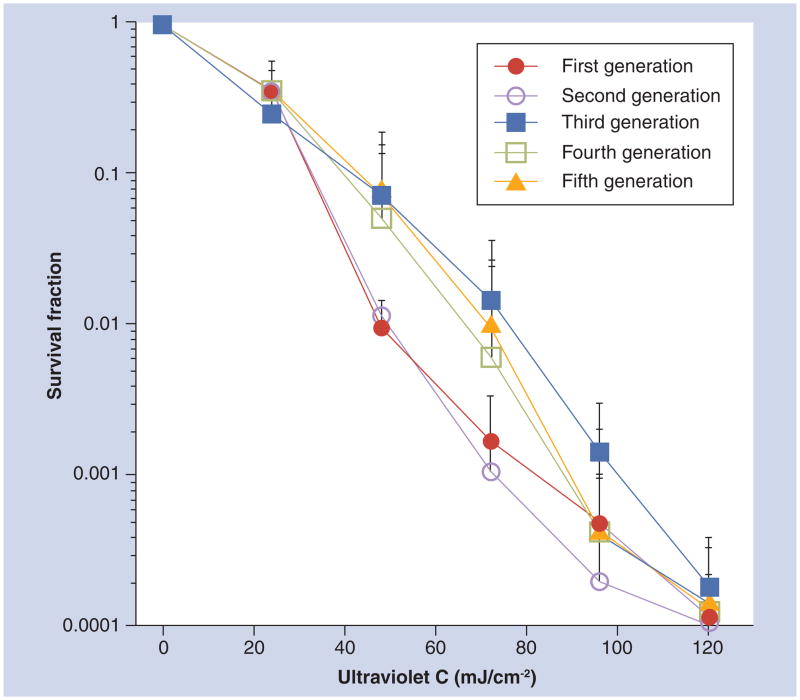
In a study on the use of UVC to treat onychomycosis, Dai et al. tested whether T. rubrum can develop resistance to UVC irradiation at 254 nm by carrying out five consecutive sublethal UVC inactivation cycles in vitro [14]. No significant difference was found in cell inactivation rates among the five consecutive cycles when 120 mJ/cm2 UVC had been delivered (p = 0.66) (Figure 6). This indicates that resistance to UVC irradiation is not rapidly acquired by T. rubrum cells that are repeatedly exposed to sublethal UVC irradiation.
Figure 6. Dose responses of Trichophyton rubrum in vitro to five consecutive sublethal ultraviolet C inactivation cycles.
The data are representative experiments performed in triplicate and are displayed as mean plus standard deviation.
Reprinted with permission from [14].
Alcantara-Diaz et al. investigated the divergent adaptation of E. coli to repeated cycles of UVC irradiation at 254 nm [41]. Five cultures of wild-type E. coli PQ30 were exposed to 80 consecutive growth–irradiation cycles of UVC light. Each growth–irradiation cycle was carried out as follows. An aliquot of 1 ml from an early stationary culture (~5 × 108–109 cells) was irradiated with a UVC germicidal lamp at an irradiance of 0.1 mW/cm2. Following irradiation, 0.1 ml of this suspension was inoculated into 1 ml of fresh lysogeny broth and incubated at 37°C for approximately 6 h to early stationary phase. Starting with a dose of 1 mJ/cm2 for each cycle, dose was increased twofold every ten growth–irradiation cycles. Quantitative dose–response curves were obtained by exposing exponentially growing cells, suspended in phosphate buffer, to several UV doses. Immediately after irradiation, cells were diluted in phosphate buffer and spread on LB agar. At the end, according to the quantitative dose–response curves, all the five cultures were found to give rise to different degrees of UVC resistance. The authors found that the adaptation to cyclic UVC irradiation was a consequence of the selection of advantageous mutations arising in different genes related to repair and replication of DNA.
In summary, excessive repetition of UVC irradiation may induce resistance of microorganisms to UVC. No resistance of fungal cells to UVC irradiation was observed in the study of Dai et al. which may be due to the limited cycles of UVC inactivation carried out [14]. While permissible UVC dose exposure limits for human tissue do not exist, it is expected that there would be an acceptable maximum number of repetition times of UVC irradiation and possibly a total lifetime cumulative exposure. For wound infections, we expect that only limited numbers of repeated doses of UVC irradiation would be required, while the UV-induced carcinogenic mutation is a long-term effect.
Expert commentary
The use of UVC irradiation for the prevention and treatment of localized infections is still in the very early stages of development. Most of the studies are confined to in vitro and ex vivo levels, while in vivo animal studies and clinical studies are much rarer.
Several in vitro studies have reported that multidrug-resistant pathogenic microorganisms are highly sensitive to UVC inactivation. Bacterial cells are found to be more sensitive to UVC than fungal cells. Microorganisms are found to be more sensitive to UVC than mammalian cells.
One advantage of using UVC over antibiotics is that UVC can eradicate microorganisms in a much faster manner. UVC may also be much more cost effective than the commonly used antibiotics.
UVC is generally much more effective and also safer for prophylaxis of wound infections than for treatment of already infected wounds. This is because when the infections get established, pathogenic microorganisms penetrate deep into the tissue and biofilms can often be formed. Higher doses of irradiation are needed to treat established infections (e.g., as reported by Dai et al. [15]) because irradiation is quickly attenuated when penetrating into tissue. Similarly, higher irradiation doses are needed when inactivating microorganisms within biofilm than are needed for their plank-tonic counterparts. It is likely that the use of higher irradiation doses is accompanied by higher side effects by compromising the inactivation selectivity and increasing the UVC-induced tissue damage.
The effects of UVC irradiation on wound healing have also been investigated, and variable results have been reported. While pathogenic microorganisms impede the healing of infected wounds, one can expect that the eradication of microorganisms by UVC would enhance wound healing in infected wounds.
Studies found that UVC at the effective antimicrobial doses can cause DNA damage to mammalian cells to some extent. However, it has also been found that the UVC-induced DNA damages can be rapidly repaired by the DNA repairing enzymes.
In contrast to the large amount of studies regarding the chronic effects of UVB on human skin and tissue, there has been no similar report on the chronic effects of UVC. However, it has been suggested in an animal study that UVC is less carcinogenic than UVB [38] because of its more superficial penetration depth. The authors of this paper state that, “Abnormal differentiation of a layer of cells that is committed to being sloughed off anyway (UVC) is not harmful, whereas mutation of the basal cells (UVA or UVB) may result in skin cancer.” [38]. On the other hand, it has been reported that UVB treatment is an effective options for a large number of cutaneous disorders in humans with excellent safety profiles. A retrospective study of 195 psoriasis patients treated with UVB did not provide evidence for increased skin cancer risk with up to 9 years of follow-up [33]. An analysis of 3867 patients receiving UVB over an 18-year period, with a median number of 29 treatments and 352 patients receiving 100 or more treatments with more than 6 months of follow-up for each patient, showed no increase in skin cancers of any kind [34].
It has been found that resistance of microorganisms to UVC may develop after excessive repetition (e.g., 80 cycles) of UVC irradiation [41]. Therefore, similar to traditional antibiotics, excessive or long- term use of UVC should also be avoided.
It is worth noting that the use of UVC for sun-sensitive patients (or lupus erythematosus patients) should be cautious. It is well known that solar irradiation, mainly the part of UVA and UVB, cause photosensitivity in lupus erythematosus patients [42], which is an abnormal reaction of human skin to solar irradiation. It was found that similar effects can also be induced by UVC [43].
It is also worth noting that the penetration of UVC irradiation in human skin and tissue is limited, and as a result, topical irradiation of UVC may not be sufficient to reach deeply located infections and subsequently inactivate pathogenic microorganisms. However, with the advancement of modern optical fiber technologies [44,45], this limitation could be overcome by delivering the UVC irradiation interstitially to the infected sites. In addition, optical clearing techniques [46,47], which have attracted extensive attention recently, have provided another potential technique for improving the UVC penetration in human skin and tissue.
In conclusion, we believe there are situations where the risk– benefit ratio is favorable for the use of UVC for treating localized infections, particularly when the microorganisms responsible are antibiotic resistant. While permissible UVC dose exposure limits for human tissue do not exist, it is expected that there would be an acceptable maximum number of repetition times for UVC irradiation and possibly a total lifetime cumulative exposure. For wound infections, we expect that only limited numbers of repeated doses of UVC irradiation would be required, while the UV-induced carcinogenic mutation is a long-term effect of prolonged use of UVC.
Five-year view
The increasing emergence of antibiotic resistance of pathogens presents a serious clinical challenge in the future. UVC should be investigated as an alternative approach for prophylaxis and treatment of localized infectious diseases, especially those caused by antibiotic-resistant pathogens. UVC should be used in a way where the side effects would be minimized and the resistance of microorganisms to UVC avoided. As a result, more extensive animal studies and clinical studies need to be carried out to investigate and optimize UVC treatment, for example the minimal effective antimicrobial doses of UVC irradiation for localized infections that varied in both stage (early, middle and late) and severity (infectious burden). Light-delivery technologies, such as optical fibers, and optical clearing techniques should be investigated to improve the penetration of UVC irradiation in human skin and tissue. Technologies that help reduce the side effects (e.g., enhanced repair of UVC-induced DNA damage to human cells, selective protection of human tissue and cells from UVC irradiation during UVC treatment) of UVC treatment are also worthy of being further investigated. In addition to epidermal cells, effects of UVC on other human bone, vessels, nerves (which may be exposed to UVC irradiation in open wounds) and leukocytes (which are important in the local defense mechanism against dissemination of infections) should also be studied.
Key issues.
Multidrug-resistant microorganisms are highly susceptible to ultraviolet C (UVC) inactivation.
With appropriate doses, UVC can selectively inactivate microorganisms while preserving viability of mammalian cells and, moreover, is reported to promote wound healing.
Animal studies have shown that UVC is less damaging to human tissue than UVB, which is an accepted option for a large number of cutaneous disorders in humans with an excellent safety profile.
Under excessive repeated UVC irradiation, resistance of microorganisms to UVC inactivation may develop.
UVC should be investigated as an alternative approach for prophylaxis and treatment of localized infectious diseases, especially those caused by multidrug-resistant pathogens.
UVC should be used in a manner such that side effects are minimized and development of resistance of microorganisms to UVC avoided.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This study was supported in part by an Airlift Research Foundation Extremity Trauma Research Grant (grant #109421 to T Dai) and the NIH (grant RO1AI050875 to MR Hamblin). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Bell SG. Antibiotic resistance: is the end of an era near? Neonatal Netw. 2003;22(6):47–54. doi: 10.1891/0730-0832.22.6.47. [DOI] [PubMed] [Google Scholar]
- 2.Park A. Antibiotics. NDM-1 how dangerous is the mutation? Time. 2010;176(14):20. [PubMed] [Google Scholar]
- 3.Prevention CfDCa. Detection of Enterobacteriaceae isolates carrying metallobeta- lactamase – United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59(24):750. [PubMed] [Google Scholar]
- 4.Vázquez M, Hanslmeier A. Ultraviolet Radiation in the Solar System. Springer; Dordrecht, The Netherlands: 2006. [Google Scholar]
- 5.Chang JC, Ossoff SF, Lobe DC, et al. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 1985;49(6):1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurzadyan GG, Gorner H, Schulte- Frohlinde D. Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat Res. 1995;141(3):244–251. [PubMed] [Google Scholar]
- 7••.Conner-Kerr TA, Sullivan PK, Gaillard J, Franklin ME, Jones RM. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound Manage. 1998;44(10):50–56. Important study demonstrated that antibiotic-resistant bacteria are sensitive to ultraviolet C (UVC) inactivation. [PubMed] [Google Scholar]
- 8.Taylor GJ, Leeming JP, Bannister GC. Effect of antiseptics, ultraviolet light and lavage on airborne bacteria in a model wound. J Bone Joint Surg Br. 1993;75(5):724–730. doi: 10.1302/0301-620X.75B5.8376427. [DOI] [PubMed] [Google Scholar]
- 9.Rao BK, Kumar P, Rao S, Gurung B. Bactericidal effect of ultraviolet C (UVC), direct and filtered through transparent plastic, on Gram-positive cocci: an in vitro study. Ostomy Wound Manage. 2011;57(7):46–52. [PubMed] [Google Scholar]
- 10.Dean SJ, Petty A, Swift S, et al. Efficacy and safety assessment of a novel ultraviolet C device for treating corneal bacterial infections. Clin Experiment Ophthalmol. 2011;39(2):156–163. doi: 10.1111/j.1442-9071.2010.02471.x. [DOI] [PubMed] [Google Scholar]
- 11.Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling. 2009;25(4):289–296. doi: 10.1080/08927010802716623. [DOI] [PubMed] [Google Scholar]
- 12.Mohr H, Steil L, Gravemann U, et al. A novel approach to pathogen reduction in platelet concentrates using short-wave ultraviolet light. Transfusion. 2009;49(12):2612–2624. doi: 10.1111/j.1537-2995.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan PK, Conner-Kerr TA. A comparative study of the effects of UVC irradiation on select procaryotic and eucaryotic wound pathogens. Ostomy Wound Manage. 2000;46(10):28–34. [PubMed] [Google Scholar]
- 14.Dai T, Tegos GP, Rolz-Cruz G, Cumbie WE, Hamblin MR. Ultraviolet C inactivation of dermatophytes: implications for treatment of onychomycosis. Br J Dermatol. 2008;158(6):1239–1246. doi: 10.1111/j.1365-2133.2008.08549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Dai T, Kharkwal GB, Zhao J, et al. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem Photobiol. 2011;87(2):342–349. doi: 10.1111/j.1751-1097.2011.00886.x. The only published animal study showing that UVC is effective in prophylaxis and treatment of Candida albicans burn infection in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor GJ, Bannister GC, Leeming JP. Wound disinfection with ultraviolet radiation. J Hosp Infect. 1995;30(2):85–93. doi: 10.1016/0195-6701(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 17.Shimomura A, Tahara D, Tominaga M, et al. The effect of ultraviolet rays on the prevention of exit-site infections. Adv Perit Dial. 1995;11:152–156. [PubMed] [Google Scholar]
- 18.Thai TP, Houghton PE, Campbell KE, Woodbury MG. Ultraviolet light C in the treatment of chronic wounds with MRSA: a case study. Ostomy Wound Manage. 2002;48(11):52–60. [PubMed] [Google Scholar]
- 19••.Thai TP, Keast DH, Campbell KE, Woodbury MG, Houghton PE. Effect of ultraviolet light C on bacterial colonization in chronic wounds. Ostomy Wound Manage. 2005;51(10):32–45. Important clinical study showing the efficacy of UVC in treating chronic bacterial infections in pressure ulcers. [PubMed] [Google Scholar]
- 20.Boker A, Rolz-Cruz G, Cumbie B, Kimball AB. A single-center, prospective, open-label, pilot study of the safety, local tolerability, and efficacy of ultraviolet-C (UVC) phototherapy for the treatment of great toenail onychomycosis. J Am Acad Dermatol. 2007;58(2):AB82. [Google Scholar]
- 21.Kloth LC. Physical modalities in wound management: UVC, therapeutic heating and electrical stimulation. Ostomy Wound Manage. 1995;41(5):18–20. 22–14, 26–17. [PubMed] [Google Scholar]
- 22•.Rennekampff HO, Busche MN, Knobloch K, Tenenhaus M. Is UV radiation beneficial in postburn wound healing? Med Hypotheses. 2010;75(5):436–438. doi: 10.1016/j.mehy.2010.04.017. Hypothesized the mechanisms of beneficial UVC effects on wound healing. [DOI] [PubMed] [Google Scholar]
- 23.Ennis WJ, Lee C, Meneses P. A biochemical approach to wound healing through the use of modalities. Clin Dermatol. 2007;25(1):63–72. doi: 10.1016/j.clindermatol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Morykwas MJ, Mark MW. Effects of ultraviolet light on fibroblast fibronectin production and lattice contraction. Wounds. 1998;10(4):111–117. [Google Scholar]
- 25.Basford JR, Hallman HO, Sheffield CG, Mackey GL. Comparison of cold-quartz ultraviolet, low-energy laser, and occlusion in wound healing in a swine model. Arch Phys Med Rehabil. 1986;67(3):151–154. doi: 10.1016/0003-9993(86)90053-5. [DOI] [PubMed] [Google Scholar]
- 26.Suo W, Wang X, Wang D. Effect of ultraviolet C irradiation on expression of transforming growth factor-β in wound. Chinese J Rehabil Theory Pract. 2002;8(1):5–7. [Google Scholar]
- 27.Suo W, Guo H, Wang X, Wang D. Effect of ultraviolet C light on the expression of basic fibroblast growth factor in rat wounds. Chinese J Phys Med Rehabil. 2003;25(11):651–654. [Google Scholar]
- 28.Freytes HA, Fernandez B, Fleming WC. Ultraviolet light in the treatment of indolent ulcers. South Med J. 1965;58(2):223–226. doi: 10.1097/00007611-196502000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Nussbaum EL, Biemann I, Mustard B. Comparison of ultrasound/ultraviolet-C and laser for treatment of pressure ulcers in patients with spinal cord injury. Phys Ther. 1994;74(9):812–823. doi: 10.1093/ptj/74.9.812. discussion 824–815. [DOI] [PubMed] [Google Scholar]
- 30.Ichihashi M, Ueda M, Budiyanto A, et al. UV-induced skin damage. Toxicology. 2003;189(1–2):21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 31.Albert MR, Ostheimer KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 2. J Am Acad Dermatol. 2003;48(6):909–918. doi: 10.1067/mjd.2003.272. [DOI] [PubMed] [Google Scholar]
- 32.Sage RJ, Lim HW. UV-based therapy and vitamin D. Dermatol Ther. 2010;23(1):72–81. doi: 10.1111/j.1529-8019.2009.01292.x. [DOI] [PubMed] [Google Scholar]
- 33.Weischer M, Blum A, Eberhard F, Rocken M, Berneburg M. No evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB phototherapy: a first retrospective study. Acta Derm Venereol. 2004;84(5):370–374. doi: 10.1080/00015550410026948. [DOI] [PubMed] [Google Scholar]
- 34.Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159(4):931–935. doi: 10.1111/j.1365-2133.2008.08776.x. [DOI] [PubMed] [Google Scholar]
- 35.Trevisan A, Piovesan S, Leonardi A, et al. Unusual high exposure to ultraviolet-C radiation. Photochem Photobiol. 2006;82(4):1077–1079. doi: 10.1562/2005-10-27-ra-728. [DOI] [PubMed] [Google Scholar]
- 36••.Sosnin EA, Stoffels E, Erofeev MV, Kieft IE, Kunts SE. The effects of UV irradiation and gas plasma treatment on living mammalian cells and bacteria: a comparative approach. IEEE Trans Plasma Sci. 2004;32(4):1544–1550. Important study showing that UVC light can selectively inactivate bacteria over mammalian cells. [Google Scholar]
- 37.Dai T, Tegos GP, St Denis TG, Anderson D, Sinofsky E, Hamblin MR. Ultraviolet-C irradiation for prevention of central venous catheter-related infections: an in vitro study. Photochem Photobiol. 2011;87(1):250–255. doi: 10.1111/j.1751-1097.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Sterenborg HJ, van der Putte SC, van der Leun JC. The dose–response relationship of tumorigenesis by ultraviolet radiation of 254 nm. Photochem Photobiol. 1988;47(2):245–253. doi: 10.1111/j.1751-1097.1988.tb02722.x. Reported that UVC is less carcinogenic to mouse skin than ultraviolet B. [DOI] [PubMed] [Google Scholar]
- 39.Camouse MM, Domingo DS, Swain FR, et al. Topical application of green and white tea extracts provides protection from solar-simulated ultraviolet light in human skin. Exp Dermatol. 2009;18(6):522–526. doi: 10.1111/j.1600-0625.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 40.Wolf P, Maier H, Mullegger RR, et al. Topical treatment with liposomes containing T4 endonuclease V protects human skin in vivo from ultraviolet-induced upregulation of interleukin-10 and tumor necrosis factor-alpha. J Invest Dermatol. 2000;114(1):149–156. doi: 10.1046/j.1523-1747.2000.00839.x. [DOI] [PubMed] [Google Scholar]
- 41••.Alcantara-Diaz D, Brena-Valle M, Serment-Guerrero J. Divergent adaptation of Escherichia coli to cyclic ultraviolet light exposures. Mutagenesis. 2004;19(5):349–354. doi: 10.1093/mutage/geh039. Demonstrated that excessive exposures of repeated UVC irradiation may induce bacterial resistance to UVC. [DOI] [PubMed] [Google Scholar]
- 42.Bijl M, Kallenberg CG. Ultraviolet light and cutaneous lupus. Lupus. 2006;15(11):724–727. doi: 10.1177/0961203306071705. [DOI] [PubMed] [Google Scholar]
- 43.Cripps DJ, Rankin J. Action spectra of lupus erythematosus and experimental immunofluorescence. Arch Dermatol. 1973;107(4):563–567. [PubMed] [Google Scholar]
- 44.Parpura V, Haydon PG. ‘Uncaging’ using optical fibers to deliver UV light directly to the sample. Croat Med J. 1999;40(3):340–345. [PubMed] [Google Scholar]
- 45.Oto M, Kikugawa S, Sarukura N, Hirano M, Hosono H. Optical fiber for deep ultraviolet light. IEEE Photonics Technol Lett. 2001;13(9):978–980. [Google Scholar]
- 46.Khan MH, Choi B, Chess S, Kelly MK, McCullough J, Nelson JS. Optical clearing of in vivo human skin: implications for light-based diagnostic imaging and therapeutics. Lasers Surg Med. 2004;34(2):83–85. doi: 10.1002/lsm.20014. [DOI] [PubMed] [Google Scholar]
- 47.Tuchin VV. Optical Clearing of Tissues and Blood. SPIE Press; WA, USA: 2006. [Google Scholar]