Abstract
Impaired aerobic exercise capacity and skeletal muscle dysfunction are associated with cardiometabolic diseases. Acute administration of capsaicin enhances exercise endurance in rodents, but the long-term effect of dietary capsaicin is unknown. The capsaicin receptor, the transient receptor potential vanilloid 1 (TRPV1) cation channel has been detected in skeletal muscle, the role of which remains unclear. Here we report the function of TRPV1 in cultured C2C12 myocytes and the effect of TRPV1 activation by dietary capsaicin on energy metabolism and exercise endurance of skeletal muscles in mice. In vitro, capsaicin increased cytosolic free calcium and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) expression in C2C12 myotubes through activating TRPV1. In vivo, PGC-1α in skeletal muscle was upregulated by capsaicin-induced TRPV1 activation or genetic overexpression of TRPV1 in mice. TRPV1 activation increased the expression of genes involved in fatty acid oxidation and mitochondrial respiration, promoted mitochondrial biogenesis, increased oxidative fibers, enhanced exercise endurance and prevented high-fat diet-induced metabolic disorders. Importantly, these effects of capsaicin were absent in TRPV1-deficient mice. We conclude that TRPV1 activation by dietary capsaicin improves energy metabolism and exercise endurance by upregulating PGC-1α in skeletal muscles. The present results indicate a novel therapeutic strategy for managing metabolic diseases and improving exercise endurance.
Keywords: TRPV1, exercise endurance, PGC-1α, skeletal muscle, energy metabolism
Introduction
Epidemiological studies suggest that exercise leads to lower blood pressure, reduced adiposity and improvements in dyslipidemia, insulin sensitivity and endothelial function 1. Energy metabolism and exercise endurance, which directly influence cardiometabolic risk factors, are primarily driven by skeletal muscle, a major mass peripheral tissue 2. Impaired aerobic exercise capacity and skeletal muscle dysfunction appear to be associated with metabolic diseases, such as obesity and diabetes. Increasing oxidative fibers and exercise endurance can improve insulin action and prevent weight gain 3, 4, 5. In this context, many attempts have been made to improve endurance capacity with nutritional regimens that promote fatty acid oxidation and muscle remodeling 6.
Capsaicin, the major pungent ingredient in hot pepper, is a highly selective agonist for transient receptor potential vanilloid 1 (TRPV1), a nonselective cation channel that primarily exists in sensory neurons and responds to noxious stimuli 7, 8. TRPV1 is also present in nonneuronal tissues, such as adipose tissue and skeletal muscle 9, 10. Our previous study showed that capsaicin inhibited adipogenesis through a TRPV1-mediated Ca2+ influx in adipocytes and chronic capsaicin administration prevented obesity in mice 11. More recently, we showed that TRPV1 activation by dietary capsaicin improved endothelium-dependent vasorelaxation and regulated blood pressure in rats 12. Acute oral administration of capsaicin enhanced fatty acid utilization and endurance capacity in rodents, and increased oxygen consumption in humans 13, 14, 15. However, the long-term effects of dietary capsaicin have not been studied yet and whether capsaicin mediates these effects through skeletal muscle TRPV1 activation is still unclear.
Adult skeletal muscle shows plasticity and can convert into different fiber types in response to exercise training. The conversion of muscle fiber from glycolytic type II (fast fiber) to the more oxidative type I (slow fiber) is likely to be mediated by a Ca2+-signaling mechanism that involves calcineurin, calmodulin-dependent kinase and the transcriptional cofactor peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) 16, 17, 18. PGC-1α is a principal regulator of the expression of genes involved in mitochondrial biogenesis, respiratory function, and carbohydrate and lipid metabolism 16, 17, 18. It also participates in the pathogenesis of obesity, diabetes and cardiomyopathy 16, 19. Thus, we hypothesize that TRPV1 activation by capsaicin might improve energy metabolism and endurance capacity in skeletal muscles through Ca2+-dependent PGC-1α upregulation.
Results
TRPV1 characterization in skeletal muscles
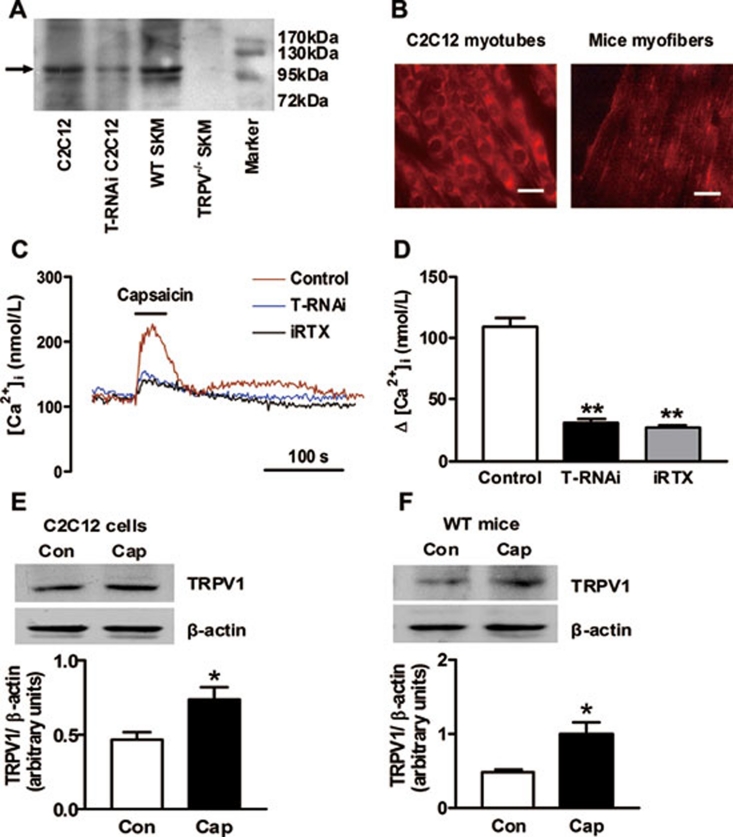
We examined TRPV1 protein expression in skeletal muscles from WT and TRPV1−/− mice and in cultured C2C12 myotubes (Figure 1A). TRPV1 protein was abundant in skeletal muscles of WT mice but was absent in TRPV1−/− mice. TRPV1 RNA interference (RNAi) reduced the expression of TRPV1 in C2C12 cells by >75% (Figure 1A). Immunofluorescence staining showed that TRPV1 was located in both the cytoplasm and plasma membranes of myocytes (Figure 1B). To determine the function of TRPV1 channels in myotubes, we examined the intracellular free calcium concentration ([Ca2+]i). Acute capsaicin stimulation caused a marked increase of [Ca2+]i in control myotubes (Figure 1C). A specific blockade of TRPV1 by 5′-iodo-resiniferatoxin (iRTX) and TRPV1 RNAi inhibited the capsaicin-induced [Ca2+]i increase by 75% and 72%, respectively (Figure 1C and 1D). TRPV1 was upregulated by capsaicin treatment. In vitro, TRPV1 protein expression was higher in C2C12 cells treated with capsaicin for 24 h compared to cells without capsaicin treatment (Figure 1E). In vivo, administering a capsaicin diet to mice for 4 months increased the TRPV1 expression in skeletal muscles (Figure 1F). These results indicate that TRPV1 is expressed in cultured myotubes and mouse skeletal muscles, which can be activated and upregulated by capsaicin treatment.
Figure 1.
TRPV1 characterization in skeletal muscles. (A) Immunoblot of TRPV1 in skeletal muscle (SKM) from wild-type (WT) and TRPV1 knockout (TRPV1−/−) mice and in C2C12 myotubes with or without TRPV1 RNAi (T-RNAi). The arrowhead indicates the band corresponding to TRPV1 protein. (B) TRPV1 localization in C2C12 myotubes and mice myofibers was shown with immunofluorescence. Bar = 50 μm. (C, D) Representative curves (C) and summary data (D) showing capsaicin (100 nM)-induced [Ca2+]i changes in cells with or without T-RNAi and cells pretreated with the specific TRPV1 inhibitor iRTX (1 μM) for 5 min. **P < 0.01 vs control. (E) Immunoblot of TRPV1 in C2C12 myotubes with (Cap) or without (Con) capsaicin (100 nM) treatment for 24 h. *P < 0.05 vs Con. (F) Immunoblot of TRPV1 in skeletal muscles of WT mice with (Cap) or without (Con) 4 months of capsaicin administration. *P < 0.05 vs Con. Summary data are means ± S.E.M. for three to four independent experiments.
TRPV1 activation increases PGC-1α expression and mitochondrial biogenesis in a Ca2+-dependent manner
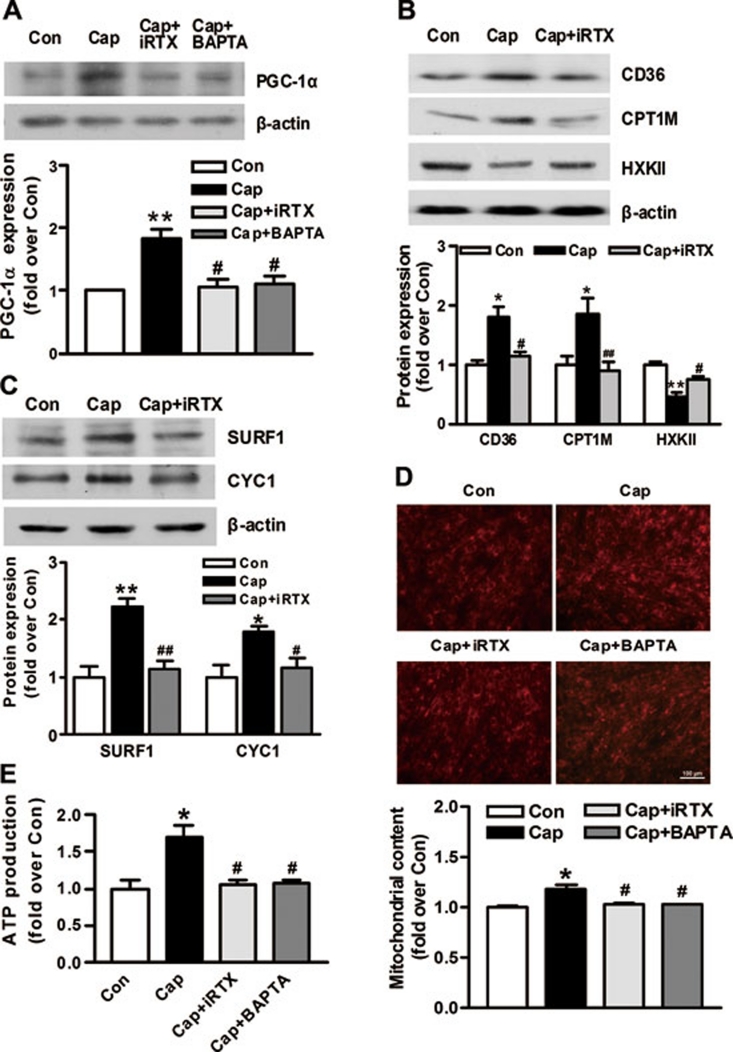
What is the physiological significance of TRPV1 channels in myotubes? Ca2+ and several Ca2+-dependent signaling pathways are involved in the induction of PGC-1α and its target genes 18, 20, 21. PGC-1α is a master regulator of lipid and glucose metabolism, mitochondrial biogenesis and muscle remodeling 22. Thus, we examined whether capsaicin exposure could upregulate PGC-1α through TRPV1-mediated Ca2+ increases. The PGC-1α expression was elevated after 24-h capsaicin treatment while this increase was reversed by the presence of iRTX and the intracellular Ca2+ chelator BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid) (Figure 2A). It is well documented that CD36 and the muscle isoform of carnitine palmityl transferase 1 are important fatty acid transporter and key enzyme in mitochondrial fatty acid oxidation 23, 24, 25, 26, 27. Here we showed that TRPV1 activation by capsaicin significantly upregulated CD36 and carnitine palmityl transferase 1, which was also abolished by iRTX (Figure 2B). In contrast to fatty acid oxidation, glycolysis is reported to be inhibited by PGC-1α activation 28. We showed here that hexokinase type II enzyme, a critical enzyme in glycolytic process 29, was significantly decreased by capsaicin and restored by the presence of iRTX (Figure 2B). To evaluate the effects of capsaicin on mitochondrial biogenesis and respiration, we detected cytochrome C1 and surfeit 1, the subunits of complex III and IV 30 in myotubes. Capsaicin increased the expressions of these mitochondrial genes, which were inhibited by iRTX (Figure 2C). We further assessed the mitochondrial mass by staining cells with a mitochondrion-selective dye and showed that the mitochondrial content was increased in capsaicin-treated cells compared with the control (Figure 2D). Likewise, the ATP production in myotubes was elevated by capsaicin treatment (Figure 2E). The effects of capsaicin on mitochondrial biogenesis and ATP production were both markedly attenuated by iRTX and BAPTA (Figure 2D and 2E). These in vitro results suggest that TRPV1 activation by capsaicin increases the PGC-1α expression, mitochondrial biogenesis and ATP production in myotubes in a Ca2+-dependent manner.
Figure 2.
TRPV1 activation increases PGC-1α expression and mitochondrial biogenesis in a Ca2+-dependent manner. (A) Immunoblot of PGC-1α in C2C12 myotubes treated with capsaicin (100 nM) in the presence or absence of the TRPV1 inhibitor iRTX (1 μM) or the intracellular Ca2+ chelator BAPTA (10 μM). (B, C) Protein expression of genes involved in fatty acid oxidation, glycolysis (B) and mitochondrial respiration (C) in myotubes treated with capsaicin (100 nM) in the presence or absence of iRTX (1 μM). (D, E) Mitochondrial content (D) and ATP production (E) in myotubes. C2C12 cells were treated with capsaicin (100 nM) in the presence or absence of iRTX (1 μM) or BAPTA (10 μM) for 24 h. Data are means ± S.E.M. for three independent experiments. Con, control; Cap, capsaicin. *P < 0.05, **P < 0.01 vs Con. #P < 0.05, ##P < 0.01 vs Cap.
Activation of TRPV1 by dietary capsaicin upregulates PGC-1α and improves mitochondrial function and muscle remodeling
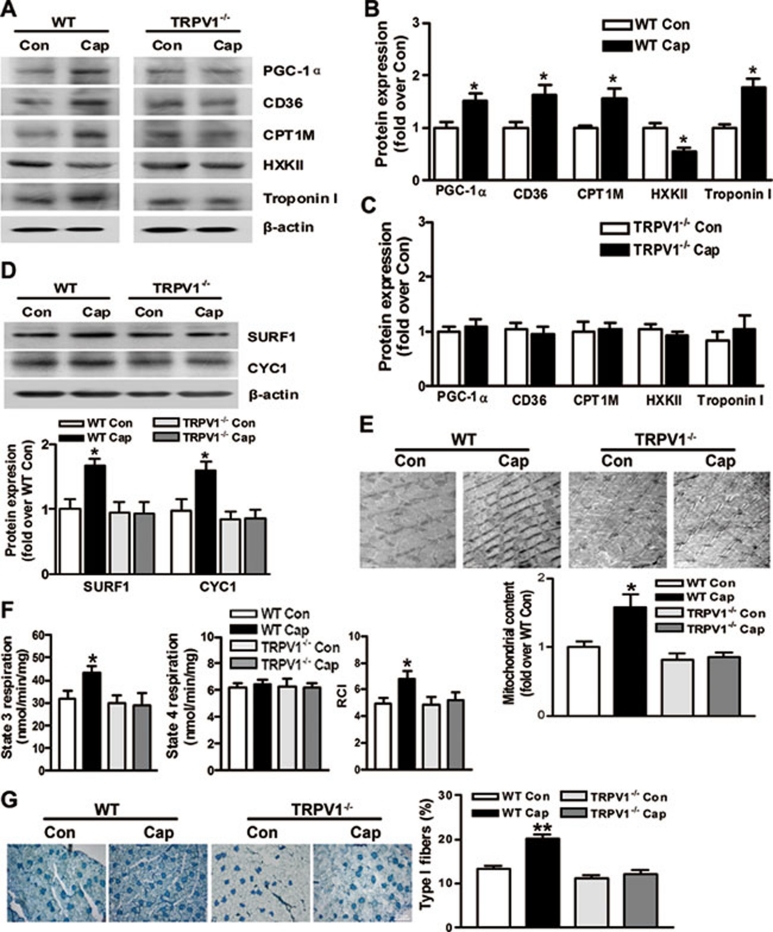
WT and TRPV1−/− mice were given either a regular chow diet or a diet containing capsaicin for 4 months. In agreement with the in vitro experiments, administration of capsaicin significantly increased the expression of PGC-1α, CD36 and carnitine palmityl transferase 1, but reduced the hexokinase type II enzyme expression in skeletal muscles (Figure 3A and 3B). The mitochondrial respiratory complex III and IV subunits and troponin I, the characteristic protein for oxidative slow fibers, were increased in mice on capsaicin diet compared to control mice (Figure 3A, 3B and 3D). By contrast, the effects of capsaicin consumption on mitochondrial respiratory complexes and troponin were absent in TRPV1−/− mice (Figure 3A-3D). The mitochondrial content in skeletal muscles was increased by dietary capsaicin in WT mice, but not in TRPV1−/− mice (Figure 3E and Supplementary information, Figure S1). The respiratory control index was markedly increased resulting from enhanced state-3 respiration and unchanged state-4 respiration in isolated mitochondria from WT mice on capsaicin diet, which was not observed in TRPV1−/− mice (Figure 3F). Skeletal muscle fiber types are partly determined by mitochondrial content, oxidative respiratory capacity and the balance between lipid oxidation and glycolysis 3, 31. We then identified the fiber types in gastrocnemius muscles and showed that there were more Type I (oxidative) fibers and fewer Type II (glycolytic) fibers in capsaicin-treated mice compared to control mice (Figure 3G). There was no difference in fiber type percentages between TRPV1−/− mice with and without dietary capsaicin consumption (Figure 3G). These results indicate that chronic activation of TRPV1 with dietary capsaicin upregulates PGC-1α, promotes mitochondrial biogenesis and oxidative respiratory capacity, and consequently improves skeletal muscle fiber transformation.
Figure 3.
Activation of TRPV1 by dietary capsaicin up-regulates PGC-1α and improves mitochondrial function and muscle remodeling. (A-D) Protein expression of genes involved in fatty acid oxidation, glycolysis, fiber specification (A-C) and mitochondrial respiration (D) in skeletal muscles from WT and TRPV1−/− mice fed a regular diet (Con) or a capsaicin diet (Cap). (E) Mitochondrial mass in gastrocnemius muscle shown by transmission electron microscopy. (F) State 3 and state 4 respiration and respiratory control index (RCI) in mitochondria isolated from fresh quadriceps femoris muscles. (G) Percentage of type I fibers in gastrocnemius muscle shown by the metachromatic staining. Oxidative (Type I) fibers were stained dark blue. Data are means ± S.E.M. n = 3-8. *P < 0.05, **P < 0.01 vs WT.
Dietary capsaicin enhances exercise endurance and reduces blood lactic acid and triglycerides through TRPV1 activation
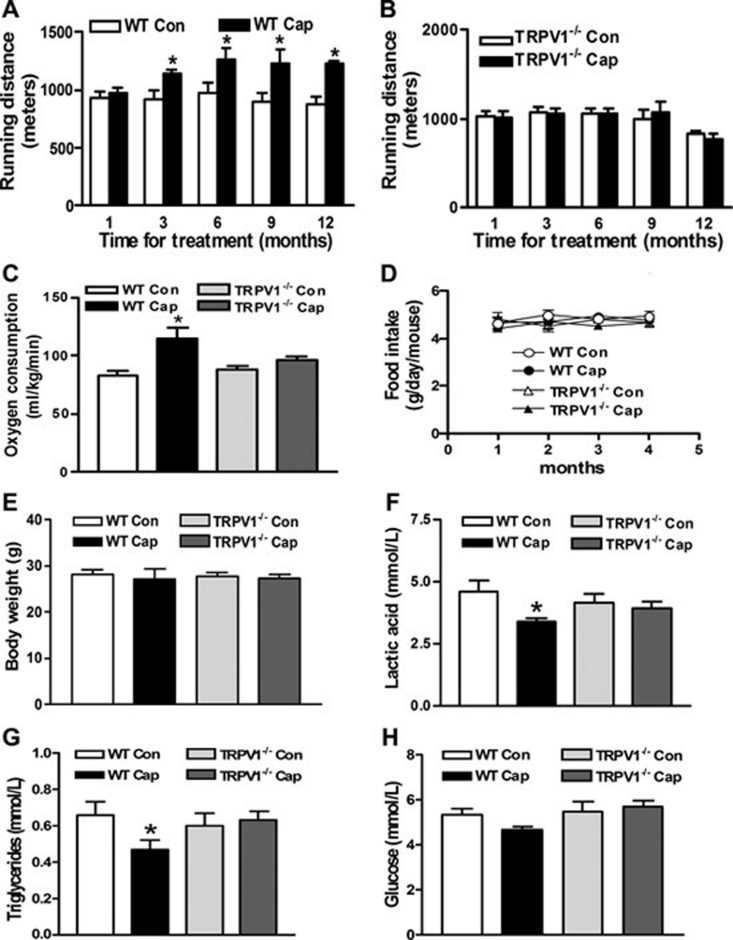
We further examined whether dietary capsaicin could improve exercise endurance and the related biochemical parameters through TRPV1 activation. Running endurance was performed with treadmill tests and oxygen consumption was measured in vivo. After 3-month capsaicin treatment, mice ran about 300 meters more before reaching exhaustion than the control (Figure 4A). Similar results were obtained for WT mice treated with capsaicin for 6, 9 and 12 months (Figure 4A). The oxygen consumption was also increased by capsaicin treatment in WT mice (Figure 4C). By contrast, administration of capsaicin did not affect exercise endurance and oxygen consumption in TRPV1−/− mice (Figure 4B and 4C). The daily food intake per month indicated that all mice tolerated the dietary capsaicin well (Figure 4D). The body weights were similar among all the groups after 4-month treatment (Figure 4E). Blood lactic acid, a product of glycolysis, is one of the key determinants for endurance performance 31. Capsaicin treatment significantly lowered the blood lactic acid and triglyceride levels in WT mice, but not in TRPV1−/− mice (Figure 4F and 4G). The blood glucose also showed a reducing tendency in capsaicin-treated mice (Figure 4H). These results support the notion that activation of TRPV1 by chronic dietary capsaicin enhances exercise endurance and improves systemic metabolic status.
Figure 4.
Dietary capsaicin enhances exercise endurance and reduces blood lactic acid and triglycerides through TRPV1 activation. (A, B) Exercise endurance test with treadmill exercise. WT (A) and TRPV1−/− mice (B) on a regular diet (Con) and a capsaicin diet (Cap) for indicated months were tested. Running distance before reaching exhaustion was recorded. Data are presented as the means ± S.E.M. for 6-10 mice. *P < 0.05 vs Con. (C) Oxygen consumption (ml/kg/min) examined when mice ran for 30 min at a speed of 10 m/min. (D) Average daily food intake (g/d) per mouse determined during the last week of each month. (E-H) Body weight and blood levels of lactic acid, triglycerides and glucose. Mice were treated with control or capsaicin diet for 4 months. Data are means ± S.E.M. for 5-9 mice. *P < 0.05 vs Con.
Transgenic TRPV1 gene increases PGC-1α expression, oxidative fibers and exercise endurance
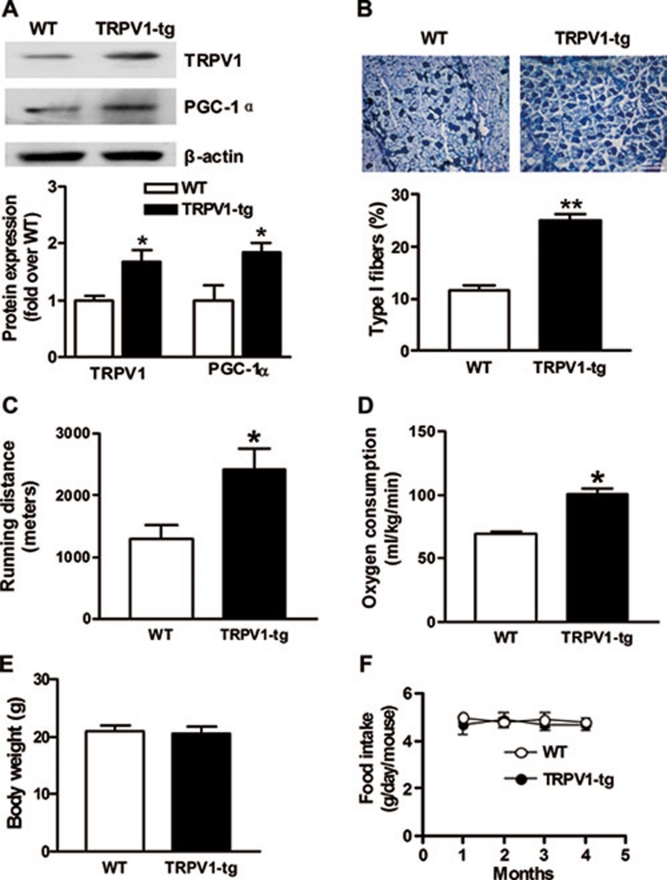
We then employed TRPV1-transgenic mice (TRPV1-tg) to further investigate whether TRPV1 mediated the increase of PGC-1α expression, oxidative fibers and exercise endurance. Compared with WT littermates, TRPV1-tg mice had significantly higher expression of TRPV1 and PGC-1α in skeletal muscles (Figure 5A). The mitochondrial content (Supplementary information, Figure S2) and the percentage of oxidative type I fibers (Figure 5B) in skeletal muscles were markedly increased in TRPV1-tg mice compared with WT littermates. Importantly, TRPV1-tg mice showed an impressive exercise performance. TRPV1-tg mice ran almost twice the distance of their WT littermates (Figure 5C). Likewise, the oxygen consumption of TRPV1-tg mice was higher than that of WT littermates (Figure 5D). However, the body weight and daily food intake did not differ between the two groups (Figure 5E and 5F). These results suggest that genetic TRPV1 overexpression produces similar metabolic changes as in capsaicin-treated mice.
Figure 5.
Transgenic TRPV1 gene increases PGC-1α expression, oxidative fibers and exercise endurance. (A) Immunoblot of TRPV1 and PGC-1α in skeletal muscle from TRPV1-tg and their wild-type littermates (WT). *P < 0.05, **P < 0.01 vs WT. (B) Metachromatic staining of gastrocnemius muscle. Type I fibers were stained dark blue. (C) Exercise endurance, (D) oxygen consumption, (E) body weight and (F) average daily food intake in TRPV1 transgenic mice and their WT littermates. *P < 0.05, **P < 0.01 vs WT. Mice were fed a regular diet until they reached 6 months of age. Data are means ± S.E.M. for 3 mice.
TRPV1 activation restores the high-fat diet (HFD)-induced PGC-1α downregulation, endurance impairment and metabolic disturbance
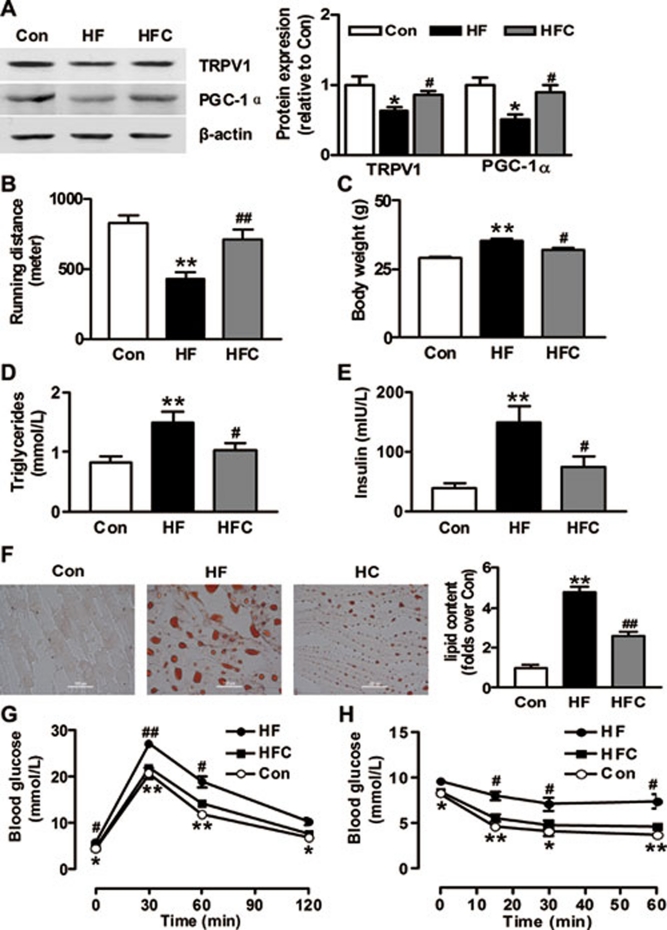
To clarify whether dietary capsaicin prevents metabolic disorders induced by HFD, mice on HFD were treated with capsaicin for 4 months. Compared with control mice, mice on HFD had lower expression of TRPV1 and PGC-1α (Figure 6A), mitochondrial content (Supplementary information, Figure S3) and percentage of type I fibers (Supplementary information, Figure S4) in skeletal muscles, which were significantly restored by dietary capsaicin. As a result, mice on HFD had a severely impaired endurance capacity, which was largely prevented by dietary capsaicin (Figure 6B). In addition, capsaicin treatment ameliorated the HFD-induced obesity, hyperlipidemia and hyperinsulinemia (Figure 6C-6E). It was noted that the skeletal muscles of mice on HFD contain much more lipids than those of control mice, but the lipid content in skeletal muscles was markedly reduced in mice on HFD plus capsaicin (Figure 6F). The intra-peritoneal glucose and insulin tolerance tests showed that capsaicin treatment significantly attenuated HFD-induced glucose intolerance and insulin resistance (Figure 6G and 6H). The present results suggest that HFD-induced endurance impairment and metabolic disorders can be prevented by dietary capsaicin consumption through TRPV1-mediated PGC-1α upregulation.
Figure 6.
TRPV1 activation restores the HFD-induced PGC-1α downregulation, endurance impairment and metabolic disorders. (A) Immunoblot of TRPV1 and PGC-1α in skeletal muscle from WT mice on normal diet (Con) and HFD without (HF) or with capsaicin supplementation (HFC). (B-E) Running endurance (B), body weight (C) and blood levels of triglycerides (D) and insulin (E) in mice. (F) Lipid contents of quadriceps femoris muscles shown by Oil-red O staining. *P < 0.05, **P < 0.01 vs Con; #P < 0.05, ##P < 0.01 vs HF. (G, H) Intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test (IPITT). *P < 0.05, **P < 0.01 vs HF; #P < 0.05, ##P < 0.01 vs HFC. Data are means ± S.E.M. for three to six mice.
Discussion
Overwhelming evidence suggests that cardiometabolic diseases are closely associated with a lack of exercise and unhealthy eating habits. Recent studies show that aerobic exercise capacity inversely correlates with cardiovascular disease and all causes of mortality across populations 32, 33, 34. Therefore, exercise and dietary interventions are highly recommended for the prevention of cardiometabolic diseases. Major findings of the present study show that dietary capsaicin consumption improves exercise endurance and energy metabolism through TRPV1-mediated PGC-1α upregulation in skeletal muscles.
Most previous studies focused on TRPV1 channel properties and their neuronal effects. However, TRPV1 has recently been reported to play different roles in many types of cells and tissues. Besides the involvement of TRPV1 in adipogenesis and vascular relaxation 11, 12, TRPV1 in neurons and smooth muscle plays opposite functional roles in the tissue-specific regulation of microvascular diameter 35. The present study reveals for the first time that chronic TRPV1 activation induces PGC-1α expression and improves mitochondrial function in a Ca2+-dependent manner in the skeletal muscle. TRPV1 is expressed in both the sarcoplasmic reticulum and plasma membrane of mouse myotubes, which is in agreement with a previous report on rat skeletal muscle 10. Ca2+ permeability is a basic property of TRPV1 channels. Capsaicin, a highly lipophilic TRPV1 agonist, is reported to exert its effects through TRPV1-mediated Ca2+ mobilization 9. The present study shows that capsaicin induced a significant increase of [Ca2+]i in myotubes, which was blocked by the specific TRPV1 antagonist iRTX. Ca2+- and calmodulin-dependent protein kinase can activate the PGC-1α gene promoter and induce PGC-1α expression, thus contributing to mitochondrial biogenesis in myocytes 18, 21, 36. We show that capsaicin exposure resulted in a significant increase in PGC-1α expression, mitochondrial biogenesis and ATP production in cultured myotubes, which can be abolished by inhibition of TRPV1 or depletion of intracellular Ca2+. It is likely that the TRPV1-mediated Ca2+ increase is critical for the capsaicin-induced PGC-1α expression in myotubes.
Several earlier studies showed that a single dose of capsaicin temporarily promoted oxygen consumption and lipid utilization and enhanced swimming capability in rodents, which was associated with sympathetic activation 13, 14. However, it is unclear whether chronic capsaicin consumption could enhance exercise endurance on a more permanent basis, whether TRPV1 in skeletal muscle is involved in these long-term changes and what the mechanisms might be. The present study shows that chronic dietary capsaicin continuously improved exercise endurance with no effect on food intake. We provide some mechanisms through which capsaicin treatment improves exercise endurance. First, the in vitro studies on cultured myotubes showed that capsaicin induced PGC-1α expression, mitochondrial biogenesis and ATP production via TRPV1-mediated Ca2+ signals. Second, dietary capsaicin administration in mice increased the expression of TRPV1 and PGC-1α in skeletal muscles, and consequently upregulated PGC-1α target genes involved in fatty acid oxidation and mitochondrial respiration, and downregulated the genes involved in glycolysis. Third, dietary capsaicin increased the mitochondrial mass and respiratory function, and promoted a fiber transformation from glycolytic type II fibers to more oxidative type I fibers. It is known that type I fibers contain more mitochondria and produce more ATP from lipid oxidation but less from glycolysis compared to type II fibers, thus providing stable energy for a longer time with less lactic acid accumulation 3, 31, 37.
Muscle fiber specification and mitochondrial function have also been associated with metabolic diseases in addition to exercise performance. Obese and diabetic patients have reduced oxidative capacity, increased glycolytic capacity and a decreased percentage of type I fibers in muscles 38, 39. Increasing the percentage of oxidative fibers can lead to improved insulin action and reduced adipocyte size 3, 4, 5. We showed that HFD not only led to various metabolic disorders but also led to downregulation of PGC-1α and reduced percentage of type 1 fibers, which is consistent with previous studies by others 30. PGC-1α downregulation was supposed to contribute to both the progression of muscle dysfunction and metabolic disorders. Increased expression of PGC-1α results in increased mitochondrial function and oxidative muscle fibers and protects from metabolic disease during aging 40, 41. The present study shows that with improved endurance capacity, dietary capsaicin also ameliorated the HFD-induced obesity, insulin resistance and glucose and lipid disorders. Both of the benefits of capsaicin on exercise endurance and energy metabolism might be attributed to the restoration of mitochondrial function and lipid oxidative capacity via TRPV1-mediated PGC-1α upregulation in skeletal muscles.
In addition, we showed that the TRPV1 is upregulated by capsaicin treatment in vitro and in vivo. We also found that exercise (Supplementary information, Figure S5) and HFD affect the protein expression of TRPV1 in muscles. It is known that TRPV1 can be activated by heat, protons and several endogenous lipid molecules, such as anandamide and eicosanoids 42. Anandamide is the most abundant endogenous endocannabinoid and regulates systemic energy metabolism 43. Anandamide in skeletal muscles participates in the regulation of TRPV1 function 44, 45. We showed that anandamide treatment significantly increased TRPV1 expression (Supplementary information, Figure S6). Thus, it is possible that some endogenous lipid signals could trigger TRPV1 in the context of muscle biology. However, this needs further investigation.
In conclusion, TRPV1 activation by dietary capsaicin improves energy metabolism and exercise endurance, which are likely driven by TRPV1-mediated Ca2+-dependent upregulation of PGC-1α and its target genes involved in the mitochondrial respiration and fatty acid oxidation. The present study reveals a new role of TRPV1 in skeletal muscle function and energy metabolism. Our novel findings also suggest that dietary capsaicin supplementation can be a promising lifestyle modification strategy to improve skeletal muscle function and prevent cardiometabolic diseases.
Materials and Methods
Cell culture
The mouse skeletal muscle cell line C2C12 (ATCC, Manassas, VA, USA) was propagated in Dulbecco's modified Eagle's medium-high glucose supplemented with 10% fetal bovine serum. C2C12 myoblasts were cultured for 48 h to reach confluence, and then the growth medium was changed to differentiation medium (Dulbecco's modified Eagle's medium supplemented with 2% horse serum) to induce myogenic differentiation. Experiments were performed after 2 days in the differentiating medium. Dulbecco's modified Eagle's medium, fetal bovine serum and horse serum were purchased from Gibco Co. (Grand Island, NY, USA). Chemicals including capsaicin, iRTX and BAPTA were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
TRPV1 gene silencing
TRPV1 was selectively silenced with RNAi using a lentiviral system according to a previously published technique 11. DNA templates for synthesizing silencing RNA were cloned into an expression plasmid for subsequent transfection. The coding sequence for TRPV1-targeting mRNA was selected using the siRNA Target Finder and Design Tool from Ambion. The potential target sequence was subjected to a BLAST search against mouse EST libraries to ensure the specificity of the target. Two oligonucleotides were synthesized:
M547-569 construct, sense, 5′-TGACAGATAGCCTGAAGCAGTTCAAGAGACTGCTTCAGGCTATCTGTCTTTTTTC-3′, antisense, 5′-TCGAGAAAAAAGACAGATAGCCTGAAGCAGTCTCTTGAACTGCTTCAGGCTATCTGTCA-3′
M1294-1316 construct, sense, 5′-TGCGCATCTTCTACTTCAACTTCAAGAGAGTTGAAGTAGAAGATGCGCTTTTTTC-3′, antisense, 5′-TCGAGAAAAAAGCGCATCTTCTACTTCAACTCTCTTGAAGTTGAAGTAGAAGATGCGCA-3′.
C2C12 myoblasts were transfected using Fugene reagent (Roche). Total protein was collected from the RNAi-transfected and empty control cells to identify the silencing of TRPV1 by immunoblotting. RNAi-transfected and control C2C12 cells were cultured and differentiated to 2-day myotubes for the in vitro study.
Immunoblot analysis
Cells were treated with capsaicin (100 nM) in the presence or absence of iRTX (1 μM) or BAPTA (10 μM) for 24 h before total protein was extracted. Tissues were homogenized and cells were lysed in high-salt extraction buffer (0.5 mol/l Tris, 1% NP-40, 1% Triton X-100, 1 g/l sodium dodecyl sulfate, 1.5 mol/l NaCl, 0.2 mol/l EDTA, 0.01 mol/l EGTA) and 0.2 mmol/l protease inhibitor, placed at −20 °C for 20 min and centrifuged at 12 000 × g at 4 °C for 20 min to remove insoluble material. Protein concentration was determined using a DC protein assay kit (Bio-Rad, Hercules, CA, USA). A total of 50-μg portions of the protein were resolved on SDS-polyacrylamide gels and electroblotted onto polyvinylidene difluoride membranes. After transfer, the membranes were blocked for 4 h at room temperature in blocking buffer (Bio-Rad). Next, the membranes were incubated overnight at 4 °C with antibodies for TRPV1 (Alamone, Israel), PGC-1α(Cell signaling, USA), CD36, carnitine palmityl transferase 1, hexokinase type II enzyme, surfeit 1, cytochrome C1 and β-actin (Santa Cruz, USA). After incubation with secondary antibodies (ZSGB-BIO, China) at room temperature for 2 h, the proteins were detected with enhanced chemiluminescence and quantified using a Gel Doc 2000 Imager (Bio-Rad). Protein expression was normalized to the internal control β-actin.
Immunofluorescence
C2C12 myotubes or cryosectioned muscle tissues were fixed with 10% formalin at room temperature for 60 min, and then bathed in 2% hydrogen peroxide methanol solution for 30 min. The cells or tissues were washed with water, then blocked with 5% bovine serum albumin for 20 min and incubated with TRPV1-specific antibodies (Alamone) overnight at 4 °C. The cells or tissues were then washed three times and incubated with fluorescent dye-labeled secondary antibodies (ZSGB-BIO) at room temperature for 30 min. Control experiments were performed in the absence of primary antibodies. Images were obtained with a TE2000-U Nikon eclipse microscope and analyzed with NIS-Elements imaging software (Nikon, Japan).
Intracellular free calcium imaging
[Ca2+]i was measured with Fura-2, as described in a previous publication 11. C2C12 myotubes grown on glass cover slips were loaded with Fura-2 (2 μmol/l) and 0.025% Pluronic F-127 in physiological saline solution containing 135 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 11 mM D-glucose and 10 mM HEPES, pH 7.4, for 40 min at room temperature in the dark. The myotubes were then washed three times with physiological saline solution. Individual cells were defined as the region of interest, and fluorescence was measured using the PTI Fluorescence Master Systems (Photon Technology International, Birmingham, NJ, USA). Fluorescence was measured at 510 nm emission, with excitation wavelengths of 340 nm and 380 nm, at baseline and after stimulation with capsaicin with or without pretreatment with iRTX (1 μM) for 5 min. Changes in [Ca2+]i were deduced from the ratios of transient increases in fluorescence intensity at 340 nm and 380 nm.
Mitochondrial staining
Mitochondria in cultured myotubes were stained with a mitochondrial probe, MitoFluor Red 589, which was reported to accumulate in mitochondria regardless of mitochondrial membrane potential, according to the manufacturer's protocol and previously reported methods 46. Briefly, C2C12 myotubes were incubated in the prewarmed (37 °C) growth medium containing 200 nM MitoFluor Red 589 for 30 min, and then washed with prewarmed PBS. Images were obtained with a TE2000-U Nikon eclipse microscope (Nikon).
Assessment of ATP production
ATP contents in C2C12 myotubes were measured using the ATP Bioluminescence Assay kit (Genmed Scientifics Inc., Shanghai, China) according to the manufacturer's protocol. The cells were kept on ice until assayed for luminescence intensity with Varioskan Flash Type 3001 (Thermo electron Co., Waltham, MA, USA). The values of luminescence intensity were normalized to the protein concentrations.
Generation of TRPV1 transgenic mice
The transgenic plasmid pcDNA3.1-rTRPV1 was generously provided by Ramon Latorre (Laboratory of Biophysics and Molecular Physiology, Chile). It was generated by cloning a cDNA fragment that encodes the open reading frame of rat TRPV1 into Kpn-Not sites of pcDNA3.1. The 8.3-kb Pvu fragment of pcDNA3.1-rTRPV1 was then isolated and injected into the pronucleus of fertilized oocytes from C57BL/6J × CBA F1 mice, and the oocytes were implanted into pseudopregnant females. A total of 400 pronuclear injections were performed, resulting in the generation of 120 pups. Tail DNA PCR screening was performed to identify founder mice that harbored the integrated transgene. A screening of the 120 pups identified five pups as founders. This job was completed by the Shanghai Research Center for Biomodel Organism. To maintain an isogenic strain, heterozygous transgenic mice were propagated through breeding with wild-type C57BL/6J mice for two generations. Transgenic mice were identified among the offspring using tail DNA PCR screening. These transgenic mice and their non-transgenic littermates were all fed with a standard laboratory chow. Exercise endurance and oxygen consumption were measured when the mice were 6-month old.
To screen transgenic mice, genomic DNA was purified from tails of 2-week-old mice and used for PCR with the following primers: 5′-GCGTGGATAGCGGTTTGA-3′ 5′-CGACTCCTGGATGTGAAGATG-3′. The tail DNA of TRPV1 transgenic mice produced a PCR product of 538 bp. TRPV1 protein expression was determined by western blot analysis when the mice were sacrificed at 6-month old.
Animal care
The C57BL/6J wild-type mice and TRPV1 knockout mice were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Both types of male mice aged 6-8 weeks were divided into two groups (n = 12, 4 mice/cage). All mice were housed in colony cages with a 12-h light/12-h dark cycle and allowed unlimited access to water and standard laboratory chow. Control animals were fed with this regular diet alone. Treated animals were fed with this diet supplemented with 0.01% capsaicin. Body weight was recorded every 10 days. The mean daily food intake was determined the last week of each month by subtracting the amount of food left uneaten each day from the amount provided the previous day. Food spillage into the cage was assessed and found to be negligible. Six mice from each group were kept on their respective diets for 4 months, and then sacrificed for blood and tissue examinations. The remaining six mice in each group were kept on their respective diets for 12 months for exercise endurance tests. The male C57BL/6J mice aged 6-8 weeks were also randomly divided into three groups, respectively, fed with regular chow diet, HFD and HFD plus 0.01% capsaicin for 4 months. Body weight, running endurance, glucose and insulin tolerance tests and blood parameters were measured. All the animals were cared for and studied according to institutional guidelines after approval from our Institutional Animal Care and Research Advisory Committee.
Exercise endurance tests
The mice engaged in strenuous exercise on a rodent treadmill (Columbus Instruments, OH, USA) and standard running tests to evaluate maximal running endurance. Prior to the exercise performance test, the mice were acclimated to the treadmill with a 5-min run at 7 m/min and a 5-min run at 10 m/min once daily for 2 days. The endurance test regimen was 10 m/min for the first 60 min, followed by 1 m/min increment increases at 15-min intervals according to the method described in a previous publication 3. The mice were considered exhausted when they were unable to avoid repeated electrical shocks. Exercise endurance capacity was equated with the total running distance achieved before exhaustion.
Oxygen consumption measurement
Mice were subjected to oxygen consumption measurements immediately after running for 30 min at 10 m/min. Oxygen consumption was measured with a respiration package (Qubit Systems Inc., Canada) consisting of an air pump, a manometer, a metabolic chamber, an oxygen analyzer and a data collecting system. A mouse was placed in the metabolic chamber, and room air was pumped through the chamber. Expired air went through a column filled with a drying agent before it entered the oxygen analyzer. The oxygen percentage data were collected at one point per second and analyzed with the Logger Pro 3.2 Software (Vernier LabPro., Oregon). Oxygen consumption (ml/min) per mouse was calculated as the starting oxygen percentage minus the oxygen percentage at platform multiplied by the air-flow rate (ml/min). The data are expressed as oxygen consumption per kilogram body weight (ml/kg/min).
Blood analysis
After fasting for 12 h and then running for 30 min at a speed of 10 m/min, the mice were immediately anesthetized, and blood was collected from the carotid arteries. Serum concentrations of glucose, triglycerides and lactic acid were determined within 24 h using commercially available assay kits (Applygen Technologies Inc., China). For the mice on HFD, the blood levels of insulin and triglycerides were determined after fasting for 12 h.
Intraperitoneal glucose tolerance test and intraperitoneal insulin tolerance test
Intraperitoneal glucose tolerance test was carried out in mice on HFD with or without capsaicin supplementation and mice on normal chow diet as previously described 47. After an overnight fast (14 h), glucose (2 g/kg body weight) was administered via injection into the peritoneal cavity, and blood was drawn from the tail vein at 0, 30, 60, and 120 min after glucose administration. Blood glucose levels were determined using the OneTouch Ultra blood glucose meter (LifeScan, CA, USA). Intraperitoneal insulin tolerance test was evaluated in fed mice on a different day. Humulin R (0.75 U/kg body weight) (Eli Lilly and Co., IN, USA) in sterile saline was administered intraperitoneally. Glucose levels were determined in the tail blood at 0, 15, 30 and 60 min after insulin injection.
Muscle fiber typing
The gastrocnemius muscle was immediately extracted and stored in liquid nitrogen. Muscle fiber typing was performed using metachromatic dye-ATPase methods, as described in a previous publication 48.
Muscular lipid contents determination
Lipid accumulation in muscles was assessed by Oil-Red O staining 49. Briefly, a stock solution was prepared by solubilizing 300 mg of oil red O dye (Sigma, USA) in 100 ml of concentrated isopropanol (99%). Cryosectioned muscle tissues were incubated at room temperature for 10 min in 12 ml of freshly prepared stock solution (filtered through a Whatmann paper) diluted in 8 ml of distilled water, and then washed with running water for 10 min. Images were obtained with a TE2000-U Nikon eclipse microscope and lipid droplet area was analyzed with NIS-Elements imaging software (Nikon).
Electron microscopic observation of mitochondria
Fresh skeletal muscles were cut into 1 mm cubes, which were fixed in 3% glutaraldehyde for 2 h, and then fixed in 1% osmium tetroxide, stepwise dehydrated in graded acetone, and infiltrated, embedded and polymerized in EPON 812. The semi-thin sections were optically positioned and further sectioned with ultramicrotome into 50-60 nm pieces, which were collected on copper grids, double-stained with uranyl acetate and lead citrate and then observed under Hitachi H-600IV transmission electron microscope and photographed. Mitochondrial areas were calculated with NIS-Elements imaging software (Nikon).
Mitochondrial respiration measurement
Mitochondria were isolated from fresh quadriceps femoris muscles by using a mitochondria isolation kit (Genmed Scientifics Inc.). The respiratory activity of the mitochondria was potentiometrically measured at 27 C, in an oxygraph vessel with a Clark electrode (Yellow Springs Instruments, OH, USA). Mitochondria were incubated in a medium containing 75 mmol/l mannitol, 25 mmol/l sucrose, 100 mmol/l KCl, 50 μmol/l EDTA, 5 mmol/l K2HPO4 and 5 mmol/l Tris-HCl, pH 7.35. The substrates used were 6 mmol/l sodium pyruvate and 6 mmol/l sodium malate. State 3 oxygen consumption was measured in the presence of 0.6 mmol/l ADP and state 4 oxygen consumption in the absence of ADP. The values were normalized to mitochondrial protein contents. Respiratory control ratio was calculated from the value of state 4 relative to state 3.
Statistics
Data are expressed as the mean ± S.E.M. Statistical differences between groups were assessed by Student's t-test or one-way analysis of variance with Bonferroni's multiple comparison post hoc tests, as appropriate. Two-sided P-values below 0.05 were considered statistically significant.
Acknowledgments
We acknowledge Dr R Latorre (Laboratory of Biophysics and Molecular Physiology, Chile) for the gift of plasmid pcDNA3.1-rTRPV1. We thank Lijuan Wang (Chongqing Institute of Hypertension, China) for technical assistance and Wing Tak Wong (Chinese University of Hong Kong, China) for revision of the manuscript. This research was supported by grants from the 973 Program (2012CB517805 and 2011CB503902) and the National Natural Science Foundation (31000519 and 30890042).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Mitochondrial DNA (mitoDNA) content in skeletal muscles from WT and TRPV1−/− mice without (Con) or with (Cap) capsaicin administration.
Mitochondrial DNA (mitoDNA) content in skeletal muscles from TRPV1-transgenic (TRPV1-tg) mice and their wild-type littermates (WT).
Mitochondrial DNA (mitoDNA) content in skeletal muscles from WT mice fed with regular chow diet (Con), high-fat diet (HF) and HF plus capsaicin (HFC).
The percentage of type I fibers in gastrocnemius muscles from WT mice fed with regular chow diet (Con), high-fat diet (HF) and HF plus capsaicin (HFC).
TRPV1 protein expression in skeletal muscles of control mice (Control), mice deprived of food for 48 hours (Starvation) and mice subjected to treadmill running for continuous 5 days, 1 hour per day (Exercise).
TRPV1 protein expression in 3T3-L1 preadipocytes with or without 24-hours' anandamide (1 μM) treatment.
Supplementary methods
References
- Yung LM, Laher I, Yao X, Chen ZY, Huang Y, Leung FP. Exercise, vascular wall and cardiovascular diseases: an update (part 2) Sports Med. 2009;39:45–63. doi: 10.2165/00007256-200939010-00004. [DOI] [PubMed] [Google Scholar]
- Smith AG, Muscat GE. Skeletal muscle and nuclear hormone receptors: implications for cardiovascular and metabolic disease. Int J Biochem Cell Biol. 2005;37:2047–2063. doi: 10.1016/j.biocel.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder JW, Bassel-Duby R, Olson EN, Zierath JR. Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J Biol Chem. 2003;278:44298–44304. doi: 10.1074/jbc.M304510200. [DOI] [PubMed] [Google Scholar]
- Luquet S, Lopez-Soriano J, Holst D, et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17:2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Saris WH, Wagenmakers AJ. Fat metabolism during exercise: a review--part III: effects of nutritional interventions. Int J Sports Med. 1998;19:371–379. doi: 10.1055/s-2007-971932. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Caterina MJ. Vanilloid receptors take a TRP beyond the sensory afferent. Pain. 2003;105:5–9. doi: 10.1016/s0304-3959(03)00259-8. [DOI] [PubMed] [Google Scholar]
- Xin H, Tanaka H, Yamaguchi M, Takemori S, Nakamura A, Kohama K. Vanilloid receptor expressed in the sarcoplasmic reticulum of rat skeletal muscle. Biochem Biophys Res Commun. 2005;332:756–762. doi: 10.1016/j.bbrc.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Yan Liu D, Ma LQ, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- Yang D, Luo Z, Ma S, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010;12:130–141. doi: 10.1016/j.cmet.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Kawada T, Ishihara K, Inoue K, Fushiki T. Increase in swimming endurance capacity of mice by capsaicin-induced adrenal catecholamine secretion. Biosci Biotechnol Biochem. 1997;61:1718–1723. doi: 10.1271/bbb.61.1718. [DOI] [PubMed] [Google Scholar]
- Oh TW, Ohta F. Dose-dependent effect of capsaicin on endurance capacity in rats. Br J Nutr. 2003;90:515–520. doi: 10.1079/bjn2003919. [DOI] [PubMed] [Google Scholar]
- Shin KO, Moritani T. Alterations of autonomic nervous activity and energy metabolism by capsaicin ingestion during aerobic exercise in healthy men. J Nutr Sci Vitaminol (Tokyo) 2007;53:124–132. doi: 10.3177/jnsv.53.124. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Kusuhara K, Madsen K, Jensen L, Hellsten Y, Pilegaard H. Calcium signalling in the regulation of PGC-1alpha, PDK4 and HKII mRNA expression. Biol Chem. 2007;388:481–488. doi: 10.1515/BC.2007.052. [DOI] [PubMed] [Google Scholar]
- Guerfali I, Manissolle C, Durieux AC, Bonnefoy R, Bartegi A, Freyssenet D. Calcineurin A and CaMKIV transactivate PGC-1alpha promoter, but differentially regulate cytochrome C promoter in rat skeletal muscle. Pflugers Arch. 2007;454:297–305. doi: 10.1007/s00424-007-0206-6. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Hoy AJ, Turner N, et al. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway GP, Luiken JJ, Glatz JF, Spriet LL, Bonen A. Contribution of FAT/CD36 to the regulation of skeletal muscle fatty acid oxidation: an overview. Acta Physiol (Oxf) 2008;194:293–309. doi: 10.1111/j.1748-1716.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- Burgess SC, He T, Yan Z, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SC, Hausler N, Merritt M, et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem. 2004;279:48941–48949. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- Hakimi P, Yang J, Casadesus G, et al. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem. 2007;282:32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende AR, Schaeffer PJ, Parker GJ, et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J Biol Chem. 2007;282:36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- Ahn KJ, Hwang HS, Park JH, et al. Evaluation of the role of hexokinase type II in cellular proliferation and apoptosis using human hepatocellular carcinoma cell lines. J Nucl Med. 2009;50:1525–1532. doi: 10.2967/jnumed.108.060780. [DOI] [PubMed] [Google Scholar]
- Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. 2008;586:35–44. doi: 10.1113/jphysiol.2007.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- Kokkinos P, Myers J, Kokkinos JP, et al. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328:533–537. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- Kark T, Bagi Z, Lizanecz E, et al. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol. 2008;73:1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Inagaki K, Noguchi T, et al. Identification and characterization of an alternative promoter of the human PGC-1alpha gene. Biochem Biophys Res Commun. 2009;381:537–543. doi: 10.1016/j.bbrc.2009.02.077. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Saris WH, Wagenmakers AJ. Fat metabolism during exercise: a review--part II: regulation of metabolism and the effects of training. Int J Sports Med. 1998;19:293–302. doi: 10.1055/s-2007-971921. [DOI] [PubMed] [Google Scholar]
- Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- Hickey MS, Carey JO, Azevedo JL, et al. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol. 1995;268:E453–457. doi: 10.1152/ajpendo.1995.268.3.E453. [DOI] [PubMed] [Google Scholar]
- Calvo JA, Daniels TG, Wang X, et al. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Suh YG, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr Pharm Des. 2005;11:2687–2698. doi: 10.2174/1381612054546789. [DOI] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D, Liu J, Godlewski G, Batkai S. Endocannabinoids and the control of energy homeostasis. J Biol Chem. 2008;283:33021–33025. doi: 10.1074/jbc.R800012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavuoto P, McAinch AJ, Hatzinikolas G, Janovska A, Game P, Wittert GA. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem Biophys Res Commun. 2007;364:105–110. doi: 10.1016/j.bbrc.2007.09.099. [DOI] [PubMed] [Google Scholar]
- Lizanecz E, Bagi Z, Pasztor ET, et al. Phosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1. Mol Pharmacol. 2006;69:1015–1023. doi: 10.1124/mol.105.015644. [DOI] [PubMed] [Google Scholar]
- Diwan A, Matkovich SJ, Yuan QY, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Ogilvie RW, Feeback DL. A metachromatic dye-ATPase method for the simultaneous identification of skeletal muscle fiber types I, IIA, IIB and IIC. Stain Technol. 1990;65:231–241. doi: 10.3109/10520299009105613. [DOI] [PubMed] [Google Scholar]
- Lee S, Guerra N, Arslanian S. Skeletal muscle lipid content (SMLC) in black and white over-weight youth: an independent risk factor of insulin resistance. Diabetes. 2008;57:A493–A494. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mitochondrial DNA (mitoDNA) content in skeletal muscles from WT and TRPV1−/− mice without (Con) or with (Cap) capsaicin administration.
Mitochondrial DNA (mitoDNA) content in skeletal muscles from TRPV1-transgenic (TRPV1-tg) mice and their wild-type littermates (WT).
Mitochondrial DNA (mitoDNA) content in skeletal muscles from WT mice fed with regular chow diet (Con), high-fat diet (HF) and HF plus capsaicin (HFC).
The percentage of type I fibers in gastrocnemius muscles from WT mice fed with regular chow diet (Con), high-fat diet (HF) and HF plus capsaicin (HFC).
TRPV1 protein expression in skeletal muscles of control mice (Control), mice deprived of food for 48 hours (Starvation) and mice subjected to treadmill running for continuous 5 days, 1 hour per day (Exercise).
TRPV1 protein expression in 3T3-L1 preadipocytes with or without 24-hours' anandamide (1 μM) treatment.
Supplementary methods