There have been indications in the past that PKM2 might play a role in cellular growth stimulation and metabolic events. For example, PKM2 is phosphorylated on tyrosines in response to Rous sarcoma virus infection 1; PKM2 is found not only in the cytoplasm but also in the nucleus, where it is associated with chromatin 2, 3; PKM2 has been reported to exhibit cysteine-dependent histone H1 phosphorylation activity 4; and PKM2 plays a role in apoptosis mediated by somatostatin analogues 5. The Cantley laboratory has shown that the M2 splice isoform of pyruvate kinase is essential for supporting tumor growth 6, 7. They also showed that the PKM2 isoform specifically binds phosphotyrosine peptides that displace the allosteric activator fructose 1,6 of PKM2, causing channeling of accumulated glucose metabolites into biosynthetic intermediates to support anabolic growth. Although it seems counterintuitive that a reduced rate of glycolysis would be required for enhanced cell growth, the authors also showed that the ability to reduce PK activity through interaction with phosphotyrosyl (p-Tyr) peptides enhanced cell growth rates. In addition, PKM2 can itself be phosphorylated on Y105 in response to growth factors as part of an inhibitory mechanism that promotes tumor growth 8. This work has established PKM2 as a pivotal factor regulating the flux between the glycolytic and pentose phosphate pathways. Moreover, a recent publication from the Semenza laboratory indicated that PKM2 plays a transcriptional role in metabolic regulation. They reported that PKM2 associates with HIF1 to recruit the p300 transcriptional co-activator to HIF-responsive promoters, thereby enhancing the hypoxic transcriptional response 9.
Now, PKM2 is back in the spotlight as the subject of two recent reports describing new mechanisms by which PKM2 expression and activity contribute to tumor cell growth. A letter in Nature from Zhimin Lu's research group describes a role for PKM2 in transcriptional activation in response to epidermal growth factor (EGF). The authors report that PKM2 associates with p-Tyr peptides on β-catenin in response to EGF stimulation, and that this complex translocates to the nucleus and localizes to the cyclin D1 (CCND1) and c-MYC promoters 10. Significantly, PKM2 depletion abolishes almost all of the growth factor-dependent increase in proliferation. Translocation is mediated through the association of PKM2 with P-Tyr-β-catenin in a pathway that permits β-catenin binding to TCF-4 but not to other Wnt pathway factors. This PKM2-β-catenin complex causes the dissociation of HDAC3 from the CCND1 promoter and enhances cyclin D and c-MYC expression. Upregulation of c-MYC expression by PKM2 forms a feed-forward loop, since c-MYC has been shown to upregulate transcription of hetergenous nuclear ribonucleoproteins (hnRNP) that promote the alternative splicing of PKM2 over PKM1 11.
This article demonstrates that PKM2 can associate with p-Tyr peptides in β-catenin to drive c-Myc and CCND1 expression 10 in addition to the well-documented effect of PKM2 on the control of anabolic growth 6, 7. However, growth stimulation by PKM2 appears to be more subtly regulated than we could have ever imagined. The Cantley laboratory has now elegantly demonstrated that oxidative stress causes oxidation of a critical Cys358 side chain of PKM2 that results in reversible enzyme inactivation and enhanced channeling of glucose metabolites through the pentose phosphate pathway 12. This diversion serves to generate reducing potential in the form of NADPH that can be used to regenerate reduced glutathione and restore the redox balance of the cell. This mechanism of enhancing NADPH production is essential for tumor cell growth.
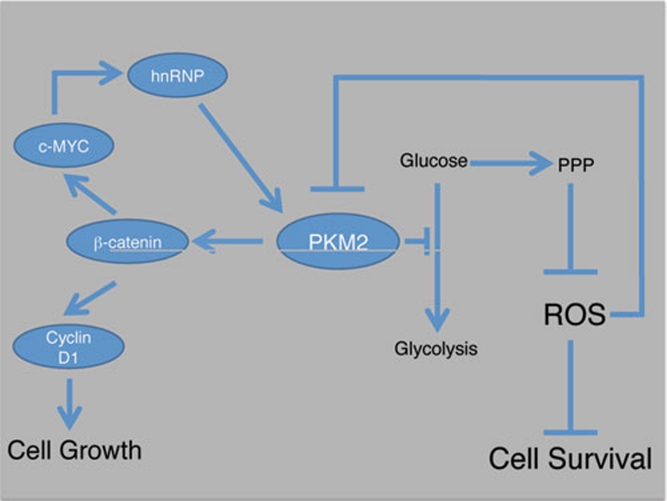
The emerging picture that we can infer from these two articles suggests that the availability of PKM2 able to associate with signaling proteins such as β-catenin in response to growth stimuli may limit the cellular response to growth factors. Whether the oxidized portion of the total PKM2 pool can affect this response by reducing PKM2 availability remains open to speculation. If so, high levels of oxidative stress could block the response to factors such as EGF by raising the level of oxidized PKM2 (Figure 1), thereby preventing highly stressed cells from responding to factors that would further tax their cellular redox management systems. Whether the oxidized form of PKM2 is capable of translocating to the nucleus and affecting transcriptional regulation is also not clear. However, an earlier report has indicated that peroxide treatment of cells does result in nuclear localization of PKM2 5. This finding also raises the question as to whether the several different post-translational modifications of PKM2 that have been reported, including tyrosyl phosphorylation 1, 8, prolyl hydroxylation 9, 13, acetylation 14, mono ubiquitination 15, sumoylation 2 and cysteine oxidation 12, are integrated to regulate PKM2's activation of transcriptional programmes 9, 10, 16 in response to cell signaling events.
Figure 1.
Pyruvate Kinase M2 (PKM2) plays a pivotal role in balancing oxidative stress and proliferation signals in response to growth factors. Growth factors stimulate glucose uptake, which is metabolized through the glycolytic and pentose phosphate pathways (PPP). High levels of ROS feed back to cause reversible inactivation of PKM2 by oxidation of Cys358. The low catalytic rate of PKM2 reduces flux through glycolysis, increasing the flux through the PPP and generating NADPH to quench oxidative stress. PKM2 associates with p-Tyr-β-catenin to increase transcription of c-MYC and cyclin D1, stimulating cell growth. C-MYC in turn stimulates expression of hnRNPs, which favors alternative splicing to generate the PKM2 isoform.
The take-home message of these two recent reports is that PKM2 plays a pivotal role in balancing growth and oxidative stress. While there is still much to learn about how PKM2 buffers the response to growth factor stimulation by providing a functional readout of the oxidative and metabolic state of the cell, the fact that growth factor responses and oxidative stress responses have both been linked to PKM2 leads one to speculate that this regulation is highly integrated. This mechanism is undoubtedly essential in embryonic tissues where PKM2 normally functions, as well as in cancer cells. These two reports suggest that we can look forward to more exciting discoveries of precisely how PKM2 drives cellular growth and survival.
References
- Presek P, Reinacher M, Eigenbrodt E. Pyruvate kinase type M2 is phosphorylated at tyrosine residues in cells transformed by Rous sarcoma virus. FEBS Lett. 1988;242:194–198. doi: 10.1016/0014-5793(88)81014-7. [DOI] [PubMed] [Google Scholar]
- Spoden GA, Morandell D, Ehehalt D, et al. The SUMO-E3 ligase PIAS3 targets pyruvate kinase M2. J Cell Biochem. 2009;107:293–302. doi: 10.1002/jcb.22125. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Hirst JA, Fujii H. Regulation of cell proliferation by interleukin3-induced nuclear translocation of pyruvate kinase. J Biol Chem. 2007;282:17706–17711. doi: 10.1074/jbc.M700094200. [DOI] [PubMed] [Google Scholar]
- Ignacak J, Stachurska MB. The dual activity of pyruvate kinase type M2 from chromatin extracts of neoplastic cells. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:425–433. doi: 10.1016/s1096-4959(02)00283-x. [DOI] [PubMed] [Google Scholar]
- Steták A, Veress R, Ovádi J, Csermely P, Kéri G, Ullrich A. Nuclear translocation of the tumor marker pyruvate kinase M2 induces programmed cell death. Cancer Res. 2007;67:1602–1608. doi: 10.1158/0008-5472.CAN-06-2870. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumor growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- Hitosugi T, Kang S, Vander Heiden MG, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxiainducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xia Y, Ji H, et al. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou D, Poulogiannis G, Asara JM, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Chen X, Ma J, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L, Li D, Zhao D, et al. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol Cell. 2011;42:719–730. doi: 10.1016/j.molcel.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Xu CW. Glucose metabolism induces mono-ubiquitination of histone H2B in mammalian cells. Biochem Biophys Res Commun. 2011;404:428–433. doi: 10.1016/j.bbrc.2010.11.138. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim HK, Han YM, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol. 2008;40:1043–1054. doi: 10.1016/j.biocel.2007.11.009. [DOI] [PubMed] [Google Scholar]